Abstract
Lenalidomide, a novel immunomodulatory drug (IMiD), is a promising therapeutic strategy for patients with relapsed/refractory chronic lymphocytic leukemia (CLL) and B-cell lymphomas. Biologically, the mechanisms responsible for lenalidomide activity are yet to be clearly defined. Based on preclinical models and early correlative studies conducted in parallel to clinical trials, lenalidomide has been found to enhance natural killer (NK)- and T-cell activity against tumor cells, alter the balance of pro- and anti-inflammatory cytokines in the tumor bed, inhibit angiogenesis, and, to a lesser degree, induce cell cycle arrest and apoptosis in cancer cells. Together, all of these biological effects appear to play a role in the activity observed in CLL or lymphoma patients treated with lenalidomide. Given the effect in NK- and T-cell function, lenalidomide is an alternative strategy to enhance the antitumor activity of monoclonal antibodies (mAbs). Clinical responses have been observed in patients with relapsed/refractory CLL, follicular lymphoma, small lymphocytic lymphoma, mantle cell lymphoma (MCL), and diffuse large B-cell lymphoma (DLBCL) treated with lenalidomide single agent. The favorable toxicity profile and route of administration made the use of lenalidomide an attractive therapy for certain types of patients (i.e. elderly, chemotherapy unfit, etc.). The erratic but serious incidence of tumor lysis syndrome and/or tumor flare reactions provides challenges in the incorporation of lenalidomide in the management of previously untreated CLL or CLL/lymphoma patients with bulky adenopathy. Correlative studies and/or retrospective analysis of lenalidomide-treated patients had identified several biomarkers associated with clinical endpoints in CLL (i.e. changes in tumor necrosis factor alpha [TNF-α] or vascular endothelial growth factor [VEGF] levels) or DLBCL (non-GCB phenotype) patients, but need to be validated. Early studies evaluating the efficacy and toxicity of lenalidomide in combination with rituximab in previously untreated indolent lymphoma are promising and warrant further study. In addition, the evaluation of lenalidomide in the maintenance setting or in combination with other target-specific agents (i.e. proteasome inhibitors) in aggressive lymphomas is being addressed in ongoing clinical trials. In summary, lenalidomide is emerging as a biologically active and novel agent in the treatment of B-cell neoplasms. Future translational and clinical studies will further define the role of lenalidomide in the management of de novo or relapsed/refractory CLL or B-cell lymphomas and identify the subset of patients most likely to gain clinical benefit.
Keywords: B-cell lymphoma, chronic lymphocytic leukemia, immunomodulatory drug, lenalidomide
Introduction
According to this year's published cancer statistics, approximately 74,030 and 14,990 new cases of lymphoma and chronic lymphocytic leukemia (CLL), respectively, will be diagnosed in 2010 [Jemal et al. 2010]. In addition, despite current available treatments, 21,530 lymphoma patients and 4390 CLL patients will die this year [Jemal et al. 2010]. Non-Hodgkin's lymphomas (NHLs) are the sixth most common cancer in the United States and depending on sex, the sixth (females) or eighth (males) most common cause of cancer-related death in the United States [Jemal et al. 2010].
The use of rituximab in combination with systemic chemotherapy has not only improved the clinical outcome of patients with B-cell lymphomas and CLL, but appears to be changing the biology of relapsed/refractory disease [Martin et al. 2008]. In order to address the challenge of managing relapsed/refractory B-cell malignancies in the rituximab era, it is important to evaluate novel therapeutic strategies targeting key regulatory pathways associated with the development, maintenance and/or progression of such conditions.
Lenalidomide is an immunomodulatory drug (IMiD) derived from thalidomide. Structurally, lenalidomide has one amino and one oxo group in its phthaloyl ring as compared with two oxo groups seen in the chemical structure of thalidomide (Figure 1). Biologically, lenalidomide appears to be 10,000 times more potent and has a better safety profile as compared with its parent compound thalidomide [Teo, 2005]. Biological antitumor effects had been demonstrated in lenalidomide preclinical models and clinical trials representing a wide spectrum of malignancies.
Figure 1.
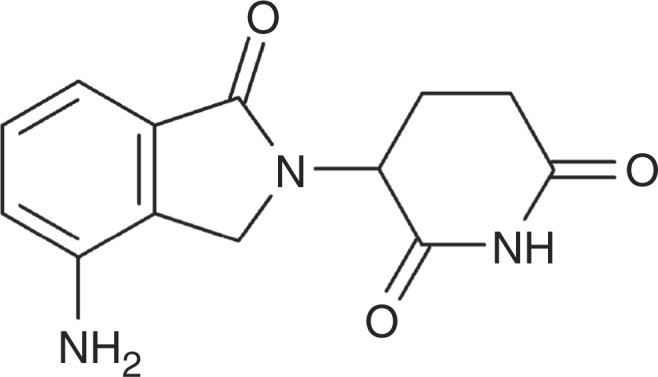
Chemical structure of lenalidomide: 3-(4-amino-1-oxo 1, 3 dihydro-2H-isoindol-2-yl) piperidine-2, 6 dione.
