Abstract
The peripheral T-cell lymphomas are a rare, heterogeneous group of non-Hodgkin's lymphomas which have an aggressive clinical course. Treatment approaches have traditionally been similar to those of diffuse large B-cell lymphomas, but outcomes have been inferior. Novel approaches involving agents and pathways developed from a better understanding of the biology of the diseases have led to therapeutic advances. The introduction of new agents, including antifolates, immunoconjugates, histone deacetylase inhibitors, monoclonal antibodies, nucleoside analogs, proteasome inhibitors, and signaling inhibitors have improved outcomes for patients with relapsed and refractory disease and are being incorporated into strategies for first-line therapy. Stem cell transplantation remains a potentially curative option for a subset of patients.
Keywords: anaplastic lymphoma kinase, human T-cell lymphotropic virus-1, natural killer/T-cell lymphoma, peripheral T-cell lymphomas, T-cell leukemia
Introduction
Peripheral T-cell lymphomas (PTCLs) are a heterogeneous group of non-Hodgkin's lymphomas of T-cell origin. They represent 12% of all lymphomas and are increasing in frequency [Abouyabis et al. 2008]. In the United States, the incidence has increased 7-8% annually. Owing to the rarity of the disease, PTCLs are poorly understood and outcomes have been inferior to those of aggressive B-cell lymphomas. The International T-Cell Lymphoma Project collected data on 1314 cases of T-cell lymphomas from 22 countries worldwide [Vose et al. 2008]. All patients presented with disease between 1990 and 2002. The most common of subtypes were PTCL not otherwise specified (PTCL-NOS, 25.9%), angioimmunoblastic T-cell lymphoma (AITL, 18.5%), natural killer (NK)/T-cell lymphoma (10.4%), adult T-cell lymphoma/leukemia (ATLL, 9.6%), and anaplastic large cell lymphoma (ALCL; anaplastic lymphoma kinase [ALK]-positive, 6.6%; ALK-negative, 5.5%). The frequency of the different subtypes varied by geographical region, with PTCL-NOS occurring more frequently in North America (34.4%) and Europe (34.3%) compared with the Far East (22.4%). In contrast, NK/T-cell lymphoma and ATLL are more frequent in the Far East (22.4% and 25% respectively). ALK-positive ALCL is more common in North America compared with Europe (16.0% versus 6.4%), and AITL rates are higher in Europe (28.7%).
Classification of T-cell lymphomas
The revised fourth edition of the 2008 World Health Organization (WHO) classification of Tumors of Hematopoietic and Lymphoid Tissues identified a number of subtypes of T-cell lymphoma and further recharacterized a number of entities [Campo et al. 2011; Harris et al. 1994]. Based on clinical features, the diseases can be divided into four subdivisions: nodal, extranodal, cutaneous, and leukemic or disseminated disease, as shown in Table 1. The nodal subtypes of PTCL include AITL, ALKpositive and ALK-negative types of ALCL (ALK-negative ALCL is considered a provisional entity), and PTCL-NOS. The extranodal PTCL subtypes are the nasal-type extranodal NK/T-cell lymphoma, enteropathy associated T-cell lymphoma, and hepatosplenic T-cell lymphoma. Several types of leukemic or disseminated types of T-cell lymphoproliferative disorders are also identified, including T-cell prolymphocytic leukemia, T-cell large granular lymphocytic leukemia, chronic lymphoproliferative disorders of NK cells (a provisional entity), aggressive NK-cell leukemia, adult T-cell lymphoma/leukemia (human T-cell lymphotropic virus-1-positive), and systemic Epstein Barr virus-positive T-cell lymphoproliferative disorders of childhood. The cutaneous group includes mycosis fungoides and the Sezary syndrome, primary cutaneous CD30-positive lymphoproliferative disorders (lymphomatoid papulosis and primary cutaneous ALCL), primary cutaneous aggressive epidermotropic CD8-positive cytotoxic T-cell lymphoma, primary cutaneous small/medium CD4-positive T-cell lymphoma (provisional), and the panniculitus-like T-cell lymphomas. The latter have been reclassified such that the αβ subtype is subcutaneous panniculitis T-cell lymphoma (SPTCL) and the δγ subtype is included in the category of primary cutaneous gamma delta (δ) T-cell lymphoma.
Table 1.
World Health Organization (WHO) 2008: the mature T-cell and natural killer cell neoplasms [Campo et al. 2011].
| T-cell prolymphocytic leukemia |
| T-cell large granular lymphocytic leukemia |
| Chronic lymphoproliferative disorder of NK-cells* |
| Aggressive NK cell leukemia |
| Systemic EBV-positive T-cell lymphoproliferative disease of childhood (associated with chronic active EBV infection) |
| Hydroa vacciniforme-like lymphoma |
| Adult T-cell leukemia/lymphoma |
| Extranodal NK/T-cell lymphoma, nasal type |
| Enteropathy-associated T-cell lymphoma |
| Hepatosplenic T-cell lymphoma |
| Subcutaneous panniculitis-like T-cell lymphoma |
| Mycosis fungoides |
| Sézary syndrome |
| Primary cutaneous CD30-positive T-cell lymphoproliferative disorder |
| Lymphomatoid papulosis |
| Primary cutaneous anaplastic large-cell lymphoma |
| Primary cutaneous aggressive epidermotropic CD8-positive cytotoxic T-cell lymphoma* |
| Primary cutaneous gamma-delta T-cell lymphoma |
| Primary cutaneous small/medium CD4-positive T-cell lymphoma* |
| Peripheral T-cell lymphoma, not otherwise specified |
| Angioimmunoblastic T-cell lymphoma |
| Anaplastic large cell lymphoma, ALK-positive |
| Anaplastic large cell lymphoma, ALK-negative* |
ALK, anaplastic lymphoma kinase; EBV, Epstein Barr virus; NK, natural killer.
These represent provisional entities or provisional subtypes of other neoplasms.
Diseases shown in italics are newly included in the 2008 WHO classification.
Outcomes of patients with PTCL
Although patients with PTCL have historically been treated with CHOP [cyclophosphamide, hydroxydaunorubicin, Oncovin (vincristine), and prednisone] and CHOP-like therapies similar to patients with diffuse large B-cell lymphoma, retrospective studies demonstrate that the outcome of patients with PTCL has been inferior with these approaches. A recent meta-analysis of 31 studies (n = 2912 patients) demonstrated that the 5-year overall survival (OS) of patients with PTCL treated with CHOP (excluding patients with ALCL due to their favorable prognosis) was 37.3% (95% CI 35.1% to 39.6%) [Abouyabis et al. 2008]. By subtype, the 5-year OS for nasal-type NK/T-cell, AITL, PTCL-NOS, and enteropathy-associated subtypes were 47.9%, 36.5%, 34% and 21% respectively. Likewise, the International T-cell Lymphoma Project which retrospectively reviewed pathology and reported outcomes on 1153 T-cell lymphoma cases demonstrated that patients who had received an anthracycline-containing regimen fared no better than those who received nonanthracycline therapy, across all T-cell lymphoma subtypes, with the exception of ALK-positive ALCL [Vose et al. 2008]. These results suggest that alternative strategies should be pursued for patients with aggressive T-cell lymphomas.
