Abstract
Activation of enteric inhibitory motor neurons causes inhibitory junctional potentials (IJPs) and muscle relaxation in mammalian gastrointestinal (GI) muscles, including humans. IJPs in many GI muscles are bi-phasic with a fast initial hyperpolarization (fIJP) due to release of a purine neurotransmitter and a slower hyperpolarization component (sIJP) due to release of nitric oxide. We sought to characterize the nature of the post-junctional receptor(s) involved in transducing purinergic neural inputs in the murine colon using mice with genetically deactivated P2ry1. Wild-type mice had characteristic biphasic IJPs and pharmacological dissection confirmed that the fIJP was purinergic and the sIJP was nitrergic. The fIJP was completely absent in P2ry1−/− mice and the P2Y1 receptor antagonist MRS2500 had no effect on electrical activity or responses to electrical field stimulation of intrinsic nerves in these mice. Contractile experiments confirmed that purinergic responses were abolished in P2ry1−/− mice. Picospritzing of neurotransmitter candidates (ATP and its primary metabolite, ADP) and β-NAD (and its primary metabolite, ADP-ribose, ADPR) caused transient hyperpolarization responses in wild-type colons, but responses to β-NAD and ADPR were completely abolished in P2ry1−/− mice. Hyperpolarization and relaxation responses to ATP and ADP were retained in colons of P2ry1−/− mice. Video imaging revealed that transit of fecal pellets was significantly delayed in colons from P2ry1−/− mice. These data demonstrate the importance of purinergic neurotransmission in regulating colonic motility and confirm pharmacological experiments suggesting that purinergic neurotransmission is mediated via P2Y1 receptors.
Key points
Normal colonic motility is regulated by excitatory and inhibitory motor neurons, and previous studies have shown that both components of neural regulation are important for normal propulsion of colonic contents.
Inhibitory neural control consists of two main components, and the major neurotransmitters have been identified as nitric oxide and purines; we investigated the nature of the receptors responsible for purine inhibitory motor control of the colon using mice with P2Y1 receptors deactivated.
Inhibitory control of the colon by purine neurotransmitters was dramatically decreased in these animals and transit of fecal pellets was delayed.
Inhibitory responses to purine neurotransmission and exogenous β-NAD, a neurotransmitter candidate, were completely abolished in P2Y1 receptor knockouts.
These studies demonstrate the importance of purinergic neural regulation of colonic motility and suggest this form of neural regulation depends upon P2Y1 receptors to receive and transduce inhibitory neural signals.
Introduction
Movements of the gastrointestinal (GI) tract are regulated by enteric excitatory and inhibitory motor neurons (Burnstock et al. 1963; Bennett, 1966; Spencer & Smith, 2001). Studies over the past several decades have determined that inhibitory neurotransmission is accomplished by release of nitric oxide (Bult et al. 1990; Sanders & Ward, 1992), purine neurotransmitter(s), and peptides, such as vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) (Crist et al. 1992; Shuttleworth & Keef, 1995; Mutafova-Yambolieva et al. 2007; Gallego et al. 2008a). There is still considerable controversy about the identity of the purine(s) that serve as neurotransmitter(s). Traditionally, most investigators have relied upon the idea that adenosine 5′-triphosphate (ATP) is the enteric inhibitory neurotransmitter (Burnstock, 1972; Crist et al. 1992; Xue et al. 1999), but recent evidence suggests that other purines, such as β-nicotinamide adenine dinucleotide (β-NAD) and/or its metabolites are better candidates for the enteric inhibitory neurotransmitter (Mutafova-Yambolieva et al. 2007; Hwang et al. 2011; Durnin et al. 2012).
Post-junctional responses to enteric inhibitory neurotransmission are typically characterized by two-component inhibitory junction potentials (Keef et al. 1993; Shuttleworth et al. 1997; Spencer et al. 1998; Gallego et al. 2006; Mutafova-Yambolieva et al. 2007). The first component is a large-amplitude, fast hyperpolarization response (fIJP) that can transiently take membrane potential close to the equilibrium potential for K+; the kinetics of the second component are at least an order of magnitude slower (sIJP) and usually less negative potentials are achieved during sIJPs. Pharmacological studies have shown that fIJPs are reduced or blocked by apamin (Banks et al. 1979; Keef et al. 1993; Spencer et al. 1998) and therefore due to activation of small-conductance Ca2+-activated K+ channels (SK channels; considered to be a hallmark of purinergic responses in GI muscles; Koh et al. 1997; Vogalis & Goyal, 1997; Kurahashi et al. 2011), and the second component is blocked by inhibitors of neuronal nitric oxide synthase and therefore considered nitrergic (Daziel et al. 1991; Stark et al. 1991; Keef et al. 1993; Shuttleworth et al. 1997). Hyperpolarization due to purinergic activation of SK channels and fJPS is coupled to reduced open probability of voltage-dependent Ca2+ channels in GI muscles and relaxation (Sanders, 2008).
Post-junctional mechanisms responsible for purinergic regulation of GI motor activity have been studied recently in greater detail and recent application of pharmacological tools with improved specificity has allowed more precise conclusions to be made about the receptors involved in mediation of responses. The post-junctional response to purinergic neurotransmission is blocked by pharmacological antagonists of P2Y receptors or by desensitization of P2Y receptors with adenosine 5′-O-2-thiodiphosphate (ADPβS) (Xue et al. 1999; Serio et al. 2003). GI smooth muscles express P2Y1 and P2Y4 receptors (Gallego et al. 2006; Monaghan et al. 2006), and receptor antagonists designed for specificity toward P2Y1 receptors block fIJPs (Gallego et al. 2006, 2008b; Mutafova-Yambolieva et al. 2007; Wang et al. 2007; Grasa et al. 2009; Hwang et al. 2011). Thus, P2Y1 receptors have been suggested as the dominant receptor mediating purinergic inhibitory neurotransmission in mammalian GI muscles. However, conclusions based purely on pharmacological tools always require further scrutiny, and this is particularly true for purinergic antagonists.
We investigated the receptors responsible for purinergic motor control using a murine model with genetic deactivation of P2ry1 (Fabre et al. 1999). Our findings support the hypothesis that P2Y1 receptors play a central role in purinergic neural regulation in the gut and validate the specificity of antagonism by MRS2500, as used in studies of enteric inhibitory neurotransmission. The results also confirm that β-NAD and ADPR mimic the inhibitory enteric neurotransmitter in the murine colon. Our data also provide further evidence that if ATP (or its metabolites) function as purinergic neurotransmitters, then these agonists cannot be released ‘in volume’ into the interstitial spaces surrounding smooth muscle cells. If this were the case, then receptors other than P2Y1 receptors would be bound and contribute to enteric inhibitory responses.
Methods
Animals
B6.129P2-P2ry1tm1Bhk/J male and female homozygotes (Fabre et al. 1999) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and mated to produce B6.129P2-P2ry1tm1Bhk/J offspring (P2ry1−/− mice). The animals were used for experiments at postnatal day (P)30 to P60. C57Bl6/6J were used as control mice as recommended by The Jackson Laboratory. Animals were killed by sedation with isoflurane followed by cervical dislocation and exsanguination. The entire GI tract, from oesophagus to the internal anal sphincter, was removed and placed in oxygenated cold Krebs–Ringer buffer (KRB) for further dissection.
Animals used for these studies were maintained and the experiments performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Institutional Animal Use and Care Committee at the University of Nevada approved all procedures used.
Electrophysiological experiments
Proximal colon segments were dissected free from the remaining GI tract and opened along the mesenteric border. After removing the mucosa by sharp dissection, the tunica muscularis (10 mm × 5 mm) was placed in a recording chamber with the circular muscle facing upward. Muscles were maintained in 37.5 ± 0.5°C by continuous perfusion of KRB solution. Circular muscle cells were impaled with microelectrodes (50–80 MΩ resistance) and transmembrane potentials were measured and analyzed as previously described (Hwang et al. 2009). Electrical recordings were made in the presence of nifedipine (1 μm) where stated, to reduce muscle contraction and maintain cellular impalements.
Electrical stimulation and pressure ejection (picospritzing) of drugs
Electrical field stimulation (EFS) was applied (0.5 ms pulses; 150 V) via parallel platinum electrodes using a square wave stimulator (Grass 588, Grass, Quincy, MA, USA). Receptor antagonists and neural toxins were added to the circulating KRB solution. Purine agonists were loaded into micropipettes, and the tips of the micropipettes were positioned by micromanipulator close to the electrical recording site and drugs were applied by picospritz ejection (10 p.s.i., 25 ms duration; General Valve, East Hanover, NJ, USA).
