Abstract
Major advances in myeloproliferative neoplasms in the last decade have cast light on their complexity. The identification of JAK2V617F briefly promised a unifying mechanism of pathogenesis with a single pathway that could be efficiently targeted. Instead, there have been major advances in understanding acquired and background genetic and epigenetic contributors to this group of disorders, with refined risk prediction models and experimental therapeutics that have provided a more nuanced model of disease. In aggregate these observations likely explain the heterogeneity of these disorders and their generally unpredictable response to therapy. Molecular studies, beginning with the identification of JAK2V617F, have led to a concept of MPN subtypes existing on a continuum, and additional discoveries such as TET2 and EZH2 mutations have provided the molecular underpinnings to begin to explain overlapping phenotypes in myeloid malignancies more generally. In many ways the pace of molecular discovery is outstripping our ability to integrate these observations into clinical care, both in terms of molecular diagnostics and medical decision making. This review will attempt to summarize, within a clinical context, our evolving understanding of myeloproliferative neoplasms. It focuses on biology, histopathology, prognostic scoring systems, stem cell transplantation as well as selected clinical/preclinical therapeutic observations.
Keywords: essential thrombocytosis, hematopoietic stem-cell transplantation, interferon, JAK2 V617F, myelofibrosis, myeloproliferative neoplasms, polycythemia vera, TET2
‘I think the next century will be the century of complexity.’
(Stephen Hawking, January 2000)
Introduction
In 1951, when William Dameshek proposed that chronic myelogenous leukemia (CML), polycythemia vera (PV), essential thrombocytosis (ET) and myelofibrosis (MF) were related disorders, ‘variable manifestations of proliferative activity of bone marrow cells perhaps due to a hitherto undiscovered stimulus’, the concept was, for some, considered radical. That MF patients receiving blood transfusions to correct cytopenias might be suffering from the same underlying aberration as PV patients dependent on blood letting? That myeloproliferative and myelodepletive afflictions were merely variations on a common theme? This was understandably a fanciful proposition [Dameshek, 1951].
A decade later, Dr Nowell and Dr Hungerford identified a one-to-one relationship between the Philadelphia chromosome and CML, and the subsequent characterization of the BCR-ABL fusion protein supported the concept that dysregulated tyrosine kinase activity might be pathogenic, that Dr Dameshek’s, ‘hitherto undiscovered stimulus’, may have finally been discovered, at least for CML [Nowell and Hungerford, 1960]. Indeed, tyrosine kinase dysregulation is today accepted as the single unifying feature of myeloproliferative neoplasms (MPN), a family of biologically and clinically related disorders now expanded to encompass diverse and relatively rare disorders including systemic mast cell disorders and chronic eosinophilic leukemia, both of which have well-characterized tyrosine kinase activation pathways [Swerdlow et al. 2008].
The specifics of the tyrosine kinase activation pathways in MF, PV and ET (the Philadelphia chromosome negative or ‘classical’ MPN), however, remained mysterious for decades. Then, in 2005, four groups identified a somatic activating point mutation in JAK2 in the majority of patients with these disorders [Baxter et al. 2005; James et al. 2005; Kralovics et al. 2005; Levine et al. 2005]. JAK2 is a nonreceptor tyrosine kinase that associates with cytokine receptors and signals canonically through STAT3 and STAT5, cytoplasmic effector proteins that can dimerize and translocate to the nucleus. More than 95% of patients with PV and approximately 60% of patients with ET and MF were found to have a G to T substitution in exon 14 of JAK2, leading to a valine to a phenylalanine change at position 617 (JAK2V617F). The mutation is in the pseudokinase domain of JAK2, which is thought to deregulate its autoinhibitory activity, although the exact mechanism by which it leads to constitutive activation remains incompletely understood. However, while targeting BCR-ABL has radically changed the natural history and treatment paradigms for CML, targeted therapies based on the discovery of JAK2V617F have had less impressive results. In addition to the pharmacology and target itself, this is most likely a reflection of the clinical heterogeneity and the biologic complexity of MPN associated with JAK2V617F.
This review is an attempt to tackle some of those complexities and their clinical (particularly their therapeutic) implications, focusing in large part on the entity of MF.
Diagnostic and histopathologic considerations
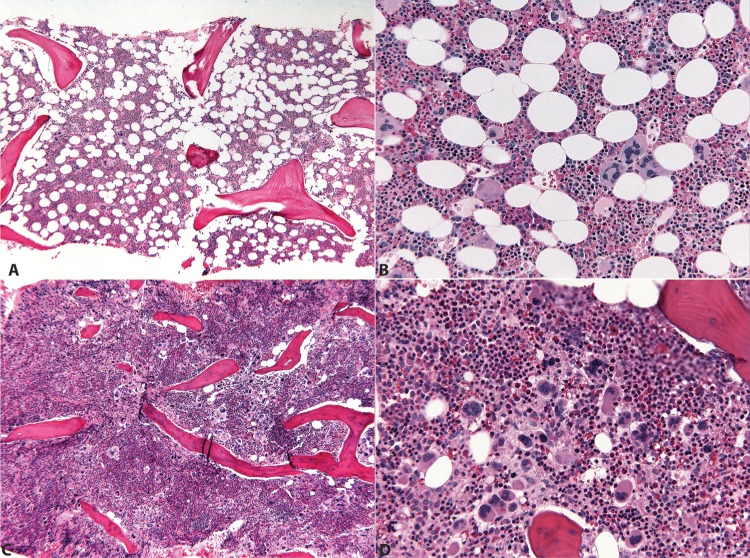
The sine qua non of MF, whether evolved from PV/ET or primary myelofibrosis (PMF), is fibrous disruption of the marrow space, typically identified by a reticulin stain, and in more advanced states of collagen fibrosis, a trichrome stain. Cytokines elaborated by the malignant clone lead to reactive stromal hyperplasia, and can also cause severe constitutional symptoms in afflicted patients. Less commonly, an early manifestation of PMF, termed prefibrotic MF, lacks marked fibrosis. Prefibrotic MF often presents with an isolated thrombocytosis, and therefore can be difficult to distinguish clinically and histologically from ET (Figure 1). In fact, making this distinction often lacks immediate clinical consequences but does have prognostic significance. True ET has a low likelihood of progression to post-ET MF, while prefibrotic MF is considered the MF prodrome. Prefibrotic MF is distinguished from ET by a constellation of bone marrow morphologic features: an ET bone marrow should be normocellular or only slightly hypercellular for age, while prefibrotic MF is typically hypercellular with expanded left-shifted granulopoiesis and decreased erythropoiesis. Perhaps the most important, and controversial, distinguishing features between these two entities are the morphology and geographic distribution of the aberrant cells held responsible for these disorders: the megakaryocytes. The megakaryocytes of ET are frequently large or giant with hyperlobated ‘staghorn’ nuclei, while those of prefibrotic MF are more variably sized (often small) and cytologically bizarre, with maturation defects and hypolobated ‘cloudlike’ nuclei. In ET, megakaryocytes are scattered singly and in small clusters throughout the marrow, while in prefibrotic MF megakaryocytes are packed densely into large aggregates and found in close proximity to the endosteum and vascular sinuses. Whether these morphologic distinctions, incorporated into the most recent (2008) World Health Organization diagnostic criteria, are truly reproducible and prognostically significant has been a matter of some debate [Spivak and Silver, 2008]. However a recent systematic re-evaluation suggests reasonable concordance and true prognostic utility for the new criteria [Thiele et al. 2011].
Figure 1.
Morphologic findings in essential thrombocytosis (ET) and myelofibrosis (MF).
(A), (B) A 39-year-old woman with JAK2V617F+ ET. Mildly hypocellular (50%) marrow (5× H&E) with balanced trilineage hematopoiesis featuring singly scattered and focally clustered normal sized and large megakaryocytes with widely spaced (‘staghorn’) nuclei (20× H&E). (C), (D) A 55-year-old woman with JAK2V617F+ MF. Markedly hypercellular (95% cellularity) marrow (5× H&E) with granulocytic hyperplasia and prominent aggregates of smaller cytologically atypical megakaryocytes with hypolobated ‘cloudlike’ nuclei (20× H&E).
