Abstract
Premna esculenta Roxb. (family Verbenaceae) is a shrub used by the ethnic people of Chittagong Hill Tracts of Bangladesh for the treatment of hepatocellular jaundice. The present study was done to evaluate the hepatoprotective and the in vivo antioxidant activity of ethanolic extracts of leaves of the plant in carbon tetrachloride-induced liver damage in rats. Hepatotoxicity was induced in rats by i.p. injection of CCl4 diluted with olive oil (1:1 v/v; 1 mL/kg body weight) on alternate days for 7 days. After 7 days of pretreatment of test extracts, the biochemical markers such as Serum Glutamate Oxaloacetate Transaminase (SGOT), Serum Glutamate Pyruvate Transaminase (SGPT), Alkaline Phosphatase (ALP), total protein, and albumin were estimated followed by the measurement of liver cytosolic antioxidant enzymes such as superoxide dismutase, catalase, and peroxidase. The data were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett's t-test. The extract both at the doses of 200 and 400 mg/kg p.o. significantly (P < 0.001) reduced the elevated levels of SGPT, SGOT, ALP and increased the reduced levels of total protein and albumin compared to the CCl4-treated animals. The extracts also showed a significant (P < 0.001) increase in the reduced levels of superoxide dismutase (SOD), catalase, and peroxidase. The effects of the extracts on these parameters were comparable with those of the standard, silymarin. The findings of the study indicate that the leaf extract of P. esculenta showed a potential hepatoprotective activity and the protective action might have manifested by restoring the hepatic SOD, catalase, and peroxidase levels. The results justify the traditional use of this plant in liver disorders.
Keywords: Antioxidant activity, carbon tetrachloride, ethanolic extracts, hepatoprotective activity, Premna esculenta, rats
INTRODUCTION
Conventional drug therapy for many common liver disorders, including nonalcoholic fatty liver disease and viral hepatitis, has limited efficacy and potentially life- threatening side effects.[1] In contrast, traditional remedies have been using by many people around the world for the treatment of liver ailments for a long period of time without significant toxic effects. Therefore, it is necessary to search for complementary and alternative medicine (CAM), especially herbal drugs for the treatment of liver disease for better efficacy and safety to replace currently used drugs.[2]
Premna esculenta Roxb. (family Verbenaceae) is a short- stemmed branching shrub which grows in forests of Chittagong and Chittagong Hill Tracts of Bangladesh and India (Assam).[3] The plant, commonly known as “Lelom pata,” has been traditionally used by tribal people of Bangladesh in the treatment of gout, hookworm infestation, hysteria, hepatocellular jaundice, leucorrhea, lipoma (tumor), edema, snake bite, stomach disorders, and ureterolithiasis.[3] In the traditional system of remedies, the leaves of the plant are applied on the affected place for the treatment of arthritis and bacterial and fungal infections. Roots are commonly used in the combination with other plants in the cure of gout, edema, and jaundice.[3] The leaves are one of the ingredients of a medicine used for jaundice in Khagrachari of Bangladesh. Leaves cooked with Nappi (a fermented paste of varieties of marine fishes) is an important diet for the patient of jaundice in Chittagong Hill Tracts.[4]
Though the leaves and roots of the plant have been traditionally used in the treatment of liver disorders like jaundice, there is no scientific document to validate its folklore uses. Besides, the plant has yet not undergone any chemical or pharmacological investigation except for the antihyperlipidemic activity of leaf and root extracts we have reported earlier.[5] Therefore, to justify the traditional claims, the present study was performed to evaluate the hepatoprotective activity of the ethanolic extract of leaves of the plant in carbon tetrachloride (CCl4)-induced liver damage in Long–Evans rats. The in vivo antioxidant activities of the extract were also evaluated in CCl4-induced hepatotoxicity and oxidative stress in rats to test whether the hepatoprotective activity results from the antioxidant activity or not. The reducing power capacity and total phenolic content were also determined because previous studies showed that phenolic compounds played an important role in antioxidant and hepatoprotective activities.
