Abstract
Background:
The impact on health related quality of life (HRQoL) of rituximab maintenance (R-M) versus observation (OBS) after induction for treatment of follicular lymphoma (FL) is unclear.
Methods:
We reviewed the charts of 137 patients (53% female, 87% White, age 61.0 ± 12.4 years) who received either R-M (n = 53) or OBS (n = 84) after chemotherapy induction for newly diagnosed FL at community oncology practices within the US. Patients (65% with advanced disease; 48% with a high FLIPI score [3–5]) had completed ≥1 Patient Care Monitor HRQoL survey in the period following front-line therapy, and were excluded if they had progressed during front-line therapy.
Results:
Linear mixed models showed that postinduction, most symptoms were stable, with patients on R-M reporting HRQoL that was equal to that reported by OBS patients.
Conclusions:
Among R-M patients, receipt of rituximab was associated with improved psychological symptoms.
Keywords: follicular lymphoma, maintenance therapy, Rituxan, rituximab, symptoms
Introduction
Follicular lymphoma (FL) is the second most common form of non-Hodgkin's lymphoma (NHL), representing 35% of adult NHL cases in the United States [Pettengell et al. 2008]. The disease is typically characterized by an indolent course and a high initial response rate, followed by relapse and recurrent progressions with successively shorter intervening intervals of stable disease [Vidal et al. 2009]. Most patients are diagnosed with advanced disease, characterized by fever, weight loss, enlarged lymph nodes, night sweats and fatigue, although patients with advanced disease may be asymptomatic [Solal-Celigny et al. 2010]. Transformation to an aggressive lymphoma subtype can occur at any stage of the disease and is associated with a very poor prognosis. Estimates of median survival are variable but approximately 8-10 years based on evidence prior to the widespread use of rituximab [Tilly and Zelenetz, 2008].
FL treatment may comprise watchful waiting, radiation, radio-immunotherapy, chemotherapy with or without the use of monoclonal antibodies, depending upon the presenting clinical characteristics [Gine et al. 2010]. The most common chemotherapy regimens, CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) and CVP (cyclophosphamide, vincristine, and prednisolone), may result in gastrointestinal distress, alopecia, mucositis, dysphagia and skin irritation [Gine et al. 2010]. Rituximab (Rituxan), a monoclonal antibody, may also be added to each of these regimens (R-CHOP, R-CVP). It is administered by IV infusion and given with chemotherapy as front-line treatment, or given as a single agent for maintenance therapy [Keating, 2010; Ghielmini et al. 2009].
The addition of rituximab to front-line chemotherapy has resulted in improved response rates, progression-free (PFS) and overall survival (OS) in several trials [Marcus et al. 2008; Hainsworth, 2002], without notable toxicity [Witzig et al. 2005; Davis et al. 2000]. In addition, the use of maintenance rituximab following chemotherapy has been shown to be superior to observation in terms of response duration among NHL patients not previously treated with rituximab [Maloney, 2008; Forstpointner et al. 2006]. Furthermore, a recent multicenter study of maintenance rituximab in FL patients showed a significant improvement in PFS after 2 years when compared with patients on observation only [Salles et al. 2011; Hochster et al. 2009]. However, the use of rituximab maintenance (R-M) after front-line chemotherapy may be associated with additional side effects compared with observation (OBS). These include infusion reaction, depletion of B cells, and increased neutropenia and infection [Vidal et al. 2009]. Because patients with minimal disease may be relatively asymptomatic, the negative impact on health-related quality of life (HRQoL) of treatment of FL might be greater than the effect of the disease [Pettengell et al. 2008]. However, the extent to which R-M incrementally impacts HRQoL is unclear, as the FL HRQoL literature in general is sparse [Cheung et al. 2009; Pettengell et al. 2008], and much of the rituximab-specific work has been done in the clinical trial setting [Witzens-Harig et al. 2009].
Pettengell and colleagues examined the impact of disease state on the health function of 222 FL patients in the UK [Pettengell et al. 2008]. The authors concluded that relapsed FL patients experienced worse HRQoL than patients who were newly diagnosed or in remission. This suggests that prolonging the time to treatment failure, perhaps via more intense induction or through use of maintenance regimens, is important to maximize HRQoL in this population.
Witzen-Harig and colleagues examined the impact of R-M on HRQoL in a prospective randomized trial of R-M versus OBS in 122 patients with CD20+ B-cell NHL [Witzens-Harig et al. 2009] and found no difference in global, functional and symptomatic health states between patients on R-M and OBS.
The goal of cancer therapies as it relates to HRQoL is to minimize the impact of disease progression and treatment-related side effects. The goal of this research was to support such decision making in patients with FL by characterizing HRQoL among patients treated in a community setting with R-M therapy compared with those who received OBS after completion of front-line therapy.
