Abstract
During ageing, immobilization periods increase and are partially responsible of sarcopaenia by inducing a muscle atrophy which is hardly recovered from. Immobilization-induced atrophy is due to an increase of muscle apoptotic and proteolytic processes and decreased protein synthesis. Moreover, previous data suggested that the lack of muscle mass recovery might be due to a defect in protein synthesis response during rehabilitation. This study was conducted to explore protein synthesis during reloading and leucine supplementation effect as a nutritional strategy for muscle recovery. Old rats (22–24 months old) were subjected to unilateral hindlimb casting for 8 days (I8) and allowed to recover for 10–40 days (R10–R40). They were fed a casein (±leucine) diet during the recovery. Immobilized gastrocnemius muscles atrophied by 20%, and did not recover even at R40. Amount of polyubiquitinated conjugates and chymotrypsin- and trypsin-like activities of the 26S proteasome increased. These changes paralleled an ‘anabolic resistance’ of the protein synthesis at the postprandial state (decrease of protein synthesis, P-S6 and P-4E-BP1). During the recovery, proteasome activities remained elevated until R10 before complete normalization and protein synthesis was slightly increased. With free leucine supplementation during recovery, if proteasome activities were normalized earlier and protein synthesis was higher during the whole recovery, it nevertheless failed in muscle mass gain. This discrepancy could be due to a ‘desynchronization’ between the leucine signal and the availability of amino acids coming from casein digestion. Thus, when supplemented with leucine-rich proteins (i.e. whey) and high protein diets, animals partially recovered the muscle mass loss.
Key points
During ageing, there is a lack of recovery of muscle mass following immobilization.
We showed, in old rats, an ‘anabolic resistance’ of muscle protein synthesis to food intake during immobilization and only a slight increase of protein synthesis during the recovery, which explain a poor muscle nitrogen balance that is insufficient to induce a muscle mass gain.
A supplementation with free leucine, an essential amino acid known to stimulate muscle protein metabolism, was efficient in inducing a greater anabolism but failed to induce muscle mass recovery.
This discrepancy was explained by a ‘desynchronization’ between the leucine signal and amino acids coming from dietary protein digestion.
An induction of a larger increase and a longer availability of amino acids in the postprandial state with rich-protein leucine (i.e. whey) and high protein diets were efficient in inducing a muscle mass recovery after immobilization.
Introduction
Sarcopaenia is the progressive loss of muscle mass and strength associated with normal ageing (Rosenberg 1989). This phenomenon is a highly predictive factor of frailty, of limited mobility, of increased susceptibility to injury and of impaired recovery (Harris 1997). Many mechanisms have been proposed to explain sarcopaenia. Besides chronic and progressive loss, periods of immobilization or acute inactivity which increase with age may contribute by themselves to muscle atrophy. Thus, the unloading-induced atrophy due to limb immobilization, chronic bed-rest or lack of physical inactivity impairs muscle mass and functions in the elderly (Bar-Shai et al. 2005). However, the effect of disuse by itself on skeletal muscle in elderly individuals has not been extensively investigated and the subsequent recovery ability has been even less studied despite the fact that we and others have shown an impaired recovery in old animals and elderly humans after immobilization-induced muscle atrophy (Chakravarthy et al. 2000; Suetta et al. 2009; Magne et al. 2011). These periods of uncompleted recoveries repeated over time may contribute to a significant muscle mass loss and then worsen sarcopaenia, and this phenomenon has been named the ‘acute catabolic crisis’ model (English & Paddon-Jones, 2010).
At a cellular level, muscle atrophy following immobilization results from an imbalance of protein turnover as the maintenance of skeletal muscle mass depends on the overall balance between rates of protein synthesis and protein breakdown. In adults, protein synthesis has been reported to be reduced at the postabsorptive state (Gibson et al. 1987) and muscle protein breakdown increased (Loughna et al. 1986; Taillandier et al. 1993, 1996; Lawler 2003; Krawiec et al. 2005; Vazeille et al. 2008) leading to the muscle protein loss. Muscle mass recovery after unloading is also dependent on the generation of a sustained positive nitrogen balance which results from normalization of changes in protein metabolism associated with an increased protein synthesis, decreased proteolysis, or simultaneous changes in both processes. Thus, during recovery in adults, muscle protein synthesis was increased (Booth 1982) and muscle protein breakdown was down-regulated, normalized or increased according to the model of immobilization used (Tilignac et al. 2002; Taillandier et al. 2003; Minnaard et al. 2005; Vazeille et al. 2008), which allowed a muscle mass recovery over time after reloading. We have shown that during ageing, mechanisms of casting-induced-atrophy were similar to those observed in adult animals, i.e. an increased ubiquitin–proteasome-dependent proteolysis and apoptosis in skeletal muscle (Magne et al. 2011). However, we recorded a lack of muscle mass recovery, which was not due to a sustained activation of these pathways after reloading as they were rapidly normalized during the recovery period. If muscle protein synthesis seems to be reduced after an immobilization period in elderly people (Kortebein et al. 2007), no data on muscle protein synthesis during recovery are available. Our previous work strongly suggests that protein synthesis could be impaired during the recovery period (Magne et al. 2011) and we have hypothesized that this alteration may explain the absence of a positive nitrogen balance and subsequently the lack of recovery of muscle mass we observed during ageing. If protein synthesis is indeed impaired during reloading, finding ways to stimulate it may be essential.
It is now well established that feeding is a robust stimulator of muscle protein synthesis (Kimball et al. 2000; Bohe et al. 2003; Moore et al. 2009). However, it is also well known that the stimulating effect of food intake on muscle anabolism is already less efficient in both old animals and elderly humans and this without any pathologies or physical inactivity (reviewed in Balage & Dardevet, 2010). These alterations of muscle protein synthesis response have been partially explained by the presence of a low grade inflammation and/or oxidative stress development with age (Marzani et al. 2008; Rieu et al. 2009). Interestingly, we and others have previously shown that immobilization generated a further increase in the intramuscular inflammatory and oxidative stress markers (Kondo et al. 1991; Biolo et al. 2008; Magne et al. 2011), which could further alter protein synthesis response to food intake and then contribute to the total lack of muscle mass recovery in the elderly after reloading. Then, during the recovery period, the postprandial phase should play a critical role since it is during this period that the major anabolic factors regarding protein metabolism are elevated.
Amino acids and particularly leucine play an important role in the stimulation of postprandial muscle protein synthesis (Anthony et al. 1999, 2001; Koopman et al. 2005). Leucine is known as a ‘nutrient signal’ as it reduces muscle protein breakdown and further stimulates muscle protein synthesis (Buse & Reid, 1975; Frexes-Steed et al. 1992; Crozier et al. 2005) particularly during ageing (Dardevet et al. 2002; Rieu et al. 2003, 2006, 2007; Combaret et al. 2005; Katsanos et al. 2006). The ‘leucine signal’ stimulates the mammalian target of rapamycin (mTOR) pathway and 70 kDa ribosomal protein S6 kinase (p70S6 kinase) activity, and enhances eIF4E-binding protein (4EBP1) phosphorylation and the association of eukaryotic initiation factor 4E (eIF4E) with eukaryotic initiative factor 4G (eIF4G) both in vitro and in vivo (Anthony et al. 2000; Dardevet et al. 2000; Bolster et al. 2004). These signalling pathways are known to become resistant to amino acid (i.e. leucine) stimulation in the case of chronic inflammation or oxidative stress (Lang et al. 2002; Marzani et al. 2008; Balage et al. 2009), phenomena that occur during immobilization in aged muscle (Magne et al. 2011). We hypothesized that following immobilization, a sustained resistance of muscle protein synthesis to leucine persisted and that increasing leucine intake could have a beneficial effect on muscle mass recovery by making the nitrogen balance positive after reloading. To our knowledge, no study has yet tested such supplementation in aged muscle recovering from immobilization. The aims of this study were to (1) determine if muscle protein synthesis response to food intake is altered during atrophy following immobilization and remains resistant to food intake stimulation during the subsequent recovery, and (2) test the effect of a leucine supplementation on muscle mass recovery by measuring muscle protein synthesis, proteolysis and signalling proteins associated with these pathways in both the postabsorptive and postprandial states.
