Abstract
In dogs, manipulation of heart rate has no effect on the exercise-induced increase in cardiac output. Whether these findings apply to humans remain uncertain, because of the large differences in cardiovascular anatomy and regulation. To investigate the role of heart rate and peripheral vasodilatation in the regulation of cardiac output during steady-state exercise, we measured central and peripheral haemodynamics in 10 healthy male subjects, with and without atrial pacing (100–150 beats min−1) during: (i) resting conditions, (ii) one-legged knee extensor exercise (24 W) and (iii) femoral arterial ATP infusion at rest. Exercise and ATP infusion increased cardiac output, leg blood flow and vascular conductance (P < 0.05), whereas cerebral perfusion remained unchanged. During atrial pacing increasing heart rate by up to 54 beats min−1, cardiac output did not change in any of the three conditions, because of a parallel decrease in stroke volume (P < 0.01). Atrial pacing increased mean arterial pressure (MAP) at rest and during ATP infusion (P < 0.05), whereas MAP remained unchanged during exercise. Atrial pacing lowered central venous pressure (P < 0.05) and pulmonary capillary wedge pressure (P < 0.05) in all conditions, whereas it did not affect pulmonary mean arterial pressure. Atrial pacing lowered the left ventricular contractility index (dP/dt) (P < 0.05) in all conditions and plasma noradrenaline levels at rest (P < 0.05), but not during exercise and ATP infusion. These results demonstrate that the elevated cardiac output during steady-state exercise is regulated by the increase in skeletal muscle blood flow and venous return to the heart, whereas the increase in heart rate appears to be secondary to the regulation of cardiac output.
Key points
During exercise, cardiac output is regulated to match oxygen delivery to the metabolic demand.
This study evaluated the role of heart rate and peripheral vasodilation in the regulation of cardiac output during exercise.
We increased heart rate by atrial pacing in 10 healthy male individuals during three different conditions: at rest, during exercise and during femoral arterial ATP infusion at rest.
Increasing the heart rate by atrial pacing by up to 54 beats min−1 did not increase cardiac output in any of the three given conditions as there was a proportional decrease in stroke volume.
These results indicate that cardiac output is regulated by changes in peripheral vasodilatation, whereas an increase in heart rate is less important.
Introduction
The mechanisms controlling the matching of cardiac output (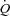 ) and skeletal muscle perfusion to the metabolic demand are thought to implicate mechanical, metabolic and neural factors (Rowell & O'Leary, 1990; Clifford & Hellsten, 2004; Saltin, 2007; Fadel & Raven, 2012). In dogs,
) and skeletal muscle perfusion to the metabolic demand are thought to implicate mechanical, metabolic and neural factors (Rowell & O'Leary, 1990; Clifford & Hellsten, 2004; Saltin, 2007; Fadel & Raven, 2012). In dogs, 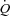 appears to be regulated by peripheral tissue O2 demand and venous return whereas the heart itself appears to play a more passive role in the regulatory process (Guyton et al. 1962; Guyton, 1967; Shepherd et al. 1973). Moreover, ventricular pacing over a wide range of heart rates (HRs) results in little or no change in
appears to be regulated by peripheral tissue O2 demand and venous return whereas the heart itself appears to play a more passive role in the regulatory process (Guyton et al. 1962; Guyton, 1967; Shepherd et al. 1973). Moreover, ventricular pacing over a wide range of heart rates (HRs) results in little or no change in 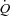 in exercising dogs (White et al. 1971; Wyss et al. 1982; Sheriff et al. 1993), indicating that a change in HR is not a regulatory mechanism of the exercise-induced increase in
in exercising dogs (White et al. 1971; Wyss et al. 1982; Sheriff et al. 1993), indicating that a change in HR is not a regulatory mechanism of the exercise-induced increase in 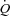 . Whether these findings in dogs apply to humans remain uncertain, because of anatomical and physiological species differences (Rowell, 2007). Firstly, and in contrast to humans, dogs have most of the blood volume above the level of the heart (Rowell, 2003). Secondly, stroke volume (SV) does not increase during exercise in dogs, whereas it increases in upright humans (Guyton et al. 1962; Åstrand et al. 1964; Vatner & Pagani, 2009). Thirdly, the redistribution of visceral blood flow observed during exercise in humans does not appear to occur in dogs (Vatner, 1975). Fourthly, the size of the heart in relation to body weight is three-fold higher in dogs compared with humans (Grande & Taylor, 1965; Gunn, 1989). HR pacing in heart patients suggest that alterations in HR are compensated for by a change in SV such that
. Whether these findings in dogs apply to humans remain uncertain, because of anatomical and physiological species differences (Rowell, 2007). Firstly, and in contrast to humans, dogs have most of the blood volume above the level of the heart (Rowell, 2003). Secondly, stroke volume (SV) does not increase during exercise in dogs, whereas it increases in upright humans (Guyton et al. 1962; Åstrand et al. 1964; Vatner & Pagani, 2009). Thirdly, the redistribution of visceral blood flow observed during exercise in humans does not appear to occur in dogs (Vatner, 1975). Fourthly, the size of the heart in relation to body weight is three-fold higher in dogs compared with humans (Grande & Taylor, 1965; Gunn, 1989). HR pacing in heart patients suggest that alterations in HR are compensated for by a change in SV such that 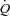 is maintained (Ross et al. 1965; Sonnenblick et al. 1965; Bevegård et al. 1967), but cardiovascular regulation is likely to be altered in heart patients. In support of a less important role of HR in the regulation of
is maintained (Ross et al. 1965; Sonnenblick et al. 1965; Bevegård et al. 1967), but cardiovascular regulation is likely to be altered in heart patients. In support of a less important role of HR in the regulation of 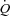 , we recently observed a similar
, we recently observed a similar 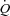 and leg blood flow (LBF) during leg exercise and ATP-induced vasodilatation despite a markedly different HR response (González-Alonso et al. 2008). An increase in HR with parasympathetic blockade increases
and leg blood flow (LBF) during leg exercise and ATP-induced vasodilatation despite a markedly different HR response (González-Alonso et al. 2008). An increase in HR with parasympathetic blockade increases 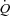 and MAP in the supine (Seifert et al. 2010), but not upright position (Boushel et al. 2001; Hopkins et al. 2003), suggesting that the central blood volume is important for HR to increase
and MAP in the supine (Seifert et al. 2010), but not upright position (Boushel et al. 2001; Hopkins et al. 2003), suggesting that the central blood volume is important for HR to increase 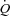 . A possibility is that peripheral vasodilatation and an increase in venous return and cardiac filling pressure are required to increase
. A possibility is that peripheral vasodilatation and an increase in venous return and cardiac filling pressure are required to increase 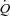 during HR pacing (Dodge et al. 1960; Guyton, 1981), but the effect of an increase in HR at rest and during conditions of increased peripheral vascular conductance such as during exercise and ATP infusion has not been investigated in healthy humans.
during HR pacing (Dodge et al. 1960; Guyton, 1981), but the effect of an increase in HR at rest and during conditions of increased peripheral vascular conductance such as during exercise and ATP infusion has not been investigated in healthy humans.
