Abstract
Allogeneic hematopoietic stem-cell transplantation (HSCT) is the most effective approach for many patients with hematologic malignancies. Unfortunately, relapse remains the most common cause of death after allogeneic HSCT, and the prognosis of relapsed disease is poor for most patients. Induction of a graft-versus-leukemia (GVL), or graft-versus-tumor, effect through the use of donor leukocyte infusion (DLI), or donor lymphocyte infusion, has been remarkably successful for relapsed chronic myelogenous leukemia. Unfortunately, response to DLI in other hematologic malignancies is much less common and depends on many factors including histology, pace and extent of relapse, and time from HSCT to relapse. Furthermore, graft-versus-host disease (GVHD) is common after DLI and often limits successful immunotherapy. Ultimately, manipulations to minimize GVHD while preserving or enhancing GVL are necessary to improve outcomes for relapse after allogeneic HSCT.
Keywords: allogeneic, donor leukocyte infusion, graft-versus-leukemia effect, hematopoietic stem-cell transplantation, relapse
Introduction
Allogeneic hematopoietic stem-cell transplantation (HSCT) is the most effective, if not the only, curative therapy for many patients with hematologic malignancies. In addition to the intensive conditioning regimen, the antileukemic property of the graft itself is critical for successful transplantation. This graft-versus-leukemia (GVL) effect was first demonstrated in animal models [Barnes et al. 1956] and later through retrospective clinical observations that showed graft-versus-host disease (GVHD) was associated with a lower risk of relapse [Sullivan et al. 1989a, 1989b; Weiden et al. 1981]. Ultimately, GVL was directly demonstrated in human transplantation when donor leukocyte infusions (DLI) effectively restored remission for relapsed chronic myelogenous leukemia (CML) [Porter et al. 1994b; Kolb et al. 1990] Since then, there have been many efforts to harness and maximize the GVL effect in the treatment of hematologic malignancies while minimizing GVHD. We review the use of allogeneic cellular adoptive immunotherapy following HSCT to treat relapsed disease as well as to treat nonrelapse complications such as posttransplantation lymphoproliferative disorder and infections. In order to further enhance this powerful antitumor effect and improve the safety of immunotherapy, novel strategies to define and manipulate both target antigens and effector cells are necessary.
Treatment of relapsed disease
Relapsed disease is the most common cause of death following allogeneic HSCT [Pasquini and Wang, 2010]. Surprisingly, there are limited data regarding the natural history of relapse [Pavletic et al. 2010]. In addition, treatment of relapse has been disappointing for most diseases other than CML [Porter et al. 2011]. The major strategy for treating relapse after allogeneic HSCT has largely been immunotherapy, by withdrawal of immune suppression, or with a second allogeneic transplantation, or with DLI. Withdrawal of immune suppression has been successful in limited case studies [Collins et al. 1992; Higano et al. 1990; Sullivan and Shulman, 1989], although is rarely effective when used alone. Second allogeneic transplantation remains an option for relapse, but is accompanied by significant treatment-related morbidity and mortality, particularly with myeloablative conditioning. In an analysis performed by the Center for International Blood and Marrow Transplant Research (CIBMTR), overall survival following a second allogeneic HSCT for acute or chronic leukemia was dependent on age and time to relapse after the first transplant. Five-year survival was 51% for young patients who relapsed more than 6 months after their first transplant but only 3% for patients over 20 years old who relapsed within 6 months. The cumulative incidence of treatment-related mortality was quite high at 30% [Eapen et al. 2004]. Given these poor options, the use of DLI has become a common therapy for recurrence after allogeneic HSCT, although GVL activity is quite disease dependent.
Disease-specific outcomes using donor leukocyte infusion to treat relapse
Chronic myelogenous leukemia
GVL induction with DLI has been dramatically effective for relapsed CML and can induce sustained long-term molecular remission in most patients who relapse with chronic phase disease [Bacigalupo et al. 1997; Kolb et al. 1990; Pavletic et al.; Porter et al. 1994b; Mackinnon et al. 1995; Van Rhee et al. 1994]. Overall, 76–79% of patients achieve a complete remission (CR) [Collins et al. 1997; Kolb et al. 1995] and most remissions are durable; recurrence rates following DLI-induced remission for chronic phase CML have been approximately 6–9% [Dazzi et al. 2000; Porter et al. 1999].
Treatment of accelerated phase or blast crisis CML with DLI contrasts sharply with that of chronic phase CML, with CR achieved in only 12–28% of patients with accelerated phase or blast crisis CML [Collins et al. 1997; Kolb et al. 1995]. In those patients who do respond, remissions frequently lack durability, with relapse rates greater than 40% [Porter et al. 1999]. Both advanced phase relapse and remission duration following HSCT <9 months were risk factors for poor response [Dazzi et al. 2000].
Tyrosine kinase inhibitors (TKIs) have been given concurrently with DLI for the treatment of relapsed CML in small numbers of patients. Although one retrospective study reports a more rapid achievement of a molecular response with this approach in both chronic and accelerated phase relapses [Savani et al. 2005], other data provide reasons for concern. For instance, TKIs may take CML precursor cells out of the active cell cycle, making them less susceptible to T-cell-mediated cytotoxicity [Jedema et al. 2009]. Furthermore, in vitro, TKIs can inhibit T-cell function and induce apoptosis of activated T cells, activities that could interfere with the cellular immune response and ultimate cure [Seggewiss et al. 2005]. In addition, many patients undergo transplantation only if they are resistant to TKI therapy and therefore it would offer an unclear benefit at the time of relapse. Prospective randomized trials would be necessary to definitively assess the safety and efficacy of concurrent TKIs and DLI. However, these studies will be difficult to perform since so few patients with CML undergo allogeneic HSCT in the modern era.
Acute leukemia and myelodysplasia
Allogeneic HSCT is performed for acute myelogenous leukemia (AML) more than any other disease in North America [Pasquini and Wang, 2010]. Relapse rates remain high, and there is an immediate need for more effective treatment of recurrent disease. Given the limited effective options, GVL induction with DLI is the most common treatment for relapsed AML. Unfortunately, the activity of DLI is disappointing in AML when compared with CML. Large retrospective analyses have reported CR rates to DLI given without chemotherapy of 15–29% [Collins et al. 1997; Kolb et al. 1995]. Patients with a CR, though small in number, can attain durable remissions [Schmid et al. 2007; Porter et al. 1999].
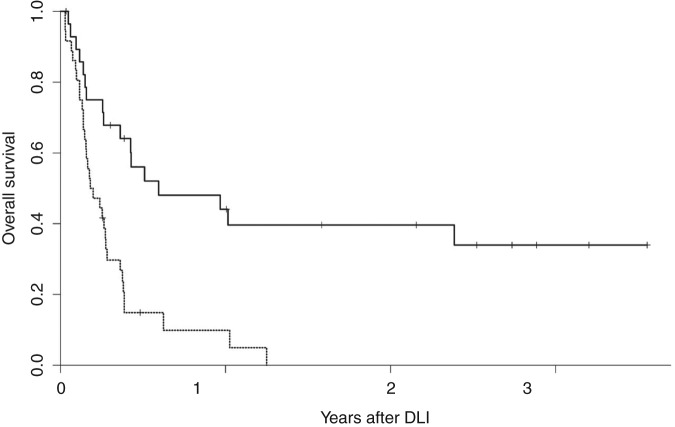
In many cases rapid proliferation of AML and high disease burden cannot be managed with GVL induction alone, which can take weeks to fully develop [Baurmann et al. 1998; Rapanotti et al. 1997]. One strategy to enhance the response rate to DLI in AML is to treat patients with cytoreductive chemotherapy before infusion. The role of pre-DLI chemotherapy was prospectively studied by treating 57 patients, most with relapsed AML following allogeneic HSCT. Induction chemotherapy was followed by DLI mobilized by granulocyte colony-stimulating factor (G-CSF) [Levine et al. 2002]; 27 patients (41%) achieved a CR, and in those who responded, 51% were alive at 1 year and 41% at 2 years. Survival in those who failed to respond to DLI was only 5% overall. In multivariate analysis, time to relapse following HSCT was most predictive of response to DLI (Figure 1). Remission was 3.7 times more likely in patients who relapsed >6 months following transplantation than in those who relapsed within 6 months of transplantation. Similar results were seen in a smaller group of patients with AML treated with chemotherapy followed by G-CSF-mobilized DLI, which confirmed the prognostic value of a remission of >6months following initial HSCT [Choi et al. 2004]. Therefore, for patients with a late relapse following HSCT there is a potent and potentially durable response to DLI plus chemotherapy.
Figure 1.
Overall survival after donor leukocyte infusion for relapsed acute myelogenous leukemia following allogeneic hematopoietic stem-cell transplantation (HSCT). Dashed lines, overall survival following relapse within 6 months of HSCT; solid lines, overall survival following relapse beyond 6 months after HSCT. (Reprinted with permission from Levine et al. [2002]).
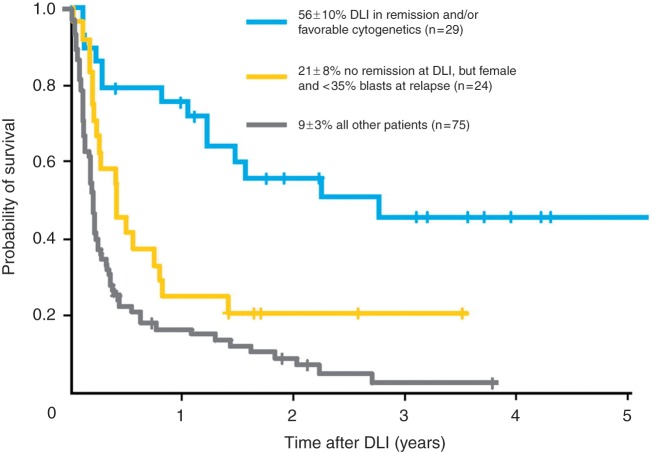
Most studies on relapse therapy for AML have included only small numbers of patients. However, the European Group for Blood and Marrow Transplantation (EBMT) recently retrospectively analyzed the outcomes of nearly 400 patients with AML, comparing patients who did and did not receive DLI for treatment of relapsed disease following allogeneic HSCT [Schmid et al. 2007]. An improved outcome was seen in those patients who had received DLI (21% overall survival at 2 years) compared with those who did not (9% overall survival at 2 years). In multivariate analysis, factors associated with improved survival in DLI recipients were a lower tumor burden at relapse (<35% bone marrow blasts), remission at time of DLI, favorable cytogenetics, and female sex. Comparing patients who were in remission at the time of DLI or those with favorable cytogenetics with patients with aplasia or active disease at the time of DLI revealed a dramatic difference in 2-year overall survival: 56% versus 9–21%, respectively (Figure 2). It is not clear if this survival benefit represents selection of patients most likely to respond to DLI or if reducing the disease burden of leukemic cells improves the success of DLI; however, it does highlight that DLI can be successful in a select group of patients with AML. Manipulations of DLI are needed to improve its efficacy, together with other new treatment strategies, particularly for high-risk patients.
Figure 2.
Overall survival in patients with different risk factors for relapse who received donor leukocyte infusion (DLI). (Reprinted with permission from Schmid et al. [2007]).