Clinically, MF can be associated with either increased or decreased white blood cell and platelet counts, but is typically associated with anemia and splenomegaly in symptomatic patients. It is also important to note that, like PV and ET, these patients have a propensity towards clotting [Barbui et al. 2010]. While this is historically well recognized in PV and ET, it was only recently established for MF, with age (over 60) and possibly JAK2V617F and/or elevated white blood cell count contributing to thrombotic risk. Unlike for PV and ET, primary and secondary prevention strategies/recommendations are not well established for MF [Barbui et al. 2011]. Vexing issues of portal hypertension, cytopenias and bleeding frequently complicate anticoagulation, cytoreductive and antiplatelet therapy in MF patients.
Prognostic scoring systems in MF: how precise can we be?
While the prognosis can be poor in MF, it is variable, ranging from a median survival of 1 to more than 15 years, depending on a variety of factors. Thus a more precise prognostic system is critical for medical decision making, particularly when aggressive treatments such as stem-cell transplantation are being considered. A short review of the evolution of prognostic scoring systems for MF illustrates the evolution of our understanding of prognostic factors. The first widely used systematic stratification of risk groups among MF patients, the Lille Score, was put forth in 1996 [Dupriez et al. 1996]. This initial system considered two factors, hemoglobin level and leukocyte count, touting a ‘simple prognostic model for survival’. Its simplicity was attractive, and indeed anemia remains one of the most predictive factors determining survival. The Lille classification system grouped patients into three risk brackets, low, intermediate and high, observing associated median survivals of 93, 26 and 13 months, respectively. But the power of the Lille system to accurately discriminate between those patients with ‘intermediate’ and ‘high’ risk levels was quickly called into question. In 1997, Cervantes and colleagues demonstrated that when applied to a new patient group the Lille scoring system could not in fact distinguish between intermediate- and high-risk patients, and proposed their own new system, adding one new prognostic element, constitutional symptoms, and replacing white blood cell count with peripheral blood blast count [Cervantes et al. 1997]. The original Cervantes system classified patients simply into low- and high-risk brackets, and while it held up over time, its weakness turned out to be not in its capacity to accurately fulfill its prognostic aims, but in distinguishing between shades of risk. Defining and refining intermediate- and high-risk groups is exceptionally useful for tailoring treatment options.
Thus, at the request of the International Working Group for MPN Research and Treatment (IWG-MRT), Cervantes and colleagues developed an entirely new prognostic system (International Prognostic Scoring System [IPSS]) using five prognostic factors: hemoglobin (<100 g/l), leukocyte count (>25 × 103/l), constitutional symptoms, circulating blasts (>1%) and, additionally, age (>65 years). This hybrid of Lille and the previous Cervantes system, with the addition of age, proved capable of accurately establishing median survival for four clearly distinct risk groups among MF patients: low (135 months), intermediate-1 (95 months), intermediate-2 (48 months) and high (27 months) [Cervantes et al. 2009]. In 2010, the IPSS system evolved further with the development of dynamic IPSS (DIPSS), which considers the same five factors as time-dependent covariates, and allows assessment of MF patients at any time during their clinical course, not merely at initial diagnosis. The DIPSS also established that risk of leukemic transformation increases with increasing risk categories [Passamonti et al. 2010]. Clinically, establishing validity over time, either for reassuring patients who are stable or for changing treatment if the prognostic category changes, can be a useful tool.
Prognostic implications for genetic events in myelofibrosis
Cytogenetics
The studies that produced the IPSS suggested that cytogenetic abnormalities contributed independently to prognosis for patients in intermediate-risk groups, although they did not formally incorporate cytogenetic criteria into the IPSS. More recently, detailed mapping of commonly affected genomic regions (on chromosomes 3, 7 and others) has identified single target genes and correlated lesions with disease progression [Klampfl et al. 2011]. Likewise, a tumor suppressor gene (L3MBTL1) found on chromosome 20, one of the most commonly deleted regions in PV specifically and MPN generally, was recently shown to be important in erythroid fate decisions, which suggested that haploinsufficiency could promote erythroid differentiation [Perna et al. 2010]. Clinically, the most recent prognostic refinement, DIPSS Plus, incorporates cytogenetic abnormalities, thrombocytopenia and transfusion-dependent anemia as additional risk factors for shortened survival. Thus, at the expense of simplicity, we are becoming better able to define risk categories in MF [Gangat et al. 2011]. Interestingly, the criteria coming into use in MF scoring systems increasingly resemble those used in myelodysplastic syndromes (MDS); these similarities likely reflect the often overlapping molecular underpinnings of these two heterogeneous disorders.
JAK2V617F: dose matters
JAK2V617F gene ‘dose’ has emerged as an important patterning event for the MPN phenotype, particularly with respect to PV and ET (see below). Dose also likely contributes to prognosis, both with respect to thrombotic risk (PV and ET) and to survival. This topic has been recently extensively reviewed in this journal [Vannucchi et al. 2011]. Notably low allele burden in PMF was observed to be associated with a ‘myelodepletive’ phenotype, i.e. bone marrow failure, associated low blood counts and increased incidence of infection with poor overall survival, when compared with those patients with PMF and high allele burdens, who tend to have a more truly myeloproliferative phenotype (constitutional symptoms, splenomegaly) [Guglielmelli et al. 2009; Tefferi et al. 2008]. That myelodepletive PMF may on some level represent a distinct biologic group is a fascinating observation, but it is also an important consideration in the interpretation of clinical trials: myelodepletive patients are more likely to be excluded from clinical trials because of cytopenias, so generalizing outcome data to this population is problematic [Verstovsek et al. 2010]. What does this suggest about biology? Does the utility of low mutant allele burden as a surrogate for worse outcome suggest that JAK2V617F is a passenger mutation, or a ‘late hit’ in a disease driven by other factors? Or is this mutation an early event which, in the proper context, dysregulates growth and/or predisposes to genomic instability? Additional studies should help to clarify the underlying biology, and ideally would include detailed analyses of patients over time. Thus far, modern prognostic systems do not take into account the presence, absence, or overall burden of the JAK2V617F mutation. How these (and other predisposing genetic factors) relate to pathogenesis, clinical presentation, and prognosis is an active area of investigation [Bejar et al. 2011; Guglielmelli et al. 2011b].
The JAK2V617F mutation: of mice and MPN
Multiple mouse models have been used to study the role of JAK2V617F in MPN, and have demonstrated the phenotypic variation associated with gene dosage. Initial studies relied on overexpression models, using retrovirally transduced bone marrow transplantation. These initial models made the fundamental observation that the JAK2V617F mutation alone was sufficient to recapitulate many of the clinicopathologic features of human PV progressing to MF. Mice transplanted with JAK2V617F transduced bone marrow display elevated hemoglobin/hematocrit, leukocytosis, and megakaryocyte hyperplasia followed by extramedullary hematopoiesis, splenomegaly and reticulin fibrosis in the bone marrow. Other somewhat more nuanced observations arose from these initial models as well. Wernig and colleagues noted that the effects of JAK2V617F bone marrow transplants were markedly different between mouse strains (Balb/c mice showing much more elevated leukocyte counts, more splenomegaly and more reticulin fibrosis than C57B1/6 mice), hinting that strain-specific modifiers might explain the phenotypic pleiotropism of MF in humans [Wernig et al. 2006].