MATERIALS AND METHODS
Drugs and chemicals
Nitroblue tetrazolium, sodium carbonate, Ethylenediaminetetraacetic acid (EDTA), potassium iodide (KI), sodium acetate, hydroxylamine hydrochloride, and Tween-80 were purchased from Sigma Chemicals Co. (USA). Carbon tetrachloride, Folin–Ciocalteu reagent, and hydrogen peroxide (H2O2) were purchased from Merck (Germany). Olive oil (P. Sasso e Figili, Oneglia, Italy) was purchased from the local market. SGPT and SGOT measuring kits were obtained from Human (Germany); SALP kits and kits for albumin (ALB) and total protein were procured from Linear Chemicals S.L. (Barcelona, Spain). Silymarin (capsule Sylbin) was obtained from Square Pharmaceuticals Ltd., Bangladesh, and all other reagents and chemicals were of BDH and E Merck analytical grade.
Plant material
The leaves of P. esculenta were collected from Rangamati, Chittagong Hill Tracts, Bangladesh, in September, 2010. The botanical identification and authentication of the plant was done by Bangladesh National Herbarium and a voucher specimen (accession no. DACB 35035) was deposited there for future reference. The leaf parts were cut into small pieces and sun dried for 7 days. The dried, cut pieces were powdered by a mechanical grinder and stored in an air-tight container.
Preparation of plant extracts
The dried and ground leaf powder (1.0 kg) was extracted with 95% ethanol (5.0 L) in an air-tight, clean, round- bottomed flask for 15 days at room temperature with occasional shaking. The whole mixture was then filtered through a cotton plug followed by Whatman's no.1 filter paper, and the filtrate thus obtained was concentrated at 45°C under reduced pressure with a Heidolph rotary evaporator. The concentrated extract was then air dried to a solid residue. The extract and standard drug silymarin were suspended in normal saline using 1.0% Tween-80 before administering to experimental animals.
Determination of the total phenolic content
The concentration of total phenol in the ethanolic extract of leaves (0.25 mg/mL) was determined using the Folin–Ciocalteu reagent and external calibration with gallic acid.[6,7] To an aliquot of the extract (0.5 mL) solution (conc. 250 μg/ mL), 2.5 mL of the Folin–Ciocalteu reagent diluted 10 times with water and 2.0 mL of Na2CO3 (7.5% w/v) solution were added. The mixture was then incubated for 20 min at room temperature for color development. For a control sample, 0.5 mL of distilled water was used. After 20 min, the absorbance was measured at 760 nm by a UV spectrophotometer. The concentration of the total phenolic content was determined as mg of GAE (gallic acid equivalent)/g of the extract by using an equation, y = 0.016x + 0.021, obtained from the gallic acid calibration curve prepared from the gallic acid solution with different concentrations (0–100 μg/mL), where y is the unit absorbance and x is the concentration of phenolic compound (GAE).
Determination of reducing power
The reducing power of the extract was evaluated according to the method of Oyaizu.[8] Volumes of 1.0 mL of different concentrations (250–7.8125 μg/mL) of the ethanolic extract of leaves prepared in methanol and ascorbic acid were mixed individually to the mixture containing 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of potassium ferricyanide K3[Fe(CN)6]; (1% w/v). The mixture was incubated at 50°C for 20 min, followed by the addition of 2.5 mL of trichloroacetic acid (1% w/v). The samples were then centrifuged at 4000 rpm for 15 min. The upper layer of the solution (2.5 mL) was mixed with 2.5 ml of distilled water and 0.5 ml of ferric chloride (0.5% w/v). The absorbance was measured at 700 nm against a blank sample. An increased absorbance of the reaction mixture indicated a higher reducing power of the plant extract.