Experience with DLI for myelodysplasia (MDS) is curiously limited given the increasing number of transplants for MDS. In several series that included only a small number of patients, response to DLI ranged between 14 and 25% (Table 1) [Campregher et al. 2007; Collins et al. 1997; Depil et al. 2004; Kolb et al. 1995]. Response is likely to vary according to how advanced MDS is at the time of relapse. Many reports combine outcomes of patients with MDS with those of AML, and it is important to note that MDS is a heterogeneous disease, making these data difficult to interpret. Further risk stratification and tailored approaches are necessary for this approach to be broadly applicable in MDS. Furthermore, the availability of new treatments for MDS raises the possibility of combination therapy, such as DLI with a methyltransferase inhibitor [Lubbert et al. 2010].
Table 1.
Response rates to donor leukocyte infusion in myelodysplasia; selected studies.
| Series | Patients (n) | CR rate (n patients) | Duration of response |
|---|---|---|---|
| Kolb et al. [1995] | 4 | 25% (1) | |
| Collins et al. [1997] | 4 | 25% (1) | |
| Depil et al. [2004] | 14 | 14% (2) | Both pts CR, one at 2 yr, the other at 5 yr |
| Campregher et al. [2007] | 16 | 21% (3) | Two remained CR, one at 65 mth, the other at 68 mth; both ultimately died of toxicity |
CR, complete remission; mth, months; yr, years; pts, patients.
Despite the fact that one of the first reports of successful DLI was for acute lymphoblastic leukemia (ALL) [Slavin et al. 2002], relapsed ALL has been notoriously refractory to treatment with DLI [Porter et al. 2011]. In patients with ALL treated with DLI alone 0–18% achieved CR, with median survival of 156 days [Collins et al. 1997; Kolb et al. 1995]. In a retrospective study specific to ALL, 3-year overall survival was 13%. Two of 13 patients treated with DLI alone attained CR, as did 5 of 25 (20%) given DLI in a chemotherapy-induced nadir. Only three patients achieved durable remission, remaining in CR for longer than 1 year [Collins et al. 2000]. A small, 10-patient prospective trial for relapsed ALL used cytoreductive therapy before DLI and also showed limited survival; although seven patients achieved a CR, only one a had durable response, remaining alive and in CR beyond 2 years [Choi et al. 2005].
These findings reveal relapsed ALL to be relatively resistant to DLI, but with a compelling and long-lasting GVL effect in certain patients. Some data suggest that DLI is more effective in children with relapsed ALL [Bader et al. 1999] and when used in the setting of minimal residual disease (MRD) [Pulsipher et al. 2009; Bader et al. 2004]. Combining DLI with TKIs for post-transplantation relapse of Philadelphia chromosome-positive ALL may also be a successful approach in certain patients [Tiribelli et al. 2009]. How to define those patients for whom DLI will be successful remains an important but unanswered question, and developing effective approaches for relapsed ALL needs to be a major research initiative.
Multiple myeloma
There is an important graft-versus-myeloma (GVM) effect associated with allogeneic HSCT [Badros et al. 2001; Bjorkstrand et al. 1996; Bruno et al. 2007]. The GVM effect was first directly demonstrated in a patient with relapsed multiple myeloma after allogeneic HSCT who was brought to a durable CR with DLI [Tricot et al. 1996]. Unfortunately, larger studies examining the use of DLI for relapsed multiple myeloma have shown variable success. Of 27 relapsed patients who were treated with DLI (13 of whom received induction chemotherapy), 8 achieved a partial response (PR) and six a CR, for total response rate of 52%. Unfortunately, only five patients (18%) were still in remission after 30 months [Lokhorst et al. 2000]. One multicenter analysis yielded even less encouraging data, with only 2 of 22 patients with multiple myeloma achieving CR with DLI alone. An additional three patients who received pre-DLI chemotherapy each had CR lasting 1–5 months, although the role of chemotherapy in these responses is unclear [Salama et al. 2000].
When used for relapse after nonmyeloablative allogeneic HSCT, DLI (given with induction chemotherapy in 12 of 63 patients) induced CR in 19% of patients and PR in 19%. Those patients with CR had a progression-free survival of 27.8 months, again suggesting that responses to DLI may be durable at least in a subset of patients [Van de Donk et al. 2006]. Immunomodulatory agents used in the treatment of multiple myeloma, such as thalidomide and lenalidomide, have been combined with DLI in the hope of enhancing its immune effect. These agents are thought to increase T-cell and natural killer (NK)-cell activation [Davies et al. 2001]. In 18 patients treated with thalidomide followed by DLI for progressive or residual disease after allogeneic transplantation, 67% responded, with 22% attaining CR [Kroger et al. 2004]. There is also interest in combining DLI with bortezomib on the basis of data suggesting that proteasome inhibition with bortezomib can provide a direct antitumor effect, inhibit alloreactive T cells, and promote myeloma cell killing [Kroger et al. 2004]. Bortezomib can be given safely after allogeneic HSCT [El-Cheikh et al. 2008]; whether a combination of bortezomib and DLI provides improved activity beyond each individual therapy remains to be determined. However, taken together, these results suggest that DLI can create a meaningful GVM effect in some patients, possibly improved by chemotherapy or immunomodulatory drugs, but toxicity and durability remain important concerns.
Non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and Hodgkin’s lymphoma
Compelling indirect evidence also supports the existence of a graft-versus-lymphoma effect [Ratanatharathorn et al. 1994; Jones et al. 1991], but few studies have examined the role DLI in relapsed lymphoma. Although no responses were seen in initial reports for non-Hodgkin’s lymphoma (NHL) [Collins et al. 1997], the EBMT reported that 6 of 14 patients responded to DLI for relapsed lymphoma [Robinson et al. 2002]. Response rates in other studies, where DLI was often given following T-cell-depleted grafts, vary between 53% and 85% [Porter et al. 2011; Thomson et al. 2010; Bishop et al. 2008; Bloor et al. 2008; Russell et al. 2005, 1996; Mandigers et al. 2003; Marks et al. 2002] (Table 2). Interestingly, in one recent study, 13 of 82 patients relapsed with follicular lymphoma after reduced-intensity conditioned allogeneic HSCT and were treated with DLI; 10 of the 13 patients achieved CR, with nine sustained responses at a median of 44 months, suggesting follicular lymphoma is a potent target for GVL induction [Thomson et al. 2010]. DLI has also been combined with rituximab in a pilot study designed to prevent relapse after reduced-intensity HSCT, and was found to be both safe and effective [Cavattoni et al. 2010]. In the future there may be a role for DLI combined with other monoclonal antibodies with multiple specificities.
Table 2.
Response rates to donor leukocyte infusion in non-Hodgkin’s lymphoma; selected studies.
| Series | Patients (n) | Conditioning for HSCT | Patients with pre-DLI chemotherapy, rituximab or radiation (n) | Response rate (n pts CR /n pts PR) | Duration of response from DLI Median (range) |
|---|---|---|---|---|---|
| Russell et al. [2005] | 17 | T-cell depleted 15 Non-T-cell depleted 2 |
10 | 65% (11 CR/0 PR) | 10 pts CR at 40 mth (12–64) |
| Thomson et al. [2010] | 13 | T-cell depleted 13 | 7 | 77% (10 CR/0 PR) | 10 pts CR at 44 mth (12–74) |
| Bloor et al. [2008] | 17 | T-cell depleted 16 | 7 | 76% (13 CR/0 PR) | 9 pts CR at 26 mth (12–60) |
| Bishop et al. [2008] | 13 | RIC 13 | 4 | 60% (8 CR/1 PR | 6 pts CR at 68 mth (42–83) |
| Marks et al. [2002] | 15 | T-cell depleted 15 | 53% (8 CR/0 PR) | 7 pts CR at 31 mth (16–40) | |
| Mandigers et al. [2003] | 7 | T-cell depleted 7 | 4 | 85% (4 CR/2 PR) | 4 pts CR at 65 mth (43–89) |
CR, complete remission; DLI, donor leukocyte infusion; HSCT, allogeneic hematopoietic stem-cell transplantation; mth, months; PR, partial response; pts, patients; RIC, reduced intensity conditioning.
Compelling evidence also suggests an important GVL effect for chronic lymphocytic leukemia (CLL). For instance, both the long-term disease control that has been attained through nonmyeloablative transplantation [Sorror et al. 2008] and the kinetics of disease response following withdrawal of immunosuppression [Ritgen et al. 2004] demonstrate GVL activity in CLL. However, the number of patients treated with DLI for CLL remains small. Response rates vary widely, from 12% to 90%, as summarized in Table 3 [Bloor et al. 2008; Hoogendoorn et al. 2007; Delgado et al. 2006; Russell et al. 2005; Sorror et al. 2005; Khouri et al. 2004; Ritgen et al. 2004; Schetelig et al. 2003; Marks et al. 2002]. These dramatically different response rates are representative of the small sample sizes, diversity of indications for DLI, disease status at the time of infusion, and the use of chemotherapy or monoclonal antibodies with DLI, but support a meaningful response to cellular immune therapy.
Table 3.
Response rates to donor leukocyte infusion in chronic lymphocytic leukemia; selected studies.
| Series | Patients receiving DLI (n) | Indication for DLI following HSCT | Patients with pre-DLI chemotherapy or rituximab (n) | Response rate (n pts CR /n pts PR) |
|---|---|---|---|---|
| Marks et al. [2002] | 8 | Persistent, progressive, or relapsed disease | 12.5% (0 CR/1 PR) | |
| Ritgen et al. [2004] | 3 | MRD by IgH RQ-PCR | 0 | 67% (2 CR/0 PR) |
| Russell et al. [2005] | 4 | 2 pts with persistent disease 2 pts with relapsed disease |
0 | 75% (3 CR/0 PR) |
| Hoogendoorn et al. [2007] | 12 | 5 pts with persistent disease 7 pts with progressive disease |
33% (4 CR/0 PR) | |
| Sorror et al. [2005] | 6 | Progressive disease | 4 | 17% (1 CR/0 PR) |
| Khouri et al. [2004] | 10 | Persistent disease | 8 | 90% (7 CR/2 PR) |
| Bloor et al. [2008] | 6 | 3 pts with progressive disease 3 pts with mixed chimerism |
1 | 83% (5 CR/0 PR) |
| Schetelig et al. [2003] | 6 | Progressive disease | 0 | 17% (1 CR/0 PR) |
| Delgado et al. [2006] | 11 | Relapsed or progressive disease | 6 | 27% (3 CR/0 PR) |
CR, complete remission; DLI, donor leukocyte infusion; HSCT, allogeneic hematopoietic stem-cell transplantation; IgH RQ-PCR, immunoglobulin heavy chain real-time quantitative polymerase chain reaction; MRD, minimal residual disease; PR, partial response; pts, patients.
Although Hodgkin’s lymphoma is also susceptible to an important allogeneic graft-versus-lymphoma effect [Porter et al. 2003], most reports of DLI for relapsed Hodgkin’s lymphoma following allogeneic HSCT include only small numbers of patients. Response rates to DLI in these patients have ranged from 15% to 75% [Peggs et al. 2009, 2007, 2005; Armand et al. 2008; Anderlini and Champlin, 2006]. Many responses are durable, however, and while DLI for relapsed Hodgkin’s disease requires further study and optimization, it probably offers the only chance and meaningful disease control for relapse after allogeneic HSCT.