Lenalidomide possess significant anti-tumor activity in various hematological malignancies including: multiple myeloma (MM), myelodysplastic syndrome (MDS), CLL, and NHL. The United States Food and Drug Administration (FDA) approved lenalidomide for the treatment of MM or MDS associated with 5q deletion. In addition, clinically significant antitumor activity has been observed in CLL and various subtypes of B-cell lymphomas. Ongoing clinical studies are seeking to further define the role of lenalidomide in lymphoid malignancies. In our current report, we provide a comprehensive review of laboratory studies aimed at understanding the mechanisms of action of lenalidomide and an update on the clinical studies evaluating lenalidomide in B-cell lymphomas.
Mechanisms of action of lenalidomide in hematological malignancies
While lenalidomide's exact mechanisms of action continue to be a subject of controversy and study, direct cytotoxicity against malignant cells and immunomodulatory effects in endothelial, stromal, and immune cells (T cells, dendritic cells [DCs], and natural killer [NK] cells) has been demonstrated in preclinical models.
Direct antitumor effects of lenalidomide in hematological malignancies
The direct effects of lenalidomide in cancer cells differ depending on experimental conditions, model tested (cell lines versus mouse models), or disease studied. In MM and MDS preclinical studies, the in vitro exposure of cell lines to lenalidomide results in decreased cell proliferation [Pellagatti et al. 2007; Bartlett et al. 2004]. Similar effects have been demonstrated in lymphoma cell lines [Hernandez-Ilizaliturri et al. 2005].
The mechanisms by which lenalidomide inhibit the proliferation of malignant cells are yet to be defined. Induction of direct apoptosis following lenalidomide exposure in vitro is inconsistent and cell dependent. Recently, several investigators had demonstrated that lenalidomide might induce cell cycle arrest in MM and lymphoma cell lines [Escoubet-Lozach et al. 2009; Gandhi et al. 2006]. Ongoing studies are characterizing the effects oflenalidomide in cell cycle regulatory proteins among different subtypes of hematological malignancies to further characterize its direct antitumor activity.
Immunomodulatory effects of lenalidomide
More consistently, investigators had demonstrated that lenalidomide activates NK cells potentiating the biological activity of monoclonal antibodies (mAbs) in B-cell lymphomas, activate cytotoxic T cells against to directly target malignant cells, alters the cytokine microenvironment in the tumor bed, and inhibit angiogenesis [Zhang et al. 2009; Reddy et al. 2008; Wu et al. 2008; Hernandez-Ilizaliturri et al. 2005; LeBlanc et al. 2004].
In recent years, scientists have become interested in characterizing the interactions between cancer cells and the tumor microenvironment, in particular, the production of cytokines by accessory cells (i.e. stromal, DC, NK and endothelial cells) that may sustain and promote tumor growth. Cytokines can influence gene activation, growth, cell differentiation, functional cell surface molecule expression, and cellular effector function. The balance between pro- and anti-inflammatory cytokines may affect the host responses against malignant cells. In vitro exposure to lenalidomide has been shown to inhibit the production of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), interleukin 6 (IL-6), and interleukin 12 (IL-12). In addition, lenalidomide drug exposure has been associated with increased levels of the anti-inflammatory cytokine interleukin 10 (IL-10) in human peripheral blood mono-nuclear cells (PBMCs) [Corral et al. 1999; Corral and Kaplan, 1999]. The capacity of lenalidomide to decrease TNF-α secretion is up to 50,000 times higher than thalidomide [Corral and Kaplan, 1999; Muller et al. 1999]. Elevated TNF-α levels had been implicated in the pathogenesis of various hematological malignancies (CLL and MM) and or ineffective hematopoiesis as seen in MDS [Symeonidis et al. 1991].
In vitro, lenalidomide downregulates the production of IL-6 by bone marrow stromal cells leading to apoptosis and a decrease in cell proliferation of MM cells [Breitkreutz et al. 2008; Lichtenstein et al. 1995].
Correlative studies reported by Ferrajoli and colleagues conducted in relapsed/refractory CLL patients treated with lenalidomide single agent have shown a decrease in TNF-α levels as early as 7 days after treatment initiation and direct correlation between changes in TNF-α levels and antitumor activity [Ferrajoli et al. 2008].