Experimental animals
Long–Evans rats (Rattus norvegicus) of either sex, weighing 150–190 g were purchased from the animal resource branch of International Centre for Diarrheal Diseases and Research (ICDDR), Bangladesh. The animals were kept under standard environmental conditions (temperature 23 ± 2°C, relative humidity 55 ± 10%, and 12-h light/dark cycle). The animals were fed with standard rat pellets (formulated by ICDDR, Bangladesh) and water ad libitum and acclimatized to laboratory conditions for 7 days before conducting experiments. The investigation was conducted on experimental animals in accordance with the international principles for laboratory animals’ use and care as found in the guidelines.[9] The study protocol was approved by the Ethical Review Committee, Faculty of Biological Science, University of Dhaka.
Hepatoprotective activity
The hepatoprotective activity was evaluated according to the method described by Ahmed et al.[10] Twenty- five rats were randomly selected and divided into five groups of five animals each. Group I served as normal controls and received only the vehicle (1% Tween-80 in normal saline) at a dose of 5 mL/kg body weight daily. Group II served as the CCl4-treated control group/negative controls and received the vehicle (5 mL/kg/ day) and CCl4 diluted with olive oil. Group III animals were administered with the standard drug silymarin at a dose of 100 mg/ kg/ day. Groups IV and V received the ethanolic extract of P. esculenta leaves (EEPEL), at a dose of 200 and 400 mg/kg/day, respectively. The vehicle or test drugs were administered orally for successive 7 days. To induce hepatotoxicity, carbon tetrachloride diluted with olive oil (1:1) was given intraperitoneally (i.p.)[11] at a dose of 1 mL/ kg body weight to all the rats except the rats in Group I on alternate days for a period of 7 days while olive oil (0.5 mL/kg i.p.) was injected into group I animals. After 24 h of the last dose of CCl4, all the rats were sacrificed by cervical decapitation; blood samples were collected through retro-orbital plexus and allowed to clot for 30 min at room temperature. The clear serum was separated by centrifugation at 4000 rpm for 10 min and serum samples were stored at -40°C until use for the determination of biochemical parameters. Hepatic tissues were carefully excised, cleaned, and homogenized in cold 1.15% KCl and10 mM phosphate buffer with EDTA (pH 7.4) and centrifuged at 10,000 rpm for 10 min. The supernatant was further centrifuged at 13,000 rpm for 60 min to obtain a cytosolic extract for the estimation of liver cytosolic superoxide dismutase (SOD), catalase, and peroxidase.
Assessment of liver function
The functional state of the liver was determined by estimating the biochemical parameters such as Serum Glutamic Oxaloacetic Transaminase (SGOT), Serum Glutamic Pyruvic Transaminase (SGPT), Serum Alkaline Phosphatase (SALP), total protein, and ALB. SGOT and SGPT were estimated by enzymatic UV kinetic methods based on the reference method of International Federation of Clinical Chemistry.[12–14] Alkaline phosphatase (ALP) was estimated by the method described by McComb and Bowers.[15] Total protein (TP) was determined by the biuret method[16] while ALB was estimated by BCG[17] involving the formation of a blue-green complex with bromocresol green at a slightly acidic pH which was measured photometrically.
Antioxidant enzymes assays in liver
Superoxide dismutase activity
SOD was assayed as described by Beauchamp and Fridovich[18] and Chidambara et al.[19] based on the reduction of nitroblue tetrazolium (NBT) to water insoluble blue formazan. The assay mixture contained 0.5 mL of liver homogenate, 1 mL of 50 mM sodium carbonate, 0.4 mL of 24 μm NBT, and 0.2 mL of 0.1 mM EDTA. The reaction was initiated by adding 0.4 mL of 1 mM hydroxylamine hydrochloride. The developed blue color in the reaction was measured at 560 nm. Zero time absorbance was taken at 560 nm followed by recording the absorbance reading every 30 s for a period of 5 min at 25°C. The above- mentioned reaction mixtures without the liver homogenate served as control. The rate of increase in absorbance units (A) per minute for the control and for the test sample(s) was determined and the percentage inhibition for the test sample(s) was calculated by the following formula:
![]()
where (A560 nm at 5 min and 30s –A560 nm at 30s)/5 min = Δ A560 nm/minute.