Donor leukocyte infusion from unrelated donors
Most studies of DLI after allogeneic HSCT include recipients of both related and unrelated donor cells. There has been concern that outcomes after unrelated donor DLI (U-DLI) may be different from those after DLI from a matched sibling. For instance, given the increased frequency of minor histocompatibility differences, there may be a higher risk of GVHD. However, it is not known if this might translate into enhanced GVL activity. Therefore it is important to consider recipients of U-DLI separately from recipients of matched sibling DLI. In addition, U-DLI is being used with increasing frequency with the increasing use of unrelated donor HSCT. Direct comparisons between sibling DLI and U-DLI are limited given the small number of cases, the frequently short follow-up period, and the use of both related and unrelated donors in many trials. In early retrospective data, outcomes appear similar to those with sibling donors, although the numbers of patients analyzed were small [Collins et al. 1997; Kolb et al. 1995]. One large retrospective analysis described 58 recipients of U-DLI identified by the National Marrow Donor Program. For those being treated with U-DLI for active disease, CR was attained in 46% of patients with CML, 42% of those with AML, and two of the four with ALL. Similar to large series of related DLI, grade II–IV acute GVHD occurred in 25% of treated patients and chronic GVHD occurred in 41% [Porter et al. 2000]. Another report of patients with CML compared related DLI with U-DLI, and found similar rates of cytogenetic remission (73% and 64%, respectively, p = 0.71). Although the rate of chronic GVHD was similar there was a trend towards more grade II–IV acute GVHD after U-DLI (58%) than after related DLI (39%), which did not reach statistical significance (p = 0.09) [Van Rhee et al. 1998].
These results imply that U-DLI is similar to related DLI and is an appropriate therapy in the setting of relapse following unrelated HSCT. In addition, there is no conclusive evidence suggesting higher toxicity or more potent GVL using U-DLI compared with sibling DLI. Therefore currently available evidence suggests that the dose of donor T cells needed to induce GVL should be similar, regardless of the donor source.
Treatment of nonrelapse complications
The administration of donor T cells can not only induce a potent GVL response but may also be useful in restoring cell-mediated immunity, which is critical to treat nonrelapse complications after transplantation. The best example is the use of DLI to treat post-transplantation Epstein–Barr virus (EBV)-associated B-cell lymphoproliferative disorder (PTLD). PTLD occurs in the setting of unrestrained EBV-driven proliferation of B cells in the absence of effective T-cell regulation; it is more common after using T-cell-depleted grafts and is most often donor derived [Loren et al. 2003; Zutter et al. 1988]. PTLD previously carried a dismal prognosis after allogeneic HSCT [Shapiro et al. 1988]. As withdrawal of immune suppression may be effective for PTLD it was logical to assume that restoring cell-mediated immunity with donor lymphocytes would directly treat PTLD. In fact, DLI has been shown to induce durable responses in most patients with PTLD after allogeneic SCT [Heslop et al. 2010; Papadopoulos et al. 1994; Porter et al. 1994a]. Between the high response rates using DLI, and the availability of rituximab, PTLD is no longer the lethal complication previously described.
DLI for PTLD is still associated with significant toxicity from GVHD. Several investigators have generated and expanded EBV-specific T cells as durable prophylaxis and treatment for EBV-related PTLD after allogeneic HSCT [Heslop et al. 2010; Gustafsson et al. 2000; Rooney et al. 1998]. This exciting work demonstrates that virus-specific (and maybe tumor-specific) T cells can be manufactured and be effective with limited GVHD and other toxicity [Melenhorst et al. 2010].
The impaired viral immunity after transplantation can lead to other life-threatening viral infections; respiratory syncytial virus [Kishi et al. 2000] and adenoviral [Leen et al. 2009; Hromas et al. 1994] infections have been successfully treated with DLI. DLI has also been used to transfer cytomegalovirus (CMV) immunity and prevent viral reactivation with CMV-specific T cells cloned from CMV-seropositive marrow donors [Walter et al. 1995; Riddell et al. 1992], and successfully treat CMV infections unresponsive to antiviral therapy [Peggs et al. 2003; Einsele et al. 2002]. These examples demonstrate the diversity of applications for DLI in the transfer of immunity from donor to host, and its ability to provide not only tumor-directed immunity but also immune responses to common infections seen after HSCT [Fujita et al. 2008].
Toxicity of donor leukocyte infusion
Successful DLI for relapse after HSCT continues to be limited by toxicity from GVHD and marrow aplasia. Both acute and chronic GVHD are common complications of DLI, occurring in 40–60% of patients [Porter and Levine, 2006]. Nevertheless, despite the relatively high dose of cells used in DLI, and lack of GVHD prophylaxis in most cases, the rate of severe acute GVHD (grade III–IV) is only 15–35%, with associated treatment-related mortality of 4–15% (reviewed by Frey and Porter [2008]). Chronic GVHD occurs in 30–60% of DLI recipients. Most initial studies found that occurrence of both acute and chronic GVHD was associated with GVL activity, although a small but significant number of patients had no GVHD and still attained CR [Collins et al. 1997; Kolb et al. 1995].
To limit GVHD while maintaining GVL, Mackinnon and colleagues used low doses of cells as initial DLI for relapsed CML, and then escalated doses until a disease response was seen [Mackinnon et al. 1995]. This approach led to very little acute GVHD. The incidence of chronic GVHD remained significant, although it was associated with continued CRs. The relative safety of this dose escalation strategy has been confirmed by additional studies [Dazzi et al. 2000; Guglielmi et al. 2002; Peggs et al. 2009; Thomson et al. 2010] and can be expected to minimize the toxicity at least of acute GVHD for patients with indolent relapses.
Pancytopenia may occur after DLI [Collins et al. 1997; Kolb et al. 1995], sometimes necessitating the need for transfusions or leading to infectious complications from neutropenia. Typically, pancytopenia reverses without therapy, although sustained marrow aplasia can occur in occasional patients, similar to transfusion-associated GVHD (TA-GVHD) [Anderson and Weinstein, 1990]. Lack of residual donor chimerism can predict aplasia following DLI [Chiorean et al. 2004; Keil et al. 1997]. In some cases marrow aplasia is due to the destruction of host hematopoiesis before or without recovery of residual donor hematopoiesis, and may be the reason pancytopenia is noted frequently after DLI for chronic phase CML [Kolb et al. 1995]. Sustained marrow aplasia may be reversed with the infusion of additional donor stem cells in some patients [Drobyski et al. 1993; Porter et al. 1994b]. Use of G-CSF-stimulated peripheral blood mononuclear cells as the source of DLI has limited the duration of neutropenia and may protect against persistent aplasia [Levine et al. 2002]. Although marrow aplasia and GVHD are significant toxicities, they are often acceptable when compared with the limited success of alternative therapies for relapsed disease following allogeneic transplantation.
Emerging strategies to enhance donor leukocyte infusion
Since the initial success of DLI to treat CML, many efforts have been made to improve the activity of DLI, particularly for diseases less susceptible to GVL induction. Numerous strategies to enhance DLI therapy have been aimed at minimizing GVHD without losing the GVL effect; others have attempted to strengthen the GVL effect by manipulating the donor product.
Reducing graft-versus-host disease
In order to facilitate the GVL effect, DLI is typically given without prophylactic immunosuppression. In patients at high risk of GHVD, such those who receive DLI from haploidentical donors, GVHD prophylaxis has been used. In a retrospective analysis GVHD prophylaxis after haploidentical DLI was found to significantly reduce the incidence of grade III–IV acute GVHD without altering complete remission rates [Huang et al. 2007]. Data on GVHD prophylaxis following DLI from human leukocyte antigen (HLA)-matched donors from the same institution has also been retrospectively described. In 48 of 70 patients who received more than 2 weeks of immunosuppression (cyclosporin or weekly methotrexate) following DLI for either relapse or prophylaxis only 7% developed acute GVHD, compared with 50% of those with no or less than 2 weeks of immunosuppression. Rates of chronic GVHD and relapse were not significantly different in this trial [Huang et al. 2009], but the heterogeneous patient population and retrospective analysis make these data difficult to interpret. Immunosuppression with DLI for patients at high risk of severe GVHD may be a reasonable approach to reduce toxicity of this therapy but may attenuate GVL responses [Imado et al. 2004].
An alternative approach to reduce GVHD has been to lower the dose of donor lymphocytes for the initial infusion and then escalate the dose of cells until complete remission is attained. This approach is based on the assumption that there is a T-cell dose at which GVL is possible but GVHD is not clinically apparent, dependent on an unknown and theoretical effector:target-cell ratio. One study using this strategy in patients with CML resulted in complete remissions in 86% of patients, with minimal acute GVHD [Mackinnon et al. 1995]. A subsequent large retrospective analysis confirmed that a lower initial cell dose reduced GVHD and improved survival [Guglielmi et al. 2002]. This approach also showed that lower doses of cells are required to induce remission in patients with a minimal disease burden, supporting the importance of an effector:target ratio [Mackinnon et al. 1995]. Interestingly this also leads to less GVHD, even in patients eventually receiving higher cumulative doses of cells. This strategy may result in delayed GVL activity and hence is best reserved for indolent malignancies, as the proliferation rate of acute leukemia may be too rapid to tolerate lower doses of cells early in the treatment course.
A more specific method to limit GVHD involves infusion of donor lymphocytes genetically modified with a suicide gene. The herpes simplex virus thymidine kinase (HSV-TK) gene may be transfected into donor cells, making them sensitive to ganciclovir. These cells have been active for PTLD and in those patients that developed GVHD ganciclovir dramatically reduced the number of HSV-TK transfected cells and effectively controlled GVHD [Bonini et al. 1997]. A phase 1–2 study of transfected HSV-TK donor cells following haploidentical HSCT led to successful control of GVHD through induction of the suicide gene in all 10 recipients who developed acute GVHD, while allowing successful immune reconstitution in 22 of 28 treated patients [Ciceri et al. 2009]. Unfortunately, immune responses to transfected cells lead to rapid disappearance of the modified cells [Berger et al. 2006]. Although this technology shows promise for many post-HSCT problems, current methods are technically difficult and time intensive, thus limiting the applicability of this approach for now.
Another method to limit GVHD after DLI would be to deplete GVHD-inducing cells. CD8+ T cells have been implicated in GVHD, and depletion of CD8+ cells in the donor transplant grafts has been associated with less GVHD than in those receiving unmodified grafts, while still retaining GVL effects [Nimer et al. 1994]. In one trial of CD8+ depleted DLI given for relapsed hematologic malignancies, 21 of 37 patients had a CR and nearly 50% of the responding patients had no evidence of GVHD [Alyea et al. 1998]. The use of CD8+ T-cell-depleted DLI for early phase CML also induces durable CRs [Shimoni et al. 2001]. Emerging data from mouse models have demonstrated that the naïve subset of CD8+ T cells leads to more significant GVHD, while the effector memory subsets (TEM) of CD8+ and CD4+ T cells maintain the GVL effect without causing GVHD [Chen et al. 2007; Beilhack et al. 2005; Chen et al. 2004; Zhang et al. 2004; Anderson et al. 2003]. Clinical trials using selected donor TEM cells to treat relapse after allogeneic HSCT are now appropriate and the results will be of great interest [Van den Brink et al. 2010].