Prognostic indices
As is the case with diffuse large B-cell lymphomas, the International Prognostic Index (IPI) has been shown to be predictive of outcome in some subsets of PTCL. The 5-year OS for IPI 0–1 versus 4–5 for PTCL-NOS was 50% versus 11%, for AITL 56% versus 25%, and for ALK-positive ALCL 90% versus 33%. A new prognostic index, the Prognostic Index for PTCL (PIT), was recently proposed specifically for the PTCL-NOS subtype [Gallamini et al. 2004]. The PIT was designed using a retrospective cohort of 385 patients. A multivariate analysis identified four factors as being significantly associated with a poor prognosis: age (>60 years; p < 0.0001), Eastern Cooperative Oncology Group (ECOG) performance status (≥2; p < 0.0001), elevated lactate dehydrogenase level (any elevation; p < 0.0001), and bone marrow involvement (any degree; p = 0.026). Using these factors, four risk groups were defined in the PIT: group 1 (zero factors), group 2 (one factor), group 3 (two factors), and group 4 (three or four factors). These groups were shown to be effective prognostic categories, with corresponding 5-year OS rates (group 1: 62.3%; group 2: 52.9%; group 3: 32.9%; group 4: 18.3%). The predictive value of PIT is now being explored in prospective clinical trials. Recently, a comparison of four prognostic scales was carried out in 121 patients with PTCL [Suzumiya et al. 2009]. In addition to the IPI and PIT scales, this comparison also evaluated the International PTCL Project (IPTCLP) score and the modified PIT (mPIT). This report determined that all four were useful in assessing PTCL patient outcome, although the authors concluded that IPTCLP could most significantly predict OS.
Molecular and immunohistochemical prognostic factors
Many clinical and laboratory findings have been evaluated in particular PTCL subtypes. Among these, ALK expression in the ALCL subtype is particularly significant; patients with ALK-positive ALCL experience a significantly prolonged OS compared with those having ALK-negative disease [Savage et al. 2008]. Both low serum albumin levels and mediastinal lymphadenopathy were independently associated with a poor OS in patients with PTCL-NOS [Chihara et al. 2009]. In addition, for patients with the PTCL-NOS subtype, CD30 expression, as well as the expression markers of proliferation such as Ki-67, have been analyzed for their prognostic ability. Two chemokine receptors, CXCR3 and CCR4, were found to be expressed in 63% and 34% of PTCL-NOS cancers, respectively [Ishida et al. 2004, 2003]. The dominant chemokine expression found in this study was CXCR3-positive/CCR4-negative; this phenotype was shown by multivariate analysis to be an independent adverse prognostic factor in both PTCL-NOS and ALK-negative ALCL.
Went and colleagues proposed a new prognostic index based on the expression of 19 markers [Went et al. 2006]. In this study, the proliferation-associated protein Ki-67 turned out to be prognostically relevant and was integrated in a new predictive score, incorporating age (>60 years), high lactate dehydrogenase, poor performance status, and Ki-67 ≥ 80%. This score was associated with the patient outcome (p < 0.0001) and was found to be more robust than PIT (p = 0.0043). However, this was demonstrated in a retrospective analysis and has yet to be confirmed in a prospective clinical trial. A number of studies are underway evaluating the potential implications of chromosomal aberrations, including their effect on gene expression, and on PTCL patient prognosis.
Therapeutic management of PTCL
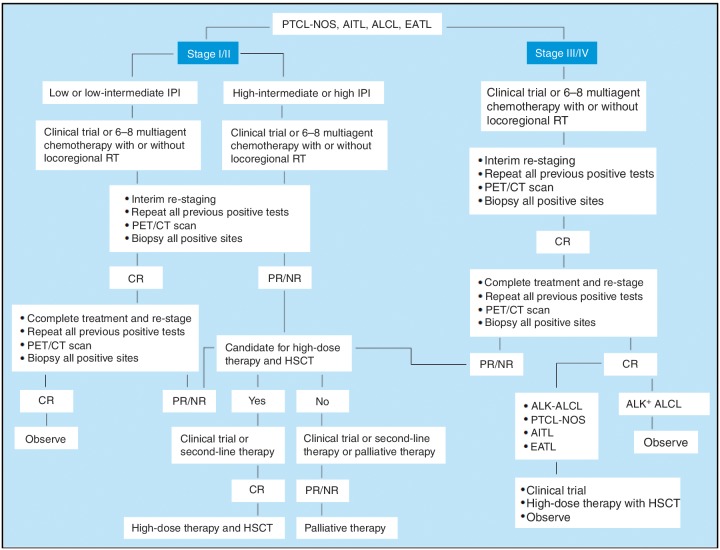
Currently, there is no standard first-line regimen for the treatment of PTCL. The National Comprehensive Cancer Network (NCCN) practice guidelines for PTCL emphasize the lack of standard treatment options (Figure 1). First-line therapy options include a variety of multiagent chemotherapy combinations, including CHOP, and consolidation recommendations consist of high-dose therapy and stem cell rescue in all patients except those with low IPI. Second-line therapy includes clinical trials, combination chemotherapy regimens, single-agent therapies, or radiation.
Figure 1.
National Comprehensive Cancer Network guidelines for the treatment of peripheral T-cell lymphoma.
AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; CR, complete response; CT, computed tomography; EATL, enteropathy-associated T-cell lymphoma; HSCT, hematopoietic stem cell transplantation; IPI, International Prognostic Index; NR, nonresponse; PET, positron emission tomography; PR, partial response; PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified; RT, radiotherapy.
Given the inferiority of CHOP, more aggressive infusional regimens, including hyper-CVAD [cyclophosphamide, vincristine, doxorubicin (Adriamycin), and dexamethasone] and hyper-CHOP, among others, were evaluated retrospectively against CHOP in patients with PTCL (n =135) at MD Anderson Cancer Center. Among those patients with non-ALCL disease, there was no significant difference in outcome between those treated with CHOP and aggressive alternatives (3-year OS 43% versus 49%). However, these results are difficult to interpret because the study was not randomized. In another small study, ESHAP (etoposide, methylprednisolone, cytarabine, and cisplatin) was administered but found to have a poor risk-benefit ratio because of the lack of complete clinical responses [Mebazaa et al. 2005].
Recently the German High Grade Non-Hodgkin's Lymphoma Study Group reported results for patients with aggressive T-cell lymphomas treated in seven trials with six to eight courses of CHOP or CHOP plus etoposide (Hi-CHOEP or MegaCHOEP) [Schmitz et al. 2010]. Of 343 patients, 70 had PTCL, 28 had AITL, 78 had ALK-positive ALCL, and 113 had ALK-ALCL. The younger patients demonstrated an improvement in event-free survival (EFS) for etoposide-containing regimens compared with the nonetoposide regimen, but increased toxicity in older patients made this approach less favorable. The improved EFS overall shifted to only a trend for improved survival when patients with ALK-positive ALCL were excluded from the analysis. The 3-year EFS for the different subtypes was 41% for PTCL, 45% for ALK- ALCL, 50% for AITL, and 76% for ALK-positive ALCL.