Units of the SOD activity were expressed as the amount of enzyme required to inhibit the reduction of NBT by 50% and the activity was expressed as units per mg of protein.[20]
Catalase activity
The activity of catalase was determined by an adaptation of the method of Aebi.[21] Briefly, 1 mL of the liver homogenate was taken in a test tube and 1.9 mL of phosphate buffer (50 mM, pH 7.4) was added to it. The reaction was initiated by the addition of 1 mL of 30 mM H2O2. A mixture of 2.9 mL of phosphate buffer and 1 mL of H2O2 without the liver homogenate served as the blank. The decrease in absorbance due to the decomposition of H2O2 was recorded at 240 nm against the blank. Units of catalase were expressed as the amount of enzyme that decomposes 1 μM of H2O2 per min at 25°C and the activity was expressed in terms of units per milligram of proteins.[20]
Peroxidase activity
Peroxidase was assayed by the method of Nicholas.[22] One milliliter of the 10 mM KI solution and 1 mL of 40 mM sodium acetate were added to 0.5 mL of the liver homogenate. Then the absorbance of potassium per iodide was read at 353 nm, which indicated the amount of peroxidase. Then 20 μL of 15 mM H2O2 was added to the reaction mixture followed by recording the change in the absorbance for a period of 5 min. Units of the peroxidase activity were expressed as the amount of enzyme required to change the optical density by 1 unit per min. The specific activity was expressed in terms of units per milligram of proteins.[20]
Statistical analysis
The results were expressed as means ± standard errors of mean (SEM). The data were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett's t-test to determine the level of significance.[23] A value of P < 0.05 was considered to be significant.[24] The statistical analysis was carried out using the SPSS program (version 17.0).
RESULTS
In the present study, hepatoprotective and in vivo antioxidant activities of EEPEL were investigated in CCl4 intoxicated rats. The total phenolic contents and reducing capacity of extract were also determined.
Total phenolic contents
The amount of total phenolic content of EEPEL was determined using the gallic acid calibration curve [Figure 1] and was found to be 15.625 mg of GAE/g of extracts [Table 1].
Figure 1.
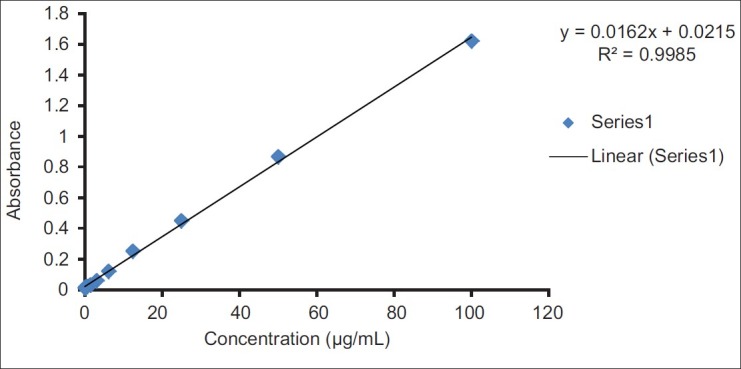
Standard curve for gallic acid. The coefficient of determination (R2) of the standard curve was 0.998
Table 1.
Total phenolics content of ethanolic extracts of Premna esculenta leaves

Reducing capacity
Figure 2 shows the reducing capability of EEPEL compared to the standard antioxidant, ascorbic acid.
Figure 2.