Regulatory T cells (Treg) may be promising for controlling GVHD while maintaining activity against tumor cells [Edinger et al. 2003]. A recent study in humans has demonstrated that by altering the conditioning regimen of allogeneic HSCT to favor proliferation of regulatory natural killer T cells (NKT), minimal GVHD (acute GVHD 2–10% and chronic GVHD 26–28%) developed while GVL was maintained [Kohrt et al. 2009], in theory related to a sustained Treg population supported by NKT cells [Pillai et al. 2009]. In the setting of relapse after allogeneic HSCT, delivery of increased numbers of Treg is feasible through the use of low-dose interleukin 2 (IL-2) given with DLI. This regimen used in a small number of patients produced increased numbers of Treg compared with patients who received DLI or IL-2 alone. No patient receiving both DLI and IL-2 developed GVHD [Zorn et al. 2009]. Brunstein and colleagues demonstrated the safety of Treg infusions following double cord blood transplants derived from a third umbilical cord blood source in a recent phase 1 trial. A total of 23 patients received 1–30 × 105/kg Treg at day +1 and, in a smaller subset of patients, at day +15. The rate of grade II–IV acute GVHD was 43%, compared with 61% in their historic controls [Brunstein et al. 2011]. Clinical trials administering Treg for patients receiving allogeneic HSCT are ongoing [Van den Brink et al. 2010].
Enhancing the graft-versus-leukemia effect
Many efforts have attempted to enhance the GVL effect of cellular therapies. Isolation and expansion of functional leukemia-reactive T cells may be the most direct way to induce GVL without GVHD. Early studies showed that it is feasible to isolate cytotoxic T cells (CTL) that demonstrate in vitro antileukemic activity and use them successfully for relapsed leukemia [Falkenburg et al. 1999; Marijt et al. 2007].
In most cases, tumor-specific target antigens have not been identified, limiting the ability to generate specific cellular immunotherapy. Potential targets for GVL induction include minor histocompatibility antigens (mHAgs) differentially expressed on hematopoietic cells [Voogt et al. 1988] and leukemia-specific antigens [Bocchia et al. 1996; Clark et al. 2001]. T cells against mismatched mHAgs that demonstrate activity in vitro against leukemic cells have been created [Marijt et al. 2003; Mutis et al. 1999]. In a recent study, patients with relapsed acute leukemia after allogeneic HCST received infusions of CD8+T-cell clones recognizing mHAgs on recipient hematopoietic cells but not recipient dermal fibroblasts [Warren et al. 2010]. Four of six patients achieved a CR, including three patients with disease refractory to chemotherapy. Although these remissions were not durable and were accompanied by unanticipated pulmonary toxicity in some patients, these findings highlight the feasibility and potential power of carefully selected tumor-specific immunotherapy. Continued challenges include the avoidance of off-target toxicity, the emergence of antigen-loss variants and limited persistence of adoptively transferred cells.
In some cases, potential tumor-associated antigens may be normal cellular antigens that are overexpressed. Proteinase 3 (PR3) is overexpressed in myeloid leukemic cells, and vaccination with the PR1 epitope of PR3 has shown considerable antileukemic activity [Molldrem, 2006; Rezvani et al. 2008]. Wilms’ tumor protein (WT1) is overexpressed on myeloid malignancy cells as well [Rosenfeld et al. 2003], and could serve as a potential target for vaccination strategies or the generation and expansion of tumor-specific DLI products [Keilholz et al. 2009]. Additional antigens considered as targets for immunotherapy have included BCR/ABL [Clark et al. 2001], NY-ESO-1, a cancer–testis antigen commonly expressed on myeloma cells [Atanackovic et al. 2007], and PRAME, an HLA-24-restricted antigen on AML cells [Greiner et al. 2006]. Further specification of CTLs to tumor cells is now being studied with genetically engineered cells with chimeric antigen receptors. Cells have been developed that demonstrate potent and persistent antileukemic properties in murine models [Milone et al. 2009], and hold potential for successful durable responses in humans.
Although feasible, the generation of tumor-specific CTL has proven time consuming and often difficult. An alternative strategy to enhance GVL activity is to use nonspecific ex vivo donor T-cell activation and expansion. Donor T cells may not be appropriately activated in vivo to induce an antitumor response, perhaps as a result of Treg, suppressive cytokines, lack of host antigen-presenting cells to present antigen, lack of costimulatory ligands on tumor cells, insufficient numbers of cytotoxic effector cells, or through other poorly defined mechanisms. To overcome many of these obstacles, T cells can be expanded ex vivo through costimulation in a physiologically appropriate manner by exposure to magnetic beads coated with anti-CD3 and anti-CD28 [Liebowitz et al. 1998; Levine et al. 1997]. In a phase 1 trial, conventional DLI was followed by escalating doses of ex vivo costimulated donor T cells (known as activated DLI or aDLI) in 18 patients with relapsed advanced hematologic malignancies after allogeneic SCT [Porter et al. 2006]. Eight of 17 evaluable patients achieved a CR, including two of four patients with AML, four of seven patients with ALL, and each patient with CLL and NHL (mantle-cell lymphoma). Activated DLI did not result in excessive toxicity: five patients developed grade I–II acute GVHD, two developed grade III acute GVHD, and four developed chronic GVHD. No patient died of complications related to GVHD. Overall, the response rates were impressive in diseases that historically have not responded well to unmanipulated DLI, suggesting that aDLI may offer an advantage for GVL induction. Future studies with aDLI will include further dose escalation, repetitive dosing of aDLI to minimize late recurrences, and attempts at activation and expansion of tumor-specific T cells.
It may also be possible to enhance the antitumor effects of cells other than donor T cells. NK cells have demonstrated potent antitumor activity, and a number of trials have demonstrated the safety and feasibility NK donor cell infusions after haploidentical HSCT [Koehl et al. 2005; Passweg et al. 2004]. Mesenchymal stromal cells (MSC) obtained from donor sources are being used successfully to treat steroid-refractory GVHD following HSCT [Le Blanc et al. 2008]. Building on these and other novel cellular strategies to enhance antitumor immune responses and modulate immune complications of transplantation should improve the safety, specificity, and success of cellular therapy following allogeneic HSCT.
Prophylactic donor leukocyte infusion
It is clear that DLI is often more successful if it is given to patients with MRD, suggesting there may be an important effector:target ratio. This suggests that DLI could be useful in the setting of MRD rather than at florid relapse. One major issue is how best to detect MRD and how to determine the clinical significance of early detection with high sensitivity methods [Kroger et al. 2010]. Numerous methods used to monitor MRD include multiparameter flow cytometry [Baer et al. 2001; Rawstron et al. 2007], real-time quantitative PCR for evidence of mixed chimerism or disease-specific markers, cytogenetics, fluorescence in situ hybridization, high-sensitivity chromosome analyses, and imaging studies (CT, PET, MRI, etc.) for tumor recurrence. The sensitivity of detection of course depends on the molecular method used. Furthermore, finding evidence of MRD with different methods may have different implications. In addition, how relapse is defined on the basis of MRD testing will have major implications for patient care, data interpretation, and future clinical trial design. A detailed review of MRD monitoring, the definition of relapse, and the clinical implications is beyond the scope of this review but has recently been reviewed in detail [Kroger et al. 2010]. Further studies to define the preferred method, optimal timing and frequency of MRD assessment, and the risk of relapse based on MRD data are necessary to determine the best use of DLI before hematologic relapse.
In CML, treating patients in early molecular relapse (defined by PCR techniques detecting recurrent BCR/ABL transcripts) with DLI has been extremely successful [Raiola et al. 2003; Mackinnon et al. 1995]. The use of PCR to detect early relapse of other diseases is much more difficult since most diseases do not express unique chimeric genes that can be targeted by PCR analysis. One example of a disease where PCR monitoring for relapse may be most successful is acute promyelocytic leukemia, where detecting recurrence of the RARa/PML transcript often heralds relapse [Gallagher et al. 2003].
Mixed chimerism is an emerging marker for residual disease and impending relapse in CML [Roth et al. 1990] and ALL, serving as a possible trigger for early use of DLI [Pulsipher et al. 2009; Lutz et al. 2008; Bader et al. 2004]. It is less clear if mixed chimerism is useful to predict relapse in AML and other diseases after transplantation.
Because GVHD is significant after DLI [Frey and Porter, 2008], studies of prophylactic DLI must include a careful assessment of GVHD risk. Timing and dose of DLI are critical. Simply limiting immune suppression after transplantation or adding additional donor T cells at the time of HSCT leads to unacceptable GVHD, at least in patients with advanced malignancies. This suggests that attempts at GVL induction very early in the transplantation course will have limited success [Sullivan et al. 1989a]. Furthermore, giving DLI immediately after lymphodepleting (or cytoreductive) chemotherapy has also led to frequent and severe GVHD, perhaps due to the homeostatic proliferation of T cells that occurs in lymphodepleted states [Miller et al. 2007] or to the proximity of the cytokine-abundant phases that occur after chemotherapy [Johnson et al. 1993; Antin and Ferrara, 1992]. In either case, delaying the administration of donor lymphocytes after transplantation (or after chemotherapy) reduces the severity of GVHD in murine models [Johnson et al. 1993; Slavin et al. 1992], suggesting that prophylactic DLI is feasible if appropriately delayed from transplantation.
Prophylactic DLI may be particularly useful in restoring GVL after T-cell-depleted transplants [Ferra et al. 2001]. T-cell depletion may limit GVHD but leads to a loss of GVL and increases the risk of relapse [Horowitz et al. 1990]. Delayed administration of DLI has been tested and confirms the importance of the timing and dose of donor T cells. In 38 patients transplanted with T-cell-depleted grafts (to prevent GVHD), followed by planned DLI, patients who received a higher dose of T cells on day 30 (1 × 107 CD3+ cells/kg) developed significantly more acute grade II or higher GVHD than those patients receiving a lower dose of T cells at day 30 (2 × 106 CD3+ cells/kg) followed by a second dose at day 45 (5 × 107 CD3+ cells/kg). In standard-risk patients there was 73% disease-free survival, similar to that seen in T-cell-replete transplants, indicating that GVL could be restored with DLI [Barrett et al. 1998]. Other studies have used planned DLI following T-cell-depleted transplants for patients with multiple myeloma. In one study, 14 patients were treated with DLI (1–3 × 107 CD4+ cells/kg) at 6–9 months following T-cell-depleted HSCT. Of the 11 patients with residual disease at the time of DLI, six attained a CR, with many responses still durable at a median of 28 months follow up. Unfortunately, GVHD occurred more commonly than expected, with 50% of patients developing either severe acute or chronic GVHD [Alyea et al. 2001].