Methods
Patients and setting
This was a retrospective chart review and database analysis conducted at seven community oncology practices in different geographic areas of the United States. Patients were eligible if they were (1) at least 18 years of age, (2) had a confirmed diagnosis of FL, (3) had received front-line therapy consisting of combination chemotherapy with or without rituximab, or consisting of rituximab monotherapy, (4) had received single-agent rituximab as single-agent maintenance therapy following front line therapy, or were followed under observation following front-line therapy, and (5) had completed at least one Patient Care Monitor (PCM) assessment after completion of front-line therapy. Administration of the PCM was part of routine care in the participating practices, and administration of the PCM was consistent across practices, always being completed prior to lab work and physician consultation. Patients were excluded if they had experienced disease progression during front line therapy or had a history of other cancer within the 5 years prior to diagnosis with FL.
Procedures
Potentially eligible patients were identified by community oncology practices affiliated with ACORN Research and through review of electronic records in the ACORN Data Warehouse. The resulting patient list was then matched with archived data in the ACORN PCM data repository. Medical charts were subsequently reviewed to determine final study eligibility according to aforementioned inclusion criteria. All patients identified as eligible were included. Completed case report forms were submitted via dedicated facsimile to the ACORN analysis center and entered into a secure database. All participating practices obtained patient permission for use of properly de-identified records, and all study procedures were approved by IntegReview Institutional Review Board of Austin, Texas.
Study measures
The primary endpoints for this study were indices of symptom burden and HRQoL as collected by the PCM. PCM, version 2.0, is an 86-item self-report measure that assesses physical symptoms, psychological symptoms and physical functioning, and asks patients to rate the severity of symptoms on an 11 point (0 to 10) Likert-type scale. The PCM is administered via touch screen tablet PC as a routine part of care at participating community oncology practices. The PCM produces standardized index scores (T scores) for six screening scales in which higher scores denote more severe symptoms. The indices are: General Physical Symptoms, Treatment Side Effects, Despair and Depression, Acute Distress, Impaired Ambulation, and Impaired Performance. The PCM has been shown to be valid for assessing HRQoL in cancer patients and has been used in a number of studies [Houts et al. 2009; Stepanski et al. 2009; Walker et al. 2009; Fortner et al. 2006]. The analysis reported in this paper included only the 38 core items used to construct the 6 index scores.
PCM surveys were included if they were collected between the end of front-line therapy and the end of either R-M or the observation period post-front line. The post-front-line period (maintenance or observation) was defined as ending when maintenance was discontinued, when observation ended (through resumption of treatment), when the patient experienced disease progression, or when the record of treatment ended. Thus, PCM surveys administered after disease progression were excluded from the analysis, and the analysis does not attempt to model the impact of progression in the study sample.
It is generally recognized that small differences or small treatment effects may be statistically significant yet clinically unimportant [Copay et al. 2007; Jaeschke et al. 1989]. The concept of the minimal important difference (MID) refers to the smallest difference that is important, and here refers specifically to the smallest change in HRQoL that is important to the patient. MID can be calculated using a variety of methods [Revicki et al. 2008]. In this study we used distributional methods based on the work by Ringash and colleagues who have suggested that 5-10% of the instrument range is the MID for HRQoL [Ringash et al. 2007]. A change of 0.5 to 1 point would therefore represent the MID for individual PCM items, and 1.5 to 3 points the MID for PCM index scores.
Statistical methods
Descriptive statistics were generated for all study variables. Kaplan-Meier analysis was used to assess the duration of R-M and of OBS in the post-front-line period, to allow for inclusion of cases for which maintenance therapy or observation was ongoing. Chi-square tests, Fisher's exact test, and independent samples t-tests were used to test for differences between maintenance and observation treatment groups on demographic, disease, and front-line treatment characteristics.
Using methods described by Littell and colleagues and Cnaan and coworkers [Littell et al. 2000; Cnaan et al. 1997], linear mixed models were employed to examine the change in symptoms and HRQoL over time following front-line chemotherapy and starting with either maintenance therapy or observation. Each model examined the interval since the end of front-line therapy (Interval). Each model also examined whether patients received R-M or OBS (Group) in the post-front-line period. Because they were central to the questions under study, each model included Interval, Group, and the interaction of Group with Interval, irrespective of statistical significance.
Results are presented graphically for two of the PCM outcomes. Although the duration of OBS or R-M varied across patients, the duration of the post-front-line period is shown at the median interval for the R-M group, about 18.5 months. This has no bearing on the underlying models, but merely simplifies the presentation of findings. Each model also examined several patient-level variables: (1) age at diagnosis, (2) race, (3) gender, (4) body mass index (BMI), (5) Eastern Cooperative Oncology Group (ECOG) performance status rating, (6) front line therapy, (7) primary insurance coverage, (8) Follicular Lymphoma International Prognostic Index (FLIPI) class, and (9) propensity score, as discussed in the following.