Methods
Animals and experimental design
All procedures were performed in accordance with institutional guidelines on animal experimentation in France and comply with the policies and regulations of The Journal of Physiology given by Drummond (2009). Male Wistar rats aged 22–24 months were housed individually under controlled environmental conditions (room temperature 22°C; 12 h light–dark cycle, light period starting at 08.00 h), fed ad libitum a standard 13% casein diet (Table 1) and given free access to water.
Table 1.
Composition of the standard and the experimental diets
| Standard diet | Control and experimental diets | ||
|---|---|---|---|
| CAS | CAS + ALA | CAS + LEU | |
| Ingredients | |||
| Casein | 166 | 166 | 166 |
| l-Cystine | 1.8 | 1.8 | 1.8 |
| Alanine1 | — | 59 | — |
| Leucine2 | — | — | 44.5 |
| Valine2 | — | — | 5.1 |
| Isoleucine | — | — | 9.8 |
| Colza oil | 30 | 30 | 30 |
| Peanut oil | 27 | 27 | 27 |
| Sunflower oil | 3 | 3 | 3 |
| Cellulose | 35 | 35 | 35 |
| Saccharose | 100 | 100 | 100 |
| lactose | 134 | 134 | 134 |
| Wheat flour | 458.2 | 399.2 | 398.8 |
| Mineral mixture AIN93 | 35 | 35 | 35 |
| Vitamins mixture AIN93 | 10 | 10 | 10 |
| given during | adaptation immobilization | recovery | recovery |
Quantities are expressed in g/kg dry matter.
Alanine was included in the control CAS+ALA diet to render the diets isonitrogenous. This amino acid has no effect on muscle protein metabolism.
Valine and isoleucine were included in the CAS+LEU diet to prevent the fall of their plasma concentrations induced by leucine supplementation. CAS: casein; CAS+ALA: casein + alanine; CAS+LEU: casein + leucine.
After a 3 week adaptation period, 143 rats were anaesthetized with isoflurane inhalation and subjected to unilateral hindlimb cast immobilization with an Orfit-soft plaque (Gibaud, France) for 8 days (I8). The foot was positioned in plantar extension to induce maximal atrophy of the gastrocnemius muscle (Goldspink, 1977; Booth, 1982; Krawiec et al. 2005; Vazeille et al. 2008; Magne et al. 2011). For muscle recovery, casts were removed and animals were allowed to recover for 10 (R10), 20 (R20), 30 (R30) or 40 (R40) days. All rats were fed the standard 13% casein diet during immobilization. Then half of the animals were fed a control diet (casein (CAS) + alanine (ALA)) and constituted the control (CON) group; the other half were fed a 4.45% leucine-supplemented diet (CAS + leucine (LEU)) and were the LEU group (Table 1). The CAS + LEU diet was supplemented with leucine to increase plasma leucine concentration. To prevent the fall of plasma valine and isoleucine concentrations induced by leucine supplementation, the CAS + LEU diet was also supplemented with appropriate amounts of these amino acids Rieu et al. 2003). Alanine, an amino acid that has no effect on muscle protein metabolism, was included in the control CAS + ALA diet to render the diets isonitrogenous.
Casted rats reduced their food intake during the immobilization period. Therefore, 39 control non-casted rats were pair-fed (PF) to the casted group (18 at I8 and 21 at R20). Seventeen rats were studied as a reference point before the immobilization period (I0).
Before immobilization (I0) and at the end of the immobilization (I8) or recovery periods (R10, R20, R30, R40), animals were killed under pentobarbital sodium anaesthesia (50 mg kg−1i.p.). On the morning of each time point studied, half of the rats in each group were not fed, so that they were in a postabsorptive state. These rats were designed ‘PA’ (PA CON and PA LEU). The others ate their respective diet (i.e. CAS + ALA or CAS + LEU) for 1 h and then were in the postprandial state; they were named ‘PP’ (PP CON and PP LEU). Non-casted pair-fed rats were also studied in the PA and the PP states using the same procedure (PA standard diet and PP standard diet).
Measurements of in vivo protein synthesis
Protein synthesis rates were measured using the flooding-dose method. Each rat was injected intravenously with [1-13C]valine (99%) (150 μmol (100 g body weight)−1), 40 min before killing (i.e. 110–140 min after the beginning of the experimental diets), to flood the precursor pool with [1-13C]valine as previously described (Mosoni et al. 1996). Rats were then killed under pentobarbital sodium anaesthesia (50 mg kg−1i.p.). Blood was withdrawn from the aorta, and hindlimb gastrocnemius muscles were carefully dissected, weighed and frozen in liquid nitrogen.
Free and bound valine enrichments were determined as follows. Muscles were powdered in liquid nitrogen in a ball mill (Dangoumeau, Prolabo, Paris, France). A 200 mg aliquot of frozen muscle powder was homogenized in 2 ml of 10% trichloroacetic acid (TCA). Homogenates were centrifuged (10,000 g, 15 min, 4°C) and supernatants, containing free amino acids, were desalted by cation-exchange chromotography (AG 50×8, 100–200 mesh, H+ form, Bio-Rad, Richmond, CA, USA) in minidisposal columns. Valine and other amino acids were eluted with 4 mol l−1 NH4OH. After evaporation of NH4OH under vacuum, free amino acids were resuspended in 0.01 mol l−1 HCl for enrichment measurements. TCA-insoluble materials were washed 3 times in 4 volumes of cold 10% TCA and once in 4 volumes of 0.2 mol l−1 perchloric acid (PCA). Resultant pellets were resuspended in 0.3 mol l−1 NaOH and incubated at 37°C for 1 h. Protein concentration was determined using the bicinchoninic procedure. Proteins were precipitated with 20% PCA overnight at 4°C, and samples centrifuged (10,000 g, 5 min, 4°C). The protein pellet was hydrolysed in 6 mol l−1 HCl at 110°C for 24 h. HCl was removed by evaporation and amino acids purified by cation-exchange chromotography as described above. Measurement of free valine enrichment was done as its t-butyldimethylsilyl derivative by gas chromatography electron impact mass spectrometry, using a gas chromatograph coupled to an organic mass spectrometer quadrupole (GC-M; Hewlett-Packard 5971A (Hewlett-Packard Co., Palo Alto, CA, USA)). Enrichment of [1-13C]valine into muscle proteins was measured as its N-acetyl-propyl derivatives by gas chromatography–combustion–isotope ratio mass spectrometry (GC-c-IRMS ‘Isoprime’ (Elementar France, Lyon, France).
Calculations
The absolute synthesis rate (ASR) was calculated from the product of the protein fractional synthesis rate (FSR) and the protein content of the tissue and expressed in mg day−1. FSR (in % day−1) was calculated from the formula: FSR =Sb× 100/Sa×t, where Sb is the enrichment at time t (minus natural basal enrichment of protein) of the protein-bound valine, t is the incorporation time in days, and Sa is the mean enrichment of free tissues valine between time 0 and t. The mean Sa enrichment was the Sa (t1/2) value calculated from the linear regression obtained in tissue between time 0 and time t.
Plasma amino acid measurements
Plasma amino acid concentrations were determined for each group at I0, I8, R20 and R40. Plasma amino acids were purified, i.e. 500 μl of plasma was added to 125 μl of sulfosalicylic acid solution (1 mol l−1 in ethanol with 0.5 mol l−1 thiodiglycol) previously completely evaporated. Norleucine (100 μl) was added as an internal standard. Amino acid concentrations were determined using an automated amino acid analyser with BTC 2410 resin (Biotronic LC 3000, Roucaire, Velizy, France).
Measurement of proteasome activities
In this pathway, two distinct steps are depicted: (1) the ubiquitination of proteins and (2) their degradation by the 26S proteasome. We first investigated the chrymotrypsin- and the trypsin-like activity of the proteasome.