We recently completed a trial aimed at restoring GVL activity with ex vivo costimulated (anti-CD3/CD28) DLI given as prophylaxis following in vivo T-cell-depleted (using alemtuzumab) allogeneic HSCT. Two planned doses were given at 4 months (1 × 107 CD3+ cells/kg) and 6 months (1 × 108 CD3+ cells/kg) post HSCT, and preliminary results show this was safe, with minimal acute GVHD [Goldstein et al. 2009]. Prophylactic DLI following T-cell-replete HLA-matched transplants has been studied as well. Sohn and colleagues treated 7 of 17 patients with advanced hematologic malignancies with prophylactic DLI 40–120 days following transplantation at 3.0–9.9 × 107 CD3+ cells/kg; 10 additional patients on the trial could not be treated because of death, relapse, or severe GVHD. Four of the seven treated patients remained alive and free of disease at a median of 597 days posttransplantation, although all with chronic GVHD [Sohn et al. 2002]. Together, these results suggest that although further work is needed to clarify the safest dose and timing, planned DLI may prevent relapse in carefully selected high-risk patients.
Conclusion
The GVL effect is critical to the success of allogeneic HSCT for hematologic malignancies. GVL induction with DLI after allogeneic transplantation is commonly used to treat relapsed disease. While effective and reliable in chronic phase CML, other diseases have shown limited responses to DLI. Predictors of response include not only type of disease, but also the extent of disease, pace of progression, and time to relapse among other factors, highlighting the need for a better understanding of disease-specific relapse and the need for earlier and more potent therapies.
Manipulations of immune effector cells to minimize GVHD and promote GVL are necessary to create more effective approaches for relapse. Numerous strategies are being tested to enhance specificity, maximize GVL, and minimize GVHD after adoptive immunotherapy. A better understanding of these effector cells and target antigens for GVL induction is clearly necessary. In addition, there is an immediate need to better study the epidemiology and natural history of relapse [Pavletic et al. 2010]. As most immune therapy is more successful in cases of MRD, better definitions of relapse, and the systematic study of prophylactic DLI may help design strategies to avoid relapse altogether. Progress in the therapeutic development of cellular immunotherapy will require carefully designed controlled trials and likely will require multinational collaborations [Porter et al. 2011]. Above all, a better understanding of factors that both guide and prevent immune response to tumor cells and efficient methods to harness this activity are necessary.
Footnotes
This work was supported in part by grants from the Leukemia & Lymphoma Society (7000-02) and NIH (K24 CA11787901) (DLP).
The authors declare no conflicts of interest in preparing this article.
References
- Alyea E., Weller E., Schlossman R., Canning C., Webb I., Doss D., et al. (2001) T-cell–depleted allogeneic bone marrow transplantation followed by donor lymphocyte infusion in patients with multiple myeloma: induction of graft-versus-myeloma effect. Blood 98: 934–939 [DOI] [PubMed] [Google Scholar]
- Alyea E.P., Soiffer R.J., Canning C., Neuberg D., Schlossman R., Pickett C., et al. (1998) Toxicity and efficacy of defined doses of CD4(+) donor lymphocytes for treatment of relapse after allogeneic bone marrow transplant. Blood 91: 3671–3680 [PubMed] [Google Scholar]
- Anderlini P., Champlin R.E. (2006) Reduced intensity conditioning for allogeneic stem-cell transplantation in relapsed and refractory Hodgkin lymphoma: where do we stand?. Biol Blood Marrow Transplant 12: 599–602 [DOI] [PubMed] [Google Scholar]
- Anderson B.E., McNiff J., Yan J., Doyle H., Mamula M., Shlomchik M.J., et al. (2003) Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest 112: 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K.C., Weinstein H.J. (1990) Transfusion-associated graft-versus-host disease. N Engl J Med 323: 315–321 [DOI] [PubMed] [Google Scholar]
- Antin J.H., Ferrara J.L. (1992) Cytokine dysregulation and acute graft-versus-host disease. Blood 80: 2964–2968 [PubMed] [Google Scholar]
- Armand P., Kim H.T., Ho V.T., Cutler C.S., Koreth J., Antin J.H., et al. (2008) Allogeneic transplantation with reduced-intensity conditioning for Hodgkin and non-Hodgkin lymphoma: Importance of histology for outcome. Biol Blood Marrow Transplant 14: 418–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanackovic D., Arfsten J., Cao Y., Gnjatic S., Schnieders F., Bartels K., et al. (2007) Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood 109: 1103–1112 [DOI] [PubMed] [Google Scholar]
- Bacigalupo A., Soracco M., Vassallo F., Abate M., Van Lint M.T., Gualandi F., et al. (1997) Donor lymphocyte infusions (DLI) in patients with chronic myeloid leukemia following allogeneic bone marrow transplantation. Bone Marrow Transplant 19: 927–932 [DOI] [PubMed] [Google Scholar]
- Bader P., Klingebiel T., Schaudt A., Theurer-Mainka U., Handgretinger R., Lang P., et al. (1999) Prevention of relapse in pediatric patients with acute leukemias and MDS after allogeneic SCT by early immunotherapy initiated on the basis of increasing mixed chimerism: a single center experience of 12 children. Leukemia 13: 2079–2086 [DOI] [PubMed] [Google Scholar]
- Bader P., Kreyenberg H., Hoelle W., Dueckers G., Handgretinger R., Lang P., et al. (2004) Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: Possible role for pre-emptive immunotherapy?. J Clin Oncol 22: 1696–1705 [DOI] [PubMed] [Google Scholar]
- Badros A., Barlogie B., Morris C., Desikan R., Martin S.R., Munshi N., et al. (2001) High response rate in refractory and poor-risk multiple myeloma after allotransplantation using a nonmyeloablative conditioning regimen and donor lymphocyte infusions. Blood 97: 2574–2579 [DOI] [PubMed] [Google Scholar]
- Baer M.R., Stewart C.C., Dodge R.K., Leget G., Sule N., Mrozek K., et al. (2001) High frequency of immunophenotype changes in acute myeloid leukemia at relapse: implications for residual disease detection (Cancer and Leukemia Group B Study 8361). Blood 97: 3574–3580 [DOI] [PubMed] [Google Scholar]
- Barnes D.W., Corp M.J., Loutit J.F., Neal F.E. (1956) Treatment of murine leukaemia with X rays and homologous bone marrow; preliminary communication. Br Med J 2: 626–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A.J., Mavroudis D., Tisdale J., Molldrem J., Clave E., Dunbar C., et al. (1998) T cell-depleted bone marrow transplantation and delayed T cell add-back to control acute GVHD and conserve a graft-versus-leukemia effect. Bone Marrow Transplant 21: 543–551 [DOI] [PubMed] [Google Scholar]
- Baurmann H., Nagel S., Binder T., Neubauer A., Siegert W., Huhn D. (1998) Kinetics of the graft-versus-leukemia response after donor leukocyte infusions for relapsed chronic myeloid leukemia after allogeneic bone marrow transplantation. Blood 92: 3582–3590 [PubMed] [Google Scholar]
- Beilhack A., Schulz S., Baker J., Beilhack G.F., Wieland C.B., Herman E.I., et al. (2005) In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood 106: 1113–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C., Flowers M.E., Warren E.H., Riddell S.R. (2006) Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood 107: 2294–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop M.R., Dean R.M., Steinberg S.M., Odom J., Pavletic S.Z., Chow C., et al. (2008) Clinical evidence of a graft-versus-lymphoma effect against relapsed diffuse large B-cell lymphoma after allogeneic hematopoietic stem-cell transplantation. Ann Oncol 19: 1935–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkstrand B.B., Ljungman P., Svensson H., Hermans J., Alegre A., Apperley J., et al. (1996) Allogeneic bone marrow transplantation versus autologous stem cell transplantation in multiple myeloma: a retrospective case-matched study from the European Group for Blood and Marrow Transplantation. Blood 88: 4711–4718 [PubMed] [Google Scholar]
- Bloor A.J., Thomson K., Chowdhry N., Verfuerth S., Ings S.J., Chakraverty R., et al. (2008) High response rate to donor lymphocyte infusion after allogeneic stem cell transplantation for indolent non-Hodgkin lymphoma. Biol Blood Marrow Transplant 14: 50–58 [DOI] [PubMed] [Google Scholar]
- Bocchia M., Korontsvit T., Xu Q., Mackinnon S., Yang S.Y., Sette A., et al. (1996) Specific human cellular immunity to bcr-abl oncogene-derived peptides. Blood 87: 3587–3592 [PubMed] [Google Scholar]
- Bonini C., Ferrari G., Verzeletti S., Servida P., Zappone E., Ruggieri L., et al. (1997) HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 276: 1719–1724 [DOI] [PubMed] [Google Scholar]
- Bruno B., Rotta M., Patriarca F., Mordini N., Allione B., Carnevale-Schianca F., et al. (2007) A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med 356: 1110–1120 [DOI] [PubMed] [Google Scholar]
- Brunstein C.G., Miller J.S., Cao Q., McKenna D.H., Hippen K.L., Curtsinger J., et al. (2011) Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117: 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campregher P.V., Gooley T., Scott B.L., Moravec C., Sandmaier B., Martin P.J., et al. (2007) Results of donor lymphocyte infusions for relapsed myelodysplastic syndrome after hematopoietic cell transplantation. Bone Marrow Transplant 40: 965–971 [DOI] [PubMed] [Google Scholar]
- Cavattoni I., Zabelina T., Ayuk F., Wolschke C., Bacher U., Zander A., et al. (2010) Pilot study of rituximab plus donor-lymphocyte infusion to prevent or treat relapse in B-cell lymphoma after allogeneic stem cell transplantation. Leuk Lymphoma 51: 146–148 [DOI] [PubMed] [Google Scholar]
- Chen B.J., Cui X., Sempowski G.D., Liu C., Chao N.J. (2004) Transfer of allogeneic CD62L- memory T cells without graft-versus-host disease. Blood 103: 1534–1541 [DOI] [PubMed] [Google Scholar]
- Chen B.J., Deoliveira D., Cui X., Le N.T., Son J., Whitesides J.F., et al. (2007) Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood 109: 3115–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiorean E.G., DeFor T.E., Weisdorf D.J., Blazar B.R., McGlave P.B., Burns L.J., et al. (2004) Donor chimerism does not predict response to donor lymphocyte infusion for relapsed chronic myelogenous leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 10: 171–177 [DOI] [PubMed] [Google Scholar]
- Choi S.J., Lee J.H., Kim S., Lee Y.S., Seol M., Ryu S.G., et al. (2005) Treatment of relapsed acute lymphoblastic leukemia after allogeneic bone marrow transplantation with chemotherapy followed by G-CSF-primed donor leukocyte infusion: a prospective study. Bone Marrow Transplant 36: 163–169 [DOI] [PubMed] [Google Scholar]