Isometric force measurements
Strips of colon muscle (approximately 10 mm × 5 mm) were cut in the long axis of the circular muscle fibres and attached to a fixed mount and to a Fort 10 (World Precision Instruments, Sarasota, FL, USA) isometric strain gauge and immersed in organ baths which were continuously perfused at 37 ± 0.5°C with oxygenated KRB. A resting force of 3 mN was applied, which has been previously shown to set the muscles at optimum resting length (Ward et al. 1998). This was followed by an equilibration period of 1 h, before experiments were initiated. Neural responses were evoked by EFS (0.3 ms, 1 and 5 Hz for 30 s) delivered via platinum ring electrodes positioned above and below the muscle. Drugs were added to tissues via the perfusion solution at the concentrations stated. Signals were recorded onto a PC via Acknowledge software (Biopac Systems Inc., Goleta, CA, USA). Areas under the trace (AUTs) were calculated using pCLAMP software (Molecular Devices LLC, Sunnyvale, CA, USA).
Video imaging and spatiotemporal mapping of artificial pellet propulsion
Entire colons were removed from wild-type and P2ry1−/− mice and immediately placed in oxygenated KRB. Colons were then gently flushed of all feces using a KRB-filled syringe and transferred to a Sylgard elastomer-lined organ bath. The whole colon was loosely pinned to the floor of the organ bath via the mesentery and continuously perfused with oxygenated KRB at 37 ± 0.5°C. A 6 mm × 2.5 mm artificial fecal pellet (compared to 6.3 ± 0.3 mm for natural pellets; n= 6) was then inserted into the proximal end of the colon. The propagation of the pellet along the entire length of the colon was recorded to a computer at 7.5 frames s−1 with a high-definition video camera (DMK 31AF03, Imaging Source, Charlotte, NC, USA) and AstroIIDC software (ASC, Calgary, Alberta, Canada). Spatiotemporal maps (STMaps) of propagating pellets were constructed from video images as described previously (Hennig et al. 1999). Briefly, greyscale-coded pixels from each frame of the movie sequence were used to construct a single row. Sequential rows were placed underneath each other in a separate image to produce an STMap of pellet propulsion. The movement of the pellet could then be visualized as the white band in the STMap and measurement of the gradient of this band reveals the velocity of the pellet movement.
Solutions and drugs
The electrophysiological and mechanical baths were constantly perfused with oxygenated KRB of the following composition (mm): NaCl 118.5; KCl 4.5; MgCl2 1.2; NaHCO3 23.8; KH2PO4 1.2; dextrose 11.0; CaCl2 2.4. The pH of the KRB was 7.3–7.4 when bubbled with 97% O2–3% CO2 at 37 ± 0.5°C. Muscles were left to equilibrate for at least 1 h before experiments were begun. Adenosine-5′-triphosphate (ATP), adenosine 5′-diphosphate (ADP), β-nicotinamide adenine dinucleotide (β-NAD), adenosine diphosphate ribose (ADPR), tetrodotoxin (TTX), atropine, Nω-Nitro-l-arginine (l-NNA) and apamin were purchased from Sigma-Aldrich (St Louis, MO, USA). MRS2500 (2- iodo-N6-methyl-(N)-methanocarba-2-deoxyadenosine-3,5-bisphosphate tetraammonium salt) was purchased from Tocris Bioscience (Ellisville, MO, USA). For some electrophysiological experiments nifedipine (Sigma-Aldrich) was dissolved in ethanol at a stock concentration of 100 μm before being added to the perfusion solution at a final concentration of 1 μm to inhibit contractile activity.
Data analysis
Data are expressed as means ± standard errors of the mean (SEM). Differences were evaluated using Student's t test. P values < 0.05 were taken as a statistically significant difference. The ‘n’ values reported in the text refer to the number of animals used for each experimental protocol. Several electrical parameters were analyzed: (i) resting membrane potential, (ii) spike complex frequency, (iii) amplitude of fast and slow inhibitory junction potentials (IJPs), (iv) duration of fast (1/2-maximal) and slow (maximum) IJP, (v) time-to-peak of IJP components, (vi) percentage of control area under the trace (AUT). AUT was calculated with pCLAMP software (Molecular Devices) and was normalized to control conditions that were taken as 100%. Figures displayed were made from digitized data using Corel Draw X3 (Corel Corp., Ontario, Canada).
Results
Comparison of spontaneous activity and responses evoked by intrinsic nerves in wild-type and P2ry1−/− colons
Proximal colon circular muscle cells of wild-type mice had resting membrane potentials (i.e. most diastolic membrane potentials) averaging −56 ± 0.8 mV and displayed spontaneous discharges of action potential complexes at a frequency of 2.2 ± 0.2 cycles min−1 (n= 10; Fig. 1A). Electrical field stimulation (single pulse 0.5 ms in duration) of muscles produced a brief excitatory junction potential (EJP) followed by a bi-phasic inhibitory junction potential, consisting of a fast transient component (fIJP) followed by a more sustained hyperpolarization (sIJP; Fig. 1B). The EJP was 15 ± 2.3 mV in amplitude with a 1/2-maximal duration of 93 ± 8 ms. The fIJP was 18 ± 3 mV in amplitude with a 1/2-maximal duration of 302 ± 13 ms, and the sIJP was 10 ± 0.2 mV with a duration of 4 ± 0.2 s (all n= 6; Fig. 1B). Atropine (1 μm) abolished the EJP but did not significantly affect the amplitude or duration of the fIJP or sIJP (Fig. 1C). In the continued presence of atropine, Nω-Nitro-l-arginine (l-NNA, 100 μm) caused depolarization from −56 ± 0.8 mV to −43 ± 0.9 mV (P < 0.0001 compared to atropine) and continuous generation of action potentials. l-NNA also abolished the sIJP and increased the amplitude of the fIJP (i.e. to 29 ± 2 mV; P= 0.002 compared to the fIJP in atropine; and a 1/2-maximal duration of 418 ± 38 ms; n= 6, Fig. 1D). Addition of the P2Y1 receptor antagonist, MRS2500 (1 μm), in the continued presence of atropine and l-NNA, did not affect membrane potential between stimuli, but it abolished the fIJP (Fig. 1E). All responses to EFS were abolished by TTX (0.3 μm) (data not shown).
Figure 1. Spontaneous electrical activity and responses to stimulation of intrinsic motor neurons in wild-type and P2ry1−/− colons.
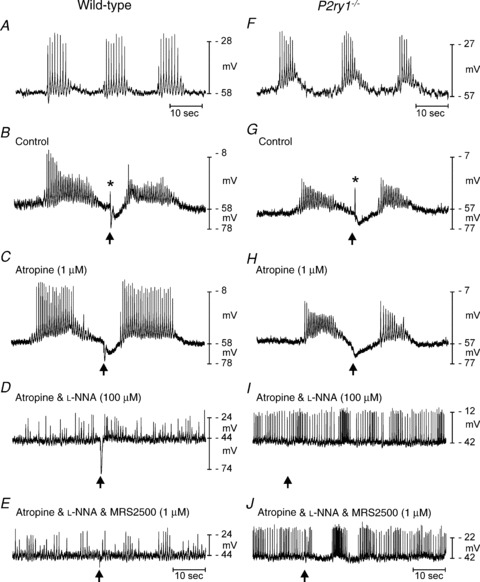
A–E, spontaneous action potential complexes and responses to EFS (single stimuli, 0.5 ms duration) in a wild-type colon. A, spontaneous action potential complexes occurred regularly in colonic muscles. B, EFS (single pulse 0.5 ms at arrow) evoked a post-junctional response consisting of an excitatory junction potential (EJP, *) and a bi-phasic inhibitory junction potential (IJP) consisting of fast (fIJP) and slow (sIJP) components. C, atropine (1 μm) blocked the EJP. D, l-NNA (100 μm) caused depolarization and action potential complexes were fused into continuous trains of action potentials. The sIJPs were blocked by l-NNA. E, MRS2500 (1 μm) blocked the fIJP. Thus, the wild-type responses to EFS are mediated by release of acetylcholine (EJP), a purine neurotransmitter (fIJP), and nitric oxide (sIJP). F–J, spontaneous electrical activity and responses to EFS in a P2ry1−/− colon. F, spontaneous action potential complexes, similar to wild-type activity, were recorded in P2ry1−/− colons. G, EFS evoked EJPs (*) that were significantly larger in amplitude than in controls. EJPs were followed by IJPs that consisted of the sIJP but lacked the fIJP component. H, atropine (1 μm) inhibited the EJP. I, l-NNA (100 μm) abolished the sIJP. J, in the presence of atropine and l-NNA, MRS2500 had no effect on membrane potential nor did it unmask any non-specific effects of this compound.