Propensity score analysis was used to balance treatment groups on demographic, disease and treatment characteristics. The propensity score (probability of being in the maintenance group) was estimated with a logistic regression model, using established methods [Dehejia and Wahba, 1999; Rosenbaum and Rubin, 1984]. The final model contained the most significant predictors among demographic, disease and treatment characteristics available from the data, to the extent allowable by the sample size. The propensity score was then used as a covariate in linear mixed models analyses.
Finally, separate analyses were conducted among patients who received R-M to examine the impact of R-M schedule, and the impact of being in the active phase of R-M therapy. Rituximab schedule was modeled as binary (4 weeks of treatment every 6 months versus all other schedules). Models also examined age, FLIPI score, interval since start of R-M, and status of each PCM survey as occurring during active rituximab therapy versus not during active rituximab therapy. Statistical analyses were conducted with SPSS version 15. All statistical tests were interpreted at α = 0.05, two tailed.
Results
Sample development
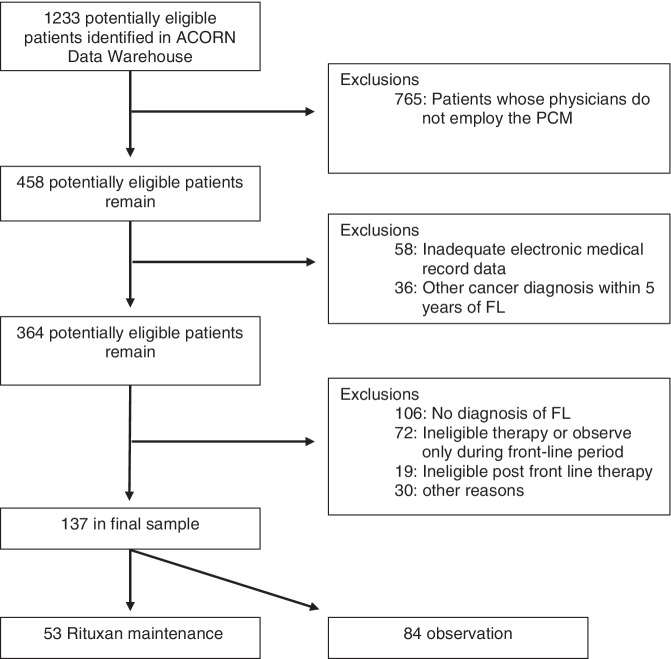
A total of 1233 potentially eligible patients were identified. Of these, 62% had no record of PCM surveys, and therefore were not eligible. This does not reflect patient refusal, but rather occurs because some physicians at participating practices do not employ the PCM survey. Primary reasons for exclusion in the remaining 458 potentially eligible subjects were insufficient evidence of FL, ineligible therapy or observation only during the front-line period, history of other cancer within 5 years of diagnosis with FL, and incomplete electronic medical record data. A total of 137 patients passed all eligibility criteria. Figure 1 depicts sample development.
Figure 1.
Flow diagram of study sample development. FL, follicular lymphoma; PCM, Patient Care Monitor.
Demographic, disease and treatment characteristics
The median date of initial diagnosis of FL in the sample was July 2005, but dates of diagnosis ranged from June 1992 to October 2008, with six patients overall diagnosed prior to 2000. The sample was largely White (86.9%) with a mean (SD) age of 61.0 (12.4) years. Fewer patients received R-M (n = 53) than OBS (n = 84) during the post-front-line period. The majority of patients were stage III (31%) or IV (34%) at the time of diagnosis, with 48% of patients classified as high risk using the FLIPI score. The most common front-line regimens were R-CHOP (34%), R-CVP (18%), and rituximab monotherapy (26%).
There was no difference between groups in age (p = 0.684), BMI (p = 0.604), gender (p = 0.861), race (p = 0.193), insurance coverage (p = 0.232) or disease stage (p = 0.350). ECOG performance status was missing for the majority of patients (63%), but did not significantly differ across treatment groups (p = 0.300). The FLIPI score, calculated and classified as per Buske and colleagues also did not significantly differ across treatment groups (p = 0.111) [Buske et al. 2006].
Start dates of front-line therapy differed significantly across treatment groups, with OBS patients having started front-line treatment on average 14 months earlier than R-M patients (p = 0.005). Front-line therapy was collapsed for analysis to any rituximab containing therapy (monotherapy or combination) versus non-rituximab containing therapy. Patients in the OBS group were more likely to have received front-line chemotherapy without rituximab (p = 0.05), but there was no difference between treatment groups in the duration of front-line therapy (p = 0.693). Additional information regarding demographic, disease, and treatment characteristics is reported in Table 1.
Table 1.