The next wave of mouse models went beyond proving the basic sufficiency of the JAK2V617F mutation in recapitulating PV/MF and examined the dose-dependent nature of its effect. Using assorted transgenic expression systems to achieve various levels of constitutive JAK2V617F expression, these models revealed that while a high level of JAK2V617F expression generated PV-MF-like symptoms as observed previously, a low level of JAK2V617F expression phenocopied essential thrombocytosis. This new understanding of JAK2V617F as a dose-dependent contributor to myeloproliferative disorders was perhaps the first credible, biologic explanation of how diseases with such variable, even sometimes opposite, pathologies could be due to the same genetic aberration. Meanwhile a parallel story was emerging in humans: simple but elegant genotyping of colony assays from cells of patients with PV and ET showed that ET patients lack progenitors homozygous for JAK2V617F, while at least some homozygous clones are typically found in individuals with PV [Scott et al. 2006]. A conditional transgenic mouse with a human version of the JAK2V617F gene under the control of the mouse Jak2 promoter develops mild elevations in hemoglobin and platelet counts [Li et al. 2010]. Interestingly, in contrast to other transgenic models, these mice demonstrate a decrease in both the size and function of the stem/progenitor cell compartment, a deficiency that does not manifest immediately, but requires prolonged exposure (26 weeks) to mutant JAK2. Stem cells display increased DNA damage, decreased cell cycling and impaired apoptotic responses. Taken together, these findings might account for the functional competitive disadvantage observed for these stem cells compared with their wild-type counterparts in primary and secondary transplantation experiments. One wonders whether the same mechanisms might account for the bone marrow failure observed in advanced ‘myelodepletive’ myelofibrotic disorders.
How does JAK2V617F associated MPN arise, who is at risk, and are there known environmental contributors?
While mouse models clearly show that JAK2V617F is sufficient for the development of an MPN phenotype, many lines of evidence suggest that this mutation might be neither the sole nor initiating event in MPN pathogenesis. The existence of rare families predisposed to developing MPN point to a heritable factor; it is notable that JAK2V617F is often present in affected family members, but always as an acquired mutation, and that both JAK2V617F and JAK2 wild-type MPN can exist within a single kindred. By contrast, elegant cytogenetic and clonal hierarchy studies within a single patient with acquired disease have confirmed multiple separate acquisitions of JAK2V617F in different clones [Olcaydu et al. 2009a]. In 2009, three groups identified a germline haplotype that increased the risk of acquisition of JAK2V617F MPN approximately four-fold. Unexpectedly, a single nucleotide polymorphism (SNP) mapped to the 3’ portion of the JAK2 gene itself on chromosome 9 and typically occurred in cis with the acquired JAK2V617F mutation [Jones et al. 2009; Kilpivaara et al. 2009; Olcaydu et al. 2009a]. Interestingly, subsequent studies confirmed not only the observed association between this SNP, commonly referred to as the 46/1 JAK2 haplotype, with JAK2V617F, but also noted an association between 46/1 and MPN-associated exon 12 mutations in JAK2 and, more surprisingly, MPN-associated mutations in Mpl, the thrombopoietin-receptor gene located on chromosome 1 [Jones et al. 2010; Olcaydu et al. 2009b]. Overall, JAK2 46/1 is estimated to contribute approximately half of the heritable risk of MPN [Campbell, 2009]. Other more circumstantial observations suggest that environmental factors may also influence the acquisition of JAK2V617F: a possible cluster of cases of PV in Southeastern Pennsylvania in a pattern that overlaps both with the distribution of waste/coal power plants and a major superfund site provides indirect evidence for an association with toxic exposure [Seaman et al. 2009]. JAK2V617F has also been shown to be present in a disproportionately high number of cases of therapy-related leukemia [Schnittger et al. 2007]. A mechanistic understanding of how these genetic and environmental factors lead to the acquisition of this mutation might one day provide insights into the prevention of the diseases associated with it.
What are the other important molecular events in MPN?
Within the JAK/STAT pathway
Recurrent activating mutations in Mpl, the thrombopoietin receptor, have been identified in a subset of patients with MF and ET. Mpl mutations rarely occur together with JAK2V617F [Pikman et al. 2006]. Mouse models of Mpl515 mutations demonstrate elevated platelet counts and develop MF with a relatively short latency [Koppikar et al. 2010]. The identification of Mpl mutations is consistent with the general model that MPN are diseases characterized by dysregulation of the cytokine receptor/JAK/STAT axis and explains, to some extent, why MF or ET without JAK2V617F is often phenotypically indistinct from JAK2V617F positive disease. By contrast, while the overwhelming majority (>95%) of patients with PV have JAK2V617F, many (if not all) of those without the mutation have mutations elsewhere in the JAK2 gene, typically exon 12, and exon 12-mutated PV is phenotypically distinct, usually presenting with an isolated erythrocytosis. How the different amino acid substitutions in JAK2 lead to an altered clinical presentation is not well established [Scott et al. 2007].
Mutations in the adaptor protein, LNK, are one example of JAK2/STAT pathway dysregulation stemming from the loss of function of a negative regulator of signaling. LNK structure/function relationships have been recently comprehensively reviewed in this journal [Oh, 2011]. LNK is an adapter protein that associates with both Mpl and JAK2 in its active form, and attenuates STAT signaling. The absence of LNK results in myeloproliferation in mouse models [Tong et al. 2005; Velazquez et al. 2002]. Missense mutations in LNK have recently been identified and characterized in two patients with MPN, one with MF and one with ET, and the mutations lead to either complete or partial loss of inhibitory activity, respectively. Interestingly STAT phosphorylation appeared to be increased in stem/progenitor cells from these patients [Oh et al. 2010].
Going nuclear
JAK2V617F may also have noncanonical oncogenic functions. Mutant JAK2 can enter the nucleus, where it has recently been shown to directly phosphorylate histones and alter the expression of the leukemic oncogene lmo2 [Dawson et al. 2009]. JAK2V617F has also been implicated in other potentially oncogenic epigenetic modifications. PRMT5, a methyltransferase, more effectively binds mutant JAK2 than wild type, both in the cytoplasm and nucleus. This interaction decreases PRMT5 activity, leading to myeloproliferation [Liu et al. 2011]. Taken together, it appears that JAK2 V617F may create genomic instability and/or epigenetic alterations relevant to the pathogenesis of MPN.
TET2
One clue to other adaptive mutations, also with epigenetic relevance, emerged with the identification of TET2 mutations in MPN. TET2 is an enzyme that modifies DNA, one of three known proteins that hydroxylates 5-methylcytosine in genomic DNA. The activity of TET2 appears to be sensitive to metabolic perturbations and important for growth regulation. TET2 mutations are present in approximately 8% of patients with MPN (with or without JAK2 V617F), 20% of patients with MDS, 12% of patients with acute myelogenous leukemia (AML), and a rather striking 42% of patients with chronic myelomonocytic leukemia (CMML) [Abdel-Wahab et al. 2009]. With this mutation cropping up in both proliferative and depletive myeloid disorders, TET2 presents itself as a potentially ‘unifying’ genetic aberration in myeloid malignancies. As we unravel the causes and effects of TET2 dysfunction in these disorders, we may begin to understand how these diseases can be situated at opposing ends of the same spectrum. Mutations in TET2 appear to have adverse prognostic significance in AML, but are not clearly established as correlating with risk in MPN at this point.
Missense mutations in TET2 occur at different sites, but collectively lead to partial or complete loss of function through inhibition of catalytic activity. Mutations in TET2 can precede the acquisition of other mutations, including JAK2 V617F [Abdel-Wahab et al. 2009; Delhommeau et al. 2009]. Much of the initial study of TET2 in MPN was dedicated to understanding a potential relationship between mutations in this gene and those in JAK2. In a small study, Delhommeau and colleagues found that the JAK2V617F mutation was preceded by mutations in TET2, although acquisition of JAK2 V617F prior to TET2 may also be possible. These observations lead to an emerging paradigm which suggests that it is not only the specific combination of molecular events but also the sequence of their acquisition that contributes to phenotype, progression and risk in these disorders.
In this vein, Delhommeau and colleagues looked at xenografts of primary CD34+ cells from MPN patients positive for JAK2V617F with or without TET2 mutations. They found a preferential expansion of human JAK2V617F cells with a TET2 mutation (with myeloid skewing) over time, and a concomitant loss of JAK2V617F cells lacking a TET2 mutation. These findings suggested the possibility that the TET2 mutation in these patients occurred in a functional stem-cell compartment, and that it conferred competitive self-renewal properties upon these cells and their offspring.