While the precise mechanism that mediates lenalidomide effects in TNF-α levels is unknown, it may be similar to thalidomide, that had been shown to promote TNF-α-mRNA degradation in monocytes [Moreira et al. 1993]. In addition to changes in the cytokine milieu, lenalidomide has been shown to affect the function of immune effector cells. In vitro studies reported by LeBlanc and colleagues suggest that lenalidomide costimulates T cells in the presence of antigen-presenting cells (APCs) via the B7-CD28 costimulatory pathway [LeBlanc et al. 2004]. Lenalidomide-mediated T-cell costimulation leads to an increase in interferon gamma (INF-α) and interleukin 2 (IL-2) production responsible for (1) autocrine T-cell proliferation, (2) paracrine NK-cell activation, and (3) increase in mAb (rituximab or trastuzumab)-mediated antibody-dependent cellular cytotoxicity (ADCC) [Reddy et al. 2008; Wu et al. 2008]. Modulation of NK-cell function had been associated with the antitumor activity observed in MDS, MM, and CLL treated with lenalidomide.
Recently, Ramsay and colleagues demonstrated that lenalidomide modulates T-cell responses in follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL). Using primary tumor cells and autologous T-cell isolated from FL or DLBCL patients, this group of investigators demonstrated that lenalidomide repaired F-actin-mediated T-cell immune synapse and downstream signaling. Moreover, ex vivo exposure of primary tumor cells and T cells to lenalidomide restored T-cell function against lymphoma cells [Ramsay et al. 2009].
Finally, lenalidomide possess anti-angiogenesis properties. In preclinical studies, exposure of MM cells to lenalidomide results in decreased production of vascular endothelial growth factor (VEGF) [Gupta et al. 2001]. Our group of investigators demonstrated that in vivo treatment of lymphoma bearing severe combined immunodeficiency (SCID) mice with lenalidomide resulted in decreased angiogenesis as determined by immunohistochemistry (IHC) [Reddy et al. 2008].
Angiogenesis inhibition had been demonstrated in bone marrow samples of −5q MDS patients treated with lenalidomide single agent and the degree of angiogenesis inhibition correlated with clinical response [Kotla et al. 2009]. A decrease in VEGF levels was also observed in CLL treated with lenalidomide [Ferrajoli et al. 2008].
Together, the data generated by several groups of investigators suggest that lenalidomide targets cancer cells directly by altering the cell cycle or apoptosis and indirectly by altering the immunological synapsis or the tumor microenvironment. Despite the advances achieved, the exact mechanisms responsible for the antitumor activity of lenalidomide observed in patients with MDS or lymphoid malignancies remains undetermined. It is possible that the primary mechanisms of action of lenalidomide could be disease specific. Ongoing translational studies are aiming to further characterize the biological effects of lenalidomide in each subtype of lymphoid malignancies in an attempt to optimize its antitumor activity and to identify biomarkers of response than can be used to identify patients that are most likely to derive a clinical benefit from this novel agent.
Clinical studies in chronic lymphocytic leukemia
The clinical activity of lenalidomide in relapsed/refractory CLL had been evaluated in two prospective phase II studies (Table 1). Chanan-Khan and colleagues reported the first clinical study evaluating the safety and efficacy of lenalidomide administered orally (p.o.) as monotherapy at 25 mg daily for 21 days every 28 days [Chanan-Khan et al. 2006]. Allopurinol was administered for the prevention of tumor lysis syndrome (TLS). A total of 45 patients with relapsed/refractory CLL were enrolled in the study, but only 29 patients were assessable for response. The median age of the patient in the study was 64 years, the median number of prior therapies was three (range 1-10), and 51% of the patients were considered fludarabine refractory.
Table 1.
Summary of prospective clinical studies evaluating efficacy of lenalidomide in the management of chronic lymphocytic leukemia (CLL).
| Study | Phase | Patient population | Treatment | ORR (%) | PFS |
|---|---|---|---|---|---|
| Chanan-Khan et al. [2006] | II | Relapsed/Refractory CLL | Lenalidomide (N = 45, evaluable for response = 29) | 47 | NR |
| Ferrajoli et al. [2008] | II | Relapsed/Refractory CLL | Lenalidomide (N = 44) | 32 | NR |
ORR, overall response rate; PFS, progression-free survival; NR, not reported.
Response was assessed using the revised 1996 National Cancer Institute (NCI) Working Group guidelines [Cheson et al. 1996]. The overall response rate (ORR) in evaluable patients was 47%, with 9% achieving a complete remission (CR). Hematological toxicities were the most common adverse event observed. TLS was observed in two patients. Tumor flare reaction (TFR), characterized by the development of painful and/or swollen lymphadenopathies occasionally associated with fever and/or bone pain, was observed in 58% of the patients [Chanan-Khan et al. 2006].
