Tuberculosis has been an elusive infectious disease. In India, two deaths occur every three minutes due to tuberculosis.[1] As modern antituberculosis therapy (ATT) can cure all patients, these deaths can be prevented. But it is imperative that these patients take ATT for the prescribed duration (minimum of six months). Hence, adherence to treatment plays an important role in the success of the treatment.
TREATMENT STRATEGY
The Revised National Tuberculosis Control Programme (RNTCP) of India is based on Directly Observed Treatment-Short course (DOTS) strategy. The RNTCP was launched as a national program in 1997 and the entire country was covered under DOTS by March 2006.[1] Even though the treatment is directly observed, there are defaulters under this strategy too. This is of concern as it can lead to the serious issue of multidrug-resistant tuberculosis (MDR TB).
RESOLVED ISSUES
Adherence to treatment is a keystone in the success of the program. The factors affecting adherence can be grouped as patient factors, disease factors, and medication characteristics. A few of the factors hindering the success were identified and rectified in the recent past.
Simplifying the regimen
Earlier, there were five categories of treatment for tuberculosis and this made the regimen complex. In 2011, this regimen has been simplified by RNTCP to include only two categories [Table 1].[2] This simplified regimen has reduced the logistic difficulties in introducing fixed-dose drug combinations (FDCs) into the regimen.
Table 1.
Revised categories of treatment for tuberculosis

Improvised formulation technology
In the early 1990s, FDCs of antitubercular drugs were not advocated because of their relatively poor bioavailability and short shelf life.[3] With the present improved formulation technologies, the currently available FDCs are bioequivalent to single drug reference products. Moreover, the stability of these FDCs even after six months in tropical countries is well established.[3] Randomized controlled trials have proved their efficacy and systematic reviews also favor the use of FDCs for tuberculosis.[3,4]
UNRESOLVED PROBLEMS
Pill burden
Presently, a category I tuberculosis patient should take six tablets and one capsule (two tablets each of isoniazid, pyrazinamide, and ethambutol, and one capsule of rifampicin) available as a combination pack for a single-day treatment [Figure 1]. Even though DOTS is an effective strategy to improve compliance, the pill burden of the current regimen hinders compliance and, thereby, the success of the treatment.
Figure 1.
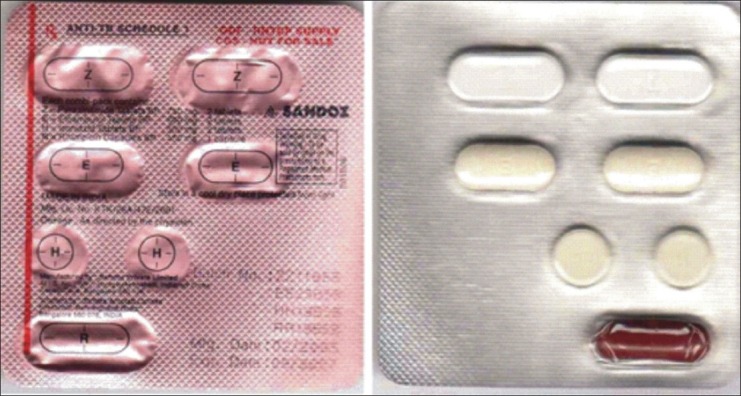
Combination pack of intensive phase category I for the treatment of tuberculosis
Discrepancy between guidelines and regimens
Standard 8 of International Standards for Tuberculosis Care and the WHO Guidelines for Treatment of Tuberculosis recommend the use of FDCs for the treatment of tuberculosis.[2,5] However, the regimens followed by RNTCP under the DOTS strategy have not yet incorporated this recommendation.
THE SOLUTION
Introducing FDCs for the treatment of tuberculosis seems to be a promising solution to the problem. Incorporating FDCs into the present regimen would offer the following benefits:
The pill burden is drastically reduced, thereby increasing acceptability by the patient.
Selection of a particular drug from the combination pack and the consequent monotherapy leading to the development of drug-resistant forms can be reduced.
Logistic difficulties such as ordering, storage, handling, and delivery of the drug can be reduced by FDCs.
Moreover, FDCs of antitubercular drugs are readily available in the market and private practitioners are using them. When we have allowed hundreds of irrational FDCs to creep into the market, why should we hesitate to introduce a rational FDC into our national program? It's right time that we introduced FDCs into the RNTCP-DOTS regimen for the treatment of tuberculosis.
REFERENCES
- 1.TBC India [Home page on internet] [Last accessed on 2012 Oct 12]. Available from: http://www.tbcindia.nic.in/key.html .
- 2.RNTCP Annual Report 2011. [Last accessed on 2012 Oct 12]. Available from: http://www.tbcindia.nic.in/documents.html .
- 3.Monedero I, Caminero JA. Evidence for promoting fixed dose combination drugs in tuberculosis treatment and control: A review. Int J Tuberc Lung Dis. 2011;15:433–9. doi: 10.5588/ijtld.09.0439. [DOI] [PubMed] [Google Scholar]
- 4.Lienhardt C, Cook SV, Burgos M, Yorke-Edwards V, Rigouts L, Anyo G, et al. Efficacy and safety of a 4-drug fixed-dose combination regimen compared with separate drugs for treatment of pulmonary tuberculosis: The Study C randomized controlled trial. JAMA. 2011;305:1415–23. doi: 10.1001/jama.2011.436. [DOI] [PubMed] [Google Scholar]
- 5.WHO Guidelines on tuberculosis. [Last accessed on 2012 Oct 12]. Available from: http://www.who.int/publications/guidelines/tuberculosis/en/index.html .