The purpose of this study was to investigate the isolated effect of an increase in HR on 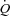 , blood pressures and limb and brain perfusion at rest and during exercise and ATP-induced hyperaemia. To accomplish these aims, we measured central and peripheral haemodynamics during atrial pacing at rest, one-legged knee extensor exercise and femoral arterial ATP infusion at rest in healthy, young men. We hypothesized that atrial pacing would lower SV because of a decrease in central venous and left ventricular filling pressure.
, blood pressures and limb and brain perfusion at rest and during exercise and ATP-induced hyperaemia. To accomplish these aims, we measured central and peripheral haemodynamics during atrial pacing at rest, one-legged knee extensor exercise and femoral arterial ATP infusion at rest in healthy, young men. We hypothesized that atrial pacing would lower SV because of a decrease in central venous and left ventricular filling pressure.
Methods
Ten healthy and recreationally active male subjects participated in this study (age 23 ± 4 years, body weight 77.7 ± 11 kg, height 185 ± 7 cm and maximal oxygen uptake (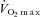 ) 3.8 ± 0.4 l min−1; mean ± SD). All subjects were normotensive, had a normal ECG and were not taking any medication. The subjects received oral and written information about potential risks and discomforts associated with the trial before giving their informed consent to participate. The study was approved by the Ethics Committee of The Capital Region of Denmark and conducted in accordance with the guidelines of the Declaration of Helsinki.
) 3.8 ± 0.4 l min−1; mean ± SD). All subjects were normotensive, had a normal ECG and were not taking any medication. The subjects received oral and written information about potential risks and discomforts associated with the trial before giving their informed consent to participate. The study was approved by the Ethics Committee of The Capital Region of Denmark and conducted in accordance with the guidelines of the Declaration of Helsinki.
Experimental protocol
Prior to the experimental day, the subjects performed one-legged knee-extensor training for 5 weeks (3–4 times per week) with the experimental leg (randomized). The subjects were instructed to abstain from caffeine, alcohol and physical activity for 24 h prior to the experimental day. On the day of the experiment, the subjects arrived at the laboratory 2 h after ingesting a light breakfast. After 30 min of supine rest, a 20 G catheter was placed in the brachial (n= 4) or femoral (n= 6) artery of the experimental leg and a catheter (131HF7, Edwards Lifesciences, Irvine, CA, USA) was placed in the pulmonary artery via a left ante-cubital vein under pressure guidance. A screw-in pace catheter (Tendril ST, St Jude Medical, Sylmar, CA, USA) was advanced through a sheath in the right internal jugular vein to the auricle of the right atrium. Correct placement was confirmed by X-ray. Following 30 min of supine rest, the subjects were seated in the one-legged knee-extensor chair in a semi-supine position (45 deg).
Following an additional 30 min of rest, the subjects completed three trials: (i) rest (n= 8), (ii) one-leg knee-extensor exercise (24 W) (n= 10) and (iii) femoral arterial ATP infusion at rest (n= 6). The ATP (1 μmol ml−1; Sigma) infusion rate was adjusted to match the leg blood flow during exercise (range 0.5–1.2 μmol min−1). In all trials, the subjects were measured with and without atrial pacing (AAI mode) aiming at gradually increasing HR by 20–30 beats min−1 for each step. In the AAI mode, the pacemaker withholds pacing when it senses an intrinsic atrial electrical activation. If the pacing rate is higher than the intrinsic rate a pacing stimulus will be delivered and this impulse will be conducted to the ventricles through the normal conduction system, ensuring normal physiological activation and relaxation of the ventricles. In the resting trial, HR was increased by 31 ± 4 and 51 ± 2 beats min−1, in the exercising trial by 15 ± 3, 31 ± 3 and 54 ± 3 beats min−1, and in the ATP infusion trial by 18 ± 3 and 41 ± 3 beats min−1. Atrial pacing was initiated after 3 min of rest/ATP infusion/exercise conditions and HR was increased every 3 min. Blood samples were obtained simultaneously from the radial/femoral and pulmonary arteries after 2.5 min. All trials were separated by 30 min of rest and the order of the trials were randomized and counterbalanced across subjects. Due to the development of intermittent Wenckebach AV block, HR could only be increased above 120 beats min−1 in three of the subjects in the two resting trials and these data are therefore not included.
Measurements
Pulmonary 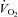 was measured online (Quark CPET system, Cosmed, Italy). HR was obtained from an electrocardiogram while arterial, pulmonary arterial mean pressure (PAMP), central venous pressure (CVP) and pulmonary capillary wedge pressure (PCWP) were monitored with transducers (Pressure Monitoring Kit, Baxter, Deerfield, IL, USA) positioned at the level of the heart and connected to a monitor (Dialogue 2000, Danica Elektronic, Copenhagen Denmark). For determination of
was measured online (Quark CPET system, Cosmed, Italy). HR was obtained from an electrocardiogram while arterial, pulmonary arterial mean pressure (PAMP), central venous pressure (CVP) and pulmonary capillary wedge pressure (PCWP) were monitored with transducers (Pressure Monitoring Kit, Baxter, Deerfield, IL, USA) positioned at the level of the heart and connected to a monitor (Dialogue 2000, Danica Elektronic, Copenhagen Denmark). For determination of 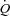 by the Fick principle (
by the Fick principle (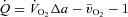 ), pulmonary arterial blood samples were withdrawn over 20 s and pulmonary
), pulmonary arterial blood samples were withdrawn over 20 s and pulmonary 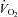 averaged for the same time period. SV was calculated as
averaged for the same time period. SV was calculated as 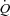 divided by HR. Systemic vascular conductance (SVC) was calculated as
divided by HR. Systemic vascular conductance (SVC) was calculated as 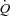 divided by the perfusion pressure (MAP – CVP) and pulse pressure was the difference between the arterial systolic and diastolic blood pressure. The left ventricular contractility index (dP/dtmax) was calculated as the peak systolic value of the first derivative of the arterial pressure curve averaged over 20 cardiac cycles. Leg vascular conductance (LVC) was the quotient of femoral arterial blood flow (LBF) to the perfusion pressure (MAP – CVP).