Demographic, disease, and treatment characteristics.
| Characteristic | R-M (N = 53) | OBS (N = 84) | p |
|---|---|---|---|
| Age at diagnosis, mean(SD) | 60.5 (12.8) | 61.4 (12.1) | 0.684 |
| Female, n (%) | 27 (50.9) | 45 (53.6) | 0.861 |
| Race, n (%) | 0.193 | ||
| Non-White | 4 (7.5) | 9 (10.7) | |
| White | 49 (92.5) | 70 (83.3) | |
| Unknown | 0 (0) | 5 (6) | |
| Primary insurance, n (%) | 0.232 | ||
| Public | 19 (35.8) | 36 (42.9) | |
| Private | 34 (64.2) | 47 (56) | |
| Other/unknown | 0 (0) | 1 (1.2) | |
| Disease stage at diagnosis, n (%) | 0.350 | ||
| I-II | 9 (17) | 24 (28.6) | |
| III-IV | 40 (75.5) | 49 (58.3) | |
| Unknown/other | 4 (7.5) | 11 (13.1) | |
| ECOG performance status, n (%) | 0.300 | ||
| 0 | 18 (34) | 19 (22.6) | |
| 1 | 4 (7.5) | 8 (9.5) | |
| 2 | 0 (0) | 2 (2.4) | |
| Missing | 31 (58.5) | 55 (65.5) | |
| FLIPI class, n (%) | 0.111 | ||
| Low risk (0-1) | 17 (32.1) | 23 (27.4) | |
| Intermediate risk (2) | 7 (13.2) | 24 (28.6) | |
| High risk (3-5) | 29 (54.7) | 37 (44) | |
| Front-line therapy*, n (%) | 0.05 | ||
| Rituximab monotherapy | 18 (33.96) | 18 (21.43) | |
| Chemotherapy without rituximab | 1 (1.9) | 10 (11.9) | |
| Chemotherapy with rituximab | 34 (64.2) | 56 (66.7) | |
| Start date of front-line therapy, mean (SD, days) | 12/9/2005 (532) | 10/8/2004 (1188) | 0.005 |
| Duration (days) of frontline therapy, mean (SD) | 104.2 (67.1) | 108.4 (56.7) | 0.693 |
| Duration (days) of R-M or OBS†, median (SE) | 448 (107.4) | 1336 (320.0) |
Collapsed to rituximab containing therapy versus non-rituximab-containing therapy for comparison.
Kaplan-Meier used to estimate median and standard error including censored observations. Duration of R-M and OBS were not compared statistically because by definition the R-M group duration was constrained by having to be on maintenance therapy.
R-M, rituximab maintenance; OBS, observation; ECOG, Eastern Cooperative Oncology Group; FLIPI, Follicular Lymphoma International Prognostic Index.
As indicated by the nonsignificant group differences reported above, the R-M and OBS groups appeared well balanced on most variables. Groups significantly differed on only two variables, start date of front-line therapy, and use of a rituximab containing regimen as part of front-line therapy. The logistic regression analysis employed to create propensity scores produced a model that included FLIPI score, race, front-line therapy with rituximab versus not, and start date of therapy. The Hosmer-Lemeshow goodness of fit test was nonsignificant, indicating good fit of the logistic model to the data.
PCM assessment
PCM surveys were available from 136 of the 137 patients in the study (all data from one patient was incomplete and could not be scored). However, 30 patients completed PCM surveys that were all outside the range for inclusion in this study (e.g. after disease progression). As a result, the effective sample for analysis of PCM survey data was 595 PCM surveys from 106 patients.
For descriptive analysis, PCM scores were aggregated at the patient level, yielding a mean score per patient for each index score. Descriptive statistics for patient level mean index scores, by treatment group, are reported in Table 2. It should be noted that although the median duration of OBS was longer than the median duration of R-M, PCM observations were not disproportionately sampled from the observation group.
Table 2.
Mean (SD) patient level Patient Care Monitor (PCM) index scores, by group.
| Treatment group | |||||
|---|---|---|---|---|---|
| PCM index score | N | #PCMs | R-M | OBS | p* |
| General Physical Symptoms | 106 | 588 | 45.4 (6.51) | 45.67 (8.12) | 0.85 |
| Treatment Side Effects | 106 | 591 | 44.64 (3.83) | 44.2 (5.3) | 0.64 |
| Acute Distress | 106 | 543 | 42.44 (6.45) | 44.18 (8.2) | 0.25 |
| Despair and Depression | 106 | 542 | 45.43 (4.97) | 46.29 (6.2) | 0.45 |
| Impaired Ambulation | 79 | 364 | 49.89 (5.35) | 51.19 (7.05) | 0.40 |
| Impaired Performance | 79 | 362 | 41.83 (5.64) | 43.07 (7.5) | 0.45 |
Independent t-test comparison of means.
PCM, Patient Care Monitor; R-M, rituximab maintenance; OBS, observation.
Linear mixed models analysis of PCM items
In general, scores reflecting physical symptoms tended to either stabilize or improve during the post-front-line period. However, the latter trends reflected a very gradual rate of change, even when statistically significant. Also, psychological symptoms and physical functioning remained stable after completion of front-line therapy. Overall, patients on R-M had PCM index scores that were generally equal to those for OBS patients.