One hundred and fifty milligrams of gastrocnemius muscle powder from pair-fed, non-immobilized and immobilized muscles at each time point was homogenized in 10 volumes of an ice-cold buffer containing 50 mm Tris-Cl (pH 7.5), 5 mm MgCl2, 250 mm sucrose, 1 mm dithiothreitol (DTT), 10 nm adenosine triphosphate (ATP) and protease inhibitors (10 μg ml−1 antipain, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin, 10 μg ml−1 pepstatin A, 20 μm phenylmethanesulphonyl fluoride (PMSF)).
Briefly, extracts were centrifuged at 10,000 g for 20 min at 4°C. Supernatants were then centrifuged at 150,000 g for 30 min at 4°C and resulting supernatants were finally centrifuged at 150,000 g for 2.5 h at 4°C. The resulting protein pellets were resuspended in 150 μl of a buffer containing 20% glycerol, 5 mm MgCl2 and 50 mm Tris-Cl (pH 7.5) (Buffer A). Protein concentration was determined on these resuspended pellets (Bio-Rad assay). The peptidase activities of the proteasome (chymotrypsin-like and trypsin-like activities) were determined by measuring the hydrolysis of the fluorogenic substrates succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin (LLVY-AMC) (Sigma, USA) and the Boc-Leu-Arg-Arg-7-amido-4-methylcoumarin (LRR-AMC) (Enzo Life Sciences, Exeter, UK), respectively. To measure the proteasome chymotrypsin-like and trypsin-like activities, 15 μg of proteins from the resuspended pellets diluted in 15 μl of Buffer A were added to 60 μl of medium containing 50 mm Tris-Cl (pH 7.5), 11.25 mm MgCl2, 1.25 mm DTT, 0.01 U apyrase and 300 μm LLVY-AMC or 800 μm LRR-AMC. Pilot experiments were performed with or without inhibitors of the chymotrypsin-like activity (MG132, Affiniti) to ensure that the activities were totally inhibited. The trypsin-like activity was measured with and without the specific inhibitor lactacystin (lactacystin, 100 μm, Sigma). Both activities were determined by measuring the accumulation of the fluorogenic cleavage product (amido-4-methylcoumarin; AMC) using a luminescence spectrometer FLX800 (Biotek, USA) during 45 min at 380 nm excitation wavelength and 440 nm emission wavelength. The time course for the accumulation of AMC after hydrolysis of the substrate was analysed by linear regression to calculate activities, i.e. the slopes of best fit of accumulation of AMC vs. time.
Analysis of muscle protein synthesis and proteolysis signalling pathways by Western blotting
Powder (300 mg) of gastrocnemius muscles was homogenized in 10 volumes of a buffer containing 1 mm dithiothreitol (DTT), 0.1 mm phenylmethanesulphonyl fluoride (PMSF), 1 mm benzamidine and 0.5 mm sodium vanadate. Extracts were then centrifuged at 9,500 rpm for 12 min at 4°C. Aliquots of supernatants were diluted in sample buffer, boiled for 5 min, and stored at −20°C until protein immunoblot analyses. Equal amounts of proteins were separated by SDS–PAGE and transferred to PVDF membranes (GE Healthcare, Orsay, France).
Muscle proteasome proteolysis pathway
To explore the first step of the ubiquitin–proteasome-dependant proteolysis (tagging of proteins by ubiquitin before their recognition by the proteasome) the anti-ubiquitinylated proteins antibody, which recognizes poly-ubiquitin chains (Millipore, USA), was used at 1:2000 dilution. Twenty-five micrograms of proteins was separated on 7% acrylamide gels. FoxO3a is a transcription factor involved in atrogene transcription. When phosphorylated, its nuclear translocation is impossible. Thus, the ratio between FoxO3a and its phosphorylated form traduces an inhibition of atrogene transcription. The abundance of transcription factor FoxO3a and its phosphorylated form phospho-FoxO3a (Ser253) was determined using appropriate antibodies (Cell Signaling Technology, Inc., Danvers, MA, USA) at 1:1000 and 1:1000 dilution, respectively. Fifty micrograms of proteins was separated on 7.5% acrylamide gels.
Muscle protein synthesis pathway
Immunoblotting was performed using appropriate antibodies: S6 and phospho-S6 (Ser235/236) (Cell Signaling Technology) at 1:6000 and 1:6000 dilution, respectively. To determine the amount of S6, 25 μg of proteins was separated on 15% acrylamide gels, and 30 μg of proteins was separated on 12% acrylamide gels to quantify amount of phospho-S6. The amount of total 4EBP1 (α, β and γ forms) was determined on 50 μg of proteins separated on 15% acrylamide gels, using an antibody (Cell Signaling Technology) at 1:4000 dilution.
Signals were detected using the ECL+ detection kit (GE Healthcare, France) after exposition onto radiographic film (Hyperfilm ECL, GE Healthcare) and quantified by densitometry using the ImageJ software. Signals were normalized against the signal obtained with a pool of samples used as reference.
Index of muscle functionality
Muscles are composed of different fibre types which have different metabolic and contractile properties. As myosin is the most abundant contractile protein, muscle functionality is linked to the muscle myosin content. Myosin content was determined on total protein extracts according to the method described by Mizunoya et al. (2008). Briefly, 5 μg of proteins was separated on 40% acrylamide–2% acrylamide-bisacrylamide gel during 15 h at 140 V. Gels were then coloured with Coomasie Blue and quantified with ImageQuant software (GE Healthcare).
Pilot experiment: whey diet and high protein diet supplementations
To improve our nutritional strategy after the free leucine supplementation, a pilot experiment was carried out. Forty-one animals were subjected to cast immobilization for 8 days and then allowed to recover during 20 (R20) and 40 (R40) days. Eight animals were killed at I8 and the others were divided in two groups: the first one (n= 16) received a soluble milk protein diet and the second one (n= 17) received a high protein diet. Seven animals were used as control before casting (I0). All animals were studied in the postprandial state.
Soluble milk proteins diet was composed of (in g kg−1 diet): 144 whey (Prolacta, Lactalis ingredients), 5.7 proline, 30 colza oil, 3 sunflower oil, 27 peanut oil, 35 cellulose, 35 AIN93 mineral mix, 10 AIN93 vitamin mix, 2.5 cholin, 100 saccharose, 126 lactose and 482 wheat flour. High protein diet was composed of (in g kg−1 diet): 156 casein (Lactalis ingredients), 144 whey (source = Prolacta, Lactalis ingredients), 30 colza oil, 3 sunflower oil, 27 peanut oil, 35 cellulose, 35 AIN93 mineral mix, 10 AIN93 vitamin mix, 2.5 cholin, 100 saccharose, 124 lactose and 334 wheat flour.
Muscle protein synthesis was measured with the flooding dose method as described previously.
Statistical analysis
All data are expressed as means ± SEM. Food intake and body weight comparisons were assessed using repeated measures analysis of variance (StatView statistical software package, v. 5, SAS Institute, Cary, NC, USA). Other measurements were analysed using a two-way ANOVA (time, diet). When significant differences were detected by ANOVA, post hoc comparisons between groups were made using Fisher's PLSD test. Significance was defined at the P < 0.05 level.
Results
Parameters of non-casted pair-fed animals
Food intake of the pair-fed group perfectly matched the one of the casted group during immobilization (11.29 ± 0.06 g day−1 at I8) and recovery (17.86 ± 1.09 g day−1 at R20). The body weight of pair-fed animals decreased slightly during the experimental protocol (−4.4% at I8 and 10.1% at R20). However, gastrocnemius muscle mass from these rats was stable at each point measured (2.498 ± 0.038 g at R20 vs. 2.553 ± 0.042 g at I8). Except the amount of polyUb conjugates which slightly increased at I8 compared to I0 (+30%, P < 0.05 using a Student's t test I8 vs. I0), before returning to I0 value, all the others parameters did not change from I0 to the end of the recovery period (data not shown).