Not surprisingly, genotypic heterogeneity appears to be associated with varying sensitivity of different clones to clinical interventions. A subsequent case report detailed a response to treatment with interferon and noted a differential effect on different subclones, with the disappearance of combined JAK2V617F/TET2-mutated clones and the persistence of their JAK2 wild-type/TET2-mutant counterparts [Kiladjian et al. 2010].
More recently, mouse models have been engineered with loss of function mutations in TET2, and have clarified its transforming role in hematopoietic malignancies [Moran-Crusio et al. 2011; Quivoron et al. 2011]. TET2-mutant homozygotes demonstrate increases in the stem/progenitor cell compartment in bone marrow and spleen, and develop a myeloproliferative/myelodysplastic syndrome reminiscent of CMML. Qualitatively, stem/progenitor cells possess increased self-renewal capacity both ex vivo and in competitive serial transplantation experiments. These differences were attenuated but maintained in TET2 haploinsufficient mice, which may be more clinically relevant, given that patients overwhelmingly maintain one wild-type allele.
Therapeutic considerations
JAK2 V617F alone in mouse models can recapitulate the MPN phenotype, but inhibiting JAK2 alone does not reverse the disease.
Tyrosine kinase inhibitors clearly have activity in patients with MF, although parallels with early clinical studies of imatinib in CML do not appear to hold. JAK2 inhibitors have been tested most extensively in advanced MF [Dolgin, 2011; Verstovsek et al. 2010], and the early normalization of blood counts that is almost invariably the outcome with initial treatment of CML in chronic phase is rarely seen [Druker et al. 2006]. Likewise, the degree of fibrosis appears to be unchanged with treatment, with only modest, if any, reductions in mutant clonal burden. In fact, the primary activity of JAK2 inhibitors in treated patients appears to be an early and sometimes dramatic reduction in spleen size, and (perhaps more impressively) improvements in quality of life that track with changes in cytokine and chemokine profiles. Our mechanistic understanding of the response (or lack thereof) to these agents is incomplete. JAK2, unlike ABL kinase, is indispensable for normal hematopoiesis [Ghaffari et al. 2001] and may therefore be a target with a finite and limited therapeutic index. The dysregulated, overactive JAK/STAT pathway in MPN cells (or their more generalized proliferative disarray) must account for selective sensitivity to inhibition, and may explain why the growth of cells from patients with and without the mutant allele is inhibited in preclinical studies [Hexner et al. 2008; Weinstein et al. 1997]. There are no published peer-reviewed data for small molecule inhibitors specific for the JAK2V617F mutant kinase, although in principle they exist in the preclinical pipeline [Dolgin, 2011]. Moreover, the published clinical trials themselves analyze biomarkers such as cytokine profiles and changes in allele burden [Pardanani et al. 2011; Verstovsek et al. 2010], but to date do not provide pharmacodynamic evidence for inhibition of the JAK2 kinase (or its downstream effectors) in treated patients. Thus, it remains formally possible that the failure to completely reverse the disease process is simply due to a failure of the drug to hit its intended target. Other schools of thought challenge the paradigm that increasing the specificity of the kinase inhibitor will improve efficacy, and instead support the concept that targeting multiple pathways within a malignant clone (or targeting nonclonal bystander cells) will enhance activity. For example, the clinical activity that has been observed with respect to changes in cytokine profiles and splenomegaly in MF could in part be attributable to JAK1 inhibition in lymphocytes or other cells within the spleen. This ‘clean’ versus ‘dirty’ drug hypothesis becomes more complicated to test or apply when one takes into account the general dearth of information available for most proprietary compounds. It is the opinion of these authors that rationally designed small molecule inhibitors should offer the greatest degree of specificity and that when possible clinical studies should include pharmacodynamic studies to measure target inhibition. This strategy would offer the most potent therapeutic intervention while minimizing off-target effects; multitarget inhibition could be achieved through combinations of small molecule inhibitors or with biologics such as interferon.
Interferon-α: how does it work?
Thus far, interferon appears to be a unique agent that can, over time, significantly decrease JAKV617F clonal burden and induce complete molecular responses with prolonged drug exposure [Kiladjian et al. 2008]. A great deal is known about the pleiotropic actions of interferons generally, but their effects on the particular pathway(s) of interest in MPN are not well understood [Kiladjian et al. 2011]. Interferons act directly on hematopoietic stem and progenitor cells, and are well known clinically to induce cytopenias. Interferon-α has recently been shown to induce murine stem cells to enter into the cell cycle, and IFN receptors may mediate self-renewal and stem-cell exhaustion/senescence [Essers et al. 2009; Sato et al. 2009]. Interferon also modulates the humoral immune response and acts upon T cells, NK cells, macrophages and dendritic cells. These bystander/immunomodulatory effects may be a key effector mechanism in MPN responses. The tumor neoantigen MPD6, for example, was found to be upregulated in cells from patients with PV in response to interferon treatment [Xiong et al. 2007]. Interferons may also modify the stem-cell niche, and are known to be anti-angiogenic. When these diverse effects are considered in toto, interferon may well be the prototypic ‘dirty drug’, arguably a more appropriate role for a biologic agent than for small molecule inhibitors.
The most promising clinical data for the use of interferon in MPN are in patients with early PV and ET; historically, trials in patients with MF have been more problematic, largely due to tolerability and, more specifically, lowering of blood counts and worsening of constitutional symptoms. That said, the pegylated formulation of IFN-2α (Pegasys™) appears to be a promising improvement both in potency and tolerability, even potentially in the MF population [Ianotto et al. 2009]: an important milestone given that interferon may also be the unique conventional therapeutic agent that can reverse and/or retard progression of bone marrow fibrosis [Silver et al. 2011]. Nonetheless, overcoming the tolerability hurdles in more advanced forms of MF is ambitious, and probably unrealistic. In PV, a major international randomized study currently underway comparing pegylated interferon with hydroxyurea has the potential to change standard practice; the study might also provide informative data with respect to progression to MF.
Hypomethylating agents and histone deacetylase (HDAC) inhibitors
5-azacitadine, a DNA methyltransferase inhibitor, has demonstrated clinical activity in patients with dysplasia or leukemia evolved from MPN [Thepot et al. 2010]. This provides further indirect clinical evidence that the epigenetic disarray proposed to play a major role in these diseases, and conferred in part upon malignant clones by mutations in TET2, EZH2, ASLX1, PRMT5, or JAK2 itself, can be modified for clinical benefit. Likewise histone deacetylase (HDAC) inhibitors have been explored in MPN, with apparent clinical activity. The single largest study (n = 29) tested Givinostat, an oral HDAC inhibitor, in an open-label pilot study in Italy [Rambaldi et al. 2010]. ET, PV and MF patients were included in the study, designed with a relatively short (24-week) exposure to drug. Freedom from phlebotomy and reduction in both spleen size and pruritis was seen in a subset of patients, with a possible modest reduction in JAKV617F for PV and ET but not MF patients. Approximately half of patients enrolled had MF, but only a minority completed the study, apparently a function of the level of illness of this patient population rather than any specific toxicity of the agent. Taken together, epigenetic modulation appears to be a promising therapeutic avenue; several investigators have proposed combining HDAC inhibitors or DNA methyltransferase inhibitors with JAK2 inhibitors although this has not been extensively modeled preclinically.
HSP90 inhibitors
HSP90, a chaperone protein known to directly bind to and stabilize proteins, including tyrosine kinases generally and JAK2 specifically, has been elegantly studied as an alternative drug target [Marubayashi et al. 2010]. PU-H71 is particularly promising, with favorable pharmacokinetic and pharmacodynamic properties. Marubayashi and colleagues showed that PU-H71 degrades JAK2, interrupting downstream pathways with specificity for JAK2 mutant cells, without disturbing JAK2 in normal tissues. This mutant specificity has been attributed to PU-H71’s prolonged and selective retention in mutant tissues. In mice PU-H71 treatment reduced white blood cell count and hematocrit levels, lowered cellularity in the bone marrow, improved extramedullary hematopoiesis and reduced clonal burden of disease.
Stem-cell transplantation in MPN: when, how, and what are the obstacles?