General Physical Symptoms
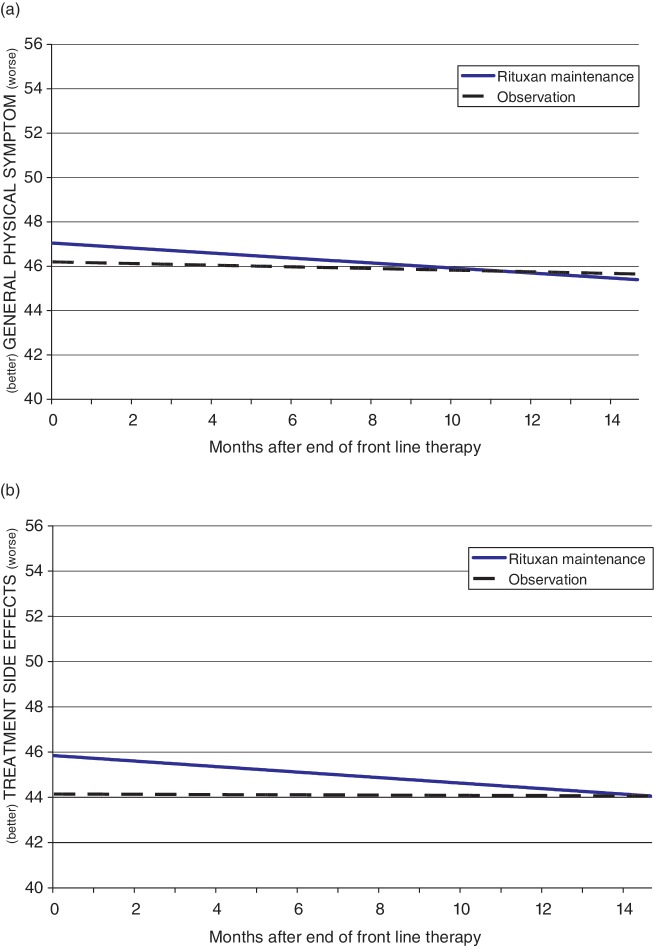
Linear mixed model results for the General Physical Symptoms index showed that patients in both the R-M and OBS groups improved (p = 0.028) significantly over the course of the period observed after cessation of front-line therapy. The R-M group had nominally worse symptoms than the OBS group at the start, but scores converged over time and the difference between groups overall was not statistically significant. The model also indicated that patients who had a history of receiving non-rituximab-containing therapy during their front-line therapy experienced significantly less (p = 0.019) physical symptom burden compared with those with a history of front-line therapy that contained rituximab. Model findings are represented in Figure 2(a).
Figure 2.
Linear mixed model of (a) General Physical Symptoms index and (b) Treatment Side Effects index.
Treatment Side Effects
Linear mixed model results for the Treatment Side Effects index showed a significant interaction between Group and Interval (p = 0.010). As shown in Figure 2(b), R-M patients started off with nominally higher (worse) Treatment Side Effects index scores, but they improved at a faster rate than patients in the OBS group. The combination of these effects was that scores for the R-M and OBS treatment groups converged by about 14 months into the post-front-line period.
Psychological symptom measures
Linear mixed model results for the Acute Distress index suggested stable scores for both groups during the post-front-line period. Similarly, linear mixed model results for the Despair and Depression index indicated that scores did not change significantly during the period following front-line therapy, and the groups did not differ significantly. The effect of front-line therapy was significant (p = 0.036), with patients who received non-rituximab-containing chemotherapy during front-line scoring lower (better) on the Despair and Depression index than patients who received rituximab.
Physical functioning measures
There was a smaller patient sample available for analysis of the Impaired Ambulation and Impaired Performance index scores (n = 79), as shown in Table 2, because some community practices exclude physical functioning outcomes in their use of the PCM.
For both R-M and OBS groups, Impaired Ambulation scores did not change significantly during the post-front-line period. Age was a statistically significant predictor for Impaired Ambulation (p = 0.002), with a 10-year increase in age associated with a 1.8-point increase (worsening) in scores.
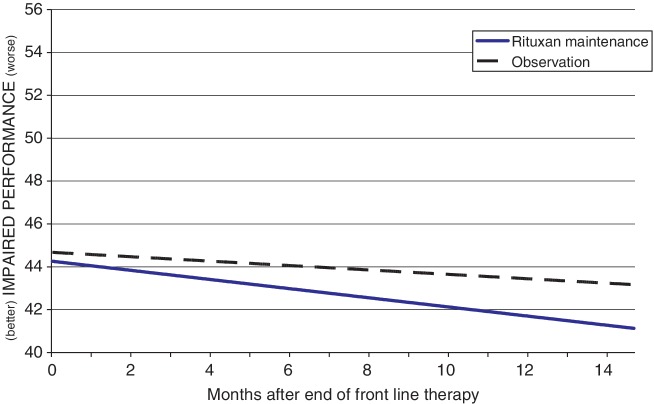
Impaired Performance scores improved significantly for the sample overall during the period following front-line therapy (p = 0.002), as shown in Figure 3. The R-M group appears in the figure to have less severe Impaired Performance index scores, but the difference between groups was nonsignificant. Age and front-line therapy were significant in predicting Impaired Performance, with younger patients (p = 0.002) and those who received a non-rituximab-containing regimen (p = 0.046) reporting less impaired functioning, as indicated by lower scores on the Impaired Performance index.