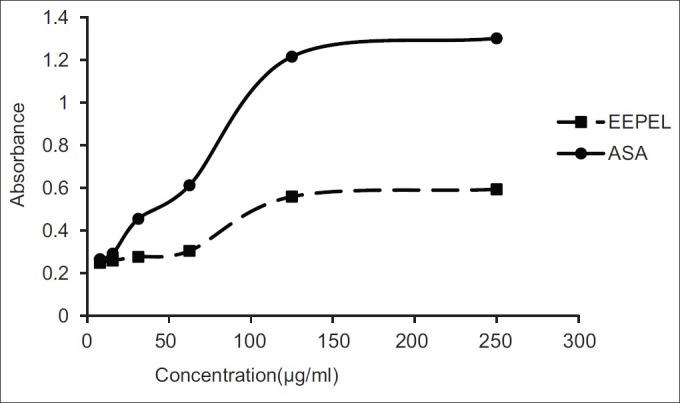
Reducing power of the ethanolic extract of leaves of Premna esculenta (EEPEL) and ascorbic acid (ASA) at different concentrations. The extract showed a moderate reducing capacity compared to that of the standard ascorbic acid
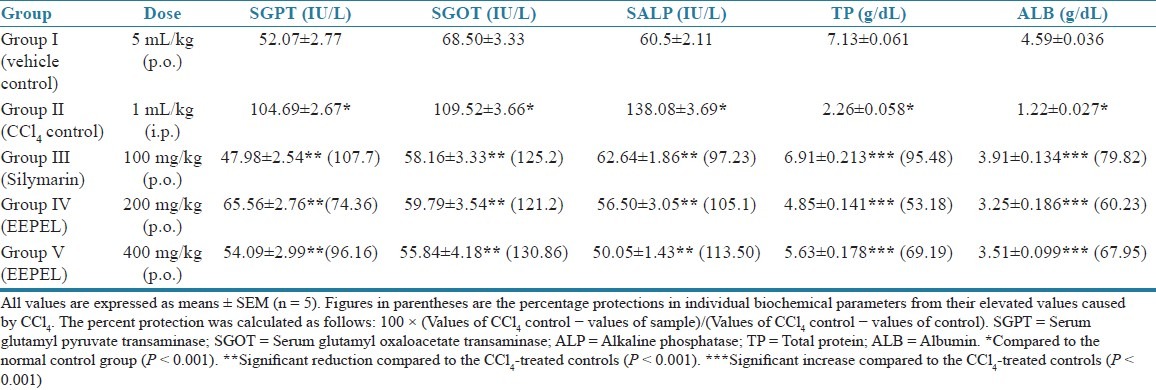
Effect of EEPEL on SGOT, SGPT, SALP, total protein and albumin concentrations in CCl4-induced liver damage in rats
The results of the investigation of the hepatoprotective activity of EEPEL are shown in Table 2. In the CCl4 control group, the significant acute hepatocellular damage was manifested by the elevated levels of SGPT, SGOT, and SALP and decreased levels of total protein and albumin when compared with those of controls. But the oral administration of EEPEL both at the doses of 200 and 400 mg/kg/day for 7 days significantly (P < 0.001) reduced the elevated levels of SGPT, SGOT, and SALP and significantly (P < 0.001) increased the reduced levels of total protein and albumin when compared with CCl4-treated rats, and these biochemical parameters were comparable with silymarin [Table 2].
Table 2.