The principle
Allogeneic hematopoietic stem-cell transplantation (HSCT) remains the only known curative intervention for MPN. In principle, it replaces the diseased hematopoietic stem-cell compartment with a healthy ‘organ’ and provides the recipient with a new immune system, which has the potential to eradicate otherwise chemoresistant disease, and can protect its new host over the long run against emerging disease via immune surveillance.
Who needs it?
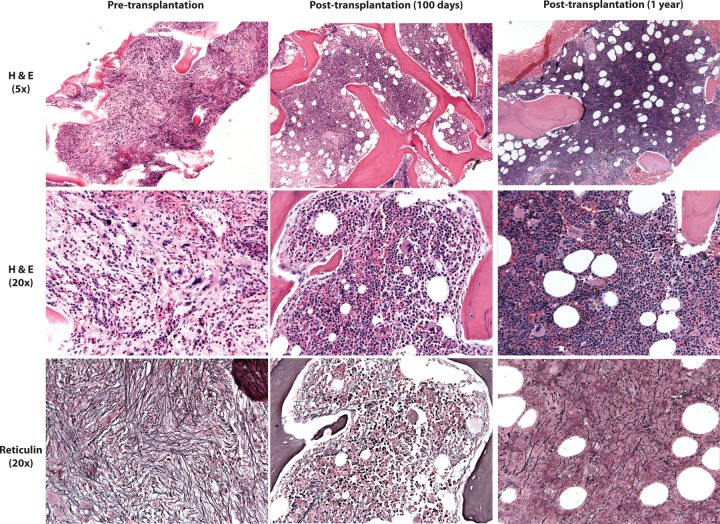
HSCT is also arguably one of the riskiest interventions in modern medicine, so careful patient selection is of paramount importance. Transplantation for PV and ET, overall associated with a normal or near-normal life expectancy, is not indicated. On the other hand, stem-cell transplantation should be an initial consideration for all patients with MF when first evaluated, and is the treatment of choice for high-risk symptomatic younger patients. However, with MF more typically presenting in the sixth or seventh decades, decisions with respect to stem-cell transplantation are rarely straightforward. The scarcity of truly effective conventional therapeutic options introduces even more complexity into clinical decision making in this setting. In addition, initial and unusually high rates of transplant-related mortality in studies using conventional myeloablative approaches in MF patients [Guardiola et al. 1999] have led to their exclusion in most prospective studies of alternative or novel conditioning/immunosuppressive regimens or donor sources. It is difficult, therefore, to know whether incremental advances in HSCT apply to patients with MF. And yet, in the right patient, HSCT is clearly feasible and often therapeutic. What is fascinating and tantalizing in such cases is the capacity for this approach to restore normal trilineage hematopoiesis in a grossly perturbed marrow microenvironment, with rapid and striking reversal of the fibrosis that is the hallmark of this neoplasm (Figure 2).
Figure 2.
Reversal of fibrosis following reduced intensity hematopoietic stem-cell transplantation (HSCT).
A 63-year-old woman with primary myelofibrosis (PMF). Pre-transplant: H&E sections (5×) show markedly fibrotic marrow. While myelopoiesis and especially erythropoiesis are decreased, megakaryopoiesis is relatively increased and cytologically atypical (20×), with numerous clustered forms that range from small and hypolobated to large and bizarre. A reticulin stain (20×) highlights extensive diffuse reticulin fibrosis. Day 100 post-transplant: H&E sections (5×) show a substantial decrease in fibrosis, with marrow that is hypercellular for age (80%) and demonstrates normal trilineage hematopoiesis. Megakaryocytes are decreased in number and cytologically unremarkable (20×). A reticulin stain shows only mild and variable fibrosis (20×). One year post-transplant: H&E sections (5×) show marrow that is hypercellular for age (80%), with normal trilineage hematopoiesis (20×). A reticulin stain shows only mild and variable fibrosis (20×).
Table 1 summarizes a series of retrospective studies that have been published within the last 5 years. Several notable findings with respect to outcomes include confirmation that performance status <90% and the presence of circulating blasts adversely influence survival, and that Lille score is not clearly predictive of transplant outcome [Ballen et al. 2010]. A small case series from a single center observed excellent outcomes and low morbidity with sirolimus, an mTOR inhibitor, and tacrolimus as graft-versus-host disease (GVHD) prophylaxis [Snyder et al. 2010]. It bears mentioning that mTOR inhibitors are one of many classes of investigational therapies shown to have activity in MPN [Guglielmelli et al. 2011a]. A multicenter study found no significant differences with respect to resolution of fibrosis in reduced intensity compared with myeloablative stem cell transplantation [Gupta et al. 2009], a remarkable testimony to the principle of transplantation as immunotherapy.
Table 1.
Retrospective analyses of hematopoietic stem-cell transplantation, 2007 to present.
| Study | MA (n) | RIC (n) | OS (%) | NRM (%) | PFS (%) | Engraftment CI/median day | Comments |
|---|---|---|---|---|---|---|---|
| [Lissandre, 2011] | 15 | 24 | 60 | 54 | 30 | 15 days (PMN) | Multicenter (France), 1994–2008, inc 2ary AML, 1/29 1st graft failure. |
| 19 days (Plt) | |||||||
| [Robin, 2011] | 46 | 101 | 39 | 32 | 39 | 90%/18 days | Registry, France, (SFGM-TC) 1997–2008, inc 2nd AML |
| [Samuelson, 2011] | 5 | 25 | 45 (22 months) | 30 (3 years) | 40 (3 years) | 90% | 30 patients aged 60–78 (median 65) years, 1999–2007 |
| [Ciurea, 2010] | 3 | 9 | 49 (31 months) | 29 (2 years) | ND | 100%, 13 days (PMN) | Single center (MD Anderson), all with leukemic transformation; 1994–2008 |
| 21.5 days (Plts) | |||||||
| [Ballen et al. 2010] | 229 | 60 | 37 (sib, 5 years) | 35 (sib, 5 years) | 33 (sib, 5 years) | 95%, 18 days (sib) | Registry, 1989–2002 (CIBMTR); performance status <90% and presence of PB blasts predicted worse DFS; Lille score not predictive. |
| 30 (URD, 5 years) | 50 (URD, 5 years) | 27 (URD, 5 years) | 83% (URD) | ||||
| [Snyder et al. 2010] | 0 | 23 | 55.6 (CsA/MMF, 2 years); 92.9 (Tac/Rap, 2 years) | 33.3 (CsA/MMF, 100 days) | ND | 16.5 days (PMN) | Single center (City of Hope); 2000–2008;cohort 1: CsA/MMF; cohort 2: Tac/Rap |
| 0 (Tac/Rap, 100 days) | 18 days (plt) | ||||||
| [Gupta et al. 2009] | 23 | 23 | 48 (MA, 3 years) | 48 (MA, 3 years) | 43 (MA, 3 years) | 18/21 days (PMN) | Multicenter, 3 Canadian, 4 European centers 1998–2005; compares MA with RIC; no significant differences in time to resolution of fibrosis RIC versus MA |
| 68 (RIC, 3 years) | 27 (RIC, 3 years) | 58 (RIC, 3 years) | 17–23 days (plt) | ||||
| [Patriarca, 2008] | 48 | 52 | 42 | 35 (1 years) | 35 | 87% CI at 90 days | 26 Italian centers (GITMO) between 1986 and 2006 |
| 43 (3 years) | |||||||
| [Kerbauy, 2007] | 95 | 9 | 61 (5 years) | 34 (5 years) | ND | 19 days | Single center (Seattle); Patients conditioned with targeted BU/CY improved survival (68%) than other regimens |
MA, myeloablative conditioning; RIC, reduced intensity conditioning; OS, overall survival; NRM, nonrelapse mortality; PFS, progression-free survival; CI, cumulative incidence; CsA, cyclosporine A; Tac, tacrolimus; Rap, sirolimus; sib, sibling; URD, unrelated donor; ND, not described.