- Choi S.J., Lee J.H., Kim S., Seol M., Lee Y.S., Lee J.S., et al. (2004) Treatment of relapsed acute myeloid leukemia after allogeneic bone marrow transplantation with chemotherapy followed by G-CSF-primed donor leukocyte infusion: a high incidence of isolated extramedullary relapse. Leukemia 18: 1789–1797 [DOI] [PubMed] [Google Scholar]
- Ciceri F., Bonini C., Stanghellini M.T., Bondanza A., Traversari C., Salomoni M., et al. (2009) Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol 10: 489–500 [DOI] [PubMed] [Google Scholar]
- Clark R.E., Dodi I.A., Hill S.C., Lill J.R., Aubert G., Macintyre A.R., et al. (2001) Direct evidence that leukemic cells present HLA-associated immunogenic peptides derived from the BCR-ABL b3a2 fusion protein. Blood 98: 2887–2893 [DOI] [PubMed] [Google Scholar]
- Collins R.H., Jr, Goldstein S., Giralt S., Levine J., Porter D., Drobyski W., et al. (2000) Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant 26: 511–516 [DOI] [PubMed] [Google Scholar]
- Collins R.H., Jr, Rogers Z.R., Bennett M., Kumar V., Nikein A., Fay J.W. (1992) Hematologic relapse of chronic myelogenous leukemia following allogeneic bone marrow transplantation: apparent graft-versus-leukemia effect following abrupt discontinuation of immunosuppression. Bone Marrow Transplant 10: 391–395 [PubMed] [Google Scholar]
- Collins R.H., Jr, Shpilberg O., Drobyski W.R., Porter D.L., Giralt S., Champlin R., et al. (1997) Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol 15: 433–444 [DOI] [PubMed] [Google Scholar]
- Davies F.E., Raje N., Hideshima T., Lentzsch S., Young G., Tai Y.T., et al. (2001) Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 98: 210–216 [DOI] [PubMed] [Google Scholar]
- Dazzi F., Szydlo R.M., Cross N.C., Craddock C., Kaeda J., Kanfer E., et al. (2000) Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood 96: 2712–2716 [PubMed] [Google Scholar]
- Delgado J., Thomson K., Russell N., Ewing J., Stewart W., Cook G., et al. (2006) Results of alemtuzumab-based reduced-intensity allogeneic transplantation for chronic lymphocytic leukemia: a British Society of Blood and Marrow Transplantation Study. Blood 107: 1724–1730 [DOI] [PubMed] [Google Scholar]
- Depil S., Deconinck E., Milpied N., Sutton L., Witz F., Jouet J.P., et al. (2004) Donor lymphocyte infusion to treat relapse after allogeneic bone marrow transplantation for myelodysplastic syndrome. Bone Marrow Transplant 33: 531–534 [DOI] [PubMed] [Google Scholar]
- Drobyski W.R., Keever C.A., Roth M.S., Koethe S., Hanson G., McFadden P., et al. (1993) Salvage immunotherapy using donor leukocyte infusions as treatment for relapsed chronic myelogenous leukemia after allogeneic bone marrow transplantation: efficacy and toxicity of a defined T-cell dose. Blood 82: 2310–2318 [PubMed] [Google Scholar]
- Eapen M., Giralt S.A., Horowitz M.M., Klein J.P., Wagner J.E., Zhang M.J., et al. (2004) Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant 34: 721–727 [DOI] [PubMed] [Google Scholar]
- Edinger M., Hoffmann P., Ermann J., Drago K., Fathman C.G., Strober S., et al. (2003) CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 9: 1144–1150 [DOI] [PubMed] [Google Scholar]
- Einsele H., Roosnek E., Rufer N., Sinzger C., Riegler S., Loffler J., et al. (2002) Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 99: 3916–3922 [DOI] [PubMed] [Google Scholar]
- El-Cheikh J., Michallet M., Nagler A., de Lavallade H., Nicolini F.E., Shimoni A., et al. (2008) High response rate and improved graft-versus-host disease following bortezomib as salvage therapy after reduced intensity conditioning allogeneic stem cell transplantation for multiple myeloma. Haematologica 93: 455–458 [DOI] [PubMed] [Google Scholar]
- Falkenburg J.H., Wafelman A.R., Joosten P., Smit W.M., van Bergen C.A., Bongaerts R., et al. (1999) Complete remission of accelerated phase chronic myeloid leukemia by treatment with leukemia-reactive cytotoxic T lymphocytes. Blood 94: 1201–1208 [PubMed] [Google Scholar]
- Ferra C., Rodriguez-Luaces M., Gallardo D., Encuentra M., Martin-Henao G.A., Peris J., et al. (2001) Individually adjusted prophylactic donor lymphocyte infusions after CD34-selected allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant 28: 963–968 [DOI] [PubMed] [Google Scholar]
- Frey N.V., Porter D.L. (2008) Graft-versus-host disease after donor leukocyte infusions: presentation and management. Best Pract Res Clin Haematol 21: 205–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Rooney C.M., Heslop H.E. (2008) Adoptive cellular immunotherapy for viral diseases. Bone Marrow Transplant 41: 193–198 [DOI] [PubMed] [Google Scholar]
- Gallagher R.E., Yeap B.Y., Bi W., Livak K.J., Beaubier N., Rao S., et al. (2003) Quantitative real-time RT-PCR analysis of PML-RAR alpha mRNA in acute promyelocytic leukemia: assessment of prognostic significance in adult patients from intergroup protocol 0129. Blood 101: 2521–2528 [DOI] [PubMed] [Google Scholar]
- Goldstein S.C., Levine B., Smith J., Hinkle J., Luger S., Perl A., et al. (2009) First report of the prophylactic administration of ex vivo co-stimulated donor lymphocyte infusion (DLI) from related and unrelated donors after reduced intensity conditioning for high risk hematologic malignancies. Blood (ASH Annual Meeting Abstracts) 112: 468 [Google Scholar]
- Greiner J., Schmitt M., Li L., Giannopoulos K., Bosch K., Schmitt A., et al. (2006) Expression of tumor-associated antigens in acute myeloid leukemia: Implications for specific immunotherapeutic approaches. Blood 108: 4109–4117 [DOI] [PubMed] [Google Scholar]
- Guglielmi C., Arcese W., Dazzi F., Brand R., Bunjes D., Verdonck L.F., et al. (2002) Donor lymphocyte infusion for relapsed chronic myelogenous leukemia: prognostic relevance of the initial cell dose. Blood 100: 397–405 [DOI] [PubMed] [Google Scholar]
- Gustafsson A., Levitsky V., Zou J.Z., Frisan T., Dalianis T., Ljungman P., et al. (2000) Epstein–Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood 95: 807–814 [PubMed] [Google Scholar]
- Heslop H.E., Slobod K.S., Pule M.A., Hale G.A., Rousseau A., Smith C.A., et al. (2010) Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 115: 925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higano C.S., Brixey M., Bryant E.M., Durnam D.M., Doney K., Sullivan K.M., et al. (1990) Durable complete remission of acute nonlymphocytic leukemia associated with discontinuation of immunosuppression following relapse after allogeneic bone marrow transplantation. A case report of a probable graft-versus-leukemia effect. Transplantation 50: 175–177 [PubMed] [Google Scholar]
- Hoogendoorn M., Jedema I., Barge R.M., van Luxemburg-Heijs S.A., Beaumont F., Marijt E.W., et al. (2007) Characterization of graft-versus-leukemia responses in patients treated for advanced chronic lymphocytic leukemia with donor lymphocyte infusions after in vitro T-cell depleted allogeneic stem cell transplantation following reduced-intensity conditioning. Leukemia 21: 2569–2574 [DOI] [PubMed] [Google Scholar]
- Horowitz M.M., Gale R.P., Sondel P.M., Goldman J.M., Kersey J., Kolb H.J., et al. (1990) Graft-versus-leukemia reactions after bone marrow transplantation. Blood 75: 555–562 [PubMed] [Google Scholar]
- Hromas R., Cornetta K., Srour E., Blanke C., Broun E.R. (1994) Donor leukocyte infusion as therapy of life-threatening adenoviral infections after T-cell-depleted bone marrow transplantation. Blood 84: 1689–1690 [PubMed] [Google Scholar]
- Huang X.J., Liu D.H., Liu K.Y., Xu L.P., Chen H., Han W. (2007) Donor lymphocyte infusion for the treatment of leukemia relapse after HLA-mismatched/haploidentical T-cell-replete hematopoietic stem cell transplantation. Haematologica 92: 414–417 [DOI] [PubMed] [Google Scholar]
- Huang X.J., Wang Y., Liu D.H., Xu L.P., Liu K.Y., Chen H., et al. (2009) Administration of short-term immunosuppressive agents after DLI reduces the incidence of DLI-associated acute GVHD without influencing the GVL effect. Bone Marrow Transplant 44: 309–316 [DOI] [PubMed] [Google Scholar]
- Imado T., Iwasaki T., Kuroiwa T., Sano H., Hara H. (2004) Effect of FK506 on donor T-cell functions that are responsible for graft-versus-host disease and graft-versus-leukemia effect. Transplantation 77: 391–398 [DOI] [PubMed] [Google Scholar]
- Jedema I., van Dreunen L., Willemze R., Falkenburg J.H.F. (2009) Treatment with tyrosine kinase inhibitors may impair the potential curative effect of allogeneic stem cell transplantation. Blood (ASH Annual Meeting Abstracts) 114: 857 [Google Scholar]
- Johnson B.D., Drobyski W.R., Truitt R.L. (1993) Delayed infusion of normal donor cells after MHC-matched bone marrow transplantation provides an antileukemia reaction without graft-versus-host disease. Bone Marrow Transplant 11: 329–336 [PubMed] [Google Scholar]
- Jones R.J., Ambinder R.F., Piantadosi S., Santos G.W. (1991) Evidence of a graft-versus-lymphoma effect associated with allogeneic bone marrow transplantation. Blood 77: 649–653 [PubMed] [Google Scholar]
- Keil F., Haas O.A., Fritsch G., Kalhs P., Lechner K., Mannhalter C., et al. (1997) Donor leukocyte infusion for leukemic relapse after allogeneic marrow transplantation: lack of residual donor hematopoiesis predicts aplasia. Blood 89: 3113–3117 [PubMed] [Google Scholar]
- Keilholz U., Letsch A., Busse A., Asemissen A.M., Bauer S., Blau I.W., et al. (2009) A clinical and immunologic phase 2 trial of Wilms’ tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood 113: 6541–6548 [DOI] [PubMed] [Google Scholar]
- Khouri I.F., Lee M.S., Saliba R.M., Andersson B., Anderlini P., Couriel D., et al. (2004) Nonablative allogeneic stem cell transplantation for chronic lymphocytic leukemia: impact of rituximab on immunomodulation and survival. Exp Hematol 32: 28–35 [DOI] [PubMed] [Google Scholar]
- Kishi Y., Kami M., Oki Y., Kazuyama Y., Kawabata M., Miyakoshi S., et al. (2000) Donor lymphocyte infusion for treatment of life-threatening respiratory syncytial virus infection following bone marrow transplantation. Bone Marrow Transplant 26: 573–576 [DOI] [PubMed] [Google Scholar]
- Koehl U., Esser R., Zimmermann S., Tonn T., Kotchetkov R., Bartling T., et al. (2005) Ex vivo expansion of highly purified NK cells for immunotherapy after haploidentical stem cell transplantation in children. Klin Padiatr 217: 345–350 [DOI] [PubMed] [Google Scholar]
- Kohrt H.E., Turnbull B.B., Heydari K., Shizuru J.A., Laport G.G., Miklos D.B., et al. (2009) TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood 114: 1099–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H.J., Mittermuller J., Clemm C., Holler E., Ledderose G., Brehm G., et al. (1990) Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 76: 2462–2465 [PubMed] [Google Scholar]