divided by the perfusion pressure (MAP – CVP) and pulse pressure was the difference between the arterial systolic and diastolic blood pressure. The left ventricular contractility index (dP/dtmax) was calculated as the peak systolic value of the first derivative of the arterial pressure curve averaged over 20 cardiac cycles. Leg vascular conductance (LVC) was the quotient of femoral arterial blood flow (LBF) to the perfusion pressure (MAP – CVP).
Femoral arterial blood flow
LBF was determined by ultrasound Doppler (Logic E9, GE Healthcare, USA) equipped with a linear probe operating at an imaging frequency of 9 MHz and Doppler frequency of 5.0 MHz. The site of blood velocity measurements in the common femoral artery was distal to the inguinal ligament but above the bifurcation into the superficial and profound femoral branch to avoid turbulence from the bifurcation. All recordings were obtained at the lowest possible insonation angle and always below 60 deg. The sample volume was maximized according to the width of the vessel and kept clear of the vessel walls. A low-velocity filter (velocities <1.8 m s−1) rejected noises. The Doppler tracings and B-mode images were recorded continuously and Doppler tracings were averaged over 16 heart cycles. The arterial diameter was determined after each Doppler recording and assessed during the systole from arterial B-mode images with the vessel parallel to the transducer.
Middle cerebral blood flow
Mean blood velocity (Vmean) was measured in the middle cerebral artery (MCA) by transcranial Doppler (2 MHz) through the temporal ultrasound window at a depth of 48–60 mm (Multidop X, DWL, Sipplingen, Germany) (Secher et al. 2008) (rest and exercise: n= 7, ATP n= 4). Brain vascular conductance (BVC) index was expressed as MCA Vmean divided by MAP.
Blood analysis
Blood gas variables and haemoglobin were measured using an ABL725 analyser (Radiometer, Copenhagen, Denmark) and corrected for blood temperature obtained from the pulmonary artery. Plasma catecholamine concentrations were determined with a radioimmunoassay (LDN, Nordhorn, Germany).
Statistical analysis
Data were analysed in Microsoft Excel 2000 and SigmaPlot 11.0. To test for statistical significance a one-way repeated-measures of variance (ANOVA) with Tukey's honestly significant difference procedure was performed. The level of significance was set at P < 0.05. Data are shown as mean ± SEM unless stated otherwise.
Results
Systemic and peripheral haemodynamics in resting conditions
Baseline 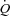 , HR and SV were 8.2 ± 0.7 l min−1, 67 ± 3 beats min−1 and 125 ± 11 ml, respectively (Fig. 1). When HR was increased with atrial pacing,
, HR and SV were 8.2 ± 0.7 l min−1, 67 ± 3 beats min−1 and 125 ± 11 ml, respectively (Fig. 1). When HR was increased with atrial pacing, 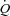 did not change because of a proportional decrease in SV (P < 0.001). MAP increased with increasing HR from 96 ± 3 mmHg (baseline) to 100 ± 3 mmHg (118 beats min−1, P < 0.05). Atrial pacing did not change SVC (90.7 ± 15.9 and 89.1 ± 14.0 ml min−1 mmHg−1 at baseline and 118 beats min−1, respectively). Atrial pacing lowered CVP, PCWP and arterial pulse pressure (P < 0.05), whereas PAMP and systolic blood pressure remained unchanged and diastolic blood pressure increased (Fig. 2). The dP/dtmax was reduced progressively with increasing HR (P < 0.05; Fig. 3).
did not change because of a proportional decrease in SV (P < 0.001). MAP increased with increasing HR from 96 ± 3 mmHg (baseline) to 100 ± 3 mmHg (118 beats min−1, P < 0.05). Atrial pacing did not change SVC (90.7 ± 15.9 and 89.1 ± 14.0 ml min−1 mmHg−1 at baseline and 118 beats min−1, respectively). Atrial pacing lowered CVP, PCWP and arterial pulse pressure (P < 0.05), whereas PAMP and systolic blood pressure remained unchanged and diastolic blood pressure increased (Fig. 2). The dP/dtmax was reduced progressively with increasing HR (P < 0.05; Fig. 3).
Figure 1. Cardiac output (A), stroke volume (B), mean arterial pressure (C) and systemic vascular conductance (D) as a function of heart rate.
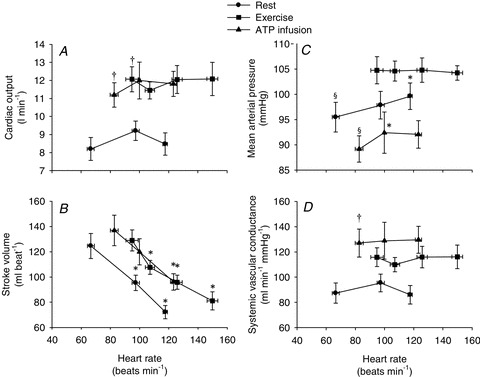
Data are means ± SEM. *Significantly different from baseline within the same trial. †Significantly different from resting conditions. §Significantly different from exercise trial. (P < 0.05).
Figure 2. Central venous pressure (A), pulmonary arterial mean pressure (B), pulmonary capillary wedge pressure (C), sysolic blood pressure (D), diastolic blood pressure (E) and pulse pressure (F) as a function of heart rate.