A subset analysis of CLL patients with high-risk cytogenetics [del(11q)(q22.3) or del(17p)(p13.1)] treated in the study, demonstrated that lenalidomide monotherapy resulted in an ORR of 39% with 19% of the high-risk cytogenetic patients achieving a CR [Sher et al. 2010].
The second clinical study was conducted at the MD Anderson Cancer Center and reported by Ferrajoli and colleagues [Ferrajoli et al. 2008]. A total of 44 relapsed/refractory advance CLL patients (Rai stage 3 or 4) were enrolled and treated with lenalidomide administered at a starting dose of 10 mg p.o. daily for 28 days until disease progression or unacceptable toxicity. The dose of lenalidomide was subsequently escalated, if tolerated, by 5 mg increments. The median age of entire group was 64 years and the median number of prior treatments was five (range 1-15). Using similar response criteria, antitumor activity was observed in 32% of the patients. Partial remission (PR) was observed in 25% of the patients, one patient achieved a nodular PR and three patients achieved a CR. Time to best response was mostly observed after 6 or 9 months of therapy.
Hematological grade 3–4 toxicities were the most common adverse events, grade 3–4 neutropenia and thrombocytopenia was observed in 41% and 16% of the courses administered to the patients, respectively. Grade 1–2 or 3–4 tumor flare reaction was observed in 10% and 2% of the courses, respectively. While the occurrence of tumor flare reaction did not correlate to clinical activity, it was more frequently observed in patients with higher tumor burden [Ferrajoli et al. 2008].
While activity was observed in heavily pre-treated CLL patients, the follow-up period in both studies is short and mature information regarding progression-free survival (PFS) and overall survival (OS) is not available.
The use of lenalidomide in previously untreated CLL was halted due to the erratic but severe incidence of TLS observed. Currently, studies are evaluating low-dose lenalidomide in highly selected untreated CLL patients to further define the role of lenalidomide in the front-line setting. Outside clinical studies, the use of lenalidomide in previously untreated CLL patients is contraindicated.
Clinical studies in non-Hodgkin's lymphoma
Lenalidomide studies in diffuse large and transformed B-cell Non-Hodgkin's lymphoma
Several studies evaluating the antitumor activity of lenalidomide have been conducted in different types of NHL (Table 2). Wiernik and colleagues reported the first phase II clinical trial (NHL-002) evaluating the safety and efficacy of lenalidomide in relapsed/refractory aggressive lymphoma (DLBCL, follicle center lymphoma, MCL, and transformed lymphoma) [Wiernik et al. 2008]. Patients received oral lenalidomide at 25 mg daily for 21 days every 28 days for 52 weeks or until disease progression or drug intolerance. The study included 49 patients of which 53% had DLBCL with a medium number of four previous treatments. Response rates were observed in 35%, including 12% complete responses/unconfirmed complete responses. The median duration of response was 6.2 months and the median PFS was 4 months [Wiernik et al. 2008].
Table 2.
Summary of clinical studies evaluating the efficacy of lenalidomide alone or in combination with rituximab in Hodgkin's (HL) or non-Hodgkin's lymphoma (NHL).
| Study | Phase | Patient population | Treatment arms | ORR (%) | PFS |
|---|---|---|---|---|---|
| Wiernik et al. [2008] | II | Relapsed/refractory aggressive | Lenalidomide | 35 | 4 m |
| NHL-002 | NHL (DLBCL, FCC, MCL, Transformed NHL) | (N = 49) | |||
| Czuczman et al. [2008] | II | Relapsed/refractory | Lenalidomide | 29 | NR |
| NHL-003 | DLBCL | (N = 73) | |||
| Zinzani et al. [2008] | II | Relapsed/refractory | Lenalidomide | 41 | NR |
| NHL-003 | MCL | (N = 39) | |||
| Witzig et al. [2009] | II | Relapsed/refractory | Lenalidomide | 23 | 4.4 m |
| Indolent NHL (FL, SLL and MZL) | (N = 43) | ||||
| Fowler et al. [2010] | II | Newly diagnosed | Lenalidomide + rituximab (N = 28) | 86 | 90% at 20 m |
| Indolent NHL (FL, SLL, MZL) | |||||
| Fehniger et al. [2009] | II | Relapsed/refractory HL | Lenalidomide (N = 38) | 34 | 4 m |
| Wang et al. [2009] | I/II | Relapsed/refractory MCL | Lenalidomide + rituximab | 53 | 14 m/18-19 m for PR/CR |
| (N = 45) |
ORR, overall response rate; NHL, non-Hodgkin's lymphoma; DLBCL, diffuse large B-cell lymphoma; FCC, follicle center lymphoma; MCL, mantle cell lymphoma; FL, follicular lymphoma; SLL, small lymphocytic lymphoma; MZL, marginal zone lymphoma; PFS, progression-free survival; NR, not reported; m, months.