Animal characteristics
Food intake was 16.89 ± 0.42 g day−1 (I0) and decreased by 65% the first day of cast-immobilization (Fig. 1A). Then, animals increased their food intake up to I8 (11.19 ± 0.29 g day−1). The nutritional intervention started the first day of the recovery period (R1). Food intake was similar in the two groups during the whole recovery period (Fig. 1A).
Figure 1. Food intake (A) and body weight (B) of casted and non-casted PF rats.
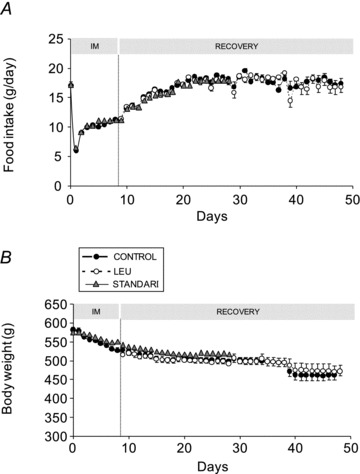
Food intake and body weight of casted and non-casted rats are similar during the whole experiment between the CON and the LEU groups and the STANDARD diet group. IM: immobilization period. Data are means ± SEM.
Casted rats exhibited a slight decrease of body weight during immobilization (−8.6% at I8, not significantly different from the pair-fed group). The decrease of body weight continued during the recovery period whatever the experimental diet given (−18.8 and −18.9% in CON and LEU group respectively at I8) (Fig. 1B).
Plasma amino acid concentrations
In rats, postprandial total amino acid concentrations and essential amino acid concentrations were not affected by the immobilization (1900.5 ± 145.0 and 1995.0 ± 144.8 μmol l−1 for total amino acid concentrations at I0 and I8, respectively) (Table 2). The nutritional intervention had no effect on total and essential amino acid concentration after 20 (R20) and 40 (R40) days of recovery. During this period, only plasma free leucine differed between the two diets. Indeed, leucine was markedly higher in the LEU group (∼×2.5 and ×3.5 at R20 and R40, respectively, P < 0.05 LEU group vs. CON group).
Table 2.
Plasma amino acid concentration in rats chronically fed casein or leucine in the postprandial state
| I0 | I8 | R20 CAS | R20 LEU | R40 CAS | R40 LEU | |
|---|---|---|---|---|---|---|
| Essential amino acids | 859.5 ± 70.6 | 885.7 ± 52.2 | 766.0 ± 40.7 | 830.3 ± 66.1 | 722.8 ± 120.6 | 863.1 ± 66.8 |
| Leucine | 62.6 ± 2.7 | 78.6 ± 6.0 | 87.7 ± 7.1 | 211.7 ± 19.3* | 60.8 ± 4.0 | 214.3 ± 27.0* |
| Total amino acids | 1900.5 ± 145.0 | 1995.0 ± 144.5 | 1792.2 ± 95.5 | 1771.5 ± 173.4 | 1569.4 ± 208.6 | 1849.6 ± 131.8 |
Amino acid concentrations are presented at the postprandial before (I0) and after (I8) the immobilization period and also during the recovery period (R20 and R40; 10 and 40 days of recovery). Values explained in μmol/L are means ± SEM. CAS: casein; LEU: leucine.
P < 0.05, LEU vs. CAS.
Effect of the leucine supplemented-diet on ubiquitin–proteasome-dependent pathways
Figure 2A shows an increased amount of polyUb conjugates at I8 (+70%, P < 0.05 vs. I0) which normalized as soon as R10. No effect of the diet was observed during the recovery period. Both chymotrypsin- (Fig. 2B) and trypsin-like activities (Fig. 2C) were increased at I8 (+42 and +27%, respectively, P < 0.05 vs. I0). Both activities were still elevated at R10 within the CON group but were largely decreased with the leucine supplementation (∼−18% and ∼−30% for chymotrypsin- and trypsin-like activity, respectively, P < 0.05, CON group vs. LEU group). Activities were then similar between the two groups. phospho-FoxO3a/FoxO3a ratio was not different between the two diets until R20. At R30, the ratio was largely increased in the LEU group (+80%, P < 0.05 vs. CON group) (Fig. 2D).
Figure 2. Ubiquitin–proteasome-dependent proteolysis in immobilized gastrocnemius muscles before and after the free leucine supplementation.
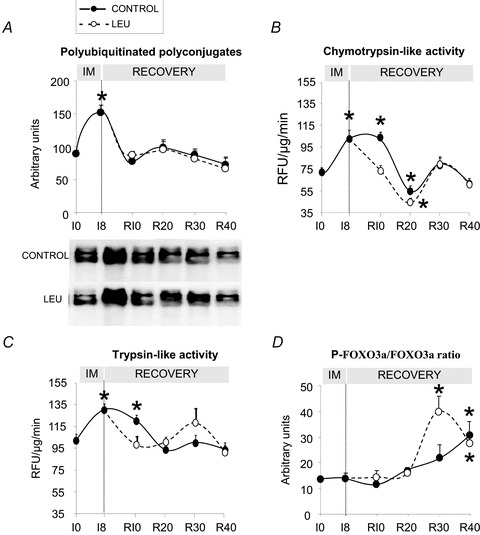
A, accumulation of polyubiquitinated polyconjugates was assessed on 25 μg of proteins by immunoblotting using an antibody that recognizes polyubiquitin chains. B and C, the chymotrypsin-like activity (B) and trypsin-like activity (C) of the proteasome were measured by using the fluorogenic substrate succinyl-LLVY-AMC and Boc-LRR-AMC as indicated in Methods. Data are expressed in relative fluorescence units (RFU μg−1 min−1). D, phospho-FoxO3a/FoxO3a ratio traduces an anti-proteolytic potential. FoxO3a and its phosphorylated form phospho-FoxO3a (Ser253) were determined using appropriate antibodies on 50 μg of proteins. IM: immobilization period; I0: before immobilization; I8: 8 days of casting; R10 to R40: 10 to 40 days of recovery. Data are means ± SEM. As no effect of the meal was observed, postabsortive and postprandial values are pooled. *P < 0.05, vs. I0. Data are means ± SEM.
Effect of the leucine supplemented diet on protein synthesis and protein synthesis pathway
At I0, before immobilization, protein synthesis increased slightly but significantly by 15.5% after food intake (P < 0.05 PA vs. PP). However at I8, the stimulatory effect of food intake disappeared as protein synthesis was not different between the PA and the PP states (13.10 ± 1.14 and 12.13 ± 0.72 mg day−1 in the PA and the PP states respectively) (Fig. 3A) demonstrating an anabolic resistance during immobilization. Indeed, immobilization did not induce any significant modifications on the ASR in the PA state but largely decreased protein synthesis in the PP state (−28% at I8, P < 0.05 vs. I0) (Fig. 3A). The stimulatory effect of food intake was only detectable at R20 in the CON group (Fig. 3B), whereas, this effect was present from R10 to R30 in the LEU group (Fig. 3C) (P < 0.05, PA vs. PP).
Figure 3. Muscle protein synthesis and protein pathway in immobilized gastrocnemius muscles before and after the free leucine supplementation.
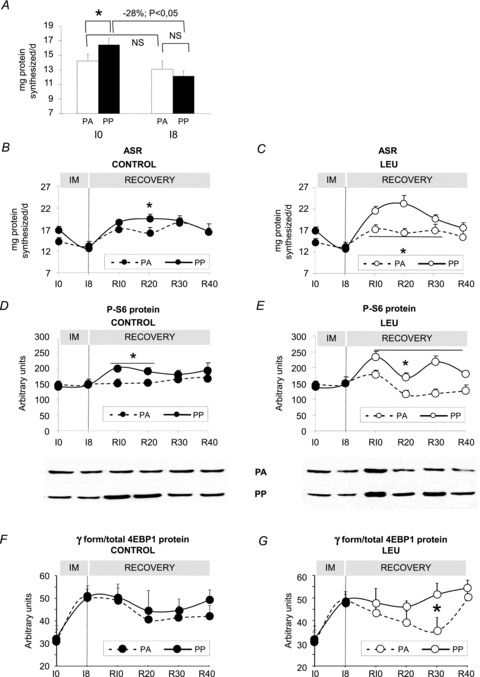
A, muscle protein synthesis at I0 and I8 in the postabsorptive and the postprandial states for each time is expressed as the absolute synthesis rate (ASR), i.e. the amount of proteins synthesized in mg day−1. B and C, ASR during immobilization and recovery in the postabsorptive (B) and the postprandial states (C). D and E, protein S6 phosphorylation in gastrocnemius for the CON group (D) and the LEU group (E) in the postabsorptive and the postprandial states. The amount of protein S6 phosphorylated was assessed by immunoblotting on 30 μg of proteins. F and G, amount of protein 4EBP1 expressed in arbitrary units as the ratio γ form/total forms in the CON group (F) and LEU group (G). IM: immobilization period; I0: before immobilization; I8: 8 days of casting; R10 to R40: 10 to 40 days of recovery; PA: postabsorptive state; PP: postprandial state. *P < 0.05, PA vs. PP. Data are means ± SEM.