Effect of the ethanolic extract of Premna esculenta leaves on serum enzymes, total proteins, and albumin in CCl4-induced liver damage in rats
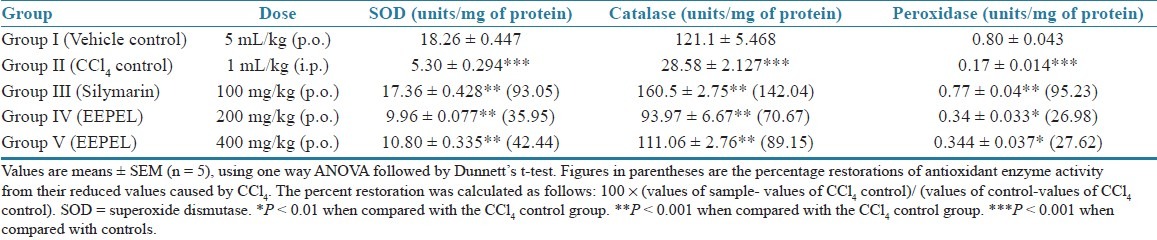
Effect of EEPEL on the levels of antioxidant enzymes in CCl4-induced liver damage in rats
As shown in Table 3, as compared to the control, CCl4- treated animals exhibited significantly (P <0.001) lower levels of SOD, catalase, and peroxidase activity in liver cytosol. Conversely, the groups which received the test drug of EEPEL both at the doses of 200 and 400 mg/kg body weight p.o. for 7 days showed a significant (P < 0.01) dose-dependent increase in the reduced levels of SOD, catalase, and peroxidase. The ethanolic extract of leaves of the plant has a moderate activity in terms of the restoration of reduced enzyme levels as compared to the standard drug, silymarin, which also significantly (P < 0.01) restored the altered levels of SOD, catalase, and peroxidase.
Table 3.
Effect of the ethanolic extract of Premna esculenta leaves on antioxidant enzymes in CCl4-induced hepatotoxicity in rats
DISCUSSION
The evaluation of the preventive action in liver damage induced by CCl4 has been widely used for hepatoprotective drug screening. CCl4 is a widely used experimental hepatotoxicant which requires metabolic activation by the liver cytochrome P-450 enzymes to form highly reactive toxic metabolites such as trichloromethyl radical (CCl3·) and peroxy trichloromethyl radical (CCl3OO·). Both trichloromethyl and its peroxy radical (CCl3–OO∙) are capable of covalently binding to proteins or lipids of cell membranes and organelles, abstracting hydrogen atoms from polyunsaturated fatty acid (PUFA), initiating lipid peroxidation thus causing damage to cell membrane, disturbing Ca2+ homeostasis, changing enzyme activities, and finally inducing hepatic injury or necrosis.[25–27] This lipid peroxidative degradation of the liver cell plasma membrane leads to the release of a variety of enzymes (GPT, GOT, ALP) normally located in the cytosol into the blood stream, and estimating the activities of these serum marker enzymes can make the assessment of liver function. The ethanolic extract of leaves of P. esculenta significantly and dose dependently reduced the elevated serum enzyme levels (GOT, GPT, and ALP) and increased the reduced serum total protein and albumin. The tendency of these enzymes to return to normality in the extract administered group is a clear manifestation of antihepatotoxic effects of the extract. The reduction in the levels of SGPT and SGOT toward the normal value indicated that the extract protected the structural integrity of hepatocyte cell membrane by stimulating the regeneration process of damaged cells. Reduction in ALP levels implies that the extract restored the stability of the biliary function during injury with CCl4. The significant increase in the total protein and albumin levels suggests the stabilization of endoplasmic reticulum leading to protein synthesis.