Hematological toxicities were the most common adverse events observed. The most common grade 4 adverse events were neutropenia (8.2%) and thrombocytopenia (8.2%); the most common grade 3 adverse events were neutropenia (24.5%) and thrombocytopenia (12.2%).
A confirmatory international phase II trial (NHL-003) of single-agent lenalidomide was undertaken in patients with relapsed/refractory aggressive NHL (n = 131) who received at least one prior treatment and had measurable disease. Lenalidomide was administered at 25 mg p.o. daily on days 1-21 of a 28-day cycle and continued until disease progression or toxicity. Data limited to the subset of patients with relapsed/refractory DLBCL (n = 73 patients, median age 67 years, range 21-87) demonstrated an overall response rate to lenalidomide of 29% (21/73): with 4% complete response (3/73), 25% partial response (18/73), and 15% (11/73) stable disease rates, respectively [Czuczman et al. 2008]. Again, grade 3 or 4 neutropenia (32%), thrombocytopenia (15%), asthenia (8%), and anemia (7%) were the most common events observed. The results of these two clinical studies confirm the activity of lenalidomide in heavily pretreated patients with relapsed or refractory DLBCL with manageable side effects.
Scientific interests are focused on identifying subsets of lymphoma patients most likely to benefit from lenalidomide therapy. We retrospectively evaluated differences in clinical outcomes for patients with germinal center B-cell-like (GCB) versus non-GCB DLBCL, treated with salvage lenalidomide at four academic institutions. The study included 40 patients with relapsed/refractory DLBCL treated under the NHL-003 study or with lenalidomide approved for off-label use. Patients were classified as GCB (n = 23) or non-GCB (n = 17) DLBCL subgroups utilizing the Han's algorithm [Hans et al. 2004]. A significant difference in clinical response to lenalidomide was observed: ORR was 53% for non-GCB patients versus 8.7% for GCB patients (p = 0.006); CR rate was 23.6% in non-GCB patients versus 4.34% in GCB patients. The PFS was 6.23 months in non-GBC patients versus 1.7 months in GCB patients (p = 0.004). No difference in OS was observed between GCB and non-GCB DLBCL patients. Our data suggest that the two major subgroups of patients with DLBCL (GCB versus non-GCB) have different antitumor responsiveness to lenalidomide in the relapsed/refractory setting [Hernandez-Ilizaliturri et al. 2010]. A large international trial is actively enrolling relapsed/refractory DLBCL patients failing high-dose chemotherapy and autologous stem cell support (HDC-ASCS) or nontransplant eligible in an attempt to prospectively validate our retrospective observations.
Lenalidomide studies in indolent B-cell lymphoma
Lenalidomide has also been studied in patients with relapsed/refractory indolent B-cell lymphoma [Witzig et al. 2009]. Witzig and colleagues prospectively evaluated the safety and efficacy of lenalidomide monotherapy in 43 patients with relapsed/refractory grade 1 or 2 FL (51%), small lymphocytic lymphoma (SLL), and marginal zone lymphoma (MZL). The median age of the patients was 63 years and the median number of prior treatment regimens was three. Patients received lenalidomide at 25 mg p.o. daily on days 1-21 every 28 days for 52 weeks or until disease progression or unacceptable toxicity. In this patient population, the ORR to lenalidomide was 23%, with three patients achieving a CR (n = 2) or unconfirmed CR (CRu, n = 1) and seven patients a PR. Activity was observed in patients with FL and SLL [Witzig et al. 2009].
The median time to antitumor response was 3.6 months (1.7-4.2 months) and the median PFS for all patients was 4.4 months (95% confidence interval [CI], 2.5-10.4 months). The type and incidence of adverse events were similar to prior CLL or lymphoma studies (i.e. hematological toxicity).
Based on preclinical studies demonstrating synergistic activity between rituximab and lenalidomide in lymphoma models, several investigators are evaluating the efficacy and tolerability of lenalidomide in combination with rituximab in indolent lymphomas.
Recently, Fowler and colleagues reported the preliminary results of lenalidomide in combination with rituximab in stage III or IV newly diagnosed indolent lymphoma patients. The preliminary report included 48 indolent lymphomas including 30 FL patients that received rituximab at 325 mg/m2 on day 1 and lenalidomide 20 mg/day p.o. on days 1-21 every 28 days for up to six cycles. The ORR of the entire cohort of patients was 86% and 93% for FL patients. CR was observed in 79% of the patients and in 86% of the FL patients. The PFS at a median follow up of 20 months is 91% [Fowler et al. 2010].
Adverse events were similar to those observed in lenalidomide single-agent studies. Of interest, no significant tumor flare reactions were observed. Currently, the study continues to enroll patients and the final results of the study with mature follow-up data are eagerly awaited.