The spontaneous electrical activity of circular smooth muscle cells from proximal colons of P2ry1−/− mice was not significantly different from age-matched wild-type mice. Resting membrane potentials in P2ry1−/− mice averaged −57 ± 0.7 mV and spontaneous action potential complexes occurred at a frequency of 2.0 ± 0.2 cycles min−1 (P= 0.24 and 0.5, respectively, comparing these values to the activity of wild-type colons; n= 10; Fig. 1F). EJPs were significantly larger in amplitude and 1/2-maximal duration in P2ry1−/− mice than in wild-type colons (27 ± 3 mV and 137 ± 12 ms, respectively; P= 0.0002 and P= 0.02, respectively, in comparison to values from wild-type mice; n= 10; Fig. 1G). The fIJP was absent as a component of the post-junctional inhibitory response in P2ry1−/− mice. The sIJP, however, was still present, averaging 11 ± 0.8 mV in amplitude and 5 ± 0.2 s in duration. The amplitude of the sIJP was not significantly different from sIJPs in wild-type mice (P= 0.53), but the duration was significantly longer in P2ry1−/− mice (P= 0.003; Fig. 1G). Addition of atropine abolished the EJP and left only the sIJP. The amplitudes of sIJPs did not change (11 ± 0.8 mV, P= 0.54) after the addition of atropine, but the duration of sIJPs increased to 5.5 ± 0.2 s (P= 0.0006 compared to wild-type and 0.113 compared to P2ry1−/− without atropine; Fig. 1H).
l-NNA caused depolarization of cells in P2ry1−/− mice (i.e. from −57 ± 0.7 mV to −42 ± 1 mV; n= 6, P < 0.0001) and resulted in the generation of continuous action potentials, as observed in wild-type colons (Fig. 1I). l-NNA abolished the sIJP, as also observed in wild-type colons (Fig. 1I); however, MRS2500 (1 μm in the continued presence of atropine and l-NNA) had no effect on responses to EFS (Fig. 1J). Superposition of post-junctional membrane responses to EFS in wild-type and P2ry1−/− colons (Fig. 2A) shows the larger EJP and the delay in the post-hyperpolarization response after cessation of the sIJP observed in P2ry1−/− mice. We did not investigate the source of this delay, but it may be due to reduced resetting of voltage-dependent inward currents that occurs during the hyperpolarization to negative potentials during the fIJP, as described previously (Baker et al. 2003).
Figure 2. Post-junctional electrical responses to enteric motor nerve stimulation in wild-type and P2ry1−/− mice.
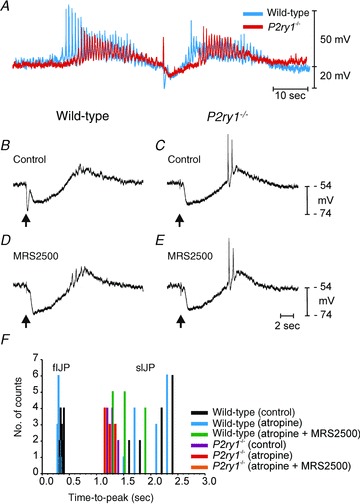
A, EFS (arrow) evoked post-junctional membrane potential responses in wild-type (blue) and P2ry1−/− mice (red). The traces lined up on the stimulus and superimposed to demonstrate differences in the kinetics of the post-junctional responses. EJPs were significantly larger in P2ry1−/− muscles because the fIJPs were absent and did not therefore compete with the EJP. Recovery from the sIJP was slower in P2ry1−/− muscles, and delayed the development of the action potential complex (post-stimulus excitation) that followed the sIJP. B, IJP under control conditions recorded from a wild-type colon muscle. Single pulse of EFS was delivered at arrow. Note prominent fIJP and sIJP components. C, response to single stimulus of colonic muscle of a P2ry1−/− mouse. Note loss of fIJP component. D and E, responses to single pulse of EFS after addition of MRS2500 (1 μm) in a wild-type and P2ry1−/− muscle. MRS2500 blocked the fIJP in the wild-type mouse and had no effect in the P2ry1−/− colon. F, histogram summarizing the times-to-peak of IJP components. Responses from wild-type or P2ry1−/− muscles and the conditions under which recordings were made are shown as coloured bars as designated in the key. Wild-type mice exhibited fIJPs in response to EFS with times-to-peak at about 300 ms before and after atropine. sIJP peaks occurred at approximately 2 s. P2ry1−/− muscles or wild-type muscles after treatment with MRS2500 displayed no IJP component with a peak at about 300 ms; only sIJP components were observed in these muscles.
We also analyzed the time courses of the fast and slow components of the IJP. The time-to-peaks of fIJPs and sIJPs were plotted as a histogram. The time-to-peak for fIJPs in wild-type muscles averaged 370 ± 10 ms under control conditions (n= 14 from 5 tissues; Fig. 2B), and the peak shifted to an average of 320 ± 20 ms after addition of atropine, but did not reach significance. Subsequent addition of MRS2500 (1 μm) completely inhibited the fIJP (Fig. 2D). Time-to-peak of the sIJP averaged 2.26 ± 0.12 s in wild-type mice and was not significantly affected by atropine (i.e. to 2.18 ± 0.02 s; P > 0.05). Addition of MRS2500 to wild-type muscles shifted the time-to-peak to 1.66 ± 0.1 s (P= 0.006). No fIJP was resolved in muscles of P2ry1−/− mice (Fig. 2C). The sIJP time-to-peak averaged 1.31 ± 0.02 s (n= 10 from 5 tissues) under control conditions in P2ry1−/− mice and this was not significantly changed after atropine and MRS2500 (i.e. 1.27 ± 0.3 s after atropine and 1.27 ± 0.02 s after MRS2500; P > 0.05 for both; Fig. 2D). Data showing examples of recordings used in the analysis of time-to-peaks of IJP components in wild-type and P2ry1−/− mice are shown in Fig. 2B–E and a summary histogram of time-to-peaks for fIJPs and sIJPs are shown in Fig. 2F.
Single stimuli were used above to allow dissection of specific components of IJPs. However, mutiple stimuli are likely to be commonplace in enteric neural regulation of the colon, and it is possible that multiple nerve action potentials could cause additional neurotransmitter release that might overflow and recruit post-junctional receptors not affected by transmitter released by single stimuli. Therefore, we also tested responses to multiple stimuli (5 Hz stimulation for 5 s). Stimulation of wild-type muscles with this protocol (in the presence of atropine) resulted in sustained hyperpolarization responses that reached a peak hyperpolarization of 22.4 ± 1.5 mV within 818 ± 68 ms and then relaxed to a sustained level of 13.6 ± 0.6 mV (Fig. 3A; n= 5) that persisted until the stimulus train was completed. Addition of l-NNA did not affect the peak hyperpolarization (22.2 ± 1.4 mV) but reduced the sustained component of the response (Fig. 3B). Further addition of MRS2500 completely blocked the hyperpolarization response during 5 Hz stimulation Fig. 3C). This protocol also initiated hyperpolarization in muscles of P2ry1−/− mice, but in these tissues the response reached a maximum of 15.2 ± 0.5 mV (Fig. 3D) and was completely blocked by l-NNA (Fig. 3E). Subsequent addition of MRS2500 had no additional effect (Fig. 3F). These experiments demonstrate that stimulation of intrinsic neurons up to 5 Hz did not release sufficient neurotransmitter to overflow onto receptors of a different class to P2Y1 receptors.
Figure 3. Membrane potential responses elicited by multiple pulse protocols.
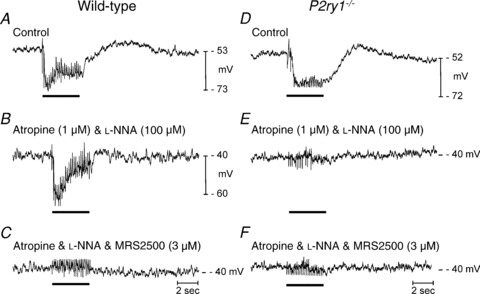
Wild-type (A–C) and P2ry1−/− muscles (D–F) were stimulated by EFS (5 Hz for 5 s; black bars in each panel). A, multiple pulse protocols under control conditions elicited sustained hyperpolarization responses that reached a peak early in the response and then relaxed to a sustained level of hyperpolarization. Noisy traces during stimulation are due to stimulus artifacts from multiple pulses of EFS. B, atropine (1 μm) and l-NNA (100 μm) blocked the EJP and reduced the sustained component, but did not block the initial peak hyperpolarization. C, further addition of MRS2500 (3 μm) blocked the entire hyperpolarization response. D, responses to multiple stimuli in muscle from a P2ry1−/− mouse under control conditions. Response is a sustained hyperpolarization without a prominent initial peak hyperpolarization. E, the initial EJP was blocked by atropine (1 μm) and the entire hyperpolarization response in P2ry1−/− muscles was blocked by l-NNA (100 μm). F, MRS2500 caused no additional effect on responses of muscles of P2ry1−/− mice.