The protective agents exert their action against CCl4- induced liver injury by the impairment of CCl4- mediated lipid peroxidation via a decreased production of CCl4- derived free radicals or through the antioxidant activity of the protective agents themselves.[28] A previous in vitro antioxidant activity study of ethanolic extracts of leaves of P. esculenta showed that the extract possesses potential DPPH, superoxide, and NO· free radical scavenging activities.[5] In the present study, the ethanolic extract of leaves of the plant showed a potential in vivo antioxidant activity as it dose dependently elevated the reduced levels of liver cytosolic SOD, catalase, and peroxidase activity. These antioxidant enzymes are involved in the reduction of reactive oxygen species (ROS) and peroxides produced in the living organism thus play a vital role in the maintenance of a balanced redox status. The restoration of the SOD activity toward a normal value indicates that the ethanolic extract can help in cellular defense mechanisms by preventing cell membrane oxidation.[29] An increase in the catalase activity with respect to CCl4 treatment indicates that the extract can play an important role in scavenging hydrogen peroxide. Similarly, an increase in the peroxidase activity indicates that the extract also helps in the restoration of vital molecules such as NAD, cytochrome, and glutathione.[29]
Therefore, the hepatoprotection of the leaf extracts of P. esculenta against CCl4- induced hepatotoxicity in rats could be attributed to its ability to restore the antioxidant enzymes SOD, catalase, and peroxidases of liver cytosol or to the free radical scavenging activity of the extract. The observed hepatoprotective activity of the leaf extract may be due to the presence of phenolic compounds [Table 1] such as phenols and tannins found in the previous preliminary phytochemical screening[5] as these compounds are believed to have the ability for scavenging and stabilizing lipid oxidation[30] and some studies suggest a correlation between phenolic content and antioxidant and hepatoprotective activities.[30–32]
CONCLUSION
In conclusion, the results of the present study indicate that the ethanolic extracts of leaves of P. esculenta exhibited a potential hepatoprotective activity against carbon tetrachloride induced hepatotoxicity and validate the traditional use of this plant in hepatocellular jaundice. Further studies are required to isolate and characterize the active principles, which are responsible for the hepatoprotective efficacy of this valuable medicinal plant.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Umer S, Asres K, Veeresham C. Hepatoprotective activities of two Ethiopian medicinal plants. Pharm Biol. 2010;48:461–8. doi: 10.3109/13880200903173593. [DOI] [PubMed] [Google Scholar]
- 2.Mitra SK, Seshadri SJ, Venkataranganna MV, Gopumadhavan S, Udupa VU, Sarma DN. Effect of HD-03-a herbal formulation in galactosamine-induced hepatopathy in rats. Indian J Physiol Pharmacol. 2000;44:82–6. [PubMed] [Google Scholar]
- 3.Uddin SN. Traditional uses of Ethnomedicinal plants of the Chittagong Hill Tracts. 1st ed. Dhaka: Bangladesh National Herbarium; 2006. [Google Scholar]
- 4.Yusuf M, Begum J, Hoque N, Chowdhury JU. Medicinal Plants of Bangladesh. 2nd ed. Chittagong: BCSIR Laboratories Chittagong; 2009. [Google Scholar]
- 5.Mahmud ZA, Bachar SC, Qais N. Antihyperlipidemic activity of leaf and root extracts of Premnae sculenta (Roxb.) in Poloxamer-407 induced hyperlipidemic mice and rats. Orient Pharm Exp Med. 2011;11:263–70. [Google Scholar]
- 6.Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51:609–14. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- 7.Sherget M, Kotnik P, Hadolin M, Hras AR, Simonic M, Knez Z. Phenol, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191–8. [Google Scholar]
- 8.Oyaizu M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 9.Council instructions about the protection of living animals used in scientific investigations. Official Journal of the European Communities (JO 86/609/CEE), L358; Brussels. 1986:1–18. [Google Scholar]