The Cancer and Leukemia Group B (CALGB) is conducting a prospective randomized phase II study evaluating the safety and efficacy of lenalidomide ± rituximab in relapsed/refractory indolent lymphoma patients previously treated with rituximab-based chemo-immunotherapy.
Lenalidomide studies in mantle cell lymphoma
The NHL-003 study also evaluated the safety and efficacy of lenalidomide in relapsed/refractory mantle cell lymphoma (MCL). Zinzani and colleagues reported the preliminary results of lenalidomide monotherapy in 39 patients with relapsed or refractory MCL enrolled in the NHL-003 study. The median age of the patients was 66 years (range 33-82), most of them males (74%), and the median number of prior treatments was three. Lenalidomide therapy resulted in an ORR of 41%, including five CR (13%). Disease stabilization was observed in 26% of the patients. As noted in prior clinical trials with lenalidomide, were grade 3 or 4 neutropenia (51%) and thrombocytopenia (25%) [Zinzani et al. 2008].
Investigators at the MD Anderson Cancer Center evaluated the combination of lenalidomide and rituximab in MCL patients. Wang and colleagues conducted a phase I/II study evaluating the maximum tolerated dose (MTD) oflenalidomide when administered in combination with 4-weekly infusions of rituximab in relapsed/refractory MCL patients [Wang et al. 2009]. Lenalidomide was administered daily from days 1-21 every 28 days at 10, 15, 20, and 25 mg dose levels until disease progression or unacceptable toxicity. Rituximab was administered weekly × 4 at 375 mg/m2/dose during the first cycle of lenalidomide. A total of 45 patients had been treated at the time of the initial report. The lenalidomide MTD was found at the 20 mg dose level. No clinical responses were seen at the 10 and 15 mg dose levels. Of interest, clinical activity was observed at higher dose-levels, with 53% of the patients achieving a clinical response (CR or PR). The median duration of response for the entire cohort of patients in the phase II component was 14 months and 18 or 19 months for those patients achieving a CR or PR respectively (Table 2) [Wang et al. 2009].
Ongoing clinical studies are evaluating the antitumor activity of lenalidomide as maintenance therapy following HDC-ASCS in de novo MCL. In previously untreated MCL patients, noneligible for HDC-ASCT, lenalidomide is been studied in combination with rituximab and bendamustine (Nordic Lymphoma Group Study MCL-4) or as maintenance following rituximab-based chemotherapy (RENEW study, NCT01021423) or rituximab—bendamustine induction (planned Intergroup study). In addition, lenalidomide is being evaluated in combination with rituximab ± bortezomib or bendamustine in the relapsed/refractory setting.
Lenalidomide studies in other histologies
Early clinical trials had evaluated lenalidomide in other less-common forms of relapsed/refractory lymphomas including Hodgkin's lymphoma (HL), peripheral T-cell lymphoma (PTCL), and cutaneous T-cell lymphoma (CTCL). Fehniger and colleagues evaluated the activity of lenalidomide in 38 patients with relapsed/refractory HL. The dose schedule administered was similar to the NHL-002 and NHL-003 studies. Of the 35 patients treated with lenalidomide, 17% achieved a radiographic response (CR or PR) and 17% had a cytostatic response (SD). The median duration of response was 4 months and the toxicity profile encounter was similar to previous studies with lenalidomide [Fehniger et al. 2009].
Lenalidomide is being evaluated in patients with T-cell lymphomas. Early results from two prospective studies evaluating the activity of lenalidomide in CTCL or PTCL were reported at the annual meeting of the American Society of Hematology. Querfeld and colleagues are evaluating the antitumor activity of lenalidomide in patients with CTCL and found early encouraging responses [Querfeld et al. 2005]. In addition, Reiman and colleagues are evaluating the activity of single-agent lenalidomide in patients with relapsed/refractory PTCL. In this Canadian multicenter study, patients are being treated with lenalidomide at 25 mg on days 1-21 every 28 days until disease progression or unacceptable toxicity. The investigators presented initial results on 10 PTCL patients and found a PR rate of 44% (4/9 patients) and disease stabilization in 56% [Reiman et al. 2007]. While the early results from these two studies are promising, further patient recruitment and follow up is necessary to allow better interpretation of the data.
In summary, lenalidomide is emerging as a promising therapeutic option for patients with relapsed/refractory CLL and various subtypes of B-cell lymphomas (FL, DLBCL, and MCL). The clinical activity observed in the relapsed/refractory setting and the acceptable toxicity profile support the use of lenalidomide in patients with limited performance status, and in those patients seeking to avoid adverse events associated with systemic chemotherapy. In the frontline setting the activity observed in FL treated with rituximab—lenalidomide is encouraging and may result in an effective therapeutic option for FL in the near future. Ongoing and future translational and clinical studies will provide insightful information that will guide physicians in the optimal use of lenalidomide as a single agent or in combination with other biological/target specific agents or chemotherapy regimens (i.e. R + CHOP). Moreover, correlative studies with lenalidomide have the potential to aid in the selection of patients based on bio-markers that are most likely to benefit from lenalidomide-based therapies.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
None declared.