The Group d'Etude des Lymphomes de l'Adulte (GELA) reported results from a combination study of ACVBP [doxorubicin (adriamycin), cyclophosphamide, vindesine, bleomycin, prednisone] with bortezomib 1.5 mg/m2 on days 1 and 5 of each ACVBP cycle, and then on days 1, 8, and 15 every 4 weeks during the consolidation phase [Delmer et al. 2009]. Of 57 eligible patients, 78% had stage III-IV disease; 81% completed induction treatment with ACVBP. The complete response (CR) rate was 45% after induction and 46% after consolidation. Thrombocytopenia was more pronounced than previously observed with ACVBP alone and the overall response rate (ORR) was not higher than previously observed with ACVBP alone.
New combination therapies for PTCL
A number of studies have investigated the combination of CHOP with novel agents. One of these has been alemtuzumab, a CD52-targeted monoclonal antibody. Up to 40% of PTCL cases have been shown to express CD52 by immunohistochemistry, although expression may vary by subtype [Piccaluga et al. 2007]. One phase II study (n = 20) evaluated CHOP combined with intravenous alemtuzumab in 3-week cycles (cycle 1: 10 mg on day 1, 20 mg on day 2; subsequent cycles: 30 mg on day 1) as frontline therapy [Gallamini et al. 2007]. Although the overall response rate (80%) was high, the 2-year EFS was 48% and OS was 53%. Nearly all patients (90%) experienced grade 4 neutropenia and approximately one-third (32%) experienced cytomegalovirus reactivation. Additionally, there were two treatment-related deaths. A prospective multicenter trial by Kim and colleagues also investigated the CHOP plus alemtuzumab combination (n = 24). In this study, the CR rate was 65%, and the ORR was 80% but 25% of patients experienced reactivation of cytomegalovirus (CMV), and this study was prematurely closed [Kim et al. 2007].
A phase I study evaluated alemtuzumab combined with dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin) in patients with PTCL [Janik et al. 2005]. In this study, alemtuzumab was administered at doses of 30, 60, or 90 mg prior to each EPOCH cycle. Significant bone marrow aplasia occurred in 2 of 3 patients at both the 60 and 90 mg dose groups; therefore, phase II study accrual is continuing at the 30 mg dose of alemtuzumab. Infections were reported in 11 of 14 patients, including bacterial, fungal and viral pathogens. Patients underwent ongoing CMV surveillance and received prophylactic therapy with acyclovir and trimethoprim-sulfamethoxazole.
The interleukin-2 fusion toxin protein denileukin diftitox has also been combined with CHOP for first-line therapy of patients with PTCL. A prospective phase II multicenter trial evaluated this combination in 49 untreated patients with aggressive PTCL subtypes [Foss et al. 2010]. The majority of these patients had nodal PTCL (23 with PTCL-NOS, 10 with AITL, and six with ALCL). In this study, denileukin diftitox was administered at a dose of 18 μg/kg/day on days 1 and 2 and CHOP was given on day 3; this was followed by growth factor support on day 4 every 21 days. The ORR (90%) and CR (76%) were high, and the median progression-free survival (PFS) was 15 months. Toxicities were generally associated with denileukin diftitox related infusion reactions. There was no increase in infectious complications or prolonged immunosuppression.
Gemcitabine has demonstrated significant activity as a single agent in patients with cutaneous T-cell lymphomas and has been used in a number of combination regimens for PTCL [Zinzani et al. 2010]. The GEM-P combination (gemcitabine, cisplatin, methylprednisolone) was tested in one phase II study, producing a 69% response rate among patients (n = 16) with aggressive T-cell lymphomas [Arkenau et al. 2007]. The combination of gemcitabine with vinorelbine and filgrastim was also found to be active in a pilot study, with an ORR of 70% in patients with PTCL (n = 10) [Spencer et al. 2007]. However, when gemcitabine was combined in a CHOP-based regimen (CHOP-EG, CHOP plus etoposide and gemcitabine), the ORR was 77% but the median EFS was disappointing at only 7 months [Kim et al. 2006].
Treatment of NK/T-cell lymphomas
One of the most difficult subtypes of PTCL to treat is NK/T-cell lymphoma. Patients with this subtype have responded poorly to anthracycline-containing regimens. Patients with localized disease (stage I or II) tend to do very well with a combination of chemotherapy and involved field radiation. The radiotherapy is an important component of managing localized NK/T-cell lymphomas and has been administered both before and after cytotoxic chemotherapy. However, once the disease becomes more advanced, outcomes are relatively poor, with 2-year OS rates of 0% for those with disseminated disease [Yamaguchi et al. 2009]. Further, the CR rate for patients with advanced-stage NK/T-cell lymphoma treated with CHOP-like regimens is relatively low, with a 5-year OS rate of less than 10% [Abouyabis et al. 2008]. A regimen of ifosfamide, methotrexate, etoposide, and prednisolone proved to be more effective with a 79% CR rate in early stage patients, but the CR rate was only 13% in advanced stage patients [Lee et al. 2006]. Furthermore, the relapse rate was high in both groups. The combination of CHOP and etoposide demonstrated a CR rate of 45% with a 3-year OS rate of 59% for nasal-type NK-cell lymphoma [Yong et al. 2009].
Recently, two groups have explored the activity of asparaginase-containing regimens. The combination of L-asparaginase with dexamethasone and methotrexate induced an ORR of 67% and a CR rate of 50% in a study of relapsed or refractory patients [Yamaguchi et al. 2008]. In another study, with a regimen of asparaginase, methotrexate and dexamethasone, response was seen in 14 of 18 evaluable patients after three cycles with 61% CR [Jaccard et al. 2011]. Based on these encouraging results, an asparaginase-containing regimen, SMILE, was studied as first-line therapy in patients with advanced NK/ T-cell lymphomas by the NK Study Group. The SMILE regimen consists of methotrexate, etoposide, ifosfamide, dexamethasone, and L-asparasginase. Of 39 patients enrolled, 21 were newly diagnosed, 13 relapsed, and five had primary refractory disease [Yamaguchi et al. 2010]. Of 29 patients who completed the therapy, the ORR was 74%, with 38% CR. The incidence of myelosuppression was high, with grade 3 or 4 infections in 41% of patients. Nevertheless, this regimen has been adopted by many centers for this difficult to treat group.
The role of autologous stem cell transplantation in PTCL
A strategy to improve outcomes in patients with PTCL in first remission is to use stem cell transplantation as a consolidation strategy. In retrospective studies, the 5-year OS has been reported to range from 40% to 50%, with 5-year disease-free survival rates from 30 to 40% [D'Amore et al. 2009; Dreger and Laport, 2008]. A prospective intent-to-transplant study of 83 patients with PTCL was recently reported by Reimer and colleagues [Reimer et al. 2009]. The treatment regimen consisted of four to six cycles of CHOP, followed by mobilizing therapy with either dexaBEAM (dexamethasone, car-mustine, melphalan, etoposide, and cytarabine) or ESHAP. Patients in complete remission or partial remission then underwent myeloablative chemoradiotherapy and autologous stem cell transplantation. Of 83 enrolled patients, 32 had PTCL-NOS and 27 had AITL. Only two-thirds (66%) of the patients had a disease response to the initial chemotherapy and went on to receive autologous stem cell transplantation. At a median follow-up time of 33 months, the estimated 3-year OS and PFS for patients in complete response were 48% and 36% respectively. Patients who did not experience a response to chemotherapy and therefore did not undergo ASCT had a very poor outcome, with a median survival of less than 2 years.