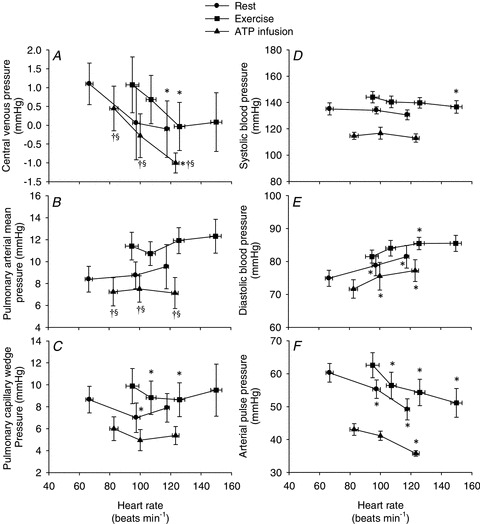
Data are means ± SEM. *Significantly different from baseline within the same trial. †Significantly different from resting conditions. §Significantly different from exercise trial. (P < 0.05).
Figure 3. The left ventricular contractility index dP/dtmax during resting conditions, exercise and femoral arterial ATP infusion with and without atrial pacing.
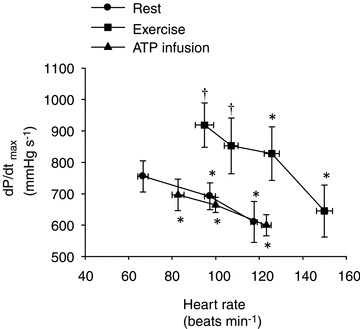
Data are means ± SEM. *Significantly different from baseline within the same trial (P < 0.05). †Significantly different from resting conditions (P < 0.05).
Femoral arterial blood flow was 0.40 ± 0.05 l min−1 at baseline, increased with atrial pacing to 0.62 ± 0.06 l min−1 (P < 0.05) at a HR of 100 beats min−1 (HR100), but returned to baseline levels at HR118 (0.53 ± 0.08 l min−1; Fig. 4). Likewise, LVC increased with atrial pacing to HR100 (4.2 ± 0.5 to 6.4 ± 0.7 ml min−1 mmHg−1, respectively (P < 0.05) and then returned to baseline levels at HR118. The MCA Vmean and brain vascular conductance index (BVC) did not change with atrial pacing.
Figure 4. Leg blood flow (A), leg vascular conductance (B), middle cerebral artery Vmean (C) and brain vascular conductance index (D) as a function of heart rate.
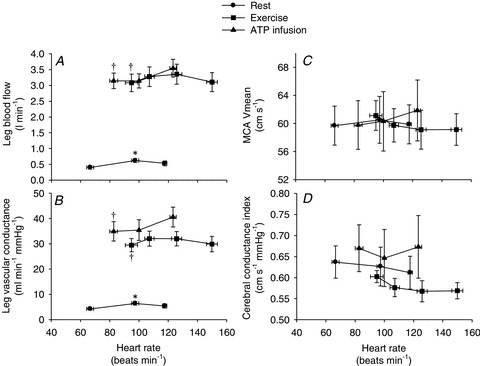
Data are means ± SEM. *Significantly different from baseline within the same trial (P < 0.05). †Significantly different from resting conditions (A: P < 0.05; B: P < 0.001).
Systemic and peripheral haemodynamics during exercise
Exercise increased 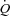 and HR to 12.1 ± 0.9 l min−1 (P < 0.05) and 95 ± 6 beats min−1 (P < 0.01), respectively, whereas SV remained unchanged. When HR was increased,
and HR to 12.1 ± 0.9 l min−1 (P < 0.05) and 95 ± 6 beats min−1 (P < 0.01), respectively, whereas SV remained unchanged. When HR was increased, 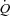 remained similar to control exercise, because of a proportional decrease in SV (P < 0.001). MAP remained constant during atrial pacing (105 ± 4 and 104 ± 2 mmHg at baseline and 150 beats min−1, respectively) and atrial pacing therefore did not cause any changes in SVC. CVP and PCWP decreased with atrial pacing (P < 0.05), whereas pacing did not alter PAMP. Atrial pacing lowered systolic blood pressure (150 beats min−1) and arterial pulse pressure (120 and 150 beats min−1) compared with exercise alone, whereas diastolic blood pressure increased at 120 and 150 beats min−1, respectively (P < 0.001). dP/dtmax was reduced progressively with increasing HR (P < 0.05).
remained similar to control exercise, because of a proportional decrease in SV (P < 0.001). MAP remained constant during atrial pacing (105 ± 4 and 104 ± 2 mmHg at baseline and 150 beats min−1, respectively) and atrial pacing therefore did not cause any changes in SVC. CVP and PCWP decreased with atrial pacing (P < 0.05), whereas pacing did not alter PAMP. Atrial pacing lowered systolic blood pressure (150 beats min−1) and arterial pulse pressure (120 and 150 beats min−1) compared with exercise alone, whereas diastolic blood pressure increased at 120 and 150 beats min−1, respectively (P < 0.001). dP/dtmax was reduced progressively with increasing HR (P < 0.05).
During exercise, leg blood flow and LVC increased to 3.1 ± 0.3 l min−1 and 30 ml min−1 mmHg−1, respectively (P < 0.001), and did not change with atrial pacing. MCA Vmean and BVC did not change with atrial pacing (Fig. 4).
Systemic and peripheral haemodynamics during arterial ATP infusion
Femoral arterial ATP infusion increased 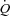 and HR to 11.2 ± 0.4 l min−1 and 83 ± 2 beats min−1, respectively (P < 0.05), whereas SV remained unchanged compared with baseline conditions.
and HR to 11.2 ± 0.4 l min−1 and 83 ± 2 beats min−1, respectively (P < 0.05), whereas SV remained unchanged compared with baseline conditions. 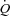 did not change when HR was raised to ∼123 beats min−1 because of a proportional decline in SV (P= 0.002). During atrial pacing, MAP and diastolic blood pressure increased (P < 0.05), whereas CVP and arterial pulse pressure decreased (P < 0.05). Atrial pacing did not change PAMP or systolic blood pressure, whereas PCWP tended (P= 0.057) to decrease. Atrial pacing did not change SVC (127.0 ± 13.5 and 129.7 ± 13.1 ml min−1 mmHg, at baseline and HR123, respectively).
