Abstract
Anemia is a major cause of morbidity in cancer patients resulting in poor physical performance, prognosis and therapy outcome. Initially, erythropoietin-stimulating agents (ESAs) were supposed to be the treatment of choice but about one third of patients turned out to be nonresponders and meta-analyses provided evidence of an increased risk of mortality if used excessively. This along with the successful use of intravenous iron for anemia in patients with chronic kidney disease prompted seven clinical studies evaluating the efficacy of intravenous iron as an adjunct to ESAs and four additional studies using intravenous iron only for anemia in cancer patients. These studies confirmed a superior response if ESAs are combined with intravenous iron and revealed iron only to be a useful option in patients with mild and absolute iron deficiency (AID). Currently, best treatment decisions for anemia in cancer might be based on measurements of serum ferritin (SF), transferrin saturation (TSAT), soluble transferrin receptor (sTfR), ferritin index (FI = sTfR/log SF), hypochromic reticulocytes (CHR) and C-reactive protein (CRP). However, there is still an urgent need for trials investigating diagnostic approaches to optimize therapy of anemia in cancer patients with iron and/or ESAs.
Keywords: anemia of chronic disease, cancer, hemoglobin, iron deficiency
Introduction
Anemia is a major cause of morbidity in cancer patients [Barrett-Lee et al. 2005; Birgegard et al. 2006]. Low hemoglobin (Hb) levels in cancer patients were shown to correlate significantly with poor physical performance [Ludwig et al. 2004; Barrett-Lee et al. 2005; Birgegard et al. 2006; Steinmetz et al. 2011], prognosis and therapy outcome [Fein et al. 1995; Dubray et al. 1996; Glaser et al. 2001; Littlewood et al. 2001; Waters et al. 2002]. There are multiple causative factors including absolute iron deficiency (AID) which may result from chronic bleeding due to gastrointestinal or gynecological lesions, blood loss from surgery, nutritional deficiencies, anemia of chronic disease (ACD), myelosuppressive effects of chemotherapy or metastatic infiltration of the bone marrow limiting erythropoiesis [Rizzo et al. 2002; Grotto, 2008]. Even in the absence of overt anemia, iron deficiency is already associated with impaired physical function, weakness and fatigue which all abate upon iron therapy [Verdon et al. 2003; Brownlie et al. 2004].
Anemia as major cause of morbidity in cancer took center stage with the approval of erythropoiesis-stimulating agents (ESAs) in 1997. At that time one did not think of functional iron deficiency (FID) in cancer patients yet and AID was generally considered of minor importance. Consequently, diagnostic procedures were not developed but therapy was initiated if three conditions were met: (1) diagnosis of cancer, (2) chemotherapy and (3) low Hb levels (< 10 g/dl). Accordingly, the first treatment guideline for cancer-associated anemia in 2002 was primarily a ‘how to use ESA’ guideline [Rizzo et al. 2002]. Only as the sixth of eight recommendations the guideline stated: ‘Baseline and periodic monitoring of iron, total iron-binding capacity, transferrin saturation, or ferritin levels and instituting iron repletion when indicated may be valuable in limiting the need for epoetin, maximizing symptomatic improvement for patients, and determining the reason for failure to respond adequately to epoetin. There is inadequate evidence to specify the optimal timing, periodicity, or testing regimen for such monitoring’. The ESA-dominated view on anemia did not change with recent guideline revisions and diagnostic procedures of anemia have not been specified at all [Bokemeyer et al. 2007; Rizzo et al. 2010a, 2010b] until the guidelines of the National Comprehensive Cancer Network (NCCN) [NCCN, 2012]. As a consequence, laboratory diagnostics are still hardly used prior to ESA therapy in daily practice [Ludwig et al. 2004; Mitchell, 2010; Steinmetz et al. 2011]. Only about 50% of physicians make use of laboratory measurements and most use ferritin only.