References
- Bartlett J.B., Dredge K., Dalgleish A.G. (2004) The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer 4: 314–322 [DOI] [PubMed] [Google Scholar]
- Breitkreutz I., Raab M.S., Vallet S., Hideshima T., Raje N., Mitsiades C., et al. (2008) Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia 22: 1925–1932 [DOI] [PubMed] [Google Scholar]
- Chanan-Khan A., Miller K.C., Musial L., Lawrence D., Padmanabhan S., Takeshita K., et al. (2006) Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: Results of a phase II study. J Clin Oncol 24: 5343–5349 [DOI] [PubMed] [Google Scholar]
- Cheson B.D., Bennett J.M., Grever M., Kay N., Keating M.J., O'Brien S., et al. (1996) National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood 87: 4990–4997 [PubMed] [Google Scholar]
- Corral L.G., Haslett P.A., Muller G.W., Chen R., Wong L.M., Ocampo C.J., et al. (1999) Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol 163: 380–386 [PubMed] [Google Scholar]
- Corral L.G., Kaplan G. (1999) Immunomodulation by thalidomide and thalidomide analogues. Ann Rheum Dis 58(Suppl. 1): 1107–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuczman M.S., Vose J.M., Zinzani P.L., Reeder C.B., Buckstein R., Haioun C., et al. (2008) Confirmation of the Efficacy and Safety of Lenalidomide Oral Monotherapy in Patients with Relapsed or Refractory Diffuse Large-B-Cell Lymphoma: Results of An International Study (NHL-003). ASH Annual Meeting Abstracts 112: 268 [Google Scholar]
- Escoubet-Lozach L., Lin I.L., Jensen-Pergakes K., Brady H.A., Gandhi A.K., Schafer P.H., et al. (2009) Pomalidomide and lenalidomide induce p21 WAF-1 expression in both lymphoma and multiple myeloma through a LSD1-mediated epigenetic mechanism. Cancer Res 69: 7347–7356 [DOI] [PubMed] [Google Scholar]
- Fehniger T., Larson S., Trinkaus K., Siegel M., Cashen A.F., Blum K.A., et al. (2009) A phase II multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood 114: 3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrajoli A., Lee B.N., Schlette E.J., O'Brien S.M., Gao H., Wen S., et al. (2008) Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood 111: 5291–5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler N.H., McLaughlin P., Hagemeister F.B., Kwak K.W., Fanale M.A., Neelapu S.S., et al. (2010) Complete response rates with lenalidomide plus rituximab for untreated indolent B-cell non-Hodgkin's lymphoma. J Clin Oncol 28: 8036 [Google Scholar]
- Gandhi A.K., Kang J., Naziruddin S., Parton A., Schafer P.H., Stirling D.I. (2006) Lenalidomide inhibits proliferation of Namalwa CSN.70 cells and interferes with Gab1 phosphorylation and adaptor protein complex assembly. Leuk Res 30: 849–858 [DOI] [PubMed] [Google Scholar]
- Gupta D., Treon S.P., Shima Y., Hideshima T., Podar K., Tai Y.T., et al. (2001) Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: Therapeutic applications. Leukemia 15: 1950–1961 [DOI] [PubMed] [Google Scholar]
- Hans C.P., Weisenburger D.D., Greiner T.C., Gascoyne R.D., Delabie J., Ott G., et al. (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103: 275–282 [DOI] [PubMed] [Google Scholar]
- Hernandez-Ilizaliturri F.J., Deeb G., Zinzani P.L., Pileri S., Malik F., Macon W.R., et al. (2010) Response of relapsed/refractory diffuse large B-cell lymphoma (DLBCL) with nongerminal center B-cell phenotype to lenalidomide (L) alone or in combination with rituximab (R). J Clin Oncol 28: 8038 [Google Scholar]
- Hernandez-Ilizaliturri F.J., Reddy N., Holkova B., Ottman E., Czuczman M.S. (2005) Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin Cancer Res 11: 5984–5992 [DOI] [PubMed] [Google Scholar]
- Jemal A., Siegel R., Xu J., Ward E. (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277–300 [DOI] [PubMed] [Google Scholar]
- Kotla V., Goel S., Nischal S., Heuck C., Vivek K., Das B., et al. (2009) Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol 2: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc R., Hideshima T., Catley L.P., Shringarpure R., Burger R., Mitsiades N., et al. (2004) Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood 103: 1787–1790 [DOI] [PubMed] [Google Scholar]