The amount of protein S6 phosphorylated is normally increased with the PA/PP transition reflecting an activation of the protein synthesis pathway. In the CON group this increase was only present at R10 and R20 (+30% and +24% respectively, P < 0.05 PA vs. PP) (Fig. 3D). In the LEU group this increase was present during the whole recovery period, from R10 to R40 with a peak at R30 (+30%, P < 0.05 PA vs. PP) (Fig. 3E). Similarly, the leucine supplementation induced a higher amount of γ form/total 4EBP1 protein (+ 44%, P < 0.05 PA vs. PP) and no effect was observed with the control diet (Fig. 3F and G).
Gastrocnemius muscle mass during immobilization and recovery
Immobilized gastrocnemius muscles atrophied by 20% at I8 (2.553 ± 0.042 g at I0 and 2.013 ± 0.059 g at I8, P < 0.05) (Fig. 4). The leucine supplementation, started after reloading and did not have any effect on muscle mass as muscle mass recovery was absent and muscle mass were similar at R40 between the two groups (2.012 ± 0.053 g and 1.963 ± 0.059 g in the CON group and the LEU group, respectively).
Figure 4. Muscle mass of gastrocnemius after immobilization and after free leucine supplementation.
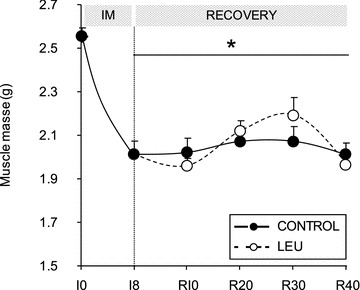
In immobilized muscles, muscle mass decreased after an 8 day immobilization period. The nutritional supplementation started after cast removal but muscle mass was not different between the CON group and the LEU group at R40 and never returned to its pre-immobilization value. IM: immobilization period; I0: before immobilization; I8: 8 days of casting; R10 to R40: 10 to 40 days of recovery. *P < 0.05, vs. I0. No significant differences between CON and LEU groups were recorded. Data are means ± SEM.
Effect of whey diet and high protein diet supplementations on muscle mass recovery
Figure 5A shows the muscle mass gain following immobilization. As mentioned before, no muscle mass gain was obtained after 40 days in the CON or the LEU groups. However, within the WHEY and HIGH PROTEIN groups, a progressive muscle mass recovery appeared as soon as 20 days after cast removal, to achieve ∼400 mg at R40. At R20, the muscle mass gain was ∼300 mg within the HIGH PROTEIN group and only ∼200 within the WHEY group (P < 0.05 vs. I8). The increase of postprandial muscle protein synthesis (% from I8) (Fig. 5B) was increased at R20 without any difference between the four diets. However, muscle protein synthesis at R40 was largely higher with WHEY and HIGH PROTEIN diets (∼+30–40%, P < 0.05 vs. CON or LEU, Student's t test). Similarly, essential amino acid concentrations were greater at R40 with WHEY diet and HIGH PROTEIN diet (∼+25–70%, P < 0.05 vs. CON or LEU) (Fig. 5C).
Figure 5. Effect of the whey and high protein diets on muscle mass gain, muscle protein synthesis and amino acid concentrations.
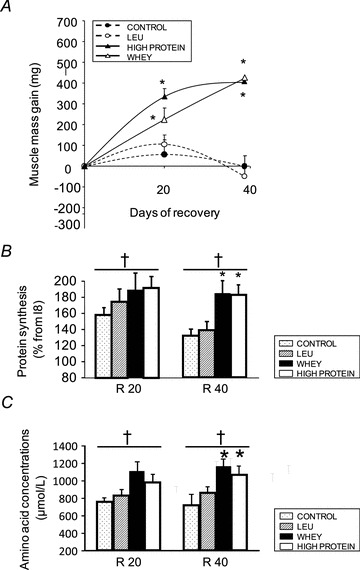
A, muscle mass gain after the nutritional intervention is presented as the gain versus I8, in mg of muscle. Four diets were tested during the recovery period: a CON diet (13% casein), a LEU diet (13% casein + 4.45% Leucine), a WHEY diet (13% whey, i.e. leucine-rich and fast digested protein diet), and a HIGH PROTEIN diet (13% casein + 13% whey). *P < 0.05, vs. I8, Student's t test. Data are means ± SEM. B, muscle protein synthesis is expressed as the percentage of the absolute synthesis rate (ASR), i.e. the amount of proteins synthesized in mg day−1, at I8. C, plasma amino acid concentrations expressed in μmol l−1 were determined on animals fed each experimental diet at R20 and R40. I8: 8 days of casting; R20 and R40: 10 and 40 days of recovery; LEU: leucine. *P < 0.05, vs. CON and LEU; †P < 0.05, vs. I8.
Muscle fibre composition
Table 3 presents the percentage of each major fibre types in gastrocnemius muscles before immobilization, after immobilization and during recovery. There was no effect of immobilization on the composition of muscle fibre types and no diet induced modification of the percentage of fibre types.
Table 3.
Effect of immobilization and diets on muscle fibres composition
| Type IIA | Type IIX | Type IIB | Type I | ||
|---|---|---|---|---|---|
| I0 | 0.2 ± 0.2 | 38.1 ± 1.5 | 57.7 ± 1.8 | 3.9 ± 0.6 | |
| I8 | 0.4 ± 0.3 | 37.8 ± 1.5 | 58.5 ± 2.0 | 2.8 ± 0.9 | |
| R20 | CON | ND | 42.9 ± 2.2 | 53.0 ± 2.4 | 4.4 ± 0.9 |
| LEU | ND | 41.4 ± 2.2 | 56.5 ± 1.8 | 3.8 ± 0.8 | |
| WHEY | ND | 41.4 ± 2.7 | 53.2 ± 3.3 | 5.3 ± 0.9 | |
| HIGH PROTEIN | ND | 35.2 ± 1.4 | 60.7 ± 1.7 | 4.1 ± 0.9 | |
| R40 | CON | ND | 41.2 ± 3.1 | 54.1 ± 3.3 | 4.7 ± 0.5 |
| LEU | ND | 38.6 ± 2.5 | 58.1 ± 3.0 | 3.3 ± 0.7 | |
| WHEY | ND | 38.3 ± 0.7 | 56.1 ± 2.1 | 5.6 ± 1.4 | |
| HIGH PROTEIN | ND | 36.7 ± 2.3 | 58.8 ± 2.1 | 4.5 ± 0.2 |
Muscle fibres composition has been assessed by electrophoresis. The composition in the 4 major types (type I, type IIA, type IIX, type IIB) is presented in % of total fibres content at I0, I8, R20 and R40 for the 4 different diets tested. CONTROL diet = 13% casein, LEU diet = (13% casein + 4.45% Leucine), WHEY diet = 13% whey, HIGH PROTEIN diet = (13% casein + 13% whey), I8: 8 days of casting; R10 and R40: 10 and 40 days of recovery. ND: non detectable. Data are means ± SEM.