did not change when HR was raised to ∼123 beats min−1 because of a proportional decline in SV (P= 0.002). During atrial pacing, MAP and diastolic blood pressure increased (P < 0.05), whereas CVP and arterial pulse pressure decreased (P < 0.05). Atrial pacing did not change PAMP or systolic blood pressure, whereas PCWP tended (P= 0.057) to decrease. Atrial pacing did not change SVC (127.0 ± 13.5 and 129.7 ± 13.1 ml min−1 mmHg, at baseline and HR123, respectively). 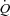 , HR and SVC were higher during ATP infusion compared with baseline conditions, CVP and PAMP were lower, whereas no differences were observed in MAP and PCWP (P < 0.05). Compared with the exercise trial,
, HR and SVC were higher during ATP infusion compared with baseline conditions, CVP and PAMP were lower, whereas no differences were observed in MAP and PCWP (P < 0.05). Compared with the exercise trial, 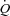 , SV and PCWP were similar, whereas HR, MAP, CVP and PAMP were lower during ATP infusion (P < 0.05). The left ventricular contractility index dP/dtmax was reduced progressively with increasing HR (P < 0.05).
, SV and PCWP were similar, whereas HR, MAP, CVP and PAMP were lower during ATP infusion (P < 0.05). The left ventricular contractility index dP/dtmax was reduced progressively with increasing HR (P < 0.05).
ATP infusion increased femoral arterial blood flow and LVC to 3.1 ± 0.2 l min−1 and 34.9 ml min−1 mmHg−1, respectively, (P < 0.001) whereas and MCA Vmean and BVC remained unchanged compared with baseline. When compared with the resting and exercising trials there were no significant differences in BVC.
Arterial blood pressure at the onset of pacing
Atrial pacing at rest caused an initial increase in MAP and diastolic blood pressure (5 s; P < 0.05), whereas after MAP, systolic and diastolic pressure were reduced (10 s; P < 0.05) and then returned to baseline levels (Fig. 5). No changes in arterial pressure were observed at the onset of pacing in the exercise trial. Atrial pacing tended (5 s; P= 0.057) to cause an initial increase in MAP in the ATP trial, but arterial pressures were then reduced (10 s; P < 0.05) and returned to baseline levels (systolic) or increased (MAP and diastolic; P < 0.05).
Figure 5. Acute changes in systolic, diastolic and mean arterial pressure in the seconds after initiation of the 1st pacing level in the three different trials: resting (A), exercising (B) and arterial ATP infusion at rest (C).
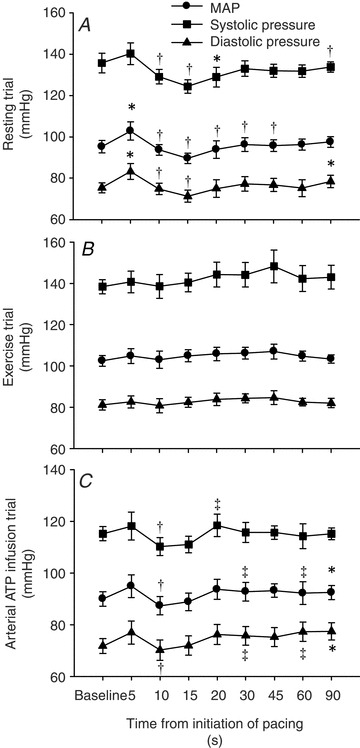
*Significantly different from baseline within the same trial (P < 0.05). †Significantly different from 5 s (P < 0.05). ‡Significantly different from 10 s (P < 0.05).
Plasma catecholamines
Plasma noradrenaline increased during exercise (P < 0.05). During ATP infusion noradrenaline levels were higher at the 1st pacing level when compared with resting conditions (Table 1, P < 0.05). Exercise and arterial ATP infusion did not change plasma adrenaline levels. Plasma noradrenaline and adrenaline decreased during the atrial pacing in the resting trial. Noradrenaline decreased from the 1st pacing level whereas adrenaline was reduced only during the 2nd pacing level (P < 0.05). Neither plasma noradrenaline nor adrenaline levels changed during atrial pacing in the exercising and arterial ATP infusion trials.
Table 1.
Plasma noradrenaline (NA) and adrenaline (A) levels during resting conditions, exercise and arterial ATP infusion with and without atrial pacing
| Baseline | 1st pacing level | 2nd pacing level | |
|---|---|---|---|
| NA rest | 0.9 ± 0.1 | 0.7 ± 0.1* | 0.7 ± 0.1* |
| NA exercise | 1.8 ± 0.3† | 1.6 ± 0.2† | 1.6 ± 0.2†‡ |
| NA ATP infusion | 1.7 ± 0.3 | 1.7 ± 0.4† | 1.1 ± 0.0 |
| A rest | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1*¶ |
| A exercise | 0.7 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 |
| A ATP infusion | 0.6 ± 0.4 | 0.6 ± 0.4 | 0.5 ± 0.3 |
All values are in nmol l−1. Data are means ± SEM.
Significantly different from baseline within the same trial.
Significantly different from 1st pacing level.
Significantly different from resting conditions.
Significantly different from arterial ATP infusion at the same pacing level.
Discussion
There are several findings in the present study in healthy humans that support the hypothesis that 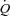 is regulated by peripheral O2 demand and vasodilatation and that a change in HR has no effect on
is regulated by peripheral O2 demand and vasodilatation and that a change in HR has no effect on 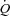 : (1) atrial pacing did not alter
: (1) atrial pacing did not alter 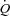 during resting conditions, exercise or arterial ATP infusion with HR increments up to 40–55 beats min−1, because of a proportional decline in SV; (2) atrial pacing caused a decrease in CVP in all three trials and PCWP was lowered at the first pacing level(s) at rest and during exercise; and (3) MAP increased at rest and during ATP infusion, but remained unchanged in the exercise trial. Collectively, these observations suggest that peripheral O2 demand and vasodilatation is the main determinant of
during resting conditions, exercise or arterial ATP infusion with HR increments up to 40–55 beats min−1, because of a proportional decline in SV; (2) atrial pacing caused a decrease in CVP in all three trials and PCWP was lowered at the first pacing level(s) at rest and during exercise; and (3) MAP increased at rest and during ATP infusion, but remained unchanged in the exercise trial. Collectively, these observations suggest that peripheral O2 demand and vasodilatation is the main determinant of 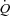 during steady-state conditions and that an increase in HR is compensated for by a reduction in SV.
during steady-state conditions and that an increase in HR is compensated for by a reduction in SV.
