Abstract
Background:
Between January 1999 and December 2008, 469 patients treated for acute myeloid leukemia (AML) were included in this single-institution study.
Methods:
We performed a case-control analysis to study the rate of obesity among patients with acute promyelocytic leukemia (APL) and non-APL AML.
Results:
A total of 81% of APL patients analyzed were obese compared with 41.7% in the non-APL group (p < 0.001). Body mass index (BMI) >30 was seen in 57% of APL patients compared with 31% for the non-APL group (p = 0.01). Neither obesity nor the chemotherapy dosing based on ideal body weight affected survival.
Conclusions:
Our findings generate the hypothesis that APL and metabolic syndromes may share a common pathogenic pathway via retinoic acid receptors (RARs), the ligand-controlled transcription factors that function as heterodimers with retinoid X receptors (RXRs) to regulate cell growth and survival. If this link is confirmed in larger studies, our data will instigate further studies using RXR and RAR modulators as a preventive strategy among obese individuals.
Keywords: acute promyelocytic leukemia, body mass index, obesity, prevention, RAR, RXR
Introduction
Acute promyelocytic leukemia (APL) is a distinct subtype of acute myeloid leukemia (AML) characterized by the chromosomal translocation t (15; 17), which produces the fusion protein PML-RARα linking the retinoic acid receptor alpha on chromosome 17 with the PML gene on chromosome 15. Increased understanding of the molecular genetics of APL has provided a rational basis for therapeutic intervention [Sanz et al. 2009; Tallman and Altman, 2009; Tallman, 2004]. Retinoic acid receptors (RARs) are ligand-controlled transcription factors that function as heterodimers with retinoid X receptors (RXRs) to regulate cell growth and survival. In APL, the fusion protein heterodimerizes with RXR and binds strongly to retinoic acid response elements, repressing transcription; high-dose alltrans retinoic acid (ATRA) can dissociate this abnormal complex [Grignani et al. 1998]. RARs have also been implicated in the pathogenesis of metabolic diseases such as obesity and type 2 diabetes mellitus [Altucci et al. 2007]. Epidemiological studies reveal that APL occurs predominantly in adults and has an increased incidence in certain regions such as Latin America and Northeastern Italy [Maule et al. 2008]. In the United States, the incidence of APL is estimated to be approximately 1000 new cases per year [Yamamoto and Goodman, 2008]. Risk factors associated with the development of APL are unclear at this time. A study by Estey and colleagues suggested that obese patients with AML were more likely to have APL [Estey et al. 1997]; however, this association has not been further studied recently in the context of the increasing prevalence of obesity over the past decade in the United States during the ATRA era. In addition, in our experience, we observed that several patients diagnosed with APL to be morbidly obese, posing a challenge in chemotherapy dosing. ATRA has changed the treatment paradigm of this once life-threatening illness to a curable disease [Tallman and Rowe, 2003]. It is plausible that therapy with ATRA not only offers a cure in a majority of APL patients but may also help control the metabolic syndrome in these patients on long-term followup.
Patients and methods
AML patients who received therapies at Vanderbilt University Medical Center from January 1999 to December 2008 were included in an Institutional Review Board-approved case-control study. Of 469 AML patients, 61 were diagnosed with cytogenetically confirmed APL and 44 APL patients matched with control subjects were included in the final analysis. Controls were selected randomly matched for age and sex over a 10-year period. Patients who were diagnosed with AML in the same month as patients with APL were included in the final analysis in a 1:2 ratio.
Height and weight were obtained at original disease presentation. Complete response rates were documented by pathological confirmation of disease status and cytogenetic analysis.
Definitions
Body mass index (BMI) was calculated using the standard formula: weight in kilograms/height in meters2. Patients were classified as underweight (BMI < 20), normal (BMI 20-25), overweight (BMI 25-30), and obese (BMI > 30).