Discussion
In this study, we have demonstrated that during ageing, muscle protein synthesis was depressed in the postprandial state by the period of disuse and only normalized during reloading. As proteolysis was also only normalized during the recovery period, the resultant nitrogen balance was then not positive enough for muscle protein gain, hence contributing to the age-related incomplete muscle mass recoveries. We also demonstrated that a dietary free leucine supplementation had no effect on muscle mass gain despite its positive anabolic effect on muscle protein metabolism during the first 20 days of the recovery period, contrarily to high protein or whey protein diets.
Sarcopaenia is the progressive loss of muscle mass and strength related to normal ageing (Rosenberg, 1989; Cruz-Jentoft et al. 2010). One of the mechanisms leading to this muscle mass loss could be the impaired muscle mass recovery following acute catabolic states observed in aged animals (Dardevet et al. 1995; Mosoni et al. 1995; Chakravarthy et al. 2000; Magne et al. 2011) or elderly humans (Suetta et al. 2009). This was recently named ‘the catabolic crisis model’ by English & Paddon-Jones (2010). At a cellular level, we have previously shown that an 8 day immobilization period by casting induced an increase of muscle proteolysis, i.e. activation of the ubiquitin–proteasome-dependent pathway (Magne et al. 2011). We have completed these observations by showing in the present study a large depression of protein synthesis in the postprandial state. This alteration could explain a large imbalance of protein metabolism, leading therefore to protein loss and ultimately muscle mass loss. During reloading we observed a normalization of protein breakdown and only a slight increase of protein synthesis above those recorded before immobilization. These phenomena may result in an insufficient positive nitrogen balance that is normally required to recover muscle mass. Immobilized muscles exhibited a decrease in responsiveness of muscle protein synthesis to anabolic stimuli (i.e. amino acids or insulin) (Phillips et al. 2009). If this impaired response of protein synthesis has already been observed during immobilization in the adult (Ferrando et al. 1996; Paddon-Jones et al. 2006; de Boer et al. 2007; Glover et al. 2008), this is the first study to demonstrate this ‘anabolic resistance’ during ageing and reloading. We and others have observed in the immobilized muscle the presence of an inflammation (Zarzhevsky et al. 2001; Magne et al. 2011) which may be responsible for this alteration of the protein synthesis pathway. Indeed, inflammation is known to be deleterious for protein synthesis during ageing (Lang et al. 2002; Balage et al. 2009) and the immobilization-induced inflammation may worsen this phenomenon, hence contributing to altered protein synthesis.
To generate the positive nitrogen balance required for protein accretion during recovery, a further increased protein synthesis, decreased proteolysis, or simultaneous changes in both processes is required. If exercise is the best way to induce these modifications to initiate muscle mass gain, it is not always possible particularly after prolonged immobilization periods in elderly people. Thus, as protein intake is a robust stimulator of muscle protein synthesis (Kimball et al. 2000; Bohe et al. 2003; Moore et al. 2009), we focused our work on this aspect. Amino acids and particularly leucine have been shown to be efficient in the stimulation of postprandial muscle protein synthesis (Anthony et al. 1999, 2001; Koopman et al. 2005). In adults, some studies have used a mix of branched amino acids during the immobilization period (Stein et al. 1999; Paddon-Jones et al. 2004; Trappe et al. 2007), but few studies have evaluated the isolated effect of leucine supplementation on muscle mass recovery after disuse situations. In 2010, Baptista et al. supplemented diets with free leucine and showed that these diets given during immobilization could attenuate the muscle mass atrophy (Baptista et al., 2010). Up to now, no study has explored its potential effect during the reloading in adult or aged animals or humans. This study was conducted to test the effect of a free leucine supplementation which is also known to stimulate protein synthesis by reactivating specific kinases implicated in pathways involved in the regulation of translation initiation, particularly during ageing (Dardevet et al. 2002; Rieu et al. 2003, 2006, 2007; Combaret et al. 2005; Katsanos et al. 2006). Its action on muscle protein metabolism by stimulating the m-TOR pathway has been well demonstrated (Anthony et al. 2000; Dardevet et al. 2000; Bolster et al. 2004) and these leucine effects could be maintained when administered chronically (Lynch et al. 2002; Rieu et al. 2003). In our study, a free leucine supplemented diet given during rehabilitation had a positive effect on the muscle protein synthesis. Indeed, free leucine supplementation induced an increased amount of phosphorylation of S6 and 4EBP1 proteins, but also a decrease of chymotrypsin- and trypsin-like activities of the 26S proteasome associated to a phospho-FoxO3a/FoxO3a ratio in favour of antiproteolytic process. This resulted in a higher protein synthesis at the postprandial state with leucine and a proteolysis normalized earlier. However, surprisingly, it failed in the muscle mass recovery.
Our measures of muscle protein synthesis were done at a single time point postprandially and confirmed that our free leucine supplementation was efficient 150–180 min after food intake. As muscle mass gain was absent, we hypothesized that the stimulation of protein synthesis was nevertheless not sustained enough to translate into a significant muscle protein accretion. We postulated that free leucine, as it is absorbed rapidly, induced an anabolic ‘leucine signal’ for protein synthesis before there was sufficient availability of amino acids coming from dietary protein digestion. Therefore, the duration or the intensity of the protein synthesis could have been insufficient. This desynchronization between the ‘leucine signal’ and the availability of substrates for protein synthesis could explain the insufficient positive nitrogen balance and the lack of protein accretion when free leucine is used.
To verify this hypothesis we improved our nutritional strategy by resynchronizing both the ‘leucine signal’ and the amino acid availability. The strategy was (1) to change the quality of proteins to use rich-leucine and fast digested proteins to induce simultaneously the ‘leucine signal’ and a large increase of amino acid concentration and (2) to increase the quantity of proteins to provide amino acids for a longer time during the postprandial state. We then used soluble milk proteins (i.e. rich-leucine and fast digested proteins) and high protein diet (i.e. casein and whey proteins), respectively. Indeed, soluble milk proteins are known to be digested faster than casein, i.e. they have a rapid absorption kinetic, but also high leucine content (Boirie et al. 1997; Dangin et al. 2001, 2003). Whey proteins have already demonstrated their positive effect in re-stimulating muscle protein accretion in defective protein synthesis stimulation during ageing (Pennings et al. 2011). A high protein diet, composed of one-half casein and the other half of soluble milk proteins, combined the properties of whey (i.e. rich-leucine and fast digested proteins) and mass effect (high quantity of amino acids for a long time; Boirie et al. 1997). We have shown in the present study that these two diets had a positive impact on muscle mass gain, inducing a gain of ∼60% of the total muscle mass loss. These two diets induced a greater protein synthesis compared to casein or free leucine-supplemented diets, associated to higher amino acid concentrations. These observations confirm data presented in the recent review of Breen & Phillips (2011), which suggest that protein-based interventions could be a good way to induce hypertrophy, particularly during ageing.
As sarcopaenia and immobilization affect muscle mass but also muscle functionality, we proposed to evaluate this functionality by measuring the muscle fibres composition. We did not record any modification of muscle fibre composition with immobilization. This could be explained by (1) a low composition of slow fibres, which are mainly affected by immobilization, and (2) a short period of (8 days), as some authors demonstrated that 10–21 days of casting are required to induce a fibre-type switch in rats (Pattisson et al. 2003; Coutinho et al. 2004). Moreover, primary effects of immobilization could be a reduction of cross-sectional area (Tomanek & Lund 1974; Nicks et al. 1989; Berg et al. 1997) before modifications of fibre type composition.
In summary, we have demonstrated here for the first time that the lack of recovery after immobilization-induced atrophy during ageing is due to an ‘anabolic resistance’ of protein synthesis to amino acids during rehabilitation. Our nutritional study showed that chronic leucine supplementation, despite a greater postprandial protein synthesis response, failed in muscle mass gain. Furthermore, this work provides an insight into the mechanisms involved in this discrepancy: our data strongly suggest the presence of a desynchronization between the stimulatory ‘leucine signal’ and the availability of amino acid substrates for protein synthesis. Our results highlight a novel approach to induce muscle mass recovery following atrophy in the elderly by giving soluble milk protein or high protein diets.