The largest prospective multicenter study to evaluate transplantation for MF was conducted through the European Group for Blood and Marrow Transplantation (EBMT) using a reduced intensity strategy [Kroger et al. 2009b]. Using the combination of fludarabine, busulfan (10 mg/kg) and antithymocyte globulin with a standard prophylactic immunosuppressive regimen, 98% of patients engrafted, with a nonrelapse mortality of 16% at 1 year. In addition, the estimated 5-year overall survival was 67%. Older age (over 55) and a mismatched donor adversely influenced survival. Subsequent post hoc analyses showed that JAK2V617F negative disease also carried adverse prognostic significance [Alchalby et al. 2010]. These generally favorable results were mirrored histologically in those patients who had serial bone marrow biopsies following transplantation. These studies showed near or complete resolution of fibrosis in 69% and 93% of patients by day 100 and day 365, respectively (see Figure 2 for a representative example of bone marrow morphology before and following reduced intensity transplantation). Not surprisingly, high-risk disease was more likely to relapse post-transplantation. An additional notable finding from the multivariable analysis was that a history of splenectomy was associated with a higher risk of relapse. While a history of splenectomy may simply be a surrogate for higher risk disease, this finding has important practical as well as hypothesis-generating implications. In practice, it puts to rest the concept that massive splenomegaly precludes engraftment, and implies that splenectomy need not be performed prior to transplantation. However, in the speculative realm, as with the results of JAK2 inhibitor studies, this observation raises questions: what, functionally, does the spleen support or provide in this disorder? And what is its function in response to treatment?
How can we use minimal residual disease monitoring?
Subsequent analyses of the EBMT cohort demonstrated that quantitative measurement of JAK2V617F predicted survival, with an undetectable level at 6 months representing an important milestone [Alchalby et al. 2010]. Furthermore, minimal residual disease-triggered pre-emptive donor leukocyte infusions can re-induce molecular remissions following HSCT [Kroger et al. 2009a]. Taken together, the observed immunotherapeutic effect of HSCT in MF is particularly striking when one considers the accepted dogma, that successful reduced intensity transplantation relies heavily on a minimal disease state prior to transplantation, and the grim reality, that because of a dearth of truly effective therapies, dense disease at time of transplantation is the rule for patients with MF [Sorror et al. 2005].
So, who to transplant?
Refinements in prognostic systems can identify those patients at highest risk for dying of disease, and those for whom the risks of transplantation are justified. Age alone is probably a crude indicator of risk, and additional tools, such as the HSCT comorbidity index can help stratify the risk of transplant-related mortality based upon other factors [Sorror et al. 2005]. Individually, patients need to be well informed about the process and willing to accept the risks of HSCT. Globally, transplantation centers and cooperative groups could become more actively engaged in facilitating the participation of patients with MF in general transplantation protocols.
Conclusions
As clinicians who follow and treat patients with this group of disorders, we are often bewildered either by their stability or by their frequently unpredictable evolution. We can start to construct stories as we imagine the sequential accumulation of molecular events. Consider the patient with stable PV, whose spleen enlarges, white blood cell count increases and phlebotomy requirements cease, who loses weight and over months to years develops anemia. Does this evolution represent the sequential acquisition of an Asxl1 mutation [Stein et al. 2011] in an epigenetically disrupted stem-cell compartment? Consider the de novo PMF patient, who presents seemingly overnight with frank and severe bone marrow failure. Is this the low JAK2V617F allele burden patient with numerous other chromosomal or molecular genetic abnormalities? Consider the patient who appears to evolve overnight to leukemia from PV without an interceding fibrotic stage, and bypassing all of our prognostic scoring systems. Does this represent an outgrowth of a pre-JAK2V617F primitive clone with a new mutation in p53, or dysregulated JAK2V617F itself wreaking nuclear havoc [Beer et al. 2010; Dawson et al. 2009]? Only carefully constructed translational studies can verify or dispel these fictionalized accounts.
The discovery of JAK2V617F unified and deepened our understanding of classical MPN, and began a cascade of subsequent molecular breakthroughs. Some initially suggested a reclassification of disease on molecular terms [Campbell and Green, 2006]. Ultimately this may be inevitable (and appropriate), but at this point in time the accumulating molecular data can seem to obscure rather than clarify our understanding of clinical phenotypes and prognosis. We increasingly recognize clinical heterogeneity within subgroups, molecularly defined or otherwise. In short, we are gaining a granular understanding of these disorders; the greatest challenge (and opportunity) may be in developing proper tools for integrating these findings in a responsible and ‘user friendly’ way into clinical investigation and practice.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Harper G. Hubbeling, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA
Dale M. Frank, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA, USA
Elizabeth O. Hexner, Department of Medicine and Abramson Cancer Center, University of Pennsylvania, 3400 Civic Center Boulevard Philadelphia, PA, 19104 USA
References
- Abdel-Wahab O., Mullally A., Hedvat C., Garcia-Manero G., Patel J., Wadleigh M., et al. (2009) Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood 114: 144–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alchalby H., Badbaran A., Zabelina T., Kobbe G., Hahn J., Wolff D., et al. (2010) Impact of JAK2V617F mutation status, allele burden, and clearance after allogeneic stem cell transplantation for myelofibrosis. Blood 116: 3572–3581 [DOI] [PubMed] [Google Scholar]
- Ballen K.K., Shrestha S., Sobocinski K.A., Zhang M.J., Bashey A., Bolwell B.J., et al. (2010) Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant 16: 358–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbui T., Barosi G., Birgegard G., Cervantes F., Finazzi G., Griesshammer M., et al. (2011) Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 29: 761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbui T., Carobbio A., Cervantes F., Vannucchi A.M., Guglielmelli P., Antonioli E., et al. (2010) Thrombosis in primary myelofibrosis: incidence and risk factors. Blood 115: 778–782 [DOI] [PubMed] [Google Scholar]
- Baxter E.J., Scott L.M., Campbell P.J., East C., Fourouclas N., Swanton S., et al. (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365: 1054–1061 [DOI] [PubMed] [Google Scholar]
- Beer P.A., Delhommeau F., LeCouedic J.P., Dawson M.A., Chen E., Bareford D., et al. (2010) Two routes to leukemic transformation after a JAK2 mutation-positive myeloproliferative neoplasm. Blood 115: 2891–2900 [DOI] [PubMed] [Google Scholar]
- Bejar R., Stevenson K., Abdel-Wahab O., Galili N., Nilsson B., Garcia-Manero G., et al. (2011) Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med 364: 2496–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P.J. (2009) Somatic and germline genetics at the JAK2 locus. Nat Genet 41: 385–386 [DOI] [PubMed] [Google Scholar]
- Campbell P.J., Green A.R. (2006) The myeloproliferative disorders. N Engl J Med 355: 2452–2466 [DOI] [PubMed] [Google Scholar]
- Cervantes F., Pereira A., Esteve J., Rafel M., Cobo F., Rozman C., et al. (1997) Identification of ‘short-lived’ and ‘long-lived’ patients at presentation of idiopathic myelofibrosis. Br J Haematol 97: 635–640 [DOI] [PubMed] [Google Scholar]
- Cervantes F., Dupriez B., Pereira A., Passamonti F., Reilly J.T., Morra E., et al. (2009) New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 113(13): 2895–2901 [DOI] [PubMed] [Google Scholar]