Contractile experiments using measurement of isometric force development in colonic muscle strips (oriented in the axis of the circular muscle) were performed to further understand the consequences of losing P2Y1 receptors on colonic motility. Circular muscle contracted spontaneously and with similar characteristics in wild-type and P2ry1−/− mice. Contractions averaged 6.9 ± 0.6 mN in amplitude and occurred at a frequency of 3.3 ± 0.3 min-1 in wild-type animals (Fig. 4A, n= 5) and 6.9 ± 0.8 mN and 3.2 ± 0.4 min-1 in P2ry1−/− colons (n= 5, P > 0.05 for both parameters, Fig. 4E). EFS (0.3 ms duration, 1 and 5 Hz for 30 s) evoked frequency-dependent reduction in the amplitude of spontaneous contractions in wild-type colonic muscles under control conditions. The decrease in contractile amplitude was accompanied by a small increase in contractile frequency. These responses resulted in a net increase in the area under the contractile traces (AUT). Atropine (1 μm) converted responses to EFS to inhibition of phasic contractions (Fig. 4B), and subsequent addition of l-NNA with continued atropine caused responses to EFS to convert to tonic contractions (i.e. reduction in inhibitory response, Fig. 4C). Finally, MRS2500 (1 μm), added with atropine and l-NNA, blocked the remaining inhibitory response and converted the response to EFS to an excitatory response (Fig. 4D). In P2ry1−/− colons EFS produced marked increase in the amplitude of contractions, and the spontaneous phasic contractions were converted to a tonic contraction lasting the duration of the stimulus at higher frequencies (Fig. 4E). Atropine blocked the excitatory response, converting the response to EFS to an inhibitory response (Fig. 4F) that was blocked by l-NNA (Fig. 4G). MRS2500 (1 μm), administered with atropine and l-NNA, had no effect on responses to EFS in P2ry1−/− colons (Fig. 4H; 1 Hz, P= 0.86; 5 Hz, P= 0.3). The contractile responses to EFS in colons of wild-type and P2ry1−/− mice are summarized in Fig. 4I and J.
Figure 4. Contractile responses to intrinsic motor neurons in colonic muscles of wild-type and P2ry1−/− mice.
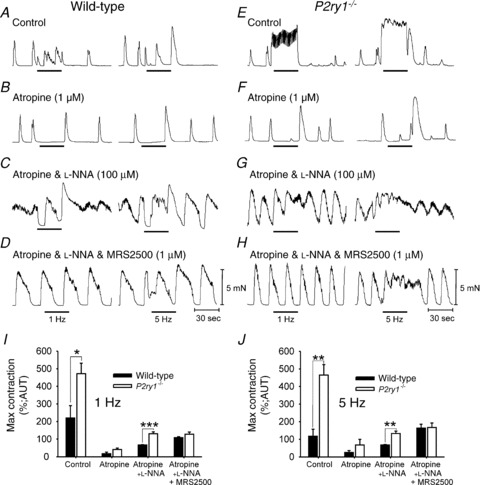
A–D, responses of wild-type colons to EFS (1 and 5 Hz, black bars). A, EFS under control conditions increased contractions. B, atropine (1 μm) converted response to EFS to total inhibition of phasic contractions. C, EFS after l-NNA (100 μm; added in addition to atropine) caused an excitatory response in which phasic contractions fused to generate a tonic contraction. At 1 Hz, the tonic contraction was preceded by a transient relaxation. With atropine and l-NNA present, addition of MRS2500 (1 μm) inhibited the initial transient relaxation to EFS and caused a frequency-dependent increase in contraction (i.e. no response to 1 Hz and excitatory response to 5 Hz). E–H, neural responses of P2ry1−/− colons. E, EFS under control conditions produced a marked increase in tone that was frequency dependent. F, atropine (1 μm) converted excitatory response to EFS to inhibition of phasic contractions. Occasional escapes from inhibition were observed in the P2ry1−/− muscles. G, l-NNA (100 μm) with atropine, caused responses to EFS to become excitatory with phasic contractions fusing to produce tone. H, in the presence of atropine and l-NNA, MRS2500 (1 μm) did not have a significant effect on neural responses (see summary in I and J). I and J, summaries of contractile responses to EFS plotted as a percentage of the maximum area under the trace (AUT) during exposures to the various pharmacological blockers used and at 1 and 5 Hz, respectively. *P < 0.05; **P < 0.01; ***P < 0.001).
Effects of purines on colons of wild-type and P2ry1−/− mice
Controversy exists regarding the identity of the neurotransmitter responsible for fIJPs. For several decades it has been assumed that ATP was the likely candidate (Burnstock, 1972; Xue et al. 1999), but recently a related purine, β-NAD (Mutafova-Yambolieva et al. 2007) or its metabolite ADPR (Durnin et al. 2012), have been shown to mimic the responses evoked by release of the authentic inhibitory neurotransmitter better than ATP (Mutafova-Yambolieva et al. 2007). Since the fIJP is absent in P2ry1−/− colons (Figs 1 and 2), these animals provide an excellent model for testing which purine is better at mimicking endogenous responses to EFS.
Picospritzing ATP and ADP onto colonic circular muscles of wild-type mice caused hyperpolarization that was reduced by apamin, but not blocked by MRS2179 (Mutafova-Yambolieva et al. 2007). Picospritzing of ATP and ADP (each 10 mm loaded in the picospritz pipettes) onto circular muscles of P2ry1−/− mice also caused hyperpolarization (i.e. 9.2 ± 0.5 mV (n= 5) and 8.4 ± 0.9 mV (n= 5; Fig. 5). Picospritzing β-NAD and ADPR caused hyperpolarization in wild-type colons (Mutafova-Yambolieva et al. 2007; Durnin et al. 2012), but did not cause hyperpolarization in P2ry1−/− (Fig. 6).
Figure 5. Hyperpolarization responses elicited by ATP and ADP in P2ry1−/− colon.
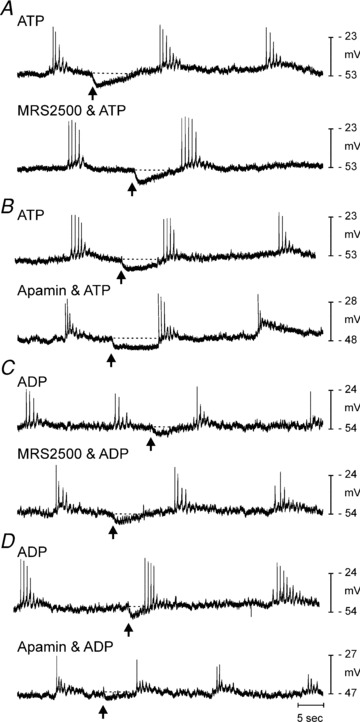
As in control mice (Mutafova-Yambolieva et al. 2007), picospritzing of ATP (A, 10 mm, 10 p.s.i. for 25 ms duration, arrows) caused transient hyperpolarization that was not blocked by MRS2500 (1 μm, lower trace). B, hyperpolarization responses to ATP were reduced by apamin (0.3 μm; lower trace). C, picospritzing of ADP (10 mm, 10 p.s.i. for 25 ms duration, arrows) under control conditions (upper trace) and in the presence of MRS2500 (lower trace) yielded hyperpolarization in P2ry1−/− colons. D, apamin also reduced the hyperpolarization response to ADP in P2ry1−/− colons.
Figure 6. Hyperpolarization to β-NAD and ADPR is absent in P2ry1−/− colons.
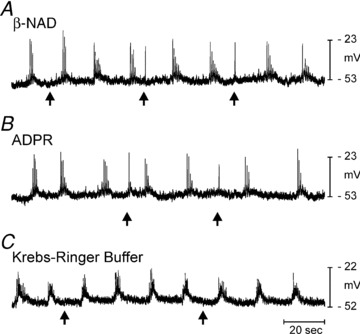
In contrast to responses in wild-type mice (Mutafova-Yambolieva et al. 2007) picospritzing β-NAD (A, 50 mm, 10 p.s.i. for 25 ms duration, arrows) failed to elicit hyperpolarization. B, ADPR (10 mm, 10 p.s.i. for 25 ms duration, arrows) also failed to elicit hyperpolarization. Both β-NAD and ADPR elicited a slight excitatory response at arrows in all muscles tested. C, spritz of KRB had no effect on membrane potential.