Statistical analysis
Summary statistics, such as proportions, means, standard deviations, 95% confidence intervals, medians and ranges, were used to describe the patient characteristics. Standard techniques in survival analysis, including Kaplan-Meier estimates and the Cox proportional hazard models, were used to estimate the time-to-event distributions of outcome variables. All patients were followed for disease relapse and survival, and Kaplan-Meier analysis was performed. Log-rank test was used to compare survival distributions among patients with various BMIs within the APL group and compared with non-APL AML cohort. Data analysis was performed using SPSS 17 for Windows (SPSS Inc., Chicago, IL) software.
Results
Patient characteristics are shown in Table 1. Of 469 patients with AML, 135 patients were included in the final analysis. For 44 APL patients included in the analysis, 91 non-APL AML patients served as controls.
Table 1.
Baseline characteristics.
| AML | APL | |
|---|---|---|
| Number of Patients | 91 | 44 |
| Gender (%) | ||
| Male | 45 (49.4) | 20 (45.4) |
| Female | 46 (50.5) | 24 (54.5) |
| Age years (%) | ||
| Mean | 46.5 | 43.5 |
| Range | (15-83) | (11-74) |
| Race (%) | ||
| White | 77 (84.6) | 30 (69) |
| African American | 11 (12) | 9 (19) |
| Others | 3 (3.2) | 5 (12) |
| Comorbidities (%) | ||
| HTN | 15 (16.4) | 8 (18) |
| CAD | 2 (2.1) | 4 (9) p = 0.03 |
| DM | 8 (8.7) | 9 (20) p = 0.09 |
| Dyslipidemia | 8 (8.7) | 4 (9) |
AML, acute myeloid leukemia; acute promyelocytic leukemia; HTN, hypertension; CAD, coronary artery disease; DM, diabetes mellitus.
The median age was 46.5 years (15-83 years) in AML patients and 43.5 years (11-74 years) in APL patients. A total of 51% of AML and 54.5% of APL patients were female.
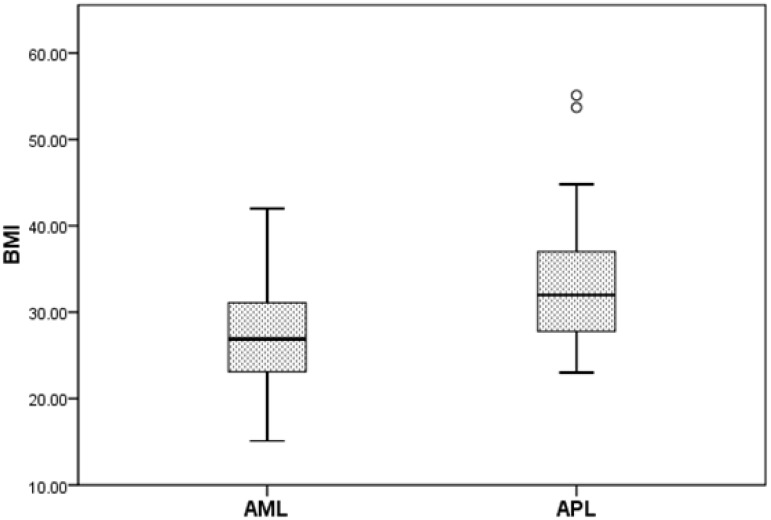
Patients with APL were significantly more obese compared with patients with a diagnosis of non-APL AML (Figure 1). The median weight of patients with APL was 96.2kg (range, 59.8-203.5) versus 81.4 kg (range, 49.6-151.5) for non-APL patients (p = 0.003). The median BMI of patients with APL was 32.0 (range 23-55.1). This was significantly higher than patients with non-APL AML, who had a median BMI of 26.9 (range, 15.1-42.0) (p < 0.001). When analyzed by gender, this trend remained significant for women (p = 0.032) but not for men (p = 0.07). BMI > 30 was seen in 57% of APL patients compared with 31% for non-APL group (p = 0.01).
Figure 1.
The median body mass index (BMI) of patients with acute promyelocytic leukemia (APL) was significantly higher compared with acute myeloid leukemia (AML) patients (p < 0.001).