- Ciurea S.O., de Lima M., Giralt S., Saliba R., Bueso-Ramos C., Andersson B.S., et al. Allogeneic stem cell transplantation for myelofibrosis with leukemic transformation. Biol Blood Marrow Transplant 16: 555–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dameshek W. (1951) Some speculations on the myeloproliferative syndromes. Blood 6: 372–375 [PubMed] [Google Scholar]
- Dawson M.A., Bannister A.J., Gottgens B., Foster S.D., Bartke T., Green A.R., et al. (2009) JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature 461: 819–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhommeau F., Dupont S., Della Valle V., James C., Trannoy S., Masse A., et al. (2009) Mutation in TET2 in myeloid cancers. N Engl J Med 360: 2289–2301 [DOI] [PubMed] [Google Scholar]
- Dolgin E. (2011) Companies hope for kinase inhibitor JAKpot. Nat Rev Drug Discov 10: 717–718 [DOI] [PubMed] [Google Scholar]
- Druker B.J., Guilhot F., O’Brien S.G., Gathmann I., Kantarjian H., Gattermann N., et al. (2006) Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 355: 2408–2417 [DOI] [PubMed] [Google Scholar]
- Dupriez B., Morel P., Demory J.L., Lai J.L., Simon M., Plantier I., et al. (1996) Prognostic factors in agnogenic myeloid metaplasia: a report on 195 cases with a new scoring system. Blood 88: 1013–1018 [PubMed] [Google Scholar]
- Essers M.A., Offner S., Blanco-Bose W.E., Waibler Z., Kalinke U., Duchosal M.A., et al. (2009) IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 458: 904–908 [DOI] [PubMed] [Google Scholar]
- Gangat N., Caramazza D., Vaidya R., George G., Begna K., Schwager S., et al. (2011) DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol 29: 392–397 [DOI] [PubMed] [Google Scholar]
- Ghaffari S., Kitidis C., Fleming M.D., Neubauer H., Pfeffer K., Lodish H.F. (2001) Erythropoiesis in the absence of janus-kinase 2: BCR-ABL induces red cell formation in JAK2(-/-) hematopoietic progenitors. Blood 98: 2948–2957 [DOI] [PubMed] [Google Scholar]
- Guardiola P., Anderson J.E., Bandini G., Cervantes F., Runde V., Arcese W., et al. (1999) Allogeneic stem cell transplantation for agnogenic myeloid metaplasia: a European Group for Blood and Marrow Transplantation, Societe Francaise de Greffe de Moelle, Gruppo Italiano per il Trapianto del Midollo Osseo, and Fred Hutchinson Cancer Research Center Collaborative Study. Blood 93: 2831–2838 [PubMed] [Google Scholar]
- Guglielmelli P., Barosi G., Rambaldi A., Marchioli R., Masciulli A., Tozzi L., et al. (2011a) Safety and efficacy of everolimus, a mTOR inhibitor, as single agent in a phase 1/2 study in patients with myelofibrosis. Blood 118: 2069–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmelli P., Barosi G., Specchia G., Rambaldi A., Lo Coco F., Antonioli E., et al. (2009) Identification of patients with poorer survival in primary myelofibrosis based on the burden of JAK2V617F mutated allele. Blood 114: 1477–1483 [DOI] [PubMed] [Google Scholar]
- Guglielmelli P., Biamonte F., Score J., Hidalgo-Curtis C., Cervantes F., Maffioli M., et al. (2011b) EZH2 mutational status predicts poor survival in myelofibrosis. Blood, in press [DOI] [PubMed] [Google Scholar]
- Gupta V., Kroger N., Aschan J., Xu W., Leber B., Dalley C., et al. (2009) A retrospective comparison of conventional intensity conditioning and reduced-intensity conditioning for allogeneic hematopoietic cell transplantation in myelofibrosis. Bone Marrow Transplant 44: 317–320 [DOI] [PubMed] [Google Scholar]
- Hexner E.O., Serdikoff C., Jan M., Swider C.R., Robinson C., Yang S., et al. (2008) Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood 111: 5663–5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianotto J.C., Kiladjian J.J., Demory J.L., Roy L., Boyer F., Rey J., et al. (2009) PEG-IFN-alpha-2a therapy in patients with myelofibrosis: a study of the French Groupe d’Etudes des Myelofibroses (GEM) and France Intergroupe des syndromes Myeloproliferatifs (FIM). Br J Haematol 146: 223–225 [DOI] [PubMed] [Google Scholar]
- James C., Ugo V., Le Couedic J.P., Staerk J., Delhommeau F., Lacout C., et al. (2005) A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434: 1144–1148 [DOI] [PubMed] [Google Scholar]
- Jones A.V., Campbell P.J., Beer P.A., Schnittger S., Vannucchi A.M., Zoi K., et al. (2010) The JAK2 46/1 haplotype predisposes to MPL-mutated myeloproliferative neoplasms. Blood 115: 4517–4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.V., Chase A., Silver R.T., Oscier D., Zoi K., Wang Y.L., et al. (2009) JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet 41: 446–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbauy D.M., Gooley T.A., Sale G.E., Flowers M.E., Doney K.C., Georges G.E., et al. (2007). Hematopoietic cell transplantation as curative therapy for idiopathic myelofibrosis, advanced polycythemia vera, and essential thrombocythemia. Biol Blood Marrow Transplant 13: 355–365 [DOI] [PubMed] [Google Scholar]
- Kiladjian J.J., Cassinat B., Chevret S., Turlure P., Cambier N., Roussel M., et al. (2008) Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 112: 3065–3072 [DOI] [PubMed] [Google Scholar]
- Kiladjian J.J., Masse A., Cassinat B., Mokrani H., Teyssandier I., le Couedic J.P., et al. (2010) Clonal analysis of erythroid progenitors suggests that pegylated interferon alpha-2a treatment targets JAK2V617F clones without affecting TET2 mutant cells. Leukemia 24: 1519–1523 [DOI] [PubMed] [Google Scholar]
- Kiladjian J.J., Mesa R.A., Hoffman R. (2011) The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood 117: 4706–4715 [DOI] [PubMed] [Google Scholar]
- Kilpivaara O., Mukherjee S., Schram A.M., Wadleigh M., Mullally A., Ebert B.L., et al. (2009) A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet 41: 455–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klampfl T., Harutyunyan A., Berg T., Gisslinger B., Schalling M., Bagienski K., et al. (2011) Genome integrity of myeloproliferative neoplasms in chronic phase and during disease progression. Blood 118: 167–176 [DOI] [PubMed] [Google Scholar]
- Koppikar P., Abdel-Wahab O., Hedvat C., Marubayashi S., Patel J., Goel A., et al. (2010) Efficacy of the JAK2 inhibitor INCB16562 in a murine model of MPLW515L-induced thrombocytosis and myelofibrosis. Blood 115: 2919–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralovics R., Passamonti F., Buser A.S., Teo S.S., Tiedt R., Passweg J.R., et al. (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 352: 1779–1790 [DOI] [PubMed] [Google Scholar]
- Kroger N., Alchalby H., Klyuchnikov E., Badbaran A., Hildebrandt Y., Ayuk F., et al. (2009a) JAK2-V617F-triggered preemptive and salvage adoptive immunotherapy with donor-lymphocyte infusion in patients with myelofibrosis after allogeneic stem cell transplantation. Blood 113: 1866–1868 [DOI] [PubMed] [Google Scholar]
- Kroger N., Holler E., Kobbe G., Bornhauser M., Schwerdtfeger R., Baurmann H., et al. (2009b) Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood 114: 5264–5270 [DOI] [PubMed] [Google Scholar]
- Levine R.L., Wadleigh M., Cools J., Ebert B.L., Wernig G., Huntly B.J., et al. (2005) Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7: 387–397 [DOI] [PubMed] [Google Scholar]
- Li J., Spensberger D., Ahn J.S., Anand S., Beer P.A., Ghevaert C., et al. (2010) JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood 116: 1528–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissandre S., Bay J.O., Cahn J.Y., Porcher R., Cacheux V., Cabrespine A., et al. Retrospective study of allogeneic haematopoietic stem-cell transplantation for myelofibrosis. Bone Marrow Transplant 46: 557–561 [DOI] [PubMed] [Google Scholar]
- Liu F., Zhao X., Perna F., Wang L., Koppikar P., Abdel-Wahab O., et al. (2011) JAK2V617F-mediated phosphorylation of PRMT5 downregulates its methyltransferase activity and promotes myeloproliferation. Cancer Cell 19: 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubayashi S., Koppikar P., Taldone T., Abdel-Wahab O., West N., Bhagwat N., et al. (2010) HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans. J Clin Invest 120: 3578–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Crusio K., Reavie L., Shih A., Abdel-Wahab O., Ndiaye-Lobry D., Lobry C., et al. (2011) Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell 20: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P.C., Hungerford D.A. (1960) Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst 25: 85–109 [PubMed] [Google Scholar]