- 10.Ahmed B, Alam T, Khan SA. Hepatoprotective activity of Luffa echinata fruits. J Ethnopharmacol. 2001;76:187–9. doi: 10.1016/s0378-8741(00)00402-5. [DOI] [PubMed] [Google Scholar]
- 11.Nishigaki I, Kuttan R, Oku H, Ashoori F, Abe H, Yagi K. Suppressive effect of curcumin on lipid peroxidation induced in rats by carbon tetrachloride or Co60 irradiation. J Clin Biochem Nutr. 1992;13:23–9. [Google Scholar]
- 12.Bergmeyer HU, Bowes GN, Jr, Horder M, Moss DW. Provisional recommendations on IFCC methods for the measurement of catalytic concentrations of enzymes Part 2. IFCC method for aspartate aminotransferase. Clin Chim Acta. 1976;70:F19–42. [PubMed] [Google Scholar]
- 13.Bergmeyer HU. IFCC methods for the measurement of catalytic concentrations of enzymes: Part 3. IFCC method for alanine aminotransferase (L-alanine: 2-oxoglutarate aminotransferase, EC 2.6.1.2) Clin Chim Acta. 1980;105:1147–54. [PubMed] [Google Scholar]
- 14.Henley KS. IFCC method for alanine aminotransferase Stage 2, Draft 1, 1979-11-09. Clin Chim Acta. 1980;105:155–66. [Google Scholar]
- 15.McComb RB, Bowers GN., Jr Study of optimum buffer conditions for measuring alkaline phosphatase activity in human serum. Clin Chem. 1972;18:97–104. [PubMed] [Google Scholar]
- 16.Peters T. Proposals for standardization of total protein assays. Clin Chem. 1968;14:1147–59. [PubMed] [Google Scholar]
- 17.Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromocresol green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 18.Beauchamp C, Fridovich I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 19.Chidambara MK, Jayaprakasha GK, Singh RP. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem. 2002;50:4791–5. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- 20.Habbu PV, Shastry RA, Mahadevan KM, Joshi H, Das SK. Hepatoprotective and antioxidant effects of Argyreia Speciosain rats. Afr J Tradit Complement Altern Med. 2008;5:158–64. doi: 10.4314/ajtcam.v5i2.31268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 22.Nicholas MA. A Spectrophotometric assay for iodide oxidation by thyroid peroxidase. Anal Biochem. 1962;4:341–5. doi: 10.1016/0003-2697(62)90097-0. [DOI] [PubMed] [Google Scholar]
- 23.Dunnet CW. New tables for multiple comparisons with a control. Biometrics. 1964;20:482–92. [Google Scholar]
- 24.Woodson RF. Probability and mathematical statistics. Chichester: Wiley; 1987. Statistical methods for the analysis of biochemical data. [Google Scholar]
- 25.Clawson GA. Mechanisms of carbon tetrachloride hepatotoxicity. Path Immunopathol Res. 1989;8:104–12. doi: 10.1159/000157141. [DOI] [PubMed] [Google Scholar]
- 26.Recknagel RO, Glender EA, Jr, Dolak JA, Walter RL. Mechanism of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–54. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 27.Boll M, Weber LW, Becker E, Stampfl A. Mechanism of carbon tetrachloride-induced hepatotoxicity. Hepatocellular damage by reactive carbon tetrachloride metabolites. Z Naturforsch C. 2001;56:649–59. doi: 10.1515/znc-2001-7-826. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed B, Alam T, Varshney M, Khan SA. Hepatoprotective activity of two plants belonging to the Apiaceae and the Euphorbiaceae family. J Ethnopharmacol. 2002;79:313–6. doi: 10.1016/s0378-8741(01)00392-0. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Lee YS, Jung SH, Kang SS, Shin KH. Anti-oxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch Pharm Res. 2003;26:719–22. doi: 10.1007/BF02976680. [DOI] [PubMed] [Google Scholar]
- 30.Yen GC, Duh PD, Tsai CL. Relationship between antioxidant activity and maturity of peanut hulls. J Agric Food Chem. 1993;41:67–70. [Google Scholar]
- 31.Chang JC, Lin CC, Wu SJ, Lin DL, Wang SS, Miaw CL, et al. Antioxidative and hepatoprotective effects of Physalis peruviana extract against acetaminophen-induced liver injury in rats. Pharm Biol. 2008;46:724–31. [Google Scholar]
- 32.Yoshikawa M, Ninomiya K, Shimoda H, Nishida N, Matsuda H. Hepatoprotective and antioxidative properties of Salacia reticulata: Preventive effects of phenolic constituents on CCl4-induced liver injury in mice. Biol Pharm Bull. 2002;25:72–6. doi: 10.1248/bpb.25.72. [DOI] [PubMed] [Google Scholar]