Cardiac output remained strikingly unaltered when HR was increased up to 55 beats min−1 in all three conditions. The unaltered 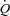 during atrial pacing is in general agreement with findings during HR pacing in resting and exercising dogs (Sheriff et al. 1993; Sheriff & Mendoza, 2004) and resting humans (Bergman et al. 2009). In all conditions, the lower SV during atrial pacing was accompanied with a lower CVP, indicating a redistribution of blood from the venous beds to the arterial and peripheral vascular beds (Sheriff et al. 1993) in a setting with otherwise constant conditions except for the increase in HR. As venous return to the heart is not increased, the SV will fall with increasing HR implying an extrathoracic venous collapse (Guyton, 1968, 1981; Thomson, 1984; Beard & Feigl, 2011). Left ventricular filling pressure was lowered with pacing at rest and during exercise and tended (P= 0.057) to be lowered during ATP infusion, suggesting that a reduced end-diastolic volume lowered SV. Interestingly, left ventricular filling pressures were preserved during ATP infusion, suggesting that peripheral vasodilatation is enough to maintain a sufficient ventricular filling despite a shift in central blood volume and without activation of the skeletal muscle pump (Tschakovsky & Sheriff, 2004; González-Alonso et al. 2008). When pacing at rest and during exercise, left ventricular filling pressure decreased at the first pacing level, but then returned to baseline levels at the highest pacing level. This observation raises the possibility that a disproportional ventricular filling time limited end-diastolic volume and thus SV at the highest pacing level. dP/dtmax was lowered with atrial pacing in all three conditions despite an unaltered circulating catecholamine level. This suggests that myocardial force production is reduced due to a lower preload (Frank–Starling mechanism) and/or altered cardiac cycle length (interval–force relationship) (Seed & Walker, 1988) rather than a reduced contractility caused by sympathetic withdrawal. The similar
during atrial pacing is in general agreement with findings during HR pacing in resting and exercising dogs (Sheriff et al. 1993; Sheriff & Mendoza, 2004) and resting humans (Bergman et al. 2009). In all conditions, the lower SV during atrial pacing was accompanied with a lower CVP, indicating a redistribution of blood from the venous beds to the arterial and peripheral vascular beds (Sheriff et al. 1993) in a setting with otherwise constant conditions except for the increase in HR. As venous return to the heart is not increased, the SV will fall with increasing HR implying an extrathoracic venous collapse (Guyton, 1968, 1981; Thomson, 1984; Beard & Feigl, 2011). Left ventricular filling pressure was lowered with pacing at rest and during exercise and tended (P= 0.057) to be lowered during ATP infusion, suggesting that a reduced end-diastolic volume lowered SV. Interestingly, left ventricular filling pressures were preserved during ATP infusion, suggesting that peripheral vasodilatation is enough to maintain a sufficient ventricular filling despite a shift in central blood volume and without activation of the skeletal muscle pump (Tschakovsky & Sheriff, 2004; González-Alonso et al. 2008). When pacing at rest and during exercise, left ventricular filling pressure decreased at the first pacing level, but then returned to baseline levels at the highest pacing level. This observation raises the possibility that a disproportional ventricular filling time limited end-diastolic volume and thus SV at the highest pacing level. dP/dtmax was lowered with atrial pacing in all three conditions despite an unaltered circulating catecholamine level. This suggests that myocardial force production is reduced due to a lower preload (Frank–Starling mechanism) and/or altered cardiac cycle length (interval–force relationship) (Seed & Walker, 1988) rather than a reduced contractility caused by sympathetic withdrawal. The similar 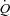 during exercise and ATP infusion was accomplished with a markedly different HR and SV responses (González-Alonso et al. 2008), indicating the leg vasodilatation drives the increase in
during exercise and ATP infusion was accomplished with a markedly different HR and SV responses (González-Alonso et al. 2008), indicating the leg vasodilatation drives the increase in 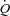 despite differences in HR response. Due to the intact AV conducting system of our healthy subjects, the effect of a reduced HR on the exercise-induced increase in
despite differences in HR response. Due to the intact AV conducting system of our healthy subjects, the effect of a reduced HR on the exercise-induced increase in 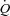 could not be investigated. However, a lowering of HR and contractility by β-blockade does not alter
could not be investigated. However, a lowering of HR and contractility by β-blockade does not alter 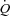 at rest or during submaximal exercise (Ekblom et al. 1972; Pawelczyk et al. 1992). Collectively, these observations suggest that peripheral O2 demand and vasodilatation control
at rest or during submaximal exercise (Ekblom et al. 1972; Pawelczyk et al. 1992). Collectively, these observations suggest that peripheral O2 demand and vasodilatation control 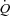 by altering venous return and left ventricular filling and that this regulation occurs independent of changes in HR, but rather by altering end-diastolic volume and contractility. At higher heart rates a reduced ventricular filling time may contribute to the reduction in SV.
by altering venous return and left ventricular filling and that this regulation occurs independent of changes in HR, but rather by altering end-diastolic volume and contractility. At higher heart rates a reduced ventricular filling time may contribute to the reduction in SV.
When pacing at rest and during ATP infusion an increased cardiac afterload due to the increased arterial pressure is also likely to have contributed to the lower SV by increasing end-systolic volume (Higginbotham et al. 1986). The initial increase in arterial pressure during atrial pacing in resting conditions and during ATP infusion suggest an immediate shift in blood volume from the venous to arterial bed (Sheriff & Mendoza, 2004). At rest, the lack of peripheral vasodilatation is likely to have contributed to the shift in blood volume. However, vascular conductance was similar during the ATP and exercise trials, suggesting that different mechanisms are also at odds. The lower contractility index and pulse pressure is likely to have reduced baroreceptor activity with atrial pacing and reduced blood pressure after the initial increase (Neil & Joels, 1961; Fadel & Raven, 2012), although a change in plasma noradrenalin could only be detected in the resting trial. During the ATP trial, the increase in MAP at the first pacing level was followed by lower MAP, pulse pressure and plasma adrenaline levels. A sympathetic withdrawal in the resting trial is likely to have caused the increase in LVC although the increased shear stress in the leg may also have caused a release of vasodilator substances (Clifford & Hellsten, 2004). During exercise and arterial ATP infusion, leg blood flow is unaffected by changes in sympathetic activation due to the ability of exercise and ATP to override sympathetic vasoconstrictor activity (Remensnyder et al. 1962; Rosenmeier et al. 2004). In contrast to the leg, cerebral perfusion and conductance index remained unchanged during atrial pacing at rest despite an increase in perfusion pressure and lower circulating plasma noradrenaline levels. This observation is in agreement with the unchanged cerebral perfusion during β-adrenergic blockade at rest (Seifert et al. 2009).