- Oh S.T. (2011) When the brakes are lost: LNK dysfunction in mice, men, and myeloproliferative neoplasms. Ther Adv Hematol 2: 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.T., Simonds E.F., Jones C., Hale M.B., Goltsev Y., Gibbs K.D., Jr, et al. (2010) Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood 116: 988–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcaydu D., Harutyunyan A., Jager R., Berg T., Gisslinger B., Pabinger I., et al. (2009a) A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet 41: 450–454 [DOI] [PubMed] [Google Scholar]
- Olcaydu D., Skoda R.C., Looser R., Li S., Cazzola M., Pietra D., et al. (2009b) The ‘GGCC’ haplotype of JAK2 confers susceptibility to JAK2 exon 12 mutation-positive polycythemia vera. Leukemia 23: 1924–1926 [DOI] [PubMed] [Google Scholar]
- Pardanani A., Gotlib J.R., Jamieson C., Cortes J.E., Talpaz M., Stone R.M., et al. (2011) Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol 29: 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti F., Cervantes F., Vannucchi A.M., Morra E., Rumi E., Pereira A., et al. (2010) A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 115: 1703–1708 [DOI] [PubMed] [Google Scholar]
- Patriarca F., Bacigalupo A., Sperotto A., Isola M., Soldano F., Bruno B., et al. (2008) Allogeneic hematopoietic stem cell transplantation in myelofibrosis: the 20-year experience of the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Haematologica 93: 1514-1522 [DOI] [PubMed] [Google Scholar]
- Perna F., Gurvich N., Hoya-Arias R., Abdel-Wahab O., Levine R.L., Asai T., et al. (2010) Depletion of L3MBTL1 promotes the erythroid differentiation of human hematopoietic progenitor cells: possible role in 20q- polycythemia vera. Blood 116: 2812–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikman Y., Lee B.H., Mercher T., McDowell E., Ebert B.L., Gozo M., et al. (2006) MPLW515L Is a Novel Somatic Activating Mutation in Myelofibrosis with Myeloid Metaplasia. PLoS Med 3(7): e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivoron C., Couronne L., Della Valle V., Lopez C.K., Plo I., Wagner-Ballon O., et al. (2011) TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell 20: 25–38 [DOI] [PubMed] [Google Scholar]
- Rambaldi A., Dellacasa C.M., Finazzi G., Carobbio A., Ferrari M.L., Guglielmelli P., et al. (2010) A pilot study of the Histone-Deacetylase inhibitor Givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol 150: 446–455 [DOI] [PubMed] [Google Scholar]
- Robin M., Tabrizi R., Mohty M., Furst S., Michallet M., Bay J.O., et al. Allogeneic haematopoietic stem cell transplantation for myelofibrosis: a report of the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC). Br J Haematol 152: 331–339 [DOI] [PubMed] [Google Scholar]
- Samuelson S., Sandmaier B.M., Heslop H.E., Popat U., Carrum G., Champlin R.E., et al. Allogeneic haematopoietic cell transplantation for myelofibrosis in 30 patients 60-78 years of age. Br J Haematol 153: 76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Onai N., Yoshihara H., Arai F., Suda T., Ohteki T. (2009) Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med 15: 696–700 [DOI] [PubMed] [Google Scholar]
- Schnittger S., Bacher U., Kern W., Haferlach C., Haferlach T. (2007) JAK2 seems to be a typical cooperating mutation in therapy-related t(8;21)/ AML1-ETO-positive AML. Leukemia 21: 183–184 [DOI] [PubMed] [Google Scholar]
- Scott L.M., Scott M.A., Campbell P.J., Green A.R. (2006) Progenitors homozygous for the V617F mutation occur in most patients with polycythemia vera, but not essential thrombocythemia. Blood 108: 2435–2437 [DOI] [PubMed] [Google Scholar]
- Scott L.M., Tong W., Levine R.L., Scott M.A., Beer P.A., Stratton M.R., et al. (2007) JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 356: 459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman V., Jumaan A., Yanni E., Lewis B., Neyer J., Roda P., et al. (2009) Use of molecular testing to identify a cluster of patients with polycythemia vera in eastern Pennsylvania. Cancer Epidemiol Biomarkers Prev 18: 534–540 [DOI] [PubMed] [Google Scholar]
- Silver R.T., Vandris K., Goldman J.J. (2011) Recombinant interferon-alpha may retard progression of early primary myelofibrosis: a preliminary report. Blood 117: 6669–6672 [DOI] [PubMed] [Google Scholar]
- Snyder D.S., Palmer J., Gaal K., Stein A.S., Pullarkat V., Sahebi F., et al. (2010) Improved outcomes using tacrolimus/sirolimus for graft-versus-host disease prophylaxis with a reduced-intensity conditioning regimen for allogeneic hematopoietic cell transplant as treatment of myelofibrosis. Biol Blood Marrow Transplant 16: 281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorror M.L., Maris M.B., Storb R., Baron F., Sandmaier B.M., Maloney D.G., et al. (2005) Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106: 2912–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak J.L., Silver R.T. (2008) The revised World Health Organization diagnostic criteria for polycythemia vera, essential thrombocytosis, and primary myelofibrosis: an alternative proposal. Blood 112: 231–239 [DOI] [PubMed] [Google Scholar]
- Stein B.L., Williams D.M., O’Keefe C., Rogers O., Ingersoll R.G., Spivak J.L., et al. (2011) Disruption of the ASXL1 gene is frequent in primary, post-essential thrombocytosis and post-polycythemia vera myelofibrosis, but not essential thrombocytosis or polycythemia vera: analysis of molecular genetics and clinical phenotypes. Haematologica 96: 1462–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow S.H., for the International Agency for Research on Cancer, World Health Organization and Louis A. Duhring Fund (2008) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. Lyon, France: International Agency for Research on Cancer, p. 439, available at: http://hdl.library.upenn.edu/1017.12/366308 [Google Scholar]
- Tefferi A., Lasho T.L., Huang J., Finke C., Mesa R.A., Li C.Y., et al. (2008) Low JAK2V617F allele burden in primary myelofibrosis, compared to either a higher allele burden or unmutated status, is associated with inferior overall and leukemia-free survival. Leukemia 22: 756–761 [DOI] [PubMed] [Google Scholar]
- Thepot S., Itzykson R., Seegers V., Raffoux E., Quesnel B., Chait Y., et al. (2010) Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood 116: 3735–3742 [DOI] [PubMed] [Google Scholar]
- Thiele J., Kvasnicka H.M., Mullauer L., Buxhofer-Ausch V., Gisslinger B., Gisslinger H. (2011) Essential thrombocythemia versus early primary myelofibrosis: a multicenter study to validate the WHO classification. Blood 117: 5710–5718 [DOI] [PubMed] [Google Scholar]
- Tong W., Zhang J., Lodish H.F. (2005) Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways. Blood 105: 4604–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucchi A.M., Pieri L., Guglielmelli P. (2011) JAK2 allele burden in the myeloproliferative neoplasms: effects on phenotype, prognosis and change with treatment. Ther Adv Hematol 2: 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez L., Cheng A.M., Fleming H.E., Furlonger C., Vesely S., Bernstein A., et al. (2002) Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J Exp Med 195: 1599–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S., Kantarjian H., Mesa R.A., Pardanani A.D., Cortes-Franco J., Thomas D.A., et al. (2010) Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 363: 1117–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J.N., Myers T.G., O’Connor P.M., Friend S.H., Fornace A.J., Jr, Kohn K.W., et al. (1997) An information-intensive approach to the molecular pharmacology of cancer. Science 275: 343–349 [DOI] [PubMed] [Google Scholar]
- Wernig G., Mercher T., Okabe R., Levine R.L., Lee B.H., Gilliland D.G. (2006) Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood 107: 4274–4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z., Yan Y., Liu E., Silver R.T., Verstovsek S., Yang F., et al. (2007) Novel tumor antigens elicit anti-tumor humoral immune reactions in a subset of patients with polycythemia vera. Clin Immunol 122: 279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]