It is possible that application of purines, even by picospritzing, could cause pre-junctional activation of motor neurons (e.g. dendrites or cell bodies of motor neurons in the myenteric plexus), and additional neurotransmitters might be released that could mimic or inhibit the post-junctional effects of purines. Therefore, we also tested the effects of ATP and ADP in the presence of atropine and l-NNA. In the presence of these neural antagonists, picospritzing of ATP or ADP (10 mm loaded in picospritz pipette) onto wild-type colons caused hyperpolarization averaging 15 ± 1.3 mV and 11.5 ± 1 mV, respectively (n= 10 for each purine; Fig. 7A–D). Application of ATP or ADP (10 mm for both) to colonic muscles of P2ry1−/− mice caused hyperpolarization responses averaging 10 ± 0.5 mV (n= 11; P= 0.001 as compared to responses in wild-type colons, Fig. 7E and F) and 10 ± 0.7 mV, respectively (n= 10; P= 0.15 as compared to wild-type colons, Fig. 7G and H). MRS2500 (1 μm) had no significant effect on hyperpolarization responses to ATP or ADP in wild-type mice, i.e. responses to ATP were 18 ± 4% (Fig. 7A; n= 5; P= 0.4) and to ADP were 17 ± 9% (Fig. 7C; n= 5; P= 0.5) reduced in the presence of MRS2500. MRS2500 also had no significant effect on responses to ATP or ADP in P2ry1−/− mice (responses to ATP were 10 ± 4%; n= 5; P= 0.8; Fig. 7E) and to ADP (11 ± 4%; n= 5; P= 0.6; Fig. 7G) reduced in the presence of MRS2500). ATP and ADP responses were reduced by apamin (0.3 μm) in wild-type and P2ry1−/− mice, i.e. by 75 ± 4% (n= 5; P= 0.0009; Fig. 7B) and 74 ± 3% (n= 5; P= 0.0008; Fig. 7D), respectively, in wild-type controls and by 83 ± 5% (n= 6; P= 0.004; Fig. 7F) and 78 ± 5% (n= 5; P= 0.005; Fig. 7H) in P2ry1−/− mice (Fig. 7B, D, F and H, respectively). These results demonstrate that ATP and ADP activate hyperpolarization responses in colonic muscles via receptors in addition to P2Y1 receptors.
Figure 7. Hyperpolarization responses to ATP and ADP in wild-type and P2ry1−/− colons in the presence of atropine and l-NNA.
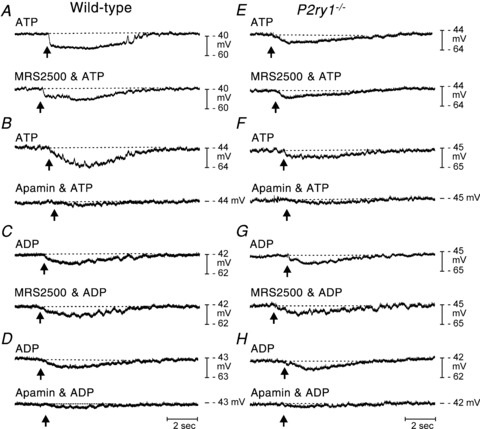
A and B, ATP picospritzed at arrow (10 mm,10 p.s.i. for 25 ms duration) caused hyperpolarization of cells in wild-type colons. Responses were not significantly affected by MRS2500 (1 μm; lower trace in A) but inhibited by apamin (0.3 μm; lower trace in B). C and D, ADP (arrow) also caused hyperpolarization in wild-type colons that were not blocked by MRS2500 (lower trace in C) but inhibited by apamin (lower trace in D). E and F, in P2ry1−/− colons ATP caused hyperpolarization that was not blocked by MRS2500 (lower trace in E) but was inhibited by apamin (lower trace in F). G and H, ADP also caused hyperpolarization in colons of P2ry1−/− mice. These responses were not blocked by MRS2500 (lower trace in G) but were reduced by apamin (lower trace in H).
Picospritzing β-NAD (50 mm in picospritz pipettes) onto colons of wild-type controls caused hyperpolarization averaging 16 ± 2 mV (n= 11; Fig. 8A and B). The hyperpolarization response was reduced by MRS2500 (79 ± 2%; n= 5; P= 0.004; Fig. 8A) and apamin (74 ± 4%; n= 6; P= 0.03; Fig. 8B). In contrast, hyperpolarization responses were absent when β-NAD was applied to colonic muscles of P2ry1−/− mice (Fig. 8E). ADPR (10 mm in picospritz pipette) also caused hyperpolarization (11 ± 0.5 mV; n= 10) in wild-type muscles (Fig. 8C and D), and this response was reduced by MRS2500 (96 ± 3%; n= 5; P= 0.0002; Fig. 7C) and apamin (74 ± 5%; n= 5; P= 0.047; Fig. 8D). No hyperpolarization occurred in response to ADPR in muscles of P2ry1−/− mice (Fig. 8F).
Figure 8. Hyperpolarization responses to β-NAD and ADPR in wild-type and P2ry1−/− colons in the presence of atropine and l-NNA.
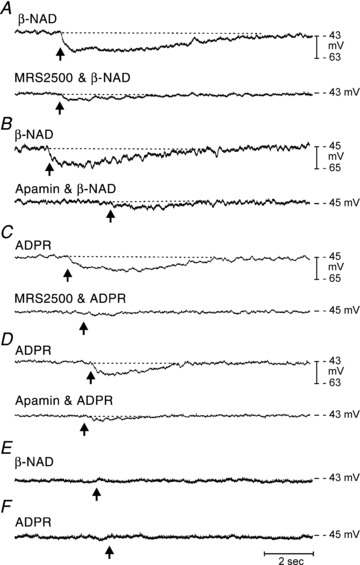
A and B, in wild-type mice β-NAD (50 mm picospritzed at arrow, 10 p.s.i. for 25 ms duration) caused hyperpolarization that was inhibited by MRS2500 (1 μm; lower trace in A) and apamin (0.3 μm; lower trace in B). C and D, picospritzed ADPR (10 mm, arrows) also caused hyperpolarization responses that were inhibited by MRS2500 (lower trace in C) and apamin (lower trace in D). E and F, hyperpolarization responses to β-NAD (E) or ADPR (F) were not observed in P2ry1−/−.
The effects of exogenous purines on wild-type and P2ry1−/− colonic muscles were further tested in mechanical experiments (Fig. 9). Application of ATP in the superfusion solution (5 mm, in the presence of atropine and l-NNA) caused inhibition of phasic contractions of wild-type circular muscle strips (by 36 ± 8%; AUT of control). The inhibitory effects of ATP were unaffected by addition of 1 μm TTX (28 ± 7%, n= 4; Fig. 9A; P= 0.46), suggesting the effects were substantially due to binding of ATP to post-junctional receptors. ATP also inhibited contractions of colonic muscles from P2ry1−/− mice (by 54 ± 8% AUT; Fig. 9B; P= 0.14 as compared to control), and this inhibition was also unaffected by TTX (52 ± 10%; P= 0.82 compared to response in absence of TTX).
Figure 9. Relaxation responses to ATP and β-NAD in wild-type and P2ry1−/− colons.
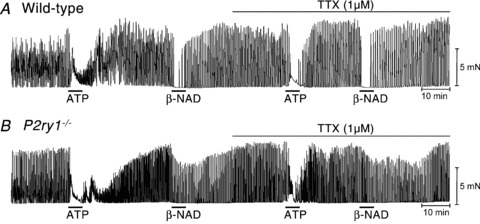
A, in wild-type mice ATP (5 mm; black bar) caused an initial contraction followed by relaxation. β-NAD (5 mm) caused relaxation. Relaxation responses were similar in the presence of tetrodotoxin (TTX; 1 μm). B, in P2ry1−/− mice ATP caused relaxation as observed in wild-type mice, but the relaxation response to β-NAD was significantly attenuated in comparison to responses in wild-type mice. Responses to ATP and β-NAD were not affected by TTX. These experiments were performed in the presence of atropine (1 μm) and l-NNA (100 μm).