Figure 3.
Linear mixed model of Impaired Performance.
Subsample analysis of rituximab maintenance data
Follow-up mixed models analysis was conducted just among R-M patients. These models were intended to address the impact of R-M in a different way, by examining maintenance schedule, and by comparing the period when R-M patients were actively getting rituximab with the period between active treatments. Results showed that there were no effects of maintenance schedule, but showed that active receipt of rituximab during maintenance therapy was associated with improved psychological symptoms relative to the intervening period between doses of R-M. The effects were large enough to be considered clinically relevant. That is, they met or exceeded the MID. The effects also were statistically significant (2.0 points and 1.7 points, p = 0.023 and p = 0.017, respectively, for the Acute Distress, and Despair and Depression index scores). No such effects were observed for the General Physical Symptoms or Treatment Side Effects index scores, the only other PCM index scores with sufficient data for modeling in this subsample.
Discussion
There is increasing evidence supporting the use of maintenance therapy with rituximab after successful induction therapy in patients with relapsed or refractory FL [van Oers et al. 2010; Ghielmini et al. 2009; Vidal et al. 2009; Witzens-Harig et al. 2009]. However, questions remain regarding the potential impact of therapy on HRQoL. The current study abstracted data from medical charts, and collected repeated HRQoL assessments from FL patients treated at seven community oncology clinics in the US. The population selected was similar in age, gender, and race to FL patients in the Surveillance, Epidemiology, and End Results database [National Cancer Institute, 2005] and to patients in the National Lymphocare Study (NLCS) [Friedberg et al. 2009]. Our findings showed that after completion of front-line therapy, most symptoms were stable, with patients on R-M reporting symptoms that were generally equal to those reported by OBS patients. Among R-M patients, active receipt of rituximab during the maintenance period was associated with improved psychological symptoms relative to the intervening period between doses of rituximab. Study findings provided no evidence of a clinically relevant adverse impact of R-M on HRQoL.
Findings from this study add to the literature on HRQoL in at least two ways. First, this study characterized changes in HRQoL over time, after completion of front-line therapy. Second, the study examined the impact on HRQoL of R-M compared with OBS. In addition, the study examined differences between patients selected for R-M and those selected for OBS in the community setting.
Response duration in the treatment of FL is improving [Boland et al. 2009; Cheung et al. 2009; Friedberg et al. 2009; Fisher et al. 2005], due in part to treatment advances such as the introduction of rituximab, and the clinical benefits of rituximab maintenance [Maloney, 2008; Forstpointner et al. 2006]. However, physicians continue to balance treatment decisions with the potential impact on patient HRQoL. Such decisions have been complicated by the paucity of data regarding the impact of treatment, including that of R-M, on HRQoL. The current study should help to clarify these issues.
The most notable finding in this study was the general absence of an adverse impact of R-M on HRQoL. Subsample analysis examining the impact of active receipt of rituximab during maintenance therapy relative to the intervening period between doses also showed no adverse impact on physical symptoms. There was an effect on psychological symptoms, but this effect indicated decreased Acute Distress and decreased Despair and Depression during active receipt of rituximab. This may reflect patients' own awareness and sense of reassurance that their disease was being actively managed, and may be counter to the potential adverse effect of watchful waiting, cited earlier [Webster and Cella, 1998]. Although this analysis was conducted with only a subsample of patients, the significant effects observed for the psychological functioning outcomes suggests that the absence of significant adverse effects on other PCM outcomes is not the result of inadequate statistical power.
As survival durations extend, the number of patients living with FL is expected to grow notably. As a result, physicians will continue to negotiate an increasing array of treatment options for this clinically heterogeneous population. Understanding the trajectory of HRQoL associated with the disease and its treatment is essential in minimizing the burden to the patient, to their caregivers and to society as a whole.
In interpreting these findings, several limitations should be considered. First, although data were collected from geographically dispersed community oncology practices, and although sample characteristics were similar to those from two national samples, the study used a convenience sample that may differ in unknown ways from the underlying population. Also, patients were not randomly assigned to treatment groups. Although propensity score modeling was employed to reduce the effects of selection bias, and although cohorts appeared well balanced, the complexity of the propensity score model was limited by the sample size in the study. As a result, some risk associated with selection bias likely remains. Third, the sample size in this study was modest, and reduced both the complexity and statistical power of tests that were conducted. This was especially true in the assessment of the effects of active treatment with rituximab, which included only the R-M sample.