In conclusion, these results demonstrate that in healthy humans the elevated 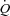 during submaximal exercise is regulated by increases in venous return that occur in parallel to the increase in skeletal muscle blood flow, whereas the HR response to exercise appears to be secondary to the regulation of
during submaximal exercise is regulated by increases in venous return that occur in parallel to the increase in skeletal muscle blood flow, whereas the HR response to exercise appears to be secondary to the regulation of 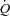 . Whether these findings also apply to maximal conditions, where the heart is operating at its limit (Saltin & Strange, 1992; Saltin & Calbet, 2006; Levine, 2008), remains unclear. In resting conditions, HR appears to play a regulatory role that is coupled to arterial blood pressure regulation. These similarities between HR pacing during exercise in humans and dogs indicate a similar role of HR in the regulation of
. Whether these findings also apply to maximal conditions, where the heart is operating at its limit (Saltin & Strange, 1992; Saltin & Calbet, 2006; Levine, 2008), remains unclear. In resting conditions, HR appears to play a regulatory role that is coupled to arterial blood pressure regulation. These similarities between HR pacing during exercise in humans and dogs indicate a similar role of HR in the regulation of 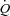 during exercise, despite the anatomical and physiological differences as well as differences in blood volume distribution and cardiac preload.
during exercise, despite the anatomical and physiological differences as well as differences in blood volume distribution and cardiac preload.
Acknowledgments
This study was supported by a grant from the Lundbeck foundation and the Danish Research Council. S.P.M was supported by a grant from the Danish Research Council. The authors have no conflicts of interest to disclose.
Glossary
Abbreviations
- BVC
brain vascular conductance
- CVP
central venous pressure
- dP/dtmax
left ventricular contractility index
- HR
heart rate
- LBF
leg blood flow
- LVC
leg vascular conductance
- MAP
mean arterial pressure
- MCA
middle cerebral artery
- MCA Vmean
brain vascular conductance index
- PAMP
pulmonary artery mean pressure
- PCWP
pulmonary capillary wedge pressure
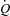
cardiac output
- SV
stroke volume
- SVC
systemic vascular conductance
- Vmean
mean blood velocity
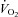
oxygen uptake
Author contributions
A.A.B.: Design of the experiments. Collection, analysis and interpretation of data. Drafting the article. J.H.S.: Collection, analysis and interpretation of data. Revising the article critically for important intellectual content. N.H.S.: Collection, analysis and interpretation of data. Revising the article critically for important intellectual content. B.S.: Collection, analysis and interpretation of data. Revising the article critically for important intellectual content. S.P.M.: Conception and design of the experiments. Collection, analysis and interpretation of data. Drafting the article. All authors approved the final version.
References
- Åstrand PO, Cuddy TE, Saltin B, Stenberg J. Cardiac output during submaxial and maximal work. J Appl Physiol. 1964;19:268–274. doi: 10.1152/jappl.1964.19.2.268. [DOI] [PubMed] [Google Scholar]
- Beard DA, Feigl EO. Understanding Guyton's venous return curves. Am J Physiol Heart Circ Physiol. 2011;3:H629–H633. doi: 10.1152/ajpheart.00228.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman BC, Tsvetkova T, Lowes B, Wolfel EE. Myocardial glucose and lactate metabolism during rest and atrial pacing in humans. J Physiol. 2009;587:2087–2099. doi: 10.1113/jphysiol.2008.168286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevegård S, Jonsson B, Karlof I, Lagergren H, Sowton E. Effect of changes in ventricular rate on cardiac output and central pressures at rest and during exercise in patients with artificial pacemakers. Cardiovasc Res. 1967;1:21–33. doi: 10.1093/cvr/1.1.21. [DOI] [PubMed] [Google Scholar]
- Boushel R, Calbet JA, Rådegran G, Sondergaard H, Wagner PD, Saltin B. Parasympathetic neural activity accounts for the lowering of exercise heart rate at high altitude. Circulation. 2001;104:1785–1791. doi: 10.1161/hc4001.097040. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- Dodge HT, Lord JD, Sandler H. Cardiovascular effects of isoproterenol in normal subjects and subjects with congestive heart failure. Am Heart J. 1960;60:94–105. doi: 10.1016/0002-8703(60)90063-6. [DOI] [PubMed] [Google Scholar]
- Ekblom B, Goldbarg AN, Kilbom A, Astrand PO. Effects of atropine and propranolol on the oxygen transport system during exercise in man. Scand J Clin Lab Invest. 1972;30:35–42. doi: 10.3109/00365517209081087. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol. 2012;97:39–50. doi: 10.1113/expphysiol.2011.057554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dufour SP. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol. 2008;586:2405–2417. doi: 10.1113/jphysiol.2008.152058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande F, Taylor HJ. Adaptive changes in the heart, vessels, and pattern of control under chronically high loads. In: Hamilton W, Dow P, editors. Handbook of Physiology, section 2, Circulation. III. Washington, DC: American Physiological Society; 1965. pp. 2615–2678. [Google Scholar]
- Gunn HM. Heart weight and running ability. J Anat. 1989;167:225–233. [PMC free article] [PubMed] [Google Scholar]
- Guyton AC. Regulation of cardiac output. N Engl J Med. 1967;277:805–812. doi: 10.1056/NEJM196710122771509. [DOI] [PubMed] [Google Scholar]
- Guyton AC. Regulation of cardiac output. Anesthesiology. 1968;29:314–326. doi: 10.1097/00000542-196803000-00016. [DOI] [PubMed] [Google Scholar]