In another recent study, 57 patients with T-cell lymphoma, including 26 with the prognostically dismal enteropathy-associated subtype, were treated with a high-dose alternative regimen (CHOP then methotrexate alternating with ifosfamide, etoposide, and epirubicin) [Jantunen et al. 2010]. Patients who achieved CR went on to transplant (n = 33). For the transplanted enteropathy-associated patients, the PFS and OS were 52% and 60% respectively, and 65% and 72% for others. These results signal that this kind of approach may be superior for this group of patients.
Allogeneic stem cell transplantation has been a potentially curative option for patients with PTCL either in first remission or after relapse. A retrospective report from the GELA reviewed 77 patients with PTCL who were transplanted, 57 of whom had ablative conditioning regimens [Le Gouill et al. 2008]. The 5-year OS and EFS rates were 57% and 53% respectively. Patients with AITL did the best with 80% OS while those with PTCL had a 63% OS at 5 years. In this study, patients with refractory disease at the time of transplant had the worse outcome. In a European Group for Blood and Marrow Transplantation (EBMT) retrospective review of 45 patients with AITL who underwent allogeneic transplant, 15 had prior autologous transplant and 12 were in first complete response (CR1) or second complete response (CR2) [Kyriakou et al. 2009]. The 3-year PFS and OS rates were favorable at 53% and 64% respectively. Corradini and colleagues also reported favorable results for 38 patients who underwent reduced intensity transplant with 3-year OS and PFS rates of 53% and 52% respectively [Corradini et al. 2004].
Recently, the results from autologous and allogeneic stem cell transplantation for patients with PTCL were reviewed by the Center for International Blood and Marrow Transplant Research [Smith et al. 2010]. There were 115 autologous and 123 allogeneic stem cell transplants. In the allogeneic group, 40% of patients had ALCL, 50% had peripheral T-cell lymphoma unspecified (PTCLu) and 10% had AITL. In the autologous group, 53% had ALCL, 34% had PTCLu and 13% had AITL. The OS and PFS for the autologous group were 59% and 47% respectively and for the allogeneic group were 47% and 36% respectively. In both groups, the number of prior chemotherapy regimens and chemorefractory disease were adverse prognostic factors. A higher incidence of disease relapse in the autologous group was balanced out by higher transplant-related mortality in the allogeneic group.
Treatment approaches for relapsed and refractory disease
The treatment for many patients with relapsed and refractory PTCL is salvage chemotherapy. The NCCN guidelines indicate that patients should be entered into a clinical trial if one is available. There are a number of single agents which have demonstrated activity in relapsed and refractory patients (Table 2). Autologous or allogeneic stem cell transplantation may be considered for selected patients who are in remission after salvage therapy.
Table 2.
Activity of single agents in relapsed/refractory peripheral T-cell lymphomas (PTCLs).
| Agent | Reference | No. PTCL | ORR (%) |
|---|---|---|---|
| Alemtuzumab | [Enblad et al. 2004] | 14 | 36 |
| Alemtuzumab | [Zinzani et al. 2005] | 6 | 60 |
| Bortezomib | [Zinzani et al. 2007] | 2 | 67 |
| Denileukin diftitox | [Dang et al. 2007] | 27 | 48 |
| Gemcitabine | [Zinzani et al. 2010] | 8 | 70 |
| Lenalidomide | [Dueck et al. 2009] | 24 | 30 |
| Cyclosporine | [Advani et al. 2007] | 12 | 66 |
| SGN-35 (brentuximab vedotin) | [Shustov et al. 2010] | 58 | 87 |
| Anti-CD4 zanolimumab | [D'Amore et al. 2010] | 21 | 24 |
| Romidepsin (NCI) | [Piekarz et al. 2009] | 43 | 39 |
| Romidepsin (pivotal) | [Coiffier et al. 2010] | 131 | 30 |
| Pralatrexate (pivotal) | [O'Connor et al. 2011] | 111 | 27 |
| Belinostat | [Pohlman et al. 2009] | 19 | 32 |
NCI, National Cancer Institute; ORR, overall response rate.
Monoclonal antibodies and conjugates
Alemtuzumab, an anti-CD52 MAb, has been shown to have activity in heavily treated patients with PTCL with an ORR of 36-52% [Piccaluga et al. 2007; Zinzani et al. 2005; Enblad et al. 2004]. Toxicities including opportunistic infections and pancytopenia occurred at a high rate and led to premature closure of one study [Enblad et al. 2004]. Alemtuzumab has been used in combination with CHOP or EPOCH (etoposide, prednisone, vincristine, cyclophophamide, and doxorubicin) with some success in PTCL, with half of patients achieving a complete response [Gallamini et al. 2007; Kim et al. 2007].
Two anti-CD30 MAbs, iratumumab and SGN-30, have shown efficacy in CD30-positive ALCL [Forero-Torres et al. 2009; Bartlett et al. 2008; Ansell et al. 2007]. In vitro studies have shown that both drugs are synergistic or additive with conventional chemotherapy [Oflazoglu et al. 2008]. An immunoconjugate of SGN-30 and monomethyl auristatin, brentuximab vetodin (SGN-35), has demonstrated an ORR of 87% with 57% CR in 58 patients with relapsed and refractory CD30-positive ALCL [Shustov et al. 2010]. The median response duration ranged from 4 to 36 weeks and was ongoing at the time of the report in 18 patients. A study is currently underway to evaluate the combination of CHOP with brentiximab vetodin in newly diagnosed patients with ALCL.
Siplizumab is an anti-CD2 MAb. CD2 is an adhesion molecule highly expressed on activated T cells and NK cells and on the majority of cells from patients with T-cell lymphoma and leukemia. Siplizumab eliminated both CD4-positive and CD8-positive T cells and NK cells without affecting B cells. In a phase I trial in patients with CD2-positive lymphoproliferative disease, siplizumab showed clinical activity, inducing CRs in two patients with large granular lymphocyte leukemia, three partial responses (PRs) in patients with ATL, and one PR in a patient with cutaneous T-cell lymphoma (CTCL) [Casale et al. 2006; O'Mahony et al. 2005]. A subsequent dose escalation study produced a PR in a patient with NK-cell large granular lymphocyte leukemia and a CR in a patient with PTCL. However, siplizumab also predisposes patients to the development of lymphoproliferative syndrome, although it may be possible to prevent that with prophylactic rituximab [O'Mahony et al. 2007].
The humanized anti-CD4 monoclonal antibody, zanolimumab, was administered to 21 patients with PTCL, with an ORR of 24% [D'Amore et al. 2007]. Bevacizumab, an antivascular endothelial growth factor MAb, has been used with success in one patient with relapsed AITL [Bruns et al. 2005], and was being studied along with CHOP in a clinical trial for patients with PTCL or NK-cell neoplasms by the Eastern Cooperative Oncology Group. However, preliminary results of this trial reported a high incidence of cardiac events related to the therapy [Advani et al. 2009].
Denileukin diftitox, a fusion protein that combines interleukin-2 receptor-binding domain with diphtheria toxin, has demonstrated activity in both cutaneous and aggressive T-cell lymphomas. In a single center phase II study at MD Anderson Cancer Center, denileukin diftitox was administered to patients with relapsed and refractory PTCL at a dose of 18 μg/kg/day for 5 days on a 21-day cycle. The ORR was 48% and responses were seen in four of 10 patients with PTCL-NOS, two of three with AITL, and two of two with ALCL [Dang et al. 2007]. In this trial, the expression of CD25 by immunohistochemistry was not predictive of response to denileukin diftitox.