β-NAD (5 mm) inhibited contractions of wild-type muscles by 86 ± 5%, and this response was insensitive to TTX (inhibition in the presence of TTX was 82 ± 9%; n= 4; Fig. 9A; P= 0.72). β-NAD also inhibited phasic contractions of circular muscles in P2ry1−/− colons by 52 ± 8%, which was less than that observed for wild-type muscles (P= 0.04). The relaxation effects of β-NAD in P2ry1−/− colons were also insensitive to TTX (41 ± 5%, n= 4; Fig. 9B, P= 0.4).
Loss of P2Y1 receptors slows colonic transit
We also tested the effects of loss of P2Y1 receptors on colonic transit. Colonic transit was measured by monitoring the movements of artificial fecal pellets using digital video recording. Transit of fecal pellets in ex vivo colons of wild-type mice was variable in the proximal colon therefore velocity measurements of transit were taken in the distal portion from 50% along the length of the colon. Colonic transit in the distal colon of wild-type mice averaged 1.12 ± 0.08 mm s−1 (n= 5). Following the addition of MRS2500 (1 μm) to the perfusion solution, fecal pellet transit stopped at a distance of 67 ± 2.8% along the length of the colon and did not proceed further during the recording period (up to 25 min; n= 5). In one experiment washout of MRS2500 for 15 min allowed for fecal pellet transit to resume. Colonic transit was delayed and altered in colons of P2ry1−/− mice. Fecal pellets were transported to a point 60.2 ± 2.1% along the colon at 0.23 ± 0.04 mm s−1 (n= 6) and then transit stopped or was delayed to an extent that the pellets did not proceed any farther during the time course of the experiments (up to 28 min; Fig. 10).
Figure 10. Colonic transit of artificial fecal pellets in wild-type and P2ry1−/− colons.
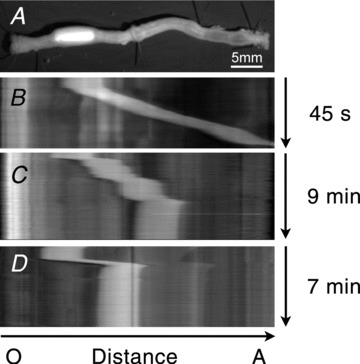
A, single video image of colon after insertion of an artificial fecal pellet (white region). B, spatio-temporal map generated from entire video of a pellet transported along the length of a wild-type colon. Fecal pellets were rapidly transported in wild-type colons (white band traversing from oral end of the colon (left) to anal end of colon (right); transit time was 45 s). C, wild-type colon after addition of MRS2500 (1 μm). In the presence of MRS2500 pellet transit stopped 67 ± 2.8% along the colon length and did not proceed further during the recording period (total time of recording in C was 9 min). D, in P2ry1−/− mice fecal pellets were transported to a point 60.2 ± 2.1% along the colon where transit was halted for the remainder of the recording (total time of recording in D was 7 min). Movements of fecal pellets are denoted by white bands in B, C and D. O and A indicate oral and anal directions of the colon, respectively.
Discussion
In the present study we have shown that mice with genetic deactivation of P2ry1 lack purinergic motor neurotransmission in colonic muscles. The fIJP component of the normal enteric inhibitory response was completely absent, and mechanical responses to repetitive field stimulation were converted to strong excitatory responses. Nitrergic inhibitory responses (sIJP) were unchanged in P2ry1−/− mice, suggesting that NO and the purine transmitter are neurotransmitters with independent response mechanisms. We also found that hyperpolarization responses to ATP and ADP were largely intact in muscles of P2ry1−/− mice while responses to β-NAD and ADPR (primary metabolite) were absent. This serves as another observation suggesting that β-NAD and ADPR more closely mimic the response to enteric inhibitory neurotransmission than ATP (Mutafova-Yambolieva et al. 2007; Hwang et al. 2011; Durnin et al. 2012). We also found that pellet transit was significantly delayed in ex vivo colons of P2yr1−/− mice. These data suggest that purinergic neural regulation, via release of neurotransmitter and binding to post-junctional P2Y1 receptors, is an important component of neural regulation of GI motility. It should be noted that the delay in pellet transport was most dramatic in the distal colon, so it is possible that there is particular dependence on purinergic motor regulation in this region.
While we detected slowed pellet transit in colons of P2ry1−/− mice, this defect was not lethal to mice homozygous for the deactivated P2ry1 transgene. Wild-type and P2ry1−/− animals at the same age were of approximately the same body weight and there were no gross differences in the appearance of the mice or in the gross dimensions or appearance of the colon. Distension of the bowel, as is imposed by the presence of fecal pellets, results in ascending excitation and descending inhibitory responses to facilitate peristalsis (Hirst, 1979; Smith et al. 1990; Waterman et al. 1994). It is clear that the excitatory component of neural regulation provides the stimulus for propulsive contractions because blocking the major excitatory input mediated by cholinergic motor neurons inhibits peristalsis (Trendelenberg, 1917). The importance of descending inhibition in peristalsis has also been evaluated (Waterman & Costa, 1994). These experiments showed that inhibition of redundant inhibitory neurotransmitters (purinergic and nitrergic) both have consequences on peristalsis, but peristaltic contractions are present when either inhibitory pathway is preserved. The redundancy of inhibitory neurotransmitters provides a ‘safety factor’ for neural regulation of motility, and probably explains the ability of mice to survive deactivation of P2ry1.
The specific effects of purinergic neural regulation on GI motility patterns have previously been studied using pharmacological tools (apamin) to block post-junctional inhibitory mechanisms (i.e. activation of small-conductance Ca2+-activated K+ channels; SK channels). Apamin has been reported to increase ejection pressures due to peristaltic contractions in the guinea-pig intestine, but unlike blocking of the nitrergic pathway, apamin did not alter the pressure threshold for initiation of peristaltic contractions (Waterman & Costa, 1994). Apamin has also altered the pattern of contractions: when peristalsis was initiated, the intestine contracted forcefully over its length rather than as a descending wave of contraction. Thus, the receptive relaxation phase of peristalsis was compromised by treatment with apamin. Our contractile experiments also demonstrate that loss of purinergic regulation in the P2ry1−/− mouse shifts the contractile pattern in response to electrical field stimulation to powerful, sustained contractions (Fig. 4), which may be equivalent to the responses noted by Waterman & Costa (1994) in their experiments in vivo. These authors noted that in spite of the change in contractile pattern after apamin, ejection of fluid loads still occurred. We monitored solid pellet propulsion in our experiments, and in this model development of sustained, long segment contractions might explain the significantly delayed pellet transport we observed (Fig. 10).
Previous studies of colonic muscles from several species, including humans, have shown that pharmacological antagonists with specificity for P2Y1 receptors cause inhibition of fIJPs (Gallego et al. 2006; Mutafova-Yambolieva et al. 2007; Wang et al. 2007; Grasa et al. 2009; Hwang et al. 2011). P2Y1 receptors have been localized to smooth muscle cells; however, smooth muscle cells also express P2Y4 receptors (Gallego et al. 2006; Monaghan et al. 2006). A recent study using quantitative PCR has shown that P2ry1 transcripts are enriched in cells expressing platelet-derived growth factor receptor α (PDGFRα+ cells), and these cells also express SK3 channels and respond to purines, such as ATP or β-NAD, with the generation of large-amplitude outward currents (Kurahashi et al. 2011). Under similar experimental conditions, purines initiated small inward currents in smooth muscle cells. PDGFRα+ cells are coupled to smooth muscles via gap junctions in GI muscles (Horiguchi & Komuro, 2000). Thus, it is possible that the P2Y1 receptors required for post-junctional responses in colonic muscles are mainly expressed by PDGFRα+ cells and purinergic hyperpolarization responses are not mediated by the binding of purines to smooth muscle receptors. Cell-specific deactivation of P2ry1 will be required to determine which cell is primarily responsible for transduction of purinergic motor inputs to GI muscles.