Despite these limitations, we believe this study provides new information regarding the experience of patients undergoing treatment of FL, and regarding the HRQoL impact of treatment. Our findings suggest that HRQoL follows a predictable course. Postinduction, most symptoms appear stable, with patients on R-M reporting symptoms that are, in general, equal to or better than those reported by OBS patients. Considering the clinical benefit of rituximab maintenance, these findings provide further support for use of R-M in patients with follicular lymphoma.
Acknowledgements
Special thanks are due to the staff of clinical research nurses at ACORN Research who assembled data for this study, and to Kathy Schulman, MS, for her contribution to the development of the manuscript.
Footnotes
The study reported in this paper was funded by Genentech, Inc. Carolina Reyes is employed by Genentech, Inc. and Sacha Satram-Hoang is a consultant to Genentech, Inc. The other authors report no declarations of interest.
References
- Boland A., Bagust A., Hockenhull J., Davis H., Chu P., Dickson R. (2009) Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-Hodgkin's lymphoma. Health Technol Assess 13(Suppl. 2): 41–48 [DOI] [PubMed] [Google Scholar]
- Buske C., Hoster E., Dreyling M., Hasford J., Unterhalt M., Hiddemann W. (2006) The Follicular Lymphoma International Prognostic Index (FLIPI) separates high-risk from intermediate- or low-risk patients with advanced-stage follicular lymphoma treated front-line with rituximab and the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with respect to treatment outcome. Blood 108: 1504–1508 [DOI] [PubMed] [Google Scholar]
- Cheung M.C., Imrie K.R., Friedlich J., Buckstein R., Lathia N., Mittmann N. (2009) The impact of follicular (FL) and other indolent non-Hodgkin's lymphomas (NHL) on work productivity-a preliminary analysis. Psychooncology 18: 554–559 [DOI] [PubMed] [Google Scholar]
- Cnaan A., Laird N.M., Slasor P. (1997) Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med 16: 2349–2380 [DOI] [PubMed] [Google Scholar]
- Copay A.G., Subach B.R., Glassman S.D., Polly D.W., Jr, Schuler T.C. (2007) Understanding the minimum clinically important difference: A review of concepts and methods. Spine J 7: 541–546 [DOI] [PubMed] [Google Scholar]
- Davis T.A., Grillo-Lopez A.J., White C.A., McLaughlin P., Czuczman M.S., Link B.K., et al. (2000) Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin's lymphoma: Safety and efficacy of re-treatment. J Clin Oncol 18: 3135–3143 [DOI] [PubMed] [Google Scholar]
- Dehejia R.H., Wahba S. (1999) Causal effects in nonexperimental studies: Reevaluating the evaluation of training programs. J Am Statist Assoc 94: 1053–1062 [Google Scholar]
- Fisher R.I., LeBlanc M., Press O.W., Maloney D.G., Unger J.M., Miller T.P. (2005) New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol 23: 8447–8452 [DOI] [PubMed] [Google Scholar]
- Forstpointner R., Unterhalt M., Dreyling M., Bock H.P., Repp R., Wandt H., et al. (2006) Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood 108: 4003–4008 [DOI] [PubMed] [Google Scholar]
- Fortner B., Baldwin S., Schwartzberg L., Houts A.C. (2006) Validation of the Cancer Care Monitor items for physical symptoms and treatment side effects using expert oncology nurse evaluation. J Pain Symptom Manage 31: 207–214 [DOI] [PubMed] [Google Scholar]
- Friedberg J.W., Taylor M.D., Cerhan J.R., Flowers C.R., Dillon H., Farber C.M., et al. (2009) Follicular lymphoma in the United States: First report of the national LymphoCare study. J Clin Oncol 27: 1202–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghielmini M.E., Schmitz S.H., Martinelli G., Peccatori F., Hess U., Fey M., et al. (2009) Longterm follow-up of patients with follicular lymphoma (FL) receiving single agent rituximab at two different schedules in study SAKK 35/98. In: Proceedings of the Annual Meeting of the American Society of Clinical Oncology, 29 May-2 June, Orlando, FL.