Acknowledgments
We would like to thank Arlette Cissoire and Philippe Lhoste from the IEN (INRA Clermont-Ferrand-Theix, France) for their excellent assistance during animal experimentations. We also thank Claire Sornet for technical assistance and Hélène Lafarge for help in the management of the bibliography. Whey proteins and Casein was kindly provided by lactalis nutrition, Le Retiers, France. This study was supported by grants from the Institut National de la Recherche Agronomique. H.M. was supported by a PhD fellowship from the Ministère de l'Enseignement Supérieur et de la Recherche.
Glossary
Abbreviations
- ASR
absolute synthesis rate
- CAS
casein
- LEU
leucine
- PA
postabsorptive
- PP
postprandial
Author contributions
H.M., I.S.A. and D.D. contributed to the conception and design of the experiments. H.M., I.S.A., C.M., M-A.P., L.C., D.R. and D.D. participated to the collection, analysis and interpretation of the data and revised the manuscript critically for important intellectual content. All authors drafted the manuscript and approved the final version for publication. All experiments were performed in the Unité de Nutrition Humaine from the Institut National de la Recherche Agronomique (Saint Genès Champanelle, France).
References
- Anthony JC, Anthony TG, Layman DK. Leucine supplementation enhances skeletal muscle recovery in rats following exercise. J Nutr. 1999;129:1102–1106. doi: 10.1093/jn/129.6.1102. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- Anthony TG, Anthony JC, Yoshizawa F, Kimball SR, Jefferson LS. Oral administration of leucine stimulates ribosomal protein mRNA translation but not global rates of protein synthesis in the liver of rats. J Nutr. 2001;131:1171–1176. doi: 10.1093/jn/131.4.1171. [DOI] [PubMed] [Google Scholar]
- Balage M, Dardevet D. Long-term effects of leucine supplementation on body composition. Curr Opin Clin Nutr Metab Care. 2010;13:265–270. doi: 10.1097/MCO.0b013e328336f6b8. [DOI] [PubMed] [Google Scholar]
- Balage M, Averous J, Remond D, Bos C, Pujos-Guillot E, Papet I, Mosoni L, Combaret L, Dardevet D. Presence of low-grade inflammation impaired postprandial stimulation of muscle protein synthesis in old rats. J Nutr Biochem. 2009;21:325–331. doi: 10.1016/j.jnutbio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Baptista IL, Leal ML, Artioli GG, Aoki MS, Fiamoncini J, Turri AO, Curi R, Miyabara EH, Moriscot AS. Leucine attenuates skeletal muscle wasting via inhibition of ubiquitin ligases. Muscle Nerve. 2010;41:800–808. doi: 10.1002/mus.21578. [DOI] [PubMed] [Google Scholar]
- Bar-Shai M, Carmeli E, Coleman R, Rozen N, Perek S, Fuchs D, Reznick AZ. The effect of hindlimb immobilization on acid phosphatase, metalloproteinases and nuclear factor-κB in muscles of young and old rats. Mech Ageing Dev. 2005;126:289–297. doi: 10.1016/j.mad.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Berg HE, Larsson L, Tesch PA. Lower limb skeletal muscle function after 6 wk of bed rest. J Appl Physiol. 1997;82:182–188. doi: 10.1152/jappl.1997.82.1.182. [DOI] [PubMed] [Google Scholar]
- Biolo G, Agostini F, Simunic B, Sturma M, Torelli L, Preiser JC, Deby-Dupont G, Magni P, Strollo F, di Prampero P, Guarnieri G, Mekjavic IB, Pisot R, Narici MV. Positive energy balance is associated with accelerated muscle atrophy and increased erythrocyte glutathione turnover during 5 wk of bed rest. Am J Clin Nutr. 2008;88:950–958. doi: 10.1093/ajcn/88.4.950. [DOI] [PubMed] [Google Scholar]
- Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol. 2003;552:315–324. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr. 1997;65:489–495. doi: 10.1093/ajcn/65.2.489. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Vary TC, Kimball SR, Jefferson LS. Leucine regulates translation initiation in rat skeletal muscle via enhanced eIF4G phosphorylation. J Nutr. 2004;134:1704–1710. doi: 10.1093/jn/134.7.1704. [DOI] [PubMed] [Google Scholar]
- Booth FW. Effect of limb immobilization on skeletal muscle. J Appl Physiol. 1982;52:1113–1118. doi: 10.1152/jappl.1982.52.5.1113. [DOI] [PubMed] [Google Scholar]
- Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab (Lond) 2011;5:68. doi: 10.1186/1743-7075-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse MG, Reid SS. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975;56:1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy MV, Davis BS, Booth FW. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol. 2000;89:1365–1379. doi: 10.1152/jappl.2000.89.4.1365. [DOI] [PubMed] [Google Scholar]
- Combaret L, Dardevet D, Rieu I, Pouch MN, Bechet D, Taillandier D, Grizard J, Attaix D. A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle. J Physiol. 2005;569:489–499. doi: 10.1113/jphysiol.2005.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho EL, Gomes AR, Franca CN, Oishi J, Salvini TF. Effect of passive stretching on the immobilized soleus muscle fiber morphology. Braz J Med Biol Res Brazilian. 2004;12:1853–1861. doi: 10.1590/s0100-879x2004001200011. [DOI] [PubMed] [Google Scholar]
- Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005;135:376–382. doi: 10.1093/jn/135.3.376. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, Ballevre O, Beaufrere B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001;280:E340–348. doi: 10.1152/ajpendo.2001.280.2.E340. [DOI] [PubMed] [Google Scholar]
- Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, Fauquant J, Ballevre O, Beaufrere B. The rate of protein digestion affects protein gain differently during ageing in humans. J Physiol. 2003;549:635–644. doi: 10.1113/jphysiol.2002.036897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Balage M, Grizard J. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr. 2000;130:2630–2635. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C, Grizard J. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal. J Nutr. 2002;132:95–100. doi: 10.1093/jn/132.1.95. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Taillandier D, Savary I, Attaix D, Grizard J. Sensitivity and protein turnover response to glucocorticoids are different in skeletal muscle from adult and old rats. Lack of regulation of the ubiquitin-proteasome proteolytic pathway in ageing. J Clin Invest. 1995;96:2113–2119. doi: 10.1172/JCI118264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol. 2007;585:241–251. doi: 10.1113/jphysiol.2007.142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. 2010;13:34–39. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab. 1996;270:E627–633. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- Frexes-Steed M, Lacy DB, Collins J, Abumrad NN. Role of leucine and other amino acids in regulating protein metabolism in vivo. Am J Physiol Endocrinol Metab. 1992;262:E925–935. doi: 10.1152/ajpendo.1992.262.6.E925. [DOI] [PubMed] [Google Scholar]
- Gibson JN, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond) 1987;72:503–509. doi: 10.1042/cs0720503. [DOI] [PubMed] [Google Scholar]
- Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586:6049–6061. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink DF. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol. 1977;264:267–282. doi: 10.1113/jphysiol.1977.sp011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. Muscle mass and strength: relation to function in population studies. J Nutr. 1997;127:1004S–1006S. doi: 10.1093/jn/127.5.1004S. [DOI] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS, Nguyen HV, Suryawan A, Bush JA, Davis TA. Feeding stimulates protein synthesis in muscle and liver of neonatal pigs through an mTOR-dependent process. Am J Physiol Endocrinol Metab. 2000;279:E1080–1087. doi: 10.1152/ajpendo.2000.279.5.E1080. [DOI] [PubMed] [Google Scholar]
- Kondo H, Kodama J, Kishibe T, Itokawa Y. Oxidative stress during recovery from muscle atrophy. FEBS letters. 1993;326:189–191. doi: 10.1016/0014-5793(93)81788-2. [DOI] [PubMed] [Google Scholar]