Histone deacetylase inhibitors
Histone deacetylase (HDAC) inhibitors are potent inducers of histone acetylation, which results in the expression of tumor suppressor genes that had been previously silenced by deacetylation. This gene expression leads to cell cycle arrest and apoptosis. There are a number of HDAC inhibitors being used or studied in T-cell lymphoma, including vorinostat, romidepsin (also known as depsipeptide), panobinostat, and belinostat. Vorinostat and romidepsin have shown single-agent activity in CTCL, and vorinostat was approved by the US Food and Drug Administration (FDA) in 2006 for the treatment of advanced and refractory CTCL [Olsen et al. 2007]. Romidepsin was also approved by the FDA in 2009 for advanced and refractory CTCL based on a demonstrated ORR in two clinical trials of 34% [Whittaker et al. 2010; Piekarz et al. 2009]. The median response duration was 15 months (range 1-20+) and median time to progression was 8.3 months in early disease and 6.4 months in more advanced disease. A phase II study of romidepsin was completed in patients with relapsed and refractory PTCL [Piekarz et al. 2009]. This phase II, open-label, multi-arm, multicenter study enrolled 43 patients with PTCL from the National Cancer Institute and nine extramural sites. Of 43 patients, 31 received at least two cycles of therapy. The mean number of prior therapies was 3.9 (range 1-12). Objective response rate was 39% overall or 55% for patients who received at least two cycles of therapy. The overall median duration of response was 8.3 months (range 1.6 months to 4.8+ years) for all patients. A multicenter, multi-national phase IIB registration study of romidepsin at the same dose and schedule in relapsed and refractory PTCL has completed accrual and results have recently been reported [Coiffier et al. 2010]. Of 130 patients with a median of two prior therapies, the ORR was 26% with 15% CR by radiographic documentation. The median response duration was 12 months, and toxicities included gastrointestinal and constitutional events and thrombocytopenia.
Belinostat, a hydroxamic acid-derived HDAC inhibitor, has been studied in both intravenous and oral formulations. Belinostat was administered intravenously at 1000 mg/m2/daily for 5 days every 3 weeks in 53 patients including 19 with refractory PTCL and 29 with refractory CTCL [Pohlman et al. 2009]. The objective response rate in PTCL was 32% with two CRs and a median response duration of 8.9+ months, and 14% in CTCL, with a response duration of 9.1 months. A multicenter phase II registration trial of belinostat in patients with relapsed PTCL is underway, and a cohort dose escalation study of oral belinostat is ongoing in patients with relapsed lymphoma.
Additive and synergistic activity has been demonstrated in vitro for combinations of HDAC inhibitors with a number of agents, including topoisomerase inhibitors, bortezomib, and cytotoxic chemotherapy drugs and clinical trials are underway to explore the activity of these combinations in T-cell lymphomas.
Antifolates
Pralatrexate is a novel folate antagonist whose activity is associated with binding to the reduced folate carrier. In a phase I/II dose escalation trial of pralatrexate in patients with refractory lymphoma, the ORR was 31%, with response durations ranging from 3 to 26 months [O'Connor et al. 2007]. The response rate in that trial was 54% for patients with T-cell lymphomas. Based on these encouraging data, the PROPEL trial was initiated [O'Connor et al. 2011]. In this trial, 111 patients with relapsed or refractory PTCL were treated with pralatrexate weekly for 6 weeks on a 7-week cycle. The median number of prior therapies was three, and 63% of patients had no response to their last line of therapy. The ORR was 27% and the median response duration was 9.4 months. Toxicities included mucusitis in 70% of patients and thrombocytopenia in 40%. Pralatrexate was approved in September 2009 by the FDA as a single agent to treat relapsed or refractory PTCL. A number of recent studies have explored the potential synergy between pralatrexate and other active agents in T-cell lymphoma. A phase I study combining pralatrexate with gemcitabine is underway.
Immunomodulators and immunosuppressants
AITL has been characterized as a disease of immune dysregulation, and in previous studies, patients have benefited from immune suppressive therapies such as methotrexate or nucleoside analogs. In a phase II trial, cyclosporine was administered to 12 patients with AITL [Advani et al. 2007]. Two-thirds (three CRs, five PRs) of the patients responded, but there were four deaths. A phase II trial of cyclosporine in AITL was conducted by ECOG but closed early because of slow accrual.
Other immune-modulating agents, including rituximab, lenalidomide, and thalidomide, are also being explored as single agents and in combination with chemotherapy. A phase II study of lenalidomide at a dose of 25 mg/m2 daily for 21 days of a 28-day cycle was conducted in 24 patients with relapsed PTCL [Dueck et al. 2009]. The ORR was 30% with a PFS of 95 days. Toxicities included neutropenia and thrombocytopenia in 20% and 33% of patients respectively.
Nucleoside analogs
Nucleoside analogs are chemotherapeutic agents that primarily inhibit DNA replication and repair. Gemcitabine is the most effective pyrimidine nucleoside analog in PTCL. It has been active both as a single agent [Zinzani et al. 2010; Marchi et al. 2005] and in combination with alemtuzumab and bortezomib. The purine nucleoside analogs include cladribine, fludarabine, clofarabine, and nelarabine. Both cladribine and fludarabine have shown efficacy in PTCL, and clofarabine and nelarabine are currently being used in several clinical trials for T-cell lymphoma [Abramson et al. 2010; Mulford et al. 2010].
The metabolic enzyme inhibitors, which include deoxycoformycin (pentostatin) and forodesine, do not incorporate into DNA, unlike the other nucleoside analogs. Pentostatin inhibits adenosine deaminase, increasing the deoxyadenosine triphosphate pool, and forodesine inhibits phosphorylase, increasing the deoxyguanosine triphosphate pool. Both agents have shown some efficacy in CTCL [Dearden, 2006]. A phase I/ II study of oral forodesine in patients with relapsed and refractory CTCL reported a 53% ORR, and a phase II trial has been completed [Korycka et al. 2007; Duvic et al. 2006].
Proteasome inhibitors
Bortezomib, a proteasome inhibitor, has been well tolerated and active as a single agent in patients with relapsed or refractory CTCL [Zinzani et al. 2007]. In a phase II study of bortezomib in patients with relapsed CTCL or PTCL, the ORR was 67% with two CRs and no grade 4 toxicity. The GELA has conducted a phase II study of bortezomib with ACVBP chemotherapy in 57 patients with untreated PTCL and has reported that 29 patients were withdrawn prematurely because of toxicity [Delmer et al. 2009]. The ORRs were similar to ACVBP alone.
Signaling inhibitors
Enzastaurin is a selective inhibitor of protein kinase C (PKC), which acts in part through the AKT pathway. By targeting the phosphatidylinositol 3-kinase (PI3K)/AKT pathways, enzastaurin inhibits cell proliferation, induces tumor cell apoptosis, and suppresses tumor-induced angiogenesis in CTCL cell lines [Querfeld et al. 2006]. Enzastaurin is currently in two phase II trials: one for patients with several types of non-Hodgkin's lymphoma, including PTCL and CTCL, and another for patients with relapsed CTCL. The recent finding of SYN/ITK translocations in PTCL tissues has suggested that syk protein tyrosine kinase inhibitors may be active in the clinic. While T-cell non-Hodgkin's lymphoma cell lines were sensitive in vitro, early clinical trial data have not been promising [Wilcox et al. 2010]. Similarly, PI3K pathways have been shown to be activated in aggressive T-cell lymphomas, and clinical trials with agents inhibiting this pathway are underway [Martin-Sanchez et al. 2010].