- Guyton AC. The relationship of cardiac output and arterial pressure control. Circulation. 1981;64:1079–1088. doi: 10.1161/01.cir.64.6.1079. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Douglas BH, Langston JB, Richardson TQ. Instantaneous increase in mean circulatory pressure and cardiac output at onset of muscular activity. Circ Res. 1962;11:431–441. doi: 10.1161/01.res.11.3.431. [DOI] [PubMed] [Google Scholar]
- Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, Bogaard HJ, Niizeki K, Yamaya Y, Ziegler MG, Wagner PD. b-Adrenergic or parasympathetic inhibition, heart rate and cardiac output during normoxic and acute hypoxic exercise in humans. J Physiol. 2003;550:605–616. doi: 10.1113/jphysiol.2003.040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BD. VO2max: what do we know, and what do we still need to know? J Physiol. 2008;586:25–34. doi: 10.1113/jphysiol.2007.147629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil E, Joels N. The impulse activity in cardiac afferent vagal fibres. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1961;240:453–460. doi: 10.1007/BF00244938. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Hanel B, Pawelczyk RA, Warberg J, Secher NH. Leg vasoconstriction during dynamic exercise with reduced cardiac output. J Appl Physiol. 1992;73:1838–1846. doi: 10.1152/jappl.1992.73.5.1838. [DOI] [PubMed] [Google Scholar]
- Remensnyder J, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, González-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Jr, Linhart JW, Braunwald E. Effects of changing heart rate in man by electrical stimulation of the right atrium: studies at rest, during exercise, and with isoproterenol. Circulation. 1965;32:549–558. doi: 10.1161/01.cir.32.4.549. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Exercise Physiology: People and Ideas. Bethesda, MD: American Physiological Society; 2003. The cardiovascular system; pp. 98–137. [Google Scholar]
- Rowell LB. Human experimentation: no accurate, quantitative data? J Appl Physiol. 2007;102:837–840. doi: 10.1152/japplphysiol.01242.2006. [DOI] [PubMed] [Google Scholar]
- Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- Saltin B. Exercise hyperaemia: magnitude and aspects on regulation in humans. J Physiol. 2007;583:819–823. doi: 10.1113/jphysiol.2007.136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Calbet JA. Point: in health and in a normoxic environment, VO2, max is limited primarily by cardiac output and locomotor muscle blood flow. J Appl Physiol. 2006;100:744–745. doi: 10.1152/japplphysiol.01395.2005. [DOI] [PubMed] [Google Scholar]
- Saltin B, Strange S. Maximal oxygen uptake: “old” and “new” arguments for a cardiovascular limitation. Med Sci Sports Exerc. 1992;24:30–37. [PubMed] [Google Scholar]
- Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol. 2008;104:306–314. doi: 10.1152/japplphysiol.00853.2007. [DOI] [PubMed] [Google Scholar]
- Seed WA, Walker JM. Review: Relation between beat interval and force of the heartbeat and its clinical implications. Cardiovasc Res. 1988;22:303–314. doi: 10.1093/cvr/22.5.303. [DOI] [PubMed] [Google Scholar]
- Seifert T, Fisher JP, Young CN, Hartwich D, Ogoh S, Raven PB, Fadel PJ, Secher NH. Glycopyrrolate abolishes the exercise-induced increase in cerebral perfusion in humans. Exp Physiol. 2010;95:1016–1025. doi: 10.1113/expphysiol.2010.054346. [DOI] [PubMed] [Google Scholar]
- Seifert T, Rasmussen P, Secher NH, Nielsen HB. Cerebral oxygenation decreases during exercise in humans with b-adrenergic blockade. Acta Physiologica. 2009;196:295–302. doi: 10.1111/j.1748-1716.2008.01946.x. [DOI] [PubMed] [Google Scholar]
- Shepherd AP, Granger HJ, Smith EE, Guyton AC. Local control of tissue oxygen delivery and its contribution to the regulation of cardiac output. Am J Physiol. 1973;225:747–755. doi: 10.1152/ajplegacy.1973.225.3.747. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Mendoza JR. Passive regulation of cardiac output during exercise by the elastic characteristics of the peripheral circulation. Exerc Sport Sci Rev. 2004;32:31–35. doi: 10.1097/00003677-200401000-00007. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Zhou XP, Scher AM, Rowell LB. Dependence of cardiac filling pressure on cardiac output during rest and dynamic exercise in dogs. Am J Physiol Heart Circ Physiol. 1993;265:H316–H322. doi: 10.1152/ajpheart.1993.265.1.H316. [DOI] [PubMed] [Google Scholar]
- Sonnenblick EH, Braunwald E, Williams JF, Jr, Glick G. Effects of exercise on myocardial force-velocity relations in intact unanesthetized man: relative roles of changes in heart rate, sympathetic activity, and ventricular dimensions. J Clin Invest. 1965;44:2051–2062. doi: 10.1172/JCI105312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson I. Cardiovascular physiology: venous return. Can Anesth Soc J. 1984;31:S31–S37. doi: 10.1007/BF03007033. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol. 2004;97:739–747. doi: 10.1152/japplphysiol.00185.2004. [DOI] [PubMed] [Google Scholar]
- Vatner S. The Peripheral Circulations. New York: Grune and Stratton; 1975. Effects of exercise on distribution of regional blood flows and resistances; pp. 211–223. [Google Scholar]
- Vatner SF, Pagani M. Cardiovascular adjustments to exercise: hemodynamics and mechanisms. Prog Cardiovasc Dis. 2009;19:91–108. doi: 10.1016/0033-0620(76)90018-9. [DOI] [PubMed] [Google Scholar]
- White S, Patrick T, Higgins CB, Vatner SF, Franklin D, Braunwald E. Effects of altering ventricular rate on blood flow distribution in conscious dogs. Am J Physiol. 1971;221:1402–1407. doi: 10.1152/ajplegacy.1971.221.5.1402. [DOI] [PubMed] [Google Scholar]
- Wyss CR, Bennett TD, Scher AM. Beat-by-beat control of cardiac output in awake dogs with atrioventricular block. Am J Physiol Heart Circ Physiol. 1982;242:H1118–H1121. doi: 10.1152/ajpheart.1982.242.6.H1118. [DOI] [PubMed] [Google Scholar]