The results of the current study shed further light on the identity of the purinergic neurotransmitter in colonic muscles. We have previously shown that β-NAD (and its metabolite ADPR) mimics responses to the purine released from enteric inhibitory motor neurons better than ATP (Mutafova-Yambolieva et al. 2007; Hwang et al. 2011; Durnin et al. 2012). fIJPs and responses to β-NAD were blocked by P2Y receptor antagonists and specific antagonists of P2Y1 receptors, but responses to ATP were not. These findings tend to suggest that ATP is not a neurotransmitter in GI muscles because it hyperpolarizes muscles via receptors other than P2Y1 receptors. However, there is also the possibility that the purine transmitter released from nerve varicosities is compartmentalized in a neuro-effector junction where P2Y1 receptors are densely expressed. If such junctions occur, then ATP cannot be ruled out as a transmitter. Observations in the current study strongly suggest that if ATP were released ‘in volume’ it would bind to receptors other than P2Y1 receptors, that are possibly on smooth muscle cells, as undoubtedly occurs with picospritzed ATP. However, these receptors appear to be unavailable to the authentic neurotransmitter because fIJPs were absent in the mice with deactivated P2ry1. On the other hand if β-NAD is the dominant neurotransmitter released, it may be selective for P2Y1 receptors over other receptors expressed by smooth muscle cells. Thus, responses to this purine could be mediated by P2Y1 receptors expressed more widely throughout smooth muscle tissues. Taken together current data favour the idea that β-NAD is the primary neurotransmitter and after release from neurons, it binds to P2Y1 receptors on PDGFRα+ cells to generate large outward currents via SK3 channels. However, additional selective gene deactivation studies are needed to fully test this hypothesis.
Analysis of the time courses of fIJPs and sIJPs revealed an interesting difference in the time-to-peak of the sIJP before and after blocking the fIJP. We found sIJPs reached peak hyperpolarization at earlier time points when fIJPs were blocked by MRS2500 in wild-type mice or in mice with genetically deactivated P2ry1. Previously we reported that repolarization of fIJPs is assisted by activation of an inward current that is reset during the fIJP hyperpolarization (Baker et al. 2003). This inward current, and the depolarizing drive it imposes on membrane potential, creates the obvious ‘notch’ between the peaks of the fIJP and sIJP and delays the peak hyperpolarization during the sIJP. Blocking the fIJP hyperpolarization blocks activation of the inward current and the delay imposed on the peak of the sIJP is therefore removed.
Consideration should be given to whether an additional P2Y receptor might participate in post-junctional purinergic responses in human colon. A previous study suggested that a component of purinergic relaxation in taenia coli of guinea pigs was mediated by P2Y11 receptors (King & Townsend-Nicholson, 2008). P2ry11 is not expressed in the mouse (Abbracchio et al. 2006), so our experiments cannot exclude the possibility of P2Y11 receptor involvement in purinergic inhibitory regulation in other species. Functional P2Y11 receptors are expressed in human tissues and couple ATP binding to Ca2+ release via activation of phospholipase Cβ and generation of inositol 1,4,5-trisphosphate (Abbracchio et al. 2006). Thus, binding of a purine transmitter to P2Y11 receptors could conceivably activate SK channels and generate post-junctional responses. β-NAD has also been shown to be an agonist for hP2Y11 receptors (Moreschi et al. 2006); however, there is no evidence that MRS2500, a highly selective P2Y1 receptor antagonist, blocks P2Y11 receptors. In fact MRS2279, another selective P2Y1 receptor antagonist with a closely related structure to MRS2500, was completely inactive on P2Y11 receptors at 30 μm (Boyer et al. 2002). IJPs in human colonic muscles were blocked quantitatively by low concentrations of MRS2500 (0.5–1 μm; Gallego et al. 2011; Hwang et al. 2011), suggesting that P2Y1 receptors also mediate IJPs exclusively in human colon.
P2Y1 receptors may also have been lost from cells in addition to post-junctional cells that mediate IJPs and purinergic motor responses in the P2ry1−/− mice. It is possible that P2Y1 receptors are present in multiple locations within the tunica muscularis. It is unlikely that ganglionic P2Y1 receptors provide a tonic stimulatory effect on motor neurons that if lost would cause a generalized anaesthetic effect on motor neurons. In our experiments both excitatory and inhibitory neurons were normal and active in colons of P2ry1−/− mice as demonstrated by cholinergic and nitrergic responses elicited by electrical field stimulation. It is also unlikely that prejunctional P2Y1 receptors exert requisite facilitation of purine release, and loss of these receptors would then shut down purinergic neurotransmission. Such a pathway would constitute positive feedback (e.g. as β-NAD or ATP are released, they would bind to a prejunctional P2Y1 receptor and facilitate release of additional purines). In fact previous studies have suggested that prejunctional autoreceptors provide feedback inhibition of purine release (Zizzo et al. 2007). Thus, purine neurotransmitter release should be enhanced in P2ry1−/− mice lacking prejunctional P2Y1 receptors.
It should also be noted that while hyperpolarization responses to β-NAD and ADPR were absent in P2ry1−/− mice, small inhibitory responses to β-NAD were noted in mechanical experiments in which muscles were exposed to β-NAD for several minutes (see Fig. 9). There are several possible explanations for the disassociation between electrical and mechanical responses. It is possible that β-NAD activated motor neurons via ganglionic stimulation and the inhibitory effects noted on contractions could be the result of a non-purine inhibitory neurotransmitter. This is unlikely because similar responses to β-NAD were noted before and after TTX, that would block output of motor neurons from ganglia (and TTX blocked post-junctional responses to electrical field stimulation). β-NAD may bind to receptors expressed in smooth muscles other than P2Y1 that are linked to mechanical inhibition but not ion channels (i.e. pharmaco-mechanical coupling). Such effects of β-NAD have not been described. Finally, β-NAD metabolites resulting from breakdown of the primary neurotransmitter in muscles (e.g. adenosine) could mediate inhibitory effects via receptors other than P2Y1 receptors. For example, β-NAD degraded to adenosine upon exposure to guinea pig taenia coli in a previous study (Burnstock & Hoyle, 1985). Adenosine, via P1 receptors, may mediate the inhibitory effects of β-NAD and ADPR on contractions in colons of P2ry1−/− mice, but further studies will be necessary to fully understand responses to metabolites.
In summary, using a genetic model in which P2ry1 was deactivated, we confirmed previous findings, using pharmacological tools, that P2Y1 receptors mediate most of the post-junctional response to enteric purinergic inhibitory neurotransmission. Purinergic neurotransmission via P2Y1 receptors is obviously an important component of neural regulation of colonic motility because pellet propulsion was significantly delayed in P2ry1−/− mice. Our data also provide further evidence that β-NAD and ADPR mimic well the enteric inhibitory purinergic neurotransmitter. Our findings also provide evidence that if ATP (and/or its metabolites) functions as a purinergic neurotransmitter, then these agonists cannot be released ‘in volume’ into the interstitial spaces surrounding smooth muscle cells. If this were the case, receptors other than P2Y1 receptors would bind ATP and contribute to enteric inhibitory responses. This scenario is not consistent with pharmacological studies or, as shown in the present study, studies of genetic models in which blockade or genetic deactivation of P2Y1 receptors causes loss of purinergic motor responses. The post-junctional site of P2Y1 receptors and/or degree of compartmentalization of the purine transmitter will be important questions to investigate in future studies of this regulatory pathway.
Acknowledgments
These studies were supported by a Program Project Grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIH; P01 DK41315-22). The authors are grateful to Nancy Horowitz for maintenance of the colony of P2ry1−/− mice.