Conclusions
The T-cell lymphomas are a rare and heterogeneous group of diseases and clinical syndromes for which treatment remains a challenge. ALK-positive ALCL remains a highly curable disease for patients with a low IPI who are treated with CHOP-like regimens. For most of the aggressive subtypes, combination chemotherapy approaches are utilized, followed by consolidation therapy with either autologous or allogeneic stem cell transplantation when appropriate. Recent advances include the use of asparaginase-containing regimens for NK lymphomas and the identification of a plethora of novel agents with activity in phase II studies. Multinational collaborations are carrying out trials to further define prognostic factors and outcomes for subsets of patients.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
None declared.
References
- Abouyabis A.N., Shenoy P.J., Lechowicz M.J., Flowers C.R. (2008) Incidence and Outcomes of the Peripheral T-Cell Lymphoma Subtypes in the United States. Leuk Lymphoma 49: 2099–2107 [DOI] [PubMed] [Google Scholar]
- Abramson J.S., Takvorian T., Jacobsen E.D., Brown J.R., Barnes J.A., Feng Y., et al. (2010) A phase I dose-escalation trial of oral clofarabine for relapsed/refractory non-Hodgkin lymphoma. Blood (ASH Annual Meeting Abstracts) 116: 1775 [Google Scholar]
- Advani R., Horwitz S., Zelenetz A., Horning S.J. (2007) Angioimmunoblastic T cell lymphoma: Treatment experience with cyclosporine. Leuk Lymphoma 48: 521–525 [DOI] [PubMed] [Google Scholar]
- Advani R.H., Hong F., Ganjoo K.N., Manola J.B., Swinnen L.J., Habermann T.M., et al. (2009) Cardiac toxicity associated with the anti-Vegf monoclonal antibody bevacizumab (Avastin) in combination with CHOP (A-CHOP) chemotherapy for peripheral T cell lymphoma (PTCL): The ECOG 2404 trial. Blood (ASH Annual Meeting Abstracts) 114: 1671 [Google Scholar]
- Ansell S.M., Horwitz S.M., Engert A., Khan K.D., Lin T., Strair R., et al. (2007) Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin's lymphoma and anaplastic large-cell lymphoma. J Clin Oncol 25: 2764–2769 [DOI] [PubMed] [Google Scholar]
- Arkenau H.T., Chong G., Cunningham D., Watkins D., Sirohi B., Chau I., et al. (2007) Gemcitabine, cisplatin and methylprednisolone for the treatment of patients with peripheral T-cell lymphoma: The Royal Marsden Hospital experience. Haematologica 92: 271–272 [DOI] [PubMed] [Google Scholar]
- Bartlett N.L., Younes A., Carabasi M.H., Forero A., Rosenblatt J.D., Leonard J.P., et al. (2008) A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood 111: 1848–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns I., Fox F., Reinecke P., Kobbe G., Kronenwett R., Jung G., et al. (2005) Complete remission in a patient with relapsed angioimmunoblastic T-cell lymphoma following treatment with bevacizumab. Leukemia 19: 1993–1995 [DOI] [PubMed] [Google Scholar]
- Campo E., Swerdlow S.H., Harris N.L., Pileri S., Stein H., Jaffe E.S. (2011) The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood, forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casale D., Bartlett N., Hurd D., Foss F., Pro B., Sokol L., et al. (2006) A phase I open label dose escalation study to evaluate MEDI-507 in patients with CD2-positive T-cell lymphoma/leukemia. Blood 108: 771a16822905 [Google Scholar]
- Chihara D., Oki Y., Ine S., Yamamoto K., Kato H., Taji H., et al. (2009) Analysis of prognostic factors in peripheral T-cell lymphoma: Prognostic value of serum albumin and mediastinal lymphadenopathy. Leuk Lymphoma 50: 1999–2004 [DOI] [PubMed] [Google Scholar]
- Coiffier B., Pro B., Prince H.M., Foss F.M., Sokol L., Greenwood M., et al. (2010) Final results from a pivotal, multicenter, international, open-label, phase 2 study of romidepsin in progressive or relapsed peripheral T-cell lymphoma (PTCL) following prior systemic therapy. Blood (ASH Annual Meeting Abstracts) 116: 114 [Google Scholar]
- Corradini P., Dodero A., Zallio F., Caracciolo D., Casini M., Bregni M., et al. (2004) Graft-versus-lymphoma effect in relapsed peripheral T-cell non-Hodgkin's lymphomas after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. J Clin Oncol 22: 2172–2176 [DOI] [PubMed] [Google Scholar]
- D'Amore F., Jantunen E., Relander T. (2009) Hemopoietic stem cell transplantation in T-cell malignancies: Who, when, and how?. Curr Hematol Malig Rep 4: 236–244 [DOI] [PubMed] [Google Scholar]
- D'Amore F., Radford J., Relander T., Jerkeman M., Tilly H., Osterborg A., et al. (2010) Phase II trial of zanolimumab (Humax-CD4) in relapsed or refractory non-cutaneous peripheral T cell lymphoma. Br J Haematol 150: 565–573 [DOI] [PubMed] [Google Scholar]
- D'Amore F., Radford J., Jerkeman M., Relander T., Tilly H., Osterborg A.K., et al. (2007) Zanolimumab (Humax-CD4™), a fully human monoclonal antibody: Efficacy and safety in patients with relapsed or treatment-refractory non-cutaneous CD4+ T-cell lymphoma. Blood 110: 999z [Google Scholar]
- Dang N.H., Pro B., Hagemeister F.B., Samaniego F., Jones D., Samuels B.I., et al. (2007) Phase II trial of denileukin diftitox for relapsed/refractory T-cell non-Hodgkin lymphoma. Br J Haematol 136: 439–447 [DOI] [PubMed] [Google Scholar]
- Dearden C.E. (2006) Role of single-agent purine analogues in therapy of peripheral T-cell lymphomas. Semin Hematol 43: S22–S26 [DOI] [PubMed] [Google Scholar]
- Delmer A., Fitoussi O.K., Gaulard P., Laurent G., Bordessoule D., Morschauser F. (2009) A phase II study of bortezomib in combination with intensified CHOP like regimen ACVBP in patients with previously untreated T-cell lymphoma. Results of a GELA LNH05-IT trial. J Clin Oncol 27: 447s [Google Scholar]
- Dreger P., Laport G.G. (2008) Controversies in lymphoma: The role of hematopoietic cell transplantation for mantle cell lymphoma and peripheral T cell lymphoma. Biol Blood Marrow Transplant 14: 100–107 [DOI] [PubMed] [Google Scholar]
- Dueck G.S., Chua N., Prasad A., Stewart D., White D., Vanderjagt R., et al. (2009) Activity of lenalidomide in a phase II trial for T-cell lymphoma: Report on the first 24 cases. ASCO Meeting Abstracts 27: 8524 [Google Scholar]
- Duvic M., Forero-Torres A., Foss F., Olsen E., Kim Y. (2007) Oral forodesine is clinically active in refractory cutaneous T-cell lymphoma. Results of a phase I/II study. Blood 108: 698a [Google Scholar]