- Kolb H.J., Schattenberg A., Goldman J.M., Hertenstein B., Jacobsen N., Arcese W., et al. (1995) Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 86: 2041–2050 [PubMed] [Google Scholar]
- Kroger N., Bacher U., Bader P., Bottcher S., Borowitz M.J., Dreger P., et al. (2010) NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on Disease-Specific Methods and Strategies for Monitoring Relapse following Allogeneic Stem Cell Transplantation. Part I: Methods, acute leukemias, and myelodysplastic syndromes. Biol Blood Marrow Transplant 16: 1187–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger N., Shimoni A., Zagrivnaja M., Ayuk F., Lioznov M., Schieder H., et al. (2004) Low-dose thalidomide and donor lymphocyte infusion as adoptive immunotherapy after allogeneic stem cell transplantation in patients with multiple myeloma. Blood 104: 3361–3363 [DOI] [PubMed] [Google Scholar]
- Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., et al. (2008) Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371: 1579–1586 [DOI] [PubMed] [Google Scholar]
- Leen A.M., Christin A., Myers G.D., Liu H., Cruz C.R., Hanley P.J., et al. (2009) Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein–Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood 114: 4283–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B.L., Bernstein W.B., Connors M., Craighead N., Lindsten T., Thompson C.B., et al. (1997) Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol 159: 5921–5930 [PubMed] [Google Scholar]
- Levine J.E., Braun T., Penza S.L., Beatty P., Cornetta K., Martino R., et al. (2002) Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol 20: 405–412 [DOI] [PubMed] [Google Scholar]
- Liebowitz D.N., Lee K.P., June C.H. (1998) Costimulatory approaches to adoptive immunotherapy. Curr Opin Oncol 10: 533–541 [DOI] [PubMed] [Google Scholar]
- Lokhorst H.M., Schattenberg A., Cornelissen J.J., van Oers M.H., Fibbe W., Russell I., et al. (2000) Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. J Clin Oncol 18: 3031–3037 [DOI] [PubMed] [Google Scholar]
- Loren A.W., Porter D.L., Stadtmauer E.A., Tsai D.E. (2003) Post-transplant lymphoproliferative disorder: a review. Bone Marrow Transplant 31: 145–155 [DOI] [PubMed] [Google Scholar]
- Lubbert M., Bertz H., Wasch R., Marks R., Ruter B., Claus R., et al. (2010) Efficacy of a 3-day, low-dose treatment with 5-azacytidine followed by donor lymphocyte infusions in older patients with acute myeloid leukemia or chronic myelomonocytic leukemia relapsed after allografting. Bone Marrow Transplant 45: 627–632 [DOI] [PubMed] [Google Scholar]
- Lutz C., Massenkeil G., Nagy M., Neuburger S., Tamm I., Rosen O., et al. (2008) A pilot study of prophylactic donor lymphocyte infusions to prevent relapse in adult acute lymphoblastic leukemias after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 41: 805–812 [DOI] [PubMed] [Google Scholar]
- Mackinnon S., Papadopoulos E.B., Carabasi M.H., Reich L., Collins N.H., Boulad F., et al. (1995) Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood 86: 1261–1268 [PubMed] [Google Scholar]
- Mandigers C.M., Verdonck L.F., Meijerink J.P., Dekker A.W., Schattenberg A.V., Raemaekers J.M. (2003) Graft-versus-lymphoma effect of donor lymphocyte infusion in indolent lymphomas relapsed after allogeneic stem cell transplantation. Bone Marrow Transplant 32: 1159–1163 [DOI] [PubMed] [Google Scholar]
- Marijt E., Wafelman A., van der Hoorn M., van Bergen C., Bongaerts R., van Luxemburg-Heijs S., et al. (2007) Phase I/II feasibility study evaluating the generation of leukemia-reactive cytotoxic T lymphocyte lines for treatment of patients with relapsed leukemia after allogeneic stem cell transplantation. Haematologica 92: 72–80 [DOI] [PubMed] [Google Scholar]
- Marijt W.A., Heemskerk M.H., Kloosterboer F.M., Goulmy E., Kester M.G., van der Hoorn M.A., et al. (2003) Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc Natl Acad Sci U S A 100: 2742–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks D.I., Lush R., Cavenagh J., Milligan D.W., Schey S., Parker A., et al. (2002) The toxicity and efficacy of donor lymphocyte infusions given after reduced-intensity conditioning allogeneic stem cell transplantation. Blood 100: 3108–3114 [DOI] [PubMed] [Google Scholar]
- Melenhorst J.J., Leen A.M., Bollard C.M., Quigley M.F., Price D.A., Rooney C.M., et al. (2010) Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood 116: 4700–4702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.S., Weisdorf D.J., Burns L.J., Slungaard A., Wagner J.E., Verneris M.R., et al. (2007) Lymphodepletion followed by donor lymphocyte infusion (DLI) causes significantly more acute graft-versus-host disease than DLI alone. Blood 110: 2761–2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milone M.C., Fish J.D., Carpenito C., Carroll R.G., Binder G.K., Teachey D., et al. (2009) Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 17: 1453–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molldrem J.J. (2006) Vaccination for leukemia. Biol Blood Marrow Transplant 12(Suppl. 1): 13–18 [DOI] [PubMed] [Google Scholar]
- Mutis T., Verdijk R., Schrama E., Esendam B., Brand A., Goulmy E. (1999) Feasibility of immunotherapy of relapsed leukemia with ex vivo-generated cytotoxic T lymphocytes specific for hematopoietic system-restricted minor histocompatibility antigens. Blood 93: 2336–2341 [PubMed] [Google Scholar]
- Nimer S.D., Giorgi J., Gajewski J.L., Ku N., Schiller G.J., Lee K., et al. (1994) Selective depletion of CD8+ cells for prevention of graft-versus-host disease after bone marrow transplantation. A randomized controlled trial. Transplantation 57: 82–87 [DOI] [PubMed] [Google Scholar]
- Papadopoulos E.B., Ladanyi M., Emanuel D., Mackinnon S., Boulad F., Carabasi M.H., et al. (1994) Infusions of donor leukocytes to treat Epstein–Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med 330: 1185–1191 [DOI] [PubMed] [Google Scholar]
- Pasquini M.C., Wang Z. (2010) Current use and outcome of hematopoietic stem cell transplantation. CIBMTR Summary Slides. Available at: http://www.cibmtr.org
- Passweg J.R., Tichelli A., Meyer-Monard S., Heim D., Stern M., Kuhne T., et al. (2004) Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia 18: 1835–1838 [DOI] [PubMed] [Google Scholar]
- Pavletic S.Z., Kumar S., Mohty M., de Lima M., Foran J.M., Pasquini M., et al. (2010) NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on the Epidemiology and Natural History of Relapse following Allogeneic Cell Transplantation. Biol Blood Marrow Transplant 16: 871–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peggs K.S., Hunter A., Chopra R., Parker A., Mahendra P., Milligan D., et al. (2005) Clinical evidence of a graft-versus-Hodgkin’s-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet 365: 1934–1941 [DOI] [PubMed] [Google Scholar]
- Peggs K.S., Sureda A., Qian W., Caballero D., Hunter A., Urbano-Ispizua A., et al. (2007) Reduced-intensity conditioning for allogeneic haematopoietic stem cell transplantation in relapsed and refractory Hodgkin lymphoma: impact of alemtuzumab and donor lymphocyte infusions on long-term outcomes. Br J Haematol 139: 70–80 [DOI] [PubMed] [Google Scholar]
- Peggs K.S., Thomson K., Morris E., Chowdhry N., Verfurth S., Kottaridis P., et al. (2009) Donor lymphocyte infusions induce high response rates and durable salvage in relapsed Hodgkin lymphoma post T cell depleted allogeneic transplantation. Blood (ASH Annual Meeting Abstracts) 114: 200 [Google Scholar]
- Peggs K.S., Verfuerth S., Pizzey A., Khan N., Guiver M., Moss P.A., et al. (2003) Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet 362: 1375–1377 [DOI] [PubMed] [Google Scholar]
- Pillai A.B., George T.I., Dutt S., Strober S. (2009) Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+Foxp3+ T regulatory cells that protects against graft-versus-host disease. Blood 113: 4458–4467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D., Levine J.E. (2006) Graft-versus-host disease and graft-versus-leukemia after donor leukocyte infusion. Semin Hematol 43: 53–61 [DOI] [PubMed] [Google Scholar]
- Porter D.L., Alyea E.P., Antin J.H., DeLima M., Estey E., Falkenburg J.H., et al. (2011) NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 16: 1467–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D.L., Collins R.H., Jr, Hardy C., Kernan N.A., Drobyski W.R., Giralt S., et al. (2000) Treatment of relapsed leukemia after unrelated donor marrow transplantation with unrelated donor leukocyte infusions. Blood 95: 1214–1221 [PubMed] [Google Scholar]
- Porter D.L., Collins R.H., Jr, Shpilberg O., Drobyski W.R., Connors J.M., Sproles A., et al. (1999) Long-term follow-up of patients who achieved complete remission after donor leukocyte infusions. Biol Blood Marrow Transplant 5: 253–261 [DOI] [PubMed] [Google Scholar]
- Porter D.L., Levine B.L., Bunin N., Stadtmauer E.A., Luger S.M., Goldstein S., et al. (2006) A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood 107: 1325–1331 [DOI] [PubMed] [Google Scholar]
- Porter D.L., Orloff G.J., Antin J.H. (1994a) Donor mononuclear cell infusions as therapy for B-cell lymphoproliferative disorder following allogeneic bone marrow transplant. Transplant Sci 4: 12–14, discussion 14–16 [PubMed] [Google Scholar]
- Porter D.L., Roth M.S., McGarigle C., Ferrara J.L., Antin J.H. (1994b) Induction of graft-versus-host disease as immunotherapy for relapsed chronic myeloid leukemia. N Engl J Med 330: 100–106 [DOI] [PubMed] [Google Scholar]
- Porter D.L., Stadtmauer E.A., Lazarus H.M. (2003) ‘GVHD’: Graft-versus-host disease or graft-versus-Hodgkin’s disease? An old acronym with new meaning. Bone Marrow Transplant 31: 739–746 [DOI] [PubMed] [Google Scholar]
- Pulsipher M.A., Bader P., Klingebiel T., Cooper L.J. (2009) Allogeneic transplantation for pediatric acute lymphoblastic leukemia: the emerging role of peritransplantation minimal residual disease/chimerism monitoring and novel chemotherapeutic, molecular, and immune approaches aimed at preventing relapse. Biol Blood Marrow Transplant 15(Suppl.): 62–71 [DOI] [PubMed] [Google Scholar]
- Raiola A.M., Van Lint M.T., Valbonesi M., Lamparelli T., Gualandi F., Occhini D., et al. (2003) Factors predicting response and graft-versus-host disease after donor lymphocyte infusions: a study on 593 infusions. Bone Marrow Transplant 31: 687–693 [DOI] [PubMed] [Google Scholar]