- Koopman R, Wagenmakers AJ, Manders RJ, Zorenc AH, Senden JM, Gorselink M, Keizer HA, van Loon LJ. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab. 2005;288:E645–653. doi: 10.1152/ajpendo.00413.2004. [DOI] [PubMed] [Google Scholar]
- Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772–1774. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- Krawiec BJ, Frost RA, Vary TC, Jefferson LS, Lang CH. Hindlimb casting decreases muscle mass in part by proteasome-dependent proteolysis but independent of protein synthesis. Am J Physiol Endocrinol Metab. 2005;289:E969–980. doi: 10.1152/ajpendo.00126.2005. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Nairn AC, MacLean DA, Vary TC. TNF-α impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab. 2002;282:E336–347. doi: 10.1152/ajpendo.00366.2001. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- Loughna P, Goldspink G, Goldspink DF. Effect of inactivity and passive stretch on protein turnover in phasic and postural rat muscles. J Appl Physiol. 1986;61:173–179. doi: 10.1152/jappl.1986.61.1.173. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Hutson SM, Patson BJ, Vaval A, Vary TC. Tissue-specific effects of chronic dietary leucine and norleucine supplementation on protein synthesis in rats. Am J Physiol Endocrinol Metab. 2002;283:E824–835. doi: 10.1152/ajpendo.00085.2002. [DOI] [PubMed] [Google Scholar]
- Magne H, Savary-Auzeloux I, Vazeille E, Claustre A, Attaix D, Anne L, Veronique SL, Philippe G, Dardevet D, Combaret L. Lack of muscle recovery after immobilization in old rats does not result from a defect in normalization of the ubiquitin-proteasome and the caspase-dependent apoptotic pathways. J Physiol. 2011;589:511–524. doi: 10.1113/jphysiol.2010.201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzani B, Balage M, Venien A, Astruc T, Papet I, Dardevet D, Mosoni L. Antioxidant supplementation restores defective leucine stimulation of protein synthesis in skeletal muscle from old rats. J Nutr. 2008;138:2205–2211. doi: 10.3945/jn.108.094029. [DOI] [PubMed] [Google Scholar]
- Minnaard R, Wagenmakers AJ, Combaret L, Attaix D, Drost MR, van Kranenburg GP, Schaart G, Hesselink MK. Ubiquitin-proteasome-dependent proteolytic activity remains elevated after zymosan-induced sepsis in rats while muscle mass recovers. Int J Biochem Cell Biol. 2005;37:2217–2225. doi: 10.1016/j.biocel.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Mizunoya W, Wakamatsu J, Tatsumi R, Ikeuchi Y. Protocol for high-resolution separation of rodent myosin heavy chain isoforms in a mini-gel electrophoresis system. Anal Biochem. 2008;377:111–113. doi: 10.1016/j.ab.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol. 2009;587:897–904. doi: 10.1113/jphysiol.2008.164087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosoni L, Valluy MC, Serrurier B, Prugnaud J, Obled C, Guezennec CY, Mirand PP. Altered response of protein synthesis to nutritional state and endurance training in old rats. Am J Physiol Endocrinol Metab. 1995;268:E328–335. doi: 10.1152/ajpendo.1995.268.2.E328. [DOI] [PubMed] [Google Scholar]
- Mosoni L, Malmezat T, Valluy MC, Houlier ML, Mirand PP. Muscle and liver protein synthesis adapt efficiently to food deprivation and refeeding in 12-month-old rats. J Nutr. 1996;126:516–522. doi: 10.1093/jn/126.2.516. [DOI] [PubMed] [Google Scholar]
- Nicks DK, Beneke WM, Key RM, Timson BF. Muscle fibre size and number following immobilisation atrophy. J Anat. 1989;163:1–5. [PMC free article] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41:215–219. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Pattison JS, Folk LC, Madsen RW, Booth FW. Selected Contribution: Identification of differentially expressed genes between young and old rat soleus muscle during recovery from immobilization-induced atrophy. J Appl Physiol. 2003;95:2171–2179. doi: 10.1152/japplphysiol.00500.2003. [DOI] [PubMed] [Google Scholar]
- Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011;93:997–1005. doi: 10.3945/ajcn.110.008102. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol. 2009;107:645–654. doi: 10.1152/japplphysiol.00452.2009. [DOI] [PubMed] [Google Scholar]
- Rieu I, Balage M, Sornet C, Debras E, Ripes S, Rochon-Bonhomme C, Pouyet C, Grizard J, Dardevet D. Increased availability of leucine with leucine-rich whey proteins improves postprandial muscle protein synthesis in ageing rats. Nutrition. 2007;23:323–331. doi: 10.1016/j.nut.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, Combaret L, Dardevet D. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol. 2009;587:5483–5492. doi: 10.1113/jphysiol.2009.178319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Sornet C, Bayle G, Prugnaud J, Pouyet C, Balage M, Papet I, Grizard J, Dardevet D. Leucine supplemented meal feeding for ten days beneficially affects postprandial muscle protein synthesis in old rats. J Nutr. 2003;133:1198–1205. doi: 10.1093/jn/133.4.1198. [DOI] [PubMed] [Google Scholar]
- Rosenberg IH. Summary comments. Am J Clin Nutr. 1989;50:1231–1233. [Google Scholar]
- Stein TP, Schluter MD, Leskiw MJ, Boden G. Attenuation of the protein wasting associated with bed rest by branched-chain amino acids. Nutrition. 1999;15:656–660. doi: 10.1016/s0899-9007(99)00120-3. [DOI] [PubMed] [Google Scholar]
- Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of ageing on human skeletal muscle after immobilization and retraining. J Appl Physiol. 2009;107:1172–1180. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- Taillandier D, Aurousseau E, Combaret L, Guezennec CY, Attaix D. Regulation of proteolysis during reloading of the unweighted soleus muscle. Int J Biochem Cell Biol. 2003;35:665–675. doi: 10.1016/s1357-2725(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Taillandier D, Aurousseau E, Meynial-Denis D, Bechet D, Ferrara M, Cottin P, Ducastaing A, Bigard X, Guezennec CY, Schmid HP, et al. Coordinate activation of lysosomal, Ca2+-activated and ATP-ubiquitin-dependent proteinases in the unweighted rat soleus muscle. Biochem J. 1996;316:65–72. doi: 10.1042/bj3160065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillandier D, Bigard X, Desplanches D, Attaix D, Guezennec CY, Arnal M. Role of protein intake on protein synthesis and fiber distribution in the unweighted soleus muscle. J Appl Physiol. 1993;75:1226–1232. doi: 10.1152/jappl.1993.75.3.1226. [DOI] [PubMed] [Google Scholar]
- Tilignac T, Temparis S, Combaret L, Taillandier D, Pouch MN, Cervek M, Cardenas DM, Le Bricon T, Debiton E, Samuels SE, Madelmont JC, Attaix D. Chemotherapy inhibits skeletal muscle ubiquitin-proteasome-dependent proteolysis. Cancer Res. 2002;62:2771–2777. [PubMed] [Google Scholar]
- Tomanek RJ, Lund DD. Degeneration of different types of skeletal muscle fibres. II. Immobilization. J Anat. 1974;118:531–541. [PMC free article] [PubMed] [Google Scholar]
- Trappe TA, Burd NA, Louis ES, Lee GA, Trappe SW. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol (Oxf) 2007;191:147–159. doi: 10.1111/j.1748-1716.2007.01728.x. [DOI] [PubMed] [Google Scholar]
- Vazeille E, Codran A, Claustre A, Averous J, Listrat A, Bechet D, Taillandier D, Dardevet D, Attaix D, Combaret L. The ubiquitin-proteasome and the mitochondria associated apoptotic pathways are sequentially downregulated during recovery after immobilization induced muscle atrophy. Am J Physiol Endocrinol Metab. 2008;295:E1181–1190. doi: 10.1152/ajpendo.90532.2008. [DOI] [PubMed] [Google Scholar]
- Zarzhevsky N, Menashe O, Carmeli E, Stein H, Reznick AZ. Capacity for recovery and possible mechanisms in immobilization atrophy of young and old animals. Ann N Y Acad Sci. 2001;928:212–225. doi: 10.1111/j.1749-6632.2001.tb05651.x. [DOI] [PubMed] [Google Scholar]