- Gine E., Gutierrez-Garcia G., Lopez-Guillermo A. (2010) Current immunochemotherapy strategies in follicular lymphoma Adv Ther, in press. [DOI] [PubMed] [Google Scholar]
- Hainsworth J.D. (2002) Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin's lymphoma: Interim follow-up of a multi-center phase II trial. Semin Oncol 29(1 Suppl. 2): 25–29 [PubMed] [Google Scholar]
- Hochster H., Weller E., Gascoyne R.D., Habermann T.M., Gordon L.I., Ryan T., et al. (2009) Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: Results of the randomized phase III ECOG1496 Study. J Clin Oncol 27: 1607–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houts A.C., Lipinski D., Olsen J.P., Baldwin S., Hasan M. (2009) Use of the patient care monitor to screen for depression in adult cancer patients interviewed with the structured clinical interview for DSM-IV. Psychooncology, in press. [DOI] [PubMed] [Google Scholar]
- Jaeschke R., Singer J., Guyatt G.H. (1989) Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 10: 407–415 [DOI] [PubMed] [Google Scholar]
- Keating G.M. (2010) Rituximab: A review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma. Drugs 70: 1445–1476 [DOI] [PubMed] [Google Scholar]
- Littell R.C., Pendergast J., Natarajan R. (2000) Modelling covariance structure in the analysis of repeated measures data. Stat Med 19: 1793–1819 [DOI] [PubMed] [Google Scholar]
- Maloney D.G. (2008) What is the role of maintenance rituximab in follicular NHL?. Oncology (Williston Park) 22: 20–26, discussion 26, 29, 33-24. [PubMed] [Google Scholar]
- Marcus R., Imrie K., Solal-Celigny P., Catalano J.V., Dmoszynska A., Raposo J.C., et al. (2008) Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol 26: 4579–4586 [DOI] [PubMed] [Google Scholar]
- National Cancer Institute (2005) SEER cancer statistics review, 1975-2006. Available at: http://www.seer.cancer.gov/csr/1975_2006/index.html
- Pettengell R., Donatti C., Hoskin P., Poynton C., Kettle P.J., Hancock B., et al. (2008) The impact of follicular lymphoma on health-related quality of life. Ann Oncol 19: 570–576 [DOI] [PubMed] [Google Scholar]
- Revicki D., Hays R.D., Cella D., Sloan J. (2008) Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 61: 102–109 [DOI] [PubMed] [Google Scholar]
- Ringash J., O'Sullivan B., Bezjak A., Redelmeier D.A. (2007) Interpreting clinically significant changes in patient-reported outcomes. Cancer 110: 196–202 [DOI] [PubMed] [Google Scholar]
- Rosenbaum P.R., Rubin D.B. (1984) Reducing bias in observational studies using subclassification on the propensity score. J Am Statist Assoc 79: 516–524 [Google Scholar]
- Salles G., Seymour J.F., Offner F., Lopez-Guillermo A., Belada D., Xerri L., et al. (2011) Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet 377: 42–51 [DOI] [PubMed] [Google Scholar]
- Solal-Celigny P., Cahu X., Cartron G. (2010) Follicular lymphoma prognostic factors in the modern era: What is clinically meaningful?. Int J Hematol 92: 246–254 [DOI] [PubMed] [Google Scholar]
- Stepanski E.J., Walker M.S., Schwartzberg L.S., Blakely L.J., Ong J.C., Houts A.C. (2009) The relation of trouble sleeping, depressed mood, pain, and fatigue in patients with cancer. J Clin Sleep Med 5: 132–136 [PMC free article] [PubMed] [Google Scholar]
- Tilly H., Zelenetz A. (2008) Treatment of follicular lymphoma: Current status. Leuk Lymphoma 49(Suppl. 1): 7–17 [DOI] [PubMed] [Google Scholar]
- van Oers M.H., Van Glabbeke M., Giurgea L., Klasa R., Marcus R.E., Wolf M., et al. (2010) Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin's lymphoma: Long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol 28: 2853–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal L., Gafter-Gvili A., Leibovici L., Dreyling M., Ghielmini M., Hsu S.F. Schmitz, et al. (2009) Rituximab maintenance for the treatment of patients with follicular lymphoma: Systematic review and metaanalysis of randomized trials. J Natl Cancer Inst 101: 248–255 [DOI] [PubMed] [Google Scholar]
- Walker M.S., Schwartzberg L.S., Stepanski E.J., Fortner B.V. (2009) A retrospective study of quality of life in a community sample of patients with early stage breast cancer. Breast Cancer Res Treat 115: 415–422 [DOI] [PubMed] [Google Scholar]
- Webster K., Cella D. (1998) Quality of life in patients with low-grade non-Hodgkin's lymphoma. Oncology (Williston Park) 12: 697–714, discussion 714, 717, 721. [PubMed] [Google Scholar]
- Witzens-Harig M., Reiz M., Heiss C., Benner A., Hensel M., Neben K., et al. (2009) Quality of life during maintenance therapy with the anti-CD20 antibody rituximab in patients with B cell non-Hodgkin's lymphoma: Results of a prospective randomized controlled trial. Ann Hematol 88: 51–57 [DOI] [PubMed] [Google Scholar]
- Witzig T.E., Vukov A.M., Habermann T.M., Geyer S., Kurtin P.J., Friedenberg W.R., et al. (2005) Rituximab therapy for patients with newly diagnosed, advanced-stage, follicular grade I non-Hodgkin's lymphoma: A phase II trial in the North Central Cancer Treatment Group. J Clin Oncol 23: 1103–1108 [DOI] [PubMed] [Google Scholar]