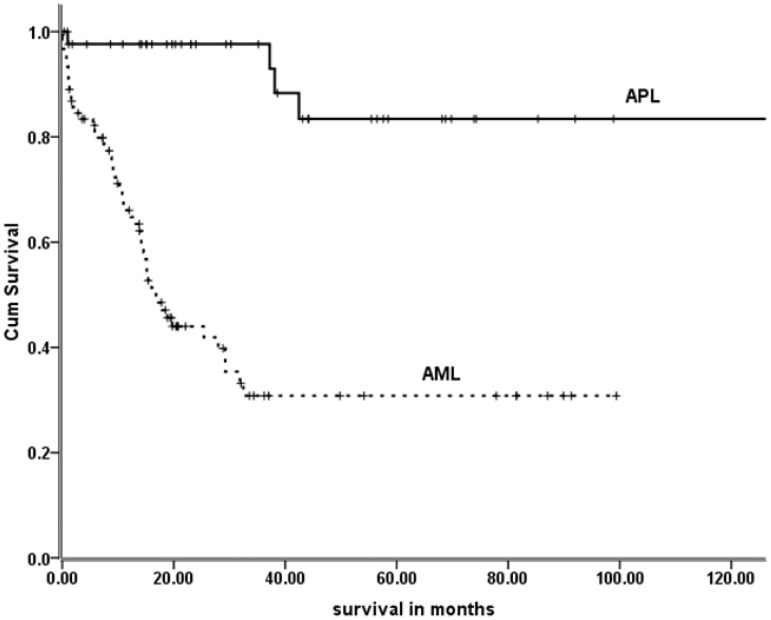
All patients with non-APL AML received standard therapy with 7 + 3i (continuous Ara-C infusion and idarubicin). A total of 37 APL patients received therapy with ATRA and an anthracycline; three received single-agent ATRA and three patients received ATRA, continuous low-dose cytarabine infusion and idarubicin. A total of 36 (81%) APL patients underwent dose adjustment based on their ideal body weight. There were statistically more significant coronary artery events in the APL group (p = 0.039). However, there were no significant differences among patients diagnosed with diabetes mellitus in either group (p = 0.09). In a univariate analysis, BMI did not significantly influence the overall outcome of patients with APL (p = 0.38). The median survival time for AML patients was 16.8 months (95% CI 29.7-49.5). The median survival for APL patients has not been reached (p < 0.001). Figure 2 shows the long-term survival of APL and AML (non-APL) group.
Figure 2.
Overall survival of acute promyelocytic leukemia (APL) and acute myeloid leukemia (AML) patients.
Discussion
Although the mechanism is unclear, our data have shown that the majority of our APL patients are either overweight or obese. Comorbid illnesses such as hypertension (HTN), diabetes mellitus and coronary artery disease (CAD) as expected were more common in obese patients (Table 1). Neither obesity nor chemotherapy dose reductions impacted the overall survival of APL patients. However, in a report by Jeddi and colleagues high BMI (>30) remained as an independent predictor of differentiation syndrome [Jeddi et al. 2010]. While confirming the report by Estey and colleagues on an increased incidence of higher BMI among APL patients [Estey et al. 1997], our report although small, offers a unique observational study in the ATRA era. This is important, especially since it is possible that APL and metabolic syndromes may share a common pathogenic pathway via RARs, to regulate cell growth and survival. If this link is true then it is possible that prolonged ATRA treatment may not only cure APL but also control the long term sequelae of metabolic syndrome.