- Lichtenstein A., Tu Y., Fady C., Vescio R., Berenson J. (1995) Interleukin-6 inhibits apoptosis of malignant plasma cells. Cell Immunol 162: 248–255 [DOI] [PubMed] [Google Scholar]
- Martin A., Conde E., Arnan M., Canales M.A., Deben G., Sancho J.M., et al. (2008) R-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: The influence of prior exposure to rituximab on outcome. A GEL/TAMO study. Haematologica 93: 1829–1836 [DOI] [PubMed] [Google Scholar]
- Moreira A.L., Sampaio E.P., Zmuidzinas A., Frindt P., Smith K.A., Kaplan G. (1993) Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med 177: 1675–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller G.W., Chen R., Huang S.Y., Corral L.G., Wong L.M., Patterson R.T., et al. (1999) Aminosubstituted thalidomide analogs: Potent inhibitors of TNF-alpha production. Bioorg Med Chem Lett 9: 1625–1630 [DOI] [PubMed] [Google Scholar]
- Pellagatti A., Jadersten M., Forsblom A.M., Cattan H., Christensson B., Emanuelsson E.K., et al. (2007) Lenalidomide inhibits the malignant clone and up-regulates the SPARC gene mapping to the commonly deleted region in 5q- syndrome patients. Proc Natl Acad Sci USA 104: 11406–11411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfeld C., Kuzel T., Guitart J., Rosen S.T. (2005) Preliminary results of a phase II study of CC-5013 (lenalidomide, Revlimid®) in patients with cutaneous T-cell lymphoma. Blood (ASH Annual Meeting Abstracts) 106: 3351 [Google Scholar]
- Ramsay A.G., Clear A.J., Kelly G., Fatah R., Matthews J., Macdougall F., et al. (2009) Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: Implications for the tumor microenvironment and immunotherapy. Blood 114: 4713–4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy N., Hernandez-Ilizaliturri F.J., Deeb G., Roth M., Vaughn M., Knight J., et al. (2008) Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol 140: 36–45 [DOI] [PubMed] [Google Scholar]
- Reiman T., Finch D., Chua N., White D.J., Stewart A.D., van der Jagt R.H., et al. (2007) First report of a phase II clinical trial of lenalidomide oral therapy for peripheral T-cell lymphoma. Blood (ASH Annual Meeting Abstracts) 110: 2579 [Google Scholar]
- Sher T., Miller K.C., Lawrence D., Whitworth A., Hernandez-Ilizaliturri F., Czuczman M.S., et al. (2010) Efficacy of lenalidomide in patients with chronic lymphocytic leukemia with high-risk cytogenetics. Leuk Lymphoma 51: 85–88 [DOI] [PubMed] [Google Scholar]
- Symeonidis A., Kourakli A., Katevas P., Perraki M., Tiniakou M., Matsouka P., et al. (1991) Immune function parameters at diagnosis in patients with myelodysplastic syndromes: Correlation with the FAB classification and prognosis. Eur J Haematol 47: 277–281 [DOI] [PubMed] [Google Scholar]
- Teo S.K. (2005) Properties of thalidomide and its analogues: Implications for anticancer therapy. AAPS J 7: E14–E19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Fayad L., Hagemeister F.B., Neelapu S., Samaniego F., McLauglin P., et al. (2009) A phase I/II study of lenalidomide in combination with rituximab in relapsed/refractory mantle cell lymphoma. Blood 114: 2719 [Google Scholar]
- Wiernik P.H., Lossos I.S., Tuscano J.M., Justice G., Vose J.M., Cole C.E., et al. (2008) Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin's lymphoma. J Clin Oncol 26: 4952–4957 [DOI] [PubMed] [Google Scholar]
- Witzig T.E., Wiernik P.H., Moore T., Reeder C., Cole C., Justice G., et al. (2009) Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin's Lymphoma. J Clin Oncol 27: 5404–5409 [DOI] [PubMed] [Google Scholar]
- Wu L., Adams M., Carter T., Chen R., Muller G., Stirling D., et al. (2008) Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res 14: 4650–4657 [DOI] [PubMed] [Google Scholar]
- Zhang L., Qian Z., Cai Z., Sun L., Wang H., Bartlett J.B., et al. (2009) Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol 84: 553–559 [DOI] [PubMed] [Google Scholar]
- Zinzani P.L., Witzig T.E., Vose J.M., Reeder C.B., Buckstein R., Haioun C., et al. (2008) Confirmation of the efficacy and safety of lenalidomide oral mono-therapy in patients with relapsed or refractory mantle-cell lymphoma: Results of an international study (NHL-003). ASH Annual Meeting Abstracts 112: 262 [Google Scholar]