- Rapanotti M.C., Arcese W., Buffolino S., Iori A.P., Mengarelli A., De Cuia M.R., et al. (1997) Sequential molecular monitoring of chimerism in chronic myeloid leukemia patients receiving donor lymphocyte transfusion for relapse after bone marrow transplantation. Bone Marrow Transplant 19: 703–707 [DOI] [PubMed] [Google Scholar]
- Ratanatharathorn V., Uberti J., Karanes C., Abella E., Lum L.G., Momin F., et al. (1994) Prospective comparative trial of autologous versus allogeneic bone marrow transplantation in patients with non-Hodgkin’s lymphoma. Blood 84: 1050–1055 [PubMed] [Google Scholar]
- Rawstron A.C., Villamor N., Ritgen M., Bottcher S., Ghia P., Zehnder J.L., et al. (2007) International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia 21: 956–964 [DOI] [PubMed] [Google Scholar]
- Rezvani K., Yong A.S., Mielke S., Savani B.N., Musse L., Superata J., et al. (2008) Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood 111: 236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell S.R., Watanabe K.S., Goodrich J.M., Li C.R., Agha M.E., Greenberg P.D. (1992) Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science 257: 238–241 [DOI] [PubMed] [Google Scholar]
- Ritgen M., Stilgenbauer S., von Neuhoff N., Humpe A., Bruggemann M., Pott C., et al. (2004) Graft-versus-leukemia activity may overcome therapeutic resistance of chronic lymphocytic leukemia with unmutated immunoglobulin variable heavy-chain gene status: implications of minimal residual disease measurement with quantitative PCR. Blood 104: 2600–2602 [DOI] [PubMed] [Google Scholar]
- Robinson S.P., Goldstone A.H., Mackinnon S., Carella A., Russell N., de Elvira C.R., et al. (2002) Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood 100: 4310–4316 [DOI] [PubMed] [Google Scholar]
- Rooney C.M., Smith C.A., Ng C.Y., Loftin S.K., Sixbey J.W., Gan Y., et al. (1998) Infusion of cytotoxic T cells for the prevention and treatment of Epstein–Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 92: 1549–1555 [PubMed] [Google Scholar]
- Rosenfeld C., Cheever M.A., Gaiger A. (2003) WT1 in acute leukemia, chronic myelogenous leukemia and myelodysplastic syndrome: therapeutic potential of WT1 targeted therapies. Leukemia 17: 1301–1312 [DOI] [PubMed] [Google Scholar]
- Roth M.S., Antin J.H., Bingham E.L., Ginsburg D. (1990) Use of polymerase chain reaction-detected sequence polymorphisms to document engraftment following allogeneic bone marrow transplantation. Transplantation 49: 714–720 [DOI] [PubMed] [Google Scholar]
- Russell L.A., Jacobsen N., Heilmann C., Simonsen A.C., Christensen L.D., Vindelov L.L. (1996) Treatment of relapse after allogeneic BMT with donor leukocyte infusions in 16 patients. Bone Marrow Transplant 18: 411–414 [PubMed] [Google Scholar]
- Russell N.H., Byrne J.L., Faulkner R.D., Gilyead M., Das-Gupta E.P., Haynes A.P. (2005) Donor lymphocyte infusions can result in sustained remissions in patients with residual or relapsed lymphoid malignancy following allogeneic haemopoietic stem cell transplantation. Bone Marrow Transplant 36: 437–441 [DOI] [PubMed] [Google Scholar]
- Salama M., Nevill T., Marcellus D., Parker P., Johnson M., Kirk A., et al. (2000) Donor leukocyte infusions for multiple myeloma. Bone Marrow Transplant 26: 1179–1184 [DOI] [PubMed] [Google Scholar]
- Savani B.N., Montero A., Kurlander R., Childs R., Hensel N., Barrett A.J. (2005) Imatinib synergizes with donor lymphocyte infusions to achieve rapid molecular remission of CML relapsing after allogeneic stem cell transplantation. Bone Marrow Transplant 36: 1009–1015 [DOI] [PubMed] [Google Scholar]
- Schetelig J., Thiede C., Bornhauser M., Schwerdtfeger R., Kiehl M., Beyer J., et al. (2003) Evidence of a graft-versus-leukemia effect in chronic lymphocytic leukemia after reduced-intensity conditioning and allogeneic stem-cell transplantation: the Cooperative German Transplant Study Group. J Clin Oncol 21: 2747–2753 [DOI] [PubMed] [Google Scholar]
- Schmid C., Labopin M., Nagler A., Bornhauser M., Finke J., Fassas A., et al. (2007) Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol 25: 4938–4945 [DOI] [PubMed] [Google Scholar]
- Seggewiss R., Lore K., Greiner E., Magnusson M.K., Price D.A., Douek D.C., et al. (2005) Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood 105: 2473–2479 [DOI] [PubMed] [Google Scholar]
- Shapiro R.S., McClain K., Frizzera G., Gajl-Peczalska K.J., Kersey J.H., Blazar B.R., et al. (1988) Epstein–Barr virus associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood 71: 1234–1243 [PubMed] [Google Scholar]
- Shimoni A., Gajewski J.A., Donato M., Martin T., O’Brien S., Talpaz M., et al. (2001) Long-term follow-up of recipients of CD8-depleted donor lymphocyte infusions for the treatment of chronic myelogenous leukemia relapsing after allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant 7: 568–575 [DOI] [PubMed] [Google Scholar]
- Slavin S., Ackerstein A., Weiss L., Nagler A., Or R., Naparstek E. (1992) Immunotherapy of minimal residual disease by immunocompetent lymphocytes and their activation by cytokines. Cancer Invest 10: 221–227 [DOI] [PubMed] [Google Scholar]
- Slavin S., Morecki S., Weiss L., Or R. (2002) Donor lymphocyte infusion: the use of alloreactive and tumor-reactive lymphocytes for immunotherapy of malignant and nonmalignant diseases in conjunction with allogeneic stem cell transplantation. J Hematother Stem Cell Res 11: 265–276 [DOI] [PubMed] [Google Scholar]
- Sohn S.K., Jung J.T., Kim D.H., Lee N.Y., Seo K.W., Chae Y.S., et al. (2002) Prophylactic growth factor-primed donor lymphocyte infusion using cells reserved at the time of transplantation after allogeneic peripheral blood stem cell transplantation in patients with high-risk hematologic malignancies. Cancer 94: 18–24 [DOI] [PubMed] [Google Scholar]
- Sorror M.L., Maris M.B., Sandmaier B.M., Storer B.E., Stuart M.J., Hegenbart U., et al. (2005) Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. J Clin Oncol 23: 3819–3829 [DOI] [PubMed] [Google Scholar]
- Sorror M.L., Storer B.E., Sandmaier B.M., Maris M., Shizuru J., Maziarz R., et al. (2008) Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol 26: 4912–4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K.M., Shulman H.M. (1989) Chronic graft-versus-host disease, obliterative bronchiolitis, and graft-versus-leukemia effect: case histories. Transplant Proc 21(Suppl. 1): 51–62 [PubMed] [Google Scholar]
- Sullivan K.M., Storb R., Buckner C.D., Fefer A., Fisher L., Weiden P.L., et al. (1989a) Graft-versus-host disease as adoptive immunotherapy in patients with advanced hematologic neoplasms. N Engl J Med 320: 828–834 [DOI] [PubMed] [Google Scholar]
- Sullivan K.M., Weiden P.L., Storb R., Witherspoon R.P., Fefer A., Fisher L., et al. (1989b) Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood 73: 1720–1728 [PubMed] [Google Scholar]
- Sun K., Welniak L.A., Panoskaltsis-Mortari A., O’Shaughnessy M.J., Liu H., Barao I., et al. (2004) Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc Natl Acad Sci U S A 101: 8120–8125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson K.J., Morris E.C., Milligan D., Parker A.N., Hunter A.E., Cook G., et al. (2010) T-cell-depleted reduced-intensity transplantation followed by donor leukocyte infusions to promote graft-versus-lymphoma activity results in excellent long-term survival in patients with multiply relapsed follicular lymphoma. J Clin Oncol 28: 3695–3700 [DOI] [PubMed] [Google Scholar]
- Tiribelli M., Sperotto A., Candoni A., Simeone E., Buttignol S., Fanin R. (2009) Nilotinib and donor lymphocyte infusion in the treatment of Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) relapsing after allogeneic stem cell transplantation and resistant to imatinib. Leuk Res 33: 174–177 [DOI] [PubMed] [Google Scholar]
- Tricot G., Vesole D.H., Jagannath S., Hilton J., Munshi N., Barlogie B. (1996) Graft-versus-myeloma effect: Proof of principle. Blood 87: 1196–1198 [PubMed] [Google Scholar]
- van de Donk N.W., Kroger N., Hegenbart U., Corradini P., San Miguel J.F., Goldschmidt H., et al. (2006) Prognostic factors for donor lymphocyte infusions following non-myeloablative allogeneic stem cell transplantation in multiple myeloma. Bone Marrow Transplant 37: 1135–1141 [DOI] [PubMed] [Google Scholar]
- van den Brink M.R., Porter D.L., Giralt S., Lu S.X., Jenq R.R., Hanash A., et al. (2010) Relapse after allogeneic hematopoietic cell therapy. Biol Blood Marrow Transplant 16(Suppl.): S138–S145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rhee F., Lin F., Cullis J.O., Spencer A., Cross N.C., Chase A., et al. (1994) Relapse of chronic myeloid leukemia after allogeneic bone marrow transplant: the case for giving donor leukocyte transfusions before the onset of hematologic relapse. Blood 83: 3377–3383 [PubMed] [Google Scholar]
- van Rhee F., Savage D., Blackwell J., Orchard K., Dazzi F., Lin F., et al. (1998) Adoptive immunotherapy for relapse of chronic myeloid leukemia after allogeneic bone marrow transplant: equal efficacy of lymphocytes from sibling and matched unrelated donors. Bone Marrow Transplant 21: 1055–1061 [DOI] [PubMed] [Google Scholar]
- Voogt P.J., Goulmy E., Veenhof W.F., Hamilton M., Fibbe W.E., Van Rood J.J., et al. (1988) Cellularly defined minor histocompatibility antigens are differentially expressed on human hematopoietic progenitor cells. J Exp Med 168: 2337–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter E.A., Greenberg P.D., Gilbert M.J., Finch R.J., Watanabe K.S., Thomas E.D., et al. (1995) Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med 333: 1038–1044 [DOI] [PubMed] [Google Scholar]
- Warren E.H., Fujii N., Akatsuka Y., Chaney C.N., Mito J.K., Loeb K.R., et al. (2010) Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood 115: 3869–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiden P.L., Sullivan K.M., Flournoy N., Storb R., Thomas E.D. (1981) Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med 304: 1529–1533 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Joe G., Zhu J., Carroll R., Levine B., Hexner E., et al. (2004) Dendritic cell-activated CD44hiCD8+ T cells are defective in mediating acute graft-versus-host disease but retain graft-versus-leukemia activity. Blood 103: 3970–3978 [DOI] [PubMed] [Google Scholar]
- Zorn E., Mohseni M., Kim H., Porcheray F., Lynch A., Bellucci R., et al. (2009) Combined CD4+ donor lymphocyte infusion and low-dose recombinant IL-2 expand FOXP3+ regulatory T cells following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 15: 382–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zutter M.M., Martin P.J., Sale G.E., Shulman H.M., Fisher L., Thomas E.D., et al. (1988) Epstein–Barr virus lymphoproliferation after bone marrow transplantation. Blood 72: 520–529 [PubMed] [Google Scholar]