Implications for RARs and RXRs modulators in APL and obesity
RARs are ligand-controlled transcription factors that function as heterodimers with RXRs to regulate cell growth and survival. RARs are implicated in the development of APL and metabolic diseases such as obesity and diabetes mellitus [Lefebvre et al. 2010]. The success of RAR modulation with differentiating agents such as ATRA in the treatment of APL has stimulated considerable interest in the development of RAR and RXR modulators. Retinoids can be either beneficial or detrimental. Retinoic acid differentiation therapy saves the lives of the majority of APL patients, but pan-retinoids are also known for their teratogenicity. With the recognition that retinoid receptors have various ligands with distinct selectivity, significant effort has been made in several areas [Jeddi et al. 2010; Tallman and Altman, 2009]. First, understanding the molecular basis of the leukemogenic events that cause APL and the mechanism(s) involved in RA action during therapy and the absence of a therapeutic potential for variant APLs and all non-APL AML patients. The second area of advancement has been in the dissection of receptor-elicited functions by mouse genetics and the use of selective ligands, including rexinoids, which are less toxic than retinoids. The third is searching for alternative therapies, to extend the use of retinoids and rexinoids beyond APL. Substantial challenges remain to exploit the therapeutic potentials of RXR ligands including the general concern of potential toxicity. A convenient way to study the effectiveness of RARs in metabolic syndrome is to study APL patients treated with ATRA. Long-term followup of these patients might indicate and will also instigate prospective studies to assess the secondary beneficial effect of ATRA on metabolic syndrome, as based on our study APL patients are predominantly either overweight or obese.
Our analysis is limited by the small sample size and inherent limitations of its retrospective nature and we need stronger evidence after ruling out causes of potential bias. This study, however, generates novel hypotheses and it is hoped will also instigate larger population-based studies or analyses of larger registry data to study whether obesity is a risk factor for developing APL and the long-term impact of ATRA therapy on metabolic syndrome. Further investigation, perhaps related to an RXR abnormality, integral to body mass gain and leukemogenesis is also warranted.
Footnotes
NR was supported by the NCRR/NIH and 5K-12 (grant number CA090625-09).
None declared.
References
- Altucci L., Leibowitz M.D., Ogilvie K.M., de Lera A.R., Gronemeyer H. (2007) RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov 6: 793–810 [DOI] [PubMed] [Google Scholar]
- Estey E., Thall P., Kantarjian H., Pierce S., Kornblau S., Keating M. (1997) Association between increased body mass index and a diagnosis of acute promyelocytic leukemia in patients with acute myeloid leukemia. Leukemia 11: 1661–1664 [DOI] [PubMed] [Google Scholar]
- Grignani F., De Matteis S., Nervi C., Tomassoni L., Gelmetti V., Cioce M., et al. (1998) Fusion proteins of the retinoic acid receptor-alpha recruit histone deacetylase in promyelocytic leukaemia. Nature 391: 815–818 [DOI] [PubMed] [Google Scholar]
- Jeddi R., Gherdira H., Mnif S., Goudier E., Fenaux P., Meddeb B. (2010) High body mass index is an independant predictor of differentiation syndrome in patients with acute promyelocytic leukemia. Leuk Res 34: 545–547 [DOI] [PubMed] [Google Scholar]
- Lefebvre P., Benomar Y., Staels B. (2010) Retinoid X receptors: Common heterodimerization partners with distinct functions. Trends Endocrinol Metab 21: 676–683 [DOI] [PubMed] [Google Scholar]
- Maule M.M., Dama E., Mosso M.L., Magnani C., Pastore G., Merletti F. (2008) High incidence of acute promyelocytic leukemia in children in northwest Italy, 1980-2003: A report from the Childhood Cancer Registry of Piedmont. Leukemia 22: 439–441 [DOI] [PubMed] [Google Scholar]
- Sanz M.A., Grimwade D., Tallman M.S., Lowenberg B., Fenaux P., Estey E.H., et al. (2009) Management of acute promyelocytic leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 113: 1875–1891 [DOI] [PubMed] [Google Scholar]
- Tallman M.S. (2004) Acute promyelocytic leukemia as a paradigm for targeted therapy. Semin Hematol 41(2 Suppl. 4): 27–32 [DOI] [PubMed] [Google Scholar]
- Tallman M.S., Altman J.K. (2009) How I treat acute promyelocytic leukemia. Blood 114: 5126–5135 [DOI] [PubMed] [Google Scholar]
- Tallman M.S., Rowe J.M. (2003) Long-term follow-up and potential for cure in acute promyelocytic leukaemia. Best Pract Res Clin Haematol 16: 535–543 [DOI] [PubMed] [Google Scholar]
- Yamamoto J.F., Goodman M.T. (2008) Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997-2002. Cancer Causes Control 19: 379–390 [DOI] [PubMed] [Google Scholar]