Glossary
Abbreviations
- ADPR
adenosine diphosphate ribose
- AUT
area under the trace
- EFS
electrical field stimulation
- EJP
excitatory junction potential
- fIJP
fast inhibitory junction potential
- GI
gastrointestinal
- KRB
Krebs–Ringer buffer
- sIJP
slow inhibitory junction potential
- TTX
tetrodotoxin
Author contributions
All the experiments were carried out in the Department of Physiology and Cell Biology, University of Nevada School of Medicine. S.J.H., P.J.B. and L.D. performed the experiments; V.M.-Y., K.M.S. and S.M.W. designed the experiments; and S.J.H., K.M.S. and S.M.W. drafted and critically revised the manuscript. All authors approved the final version of the manuscript.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII. Update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Mutafova-Yambolieva V, Monaghan K, Horowitz B, Sanders KM, Koh SD. Mechanism of active repolarization of inhibitory junction potential in murine colon. Am J Physiol Gastrointest Liver Physiol. 2003;28:G813–G821. doi: 10.1152/ajpgi.00115.2003. [DOI] [PubMed] [Google Scholar]
- Banks BE, Brown C, Burgess GM, Burnstock G, Claret M, Cocks TM, Jenkinson DH. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979;282:415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Bennett MR. Transmission from intramural excitatory nerves to the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1966;185:132–147. doi: 10.1113/jphysiol.1966.sp007976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J, Adams M, Ravi RG, Jacobson KA, Harden TK. 2-Chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine −3′,5′-bisphosphate is a selective high affinity P2Y1 receptor antagonist. Br J Pharmacol. 2002;135:2004–2010. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990;345:346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- Burnstock G, Campbell G, Bennett M, Holman M. Inhibition of the smooth muscle of the taenia coli. Nature. 1963;200:581–582. doi: 10.1038/200581a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Hoyle CH. Actions of adenine dinucleotides in the guinea-pig taenia coli: NAD acts indirectly on P1-purinoceptors; NADP acts like a P2-purinoceptor agonist. Br J Pharmacol. 1985;84:825–831. doi: 10.1111/j.1476-5381.1985.tb17376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist JR, He XD, Goyal RK. Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea-pig ileum circular muscle. J Physiol. 1992;447:119–131. doi: 10.1113/jphysiol.1992.sp018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel HH, Thornbury KD, Ward SM, Sanders KM. Involvement of nitric oxide synthetic pathway in inhibitory junction potentials in canine proximal colon. Am J Physiol Gastrointest Liver Physiol. 1991;260:G789–G792. doi: 10.1152/ajpgi.1991.260.5.G789. [DOI] [PubMed] [Google Scholar]
- Durnin L, Hwang SJ, Ward SM, Sanders KM, Mutafova-Yambolieva VN. Adenosine 5′-diphosphate-ribose (ADPR) is a neural regulator in primate and murine large intestine along with β-NAD+ J Physiol. 2012. DOI: 10.1113/jphysiol.2011.222414. [DOI] [PMC free article] [PubMed]
- Fabre JE, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, Koller BH. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- Gallego D, Gil V, Aleu J, Aulí M, Clavé P, Jiménez M. Purinergic and nitrergic junction potential in the human colon. Am J Physiol Gastrointest Liver Physiol. 2008a;295:G522–G533. doi: 10.1152/ajpgi.00510.2007. [DOI] [PubMed] [Google Scholar]
- Gallego D, Gil V, Aleu J, Martinez-Cutillas M, Clavé P, Jimenez M. Pharmacological characterization of purinergic inhibitory neuromuscular transmission in the human colon. Neurogastroenterol Motil. 2011;23:792–802. doi: 10.1111/j.1365-2982.2011.01725.x. [DOI] [PubMed] [Google Scholar]
- Gallego D, Hernández P, Clavé P, Jiménez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G584–G594. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- Gallego D, Vanden BergheP, Farré R, Tack J, Jiménez M. P2Y1 receptors mediate inhibitory neuromuscular transmission and enteric neuronal activation in small intestine. Neurogastroenterol Motil. 2008b;20:159–168. doi: 10.1111/j.1365-2982.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- Grasa L, Gil V, Gallego D, Martín MT, Jiménez M. P2Y1 receptors mediate inhibitory neuromuscular transmission in the rat colon. Br J Pharmacol. 2009;158:1641–1652. doi: 10.1111/j.1476-5381.2009.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol. 1999;517:575–590. doi: 10.1111/j.1469-7793.1999.0575t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS. Mechanisms of peristalsis. Br Med Bull. 1979;35:263–268. doi: 10.1093/oxfordjournals.bmb.a071587. [DOI] [PubMed] [Google Scholar]
- Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the WWν mouse small intestine. J Auton Nerv Syst. 2000;80:142–147. doi: 10.1016/s0165-1838(00)00089-8. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587:4887–4904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, Sanders KM, Mutafova-Yambolieva VN. β-Nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology. 2011;140:608–617. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keef KD, Du C, Ward SM, McGregor B, Sanders KM. Enteric inhibitory neural regulation of human colonic circular muscle: role of nitric oxide. Gastroenterology. 1993;105:1009–1016. doi: 10.1016/0016-5085(93)90943-7. [DOI] [PubMed] [Google Scholar]
- King BF, Townsend-Nicholson A. Involvement of P2Y1 and P2Y11 purinoceptors in parasympathetic inhibition of colonic smooth muscle. J Pharmacol Exp Ther. 2008;324:1055–1063. doi: 10.1124/jpet.107.131169. [DOI] [PubMed] [Google Scholar]
- Koh SD, Dick GM, Sanders KM. Small-conductance Ca2+-dependent K+ channels activated by ATP in murine colonic smooth muscle. Am J Physiol Cell Physiol. 1997;273:C2010–C2021. doi: 10.1152/ajpcell.1997.273.6.C2010. [DOI] [PubMed] [Google Scholar]
- Kurahashi M, Zheng H, Dwyer L, Ward SM, Don KohS, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan KP, Koh SD, Ro S, Yeom J, Horowitz B, Sanders KM. Nucleotide regulation of the voltage-dependent nonselective cation conductance in murine colonic myocytes. Am J Physiol Cell Physiol. 2006;291:C985–C994. doi: 10.1152/ajpcell.00112.2006. [DOI] [PubMed] [Google Scholar]
- Moreschi I, Bruzzone S, Nicholas RA, Fruscione F, Sturla L, Benvenuto F, Usai C, Meis S, Kassack MU, Zocchi E, De Flora A. Extracellular NAD+ is an agonist of the human P2Y11 purinergic receptor in human granulocytes. J Biol Chem. 2006;281:31419–32429. doi: 10.1074/jbc.M606625200. [DOI] [PubMed] [Google Scholar]
- Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. β-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil. 2008;20(Suppl. 1):39–53. doi: 10.1111/j.1365-2982.2008.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Ward SM. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol Gastrointest Liver Physiol. 1992;262:G379–G392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- Serio R, Alessandro M, Zizzo MG, Tamburello MP, Mulè F. Neurotransmitters involved in the fast inhibitory junction potentials in mouse distal colon. Eur J Pharmacol. 2003;460:183–190. doi: 10.1016/s0014-2999(02)02923-0. [DOI] [PubMed] [Google Scholar]
- Shuttleworth CW, Keef KD. Roles of peptides in enteric neuromuscular transmission. Regul Pept. 1995;56:101–120. doi: 10.1016/0167-0115(95)00013-2. [DOI] [PubMed] [Google Scholar]
- Shuttleworth CWR, Conlon SB, Sanders KM. Regulation of citrulline recycling in nitric oxide-dependent neurotransmission in the murine proximal colon. Br J Pharmacol. 1997;20:707–713. doi: 10.1038/sj.bjp.0700949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Distension-evoked ascending and descending reflexes in the circular muscle of guinea-pig ileum: an intracellular study. J Auton Nerv Syst. 1990;29:203–218. doi: 10.1016/0165-1838(90)90146-a. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Bywater RAR, Holman ME, Taylor GS. Spontaneous and evoked inhibitory junction potentials in the circular muscle layer of colon. J Auton Nerv Syst. 1998;69:115–121. doi: 10.1016/s0165-1838(98)00012-5. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol. 2001;533:787–799. doi: 10.1111/j.1469-7793.2001.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark ME, Bauer AJ, Szurszewski JH. Effect of nitric oxide on circular muscle of the canine small intestine. J Physiol. 1991;444:743–761. doi: 10.1113/jphysiol.1991.sp018904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg P. Physiologische und pharmakologische Versuche über die Dünndarmperistaltik. Arch Exp Pathol Pharmakol. 1917;81:51–129. doi: 10.1007/s00210-006-0052-7. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Goyal RK. Activation of small conductance Ca2+-dependent K+ channels by purinergic agonists in smooth muscle cells of the mouse ileum. J Physiol. 1997;502:497–508. doi: 10.1111/j.1469-7793.1997.497bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GD, Wang XY, Hu HZ, Liu S, Gao N, Fang X, Xia Y, Wood JD. Inhibitory neuromuscular transmission mediated by the P2Y1 purinergic receptor in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1483–G1489. doi: 10.1152/ajpgi.00450.2006. [DOI] [PubMed] [Google Scholar]
- Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- Waterman SA, Costa M. The role of enteric inhibitory motoneurons in peristalsis in the isolated guinea-pig small intestine. J Physiol. 1994;477:459–468. doi: 10.1113/jphysiol.1994.sp020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SA, Costa M, Tonini M. Enteric inhibitory reflexes mediate accommodation in the isolated guinea-pig small intestine. J Physiol. 1994;474:539–546. doi: 10.1113/jphysiol.1994.sp020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Farrugia G, Sarr MG, Szurszewski JH. ATP is a mediator of the fast inhibitory junction potential in human jejunal circular smooth muscle. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1373–G1379. doi: 10.1152/ajpgi.1999.276.6.G1373. [DOI] [PubMed] [Google Scholar]
- Zizzo MG, Mulè F, Serio R. Inhibitory purinergic transmission in mouse caecum: role for P2Y1 receptors as prejunctional modulators of ATP release. Neuroscience. 2007;150:658–664. doi: 10.1016/j.neuroscience.2007.09.055. [DOI] [PubMed] [Google Scholar]