- Enblad G., Hagberg H., Erlanson M., Lundin J., Macdonald A.P., Repp R., et al. (2004) A pilot study of alemtuzumab (Anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood 103: 2920–2924 [DOI] [PubMed] [Google Scholar]
- Forero-Torres A., Leonard J.P., Younes A., Rosenblatt J.D., Brice P., Bartlett N.L., et al. (2009) A phase II study of SGN-30 (anti-CD30 MAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol 146: 171–179 [DOI] [PubMed] [Google Scholar]
- Foss F.M., Sjak-Shie N.N., Goy A., Advani R., Jacobsen E.D. (2010) Phase II study of denileukin diftitox with CHOP chemotherapy in newly-diagnosed PTCL: Concept trial. ASCO Meeting Abstracts 28: 8045 [Google Scholar]
- Gallamini A., Stelitano C., Calvi R., Bellei M., Mattei D., Vitolo U., et al. (2004) Peripheral T-cell lymphoma unspecified (PTCL-U): A new prognostic model from a retrospective multicentric clinical study. Blood 103: 2474–2479 [DOI] [PubMed] [Google Scholar]
- Gallamini A., Zaja F., Patti C., Billio A., Specchia M.R., Tucci A., et al. (2007) Alemtuzumab (campath-1h) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: Results of a GITIL (Gruppo Italiano Terapie Innovative Nei Linfomi) prospective multicenter trial. Blood 110: 2316–2323 [DOI] [PubMed] [Google Scholar]
- Harris N.L., Jaffe E.S., Stein H., Banks P.M., Chan J.K., Cleary M.L., et al. (1994) A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood 84: 1361–1392 [PubMed] [Google Scholar]
- Ishida T., Inagaki H., Utsunomiya A., Takatsuka Y., Komatsu H., Iida S., et al. (2004) CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin Cancer Res 10: 5494–5500 [DOI] [PubMed] [Google Scholar]
- Ishida T., Utsunomiya A., Iida S., Inagaki H., Takatsuka Y., Kusumoto S., et al. (2003) Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: Its close association with skin involvement and unfavorable outcome. Clin Cancer Res 9: 3625–3634 [PubMed] [Google Scholar]
- Jaccard A., Gachard N., Marin B., Rogez S., Audrain M., Suarez F., et al. (2011) Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase II study. Blood 117: 1834–1839 [DOI] [PubMed] [Google Scholar]
- Janik J., Dunleavy K., Pittaluga S., Jaffe E., Grant N., Shovlin M., et al. (2005) A pilot study of campath-1 with dose-adjusted epoch in CD52 expressing aggressive T-cell malignancies. Blood 106: 334816051743 [Google Scholar]
- Jantunen E., Relander T., Lauritzsen G.F., Hagberg H., Anderson H., Cavallin-Stahl E., et al. (2010) Intensive induction chemotherapy followed by autologous stem cell transplantation (ASCT) in patients with enteropathy-associated T-cell lymphoma: A prospective study by the Nordic Lymphoma Group (NLG-T-01). Blood (ASH Annual Meeting Abstracts) 116: 3565 [Google Scholar]
- Kim J.G., Sohn S.K., Chae Y.S., Cho Y.Y., Yang D.H., Lee J.J., et al. (2007) Alemtuzumab plus CHOP as front-line chemotherapy for patients with peripheral T-cell lymphomas: A phase II study. Cancer Chemother Pharmacol 60: 129–134 [DOI] [PubMed] [Google Scholar]
- Kim J.G., Sohn S.K., Chae Y.S., Kim D.H., Baek J.H., Lee K.B., et al. (2006) CHOP plus etoposide and gemcitabine (CHOP-EG) as front-line chemotherapy for patients with peripheral T cell lymphomas. Cancer Chemother Pharmacol 58: 35–39 [DOI] [PubMed] [Google Scholar]
- Korycka A., Blonski J.Z., Robak T. (2007) Forodesine (BCX-1777, Immucillin H)-a new purine nucleoside analogue: Mechanism of action and potential clinical application. Mini Rev Med Chem 7: 976–983 [DOI] [PubMed] [Google Scholar]
- Kyriakou C., Canals C., Finke J., Kobbe G., Harousseau J.L., Kolb H.J., et al. (2009) Allogeneic stem cell transplantation is able to induce long-term remissions in angioimmunoblastic T-cell lymphoma: A retrospective study from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 27: 3951–3958 [DOI] [PubMed] [Google Scholar]
- Lee K.W., Yun T., Kim D.W., Im S.A., Kim T.Y., Yoon S.S., et al. (2006) First-line ifosfamide, methotrexate, etoposide and prednisolone chemotherapy +/-radiotherapy is active in stage I/II extranodal NK/T-cell lymphoma. Leuk Lymphoma 47: 1274–1282 [DOI] [PubMed] [Google Scholar]
- Le Gouill S., Milpied N., Buzyn A., De Latour R.P., Vernant J.P., Mohty M., et al. (2008) Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: A study by the Societe Francaise De Greffe De Moelle Et De Therapie Cellulaire. J Clin Oncol 26: 2264–2271 [DOI] [PubMed] [Google Scholar]
- Marchi E., Alinari L., Tani M., Stefoni V., Pimpinelli N., Berti E., et al. (2005) Gemcitabine as frontline treatment for cutaneous T-cell lymphoma: Phase II study of 32 patients. Cancer 104: 2437–2441 [DOI] [PubMed] [Google Scholar]
- Martin-Sanchez E., Rodriguez-Pinilla S.M., Lombardia L., Sanchez-Beato M., Dominguez-Gonzalez B., Romero D., et al. (2010) PI3KCA and PIM inhibitors in peripheral T-cell lymphomas. Blood (ASH Annual Meeting Abstracts) 116: 4917 [Google Scholar]
- Mebazaa A., Dupuy A., Rybojad M., Mouly F., Moulonguet I., Vignon-Pennamen M.D., et al. (2005) Eshap for primary cutaneous T-cell lymphomas: Efficacy and tolerance in 11 patients. Hematol J 5: 553–558 [DOI] [PubMed] [Google Scholar]
- Mulford D.A., Pohlman B.L., Hamlin P.A., Young F., Pamer E., Horwitz S.M. (2010) A phase I/II trial of clofarabine in patients with relapsed T-cell or NK-cell lymphomas. ASCO Meeting Abstracts 28: 8046 [Google Scholar]
- O'Connor O.A., Hamlin P.A., Portlock C., Moskowitz C.H., Noy A., Straus D.J., et al. (2007) Pralatrexate, a novel class of antifol with high affinity for the reduced folate carrier-type 1, produces marked complete and durable remissions in a diversity of chemotherapy refractory cases of T-cell lymphoma. Br J Haematol 139: 425–428 [DOI] [PubMed] [Google Scholar]
- O'Connor O.A., Pro B., Pinter-Brown L., Bartlett N., Popplewell L., Coiffier B., et al. (2011) Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: Results from the pivotal PROPEL study. J Clin Oncol, forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oflazoglu E., Kissler K.M., Sievers E.L., Grewal I.S., Gerber H. (2008) Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemotherapy improves antitumour activity in Hodgkin lymphoma. Br J Hematol 142: 69–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony D., Morris J., Moses L., O'Hagan D., Gao W., Stetler-Stevenson M., et al. (2005) Phase I trial of siplizumab in CD2-positive lymphoproliferative disease. Blood 106: 937a [Google Scholar]
- O'Mahony D., Morris M., Stetler-Stevenson M., Matthews H., Pittaluga S., Albert P., et al. (2007) EBV-related lymphoproliferative disease complicating therapy with siplizumab, a novel anti-CD2 mediated T- and NK-cell depleting agent, in patients with T-cell malignancies. Blood 110: 1043a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen E.A., Kim Y.H., Kuzel T.M., Pacheco T.R., Foss F.M., Parker S., et al. (2007) Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol 25: 3109–3115 [DOI] [PubMed] [Google Scholar]
- Piccaluga P.P., Agostinelli C., Righi S., Zinzani P.L., Pileri S.A. (2007) Expression of CD52 in peripheral T-cell lymphoma. Haematologica 92: 566–567 [DOI] [PubMed] [Google Scholar]
- Piekarz R.L., Frye R., Turner M., Wright J.J., Allen S.L., Kirschbaum M.H., et al. (2009) Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol 27: 5410–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarz R., Wright J., Frye R., Allen S.L., Joske D., Kirschbaum M., et al. (2009) Final results of a phase 2 NCI multicenter study of romidepsin in patients with relapsed peripheral T-cell lymphoma (PTCL). Blood (ASH Annual Meeting Abstracts) 114: 1657 [Google Scholar]
- Pohlman B., Advani R., Duvic M., Hymes K.B., Intragumtornchai T., Lekhakula A., et al. (2009) Final results of a phase II trial of belinostat (PXD101) in patients with recurrent or refractory peripheral or cutaneous T-cell lymphoma. Blood (ASH Annual Meeting Abstracts) 114: 920 [Google Scholar]
- Querfeld C., Rizvi M.A., Kuzel T.M., Guitart J., Rademaker A., Sabharwal S.S., et al. (2006) The selective protein kinase C beta inhibitor enzastaurin induces apoptosis in cutaneous T-cell lymphoma cell lines through the AKT pathway. J Invest Dermatol 126: 1641–1647 [DOI] [PubMed] [Google Scholar]
- Reimer P., Rudiger T., Geissinger E., Weissinger F., Nerl C., Schmitz N., et al. (2009) Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: Results of a prospective multicenter study. J Clin Oncol 27: 106–113 [DOI] [PubMed] [Google Scholar]
- Savage K.J., Harris N.L., Vose J.M., Ullrich F., Jaffe E.S., Connors J.M., et al. (2008) ALK-anaplastic large-cell lymphoma is clinically and immunopheno-typically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: Report from the International Peripheral T-Cell Lymphoma Project. Blood 111: 5496–5504 [DOI] [PubMed] [Google Scholar]
- Schmitz N., Trumper L., Ziepert M., Nickelsen M., Ho A.D., Metzner B., et al. (2010) Treatment and prognosis of mature T-cell and NK-cell lymphoma: An analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood 116: 3418–3425 [DOI] [PubMed] [Google Scholar]
- Shustov A.R., Advani R., Brice P., Bartlett N.L., Rosenblatt J.D., Illidge T., et al. (2010) Complete remissions with brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood (ASH Annual Meeting Abstracts) 116: 961 [Google Scholar]
- Smith S.B.L., Van Besin K., Lerademacher J., He W., Laport G., Montoto S., et al. (2010) Autologous vs allogeenic hematopoietic stem cell transplantion for T-NHL: A CIBMTR analysis. Blood 116: 689 [Google Scholar]
- Spencer A., Reed K., Arthur C. (2007) Pilot study of an outpatient-based approach for advanced lymphoma using vinorelbine, gemcitabine and filgrastim. Intern Med J 37: 760–766 [DOI] [PubMed] [Google Scholar]
- Suzumiya J., Ohshima K., Tamura K., Karube K., Uike N., Tobinai K., et al. (2009) The international prognostic index predicts outcome in aggressive adult T-cell leukemia/lymphoma: Analysis of 126 patients from the International Peripheral T-Cell Lymphoma Project. Ann Oncol 20: 715–721 [DOI] [PubMed] [Google Scholar]
- Vose J., Armitage J., Weisenburger D. (2008) International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma Study: Pathology Findings and Clinical Outcomes. J Clin Oncol 26: 4124–4130 [DOI] [PubMed] [Google Scholar]
- Went P., Agostinelli C., Gallamini A., Piccaluga P.P., Ascani S., Sabattini E., et al. (2006) Marker expression in peripheral T-cell lymphoma: A proposed clinical-pathologic prognostic score. J Clin Oncol 24: 2472–2479 [DOI] [PubMed] [Google Scholar]
- Whittaker S.J., Demierre M.F., Kim E.J., Rook A.H., Lerner A., Duvic M., et al. (2010) Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol 28: 4485–4491 [DOI] [PubMed] [Google Scholar]
- Wilcox R.A., Sun D.X., Novak A., Dogan A., Ansell S.M., Feldman A.L. (2010) Inhibition of Syk protein tyrosine kinase induces apoptosis and blocks proliferation in T-cell non-Hodgkin's lymphoma cell lines. Leukemia 24: 229–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Kwong Y., Maeda Y., Hashimoto C., Kim W., Suh C., et al. (2010) Phase II study of SMILE chemotherapy for newly-diagnosed stage IV, relapsed or refractory extranodal NK/T-cell lymphoma, nasal type: NKTSG study. ASCO Meeting Abstracts 28: 8044. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Suzuki R., Kwong Y.L., Kim W.S., Hasegawa Y., Izutsu K., et al. (2008) Phase I study of dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) chemotherapy for advanced-stage, relapsed or refractory extranodal natural killer (Nk)/T-cell lymphoma and leukemia. Cancer Sci 99: 1016–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Tobinai K., Oguchi M., Ishizuka N., Kobayashi Y., Isobe Y., et al. (2009) Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol 27: 5594–5600 [DOI] [PubMed] [Google Scholar]
- Yong W., Zheng W., Zhu J., Zhang Y., Wang X., Xie Y., et al. (2009) L-Asparaginase in the treatment of refractory and relapsed extranodal NK/T-cell lymphoma, nasal type. Ann Hematol 88: 647–652 [DOI] [PubMed] [Google Scholar]
- Zinzani P.L., Alinari L., Tani M., Fina M., Pileri S., Baccarani M. (2005) Preliminary observations of a phase II study of reduced-dose alemtuzumab treatment in patients with pretreated T-cell lymphoma. Haematologica 90: 702–703 [PubMed] [Google Scholar]
- Zinzani P.L., Musuraca G., Tani M., Stefoni V., Marchi E., Fina M., et al. (2007) Phase II trial of proteasome inhibitor bortezomib in patients with relapsed or refractory cutaneous T-cell lymphoma. J Clin Oncol 25: 4293–4297 [DOI] [PubMed] [Google Scholar]
- Zinzani P.L., Venturini F., Stefoni V., Fina M., Pellegrini C., Derenzini E., et al. (2010) Gemcitabine as single agent in pretreated T-cell lymphoma patients: Evaluation of the long-term outcome. Ann Oncol 21: 860–863 [DOI] [PubMed] [Google Scholar]