Routine practice of triggering ESA treatment of cancer-related anemia based on chemotherapy and low Hb-values only [Steinmetz et al. 2008] has resulted in overall response rates of about 50–60% and the need for blood transfusion has remained high, at about 20–30%. Moreover, during recent years, evidence has been increasing that aggressive ESA treatment as well as blood transfusions may increase all-cause mortality [Spahn et al. 2008; Bohlius et al. 2009] although no such evidence is available if ESAs are used according to the label with target Hb levels between 11–12 g/dl [Glaspy et al. 2010].
The poor response rates to ESA therapy alone and the positive experience with intravenous iron in chronic kidney disease prompted the first study of the combination treatment of ESAs and intravenous iron in cancer patients [Auerbach et al. 2004]. This was followed by six additional studies using iron as an adjunct to ESAs and by four studies using even iron alone in anemic cancer patients. In parallel, the understanding of the pathophysiology of cancer-related anemia has grown substantially [Grotto, 2008] and the detection of hepcidin opened new insights into the regulation of iron metabolism and hematopoiesis [Krause et al. 2000; Park et al. 2001; Goodnough et al. 2010; Thomas et al. 2011]. Consequently, the ACD which has already been known to cause anemia in cancer patients prior to the era of ESAs [Cash and Sears, 1989] came to the fore again [Weiss and Goodnough, 2005] whereas the effect of chemotherapy stood back. With the rediscovery of the concept of ACD the role of iron has changed too. While during the early ESA years iron was used in AID only, the FID described in ACD may explain benefits of iron also in patients with normal or even elevated total body iron. This review focuses on when and how to use iron in cancer-related anemia.
Pathophysiology of anemia in cancer and derived diagnostic tests
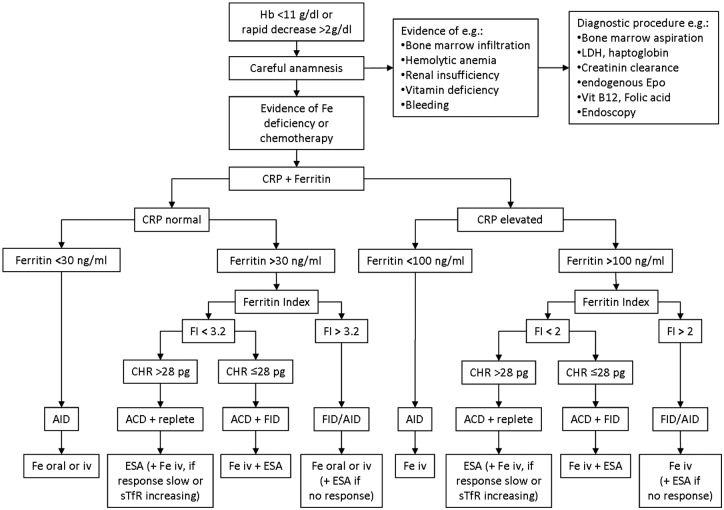
Based on WHO criteria [de Benoist et al. 2008] the definition of anemia is relatively simple: Hb <12 g/dl in nonpregnant women and Hb <13 g/dl in male subjects older than 15 years. However, the problem of anemia in cancer is complex and it is not only challenging to identify causes in any given patient, but also to evaluate the relevance of single factors in general. If indicated by a careful anamnesis, initially bone marrow infiltration, hemolytic anemia, renal insufficiency and vitamin deficiency should be ruled out by bone marrow aspiration, measurement of serum levels of lactate dehydrogenase (LDH) and haptoglobin, of creatinine clearance and endogenous erythropoietin, and of vitamin B12 and folic acid, respectively (Figure 1). However, the diagnosis of iron deficiency is not as easy, as one needs to discriminate AID and FID [NCCN, 2012].
Figure 1.
Proposal of a diagnostic and treatment algorithm (incorporating the diagnostic plot of Thomas and Thomas [2002]). ESA: erythropoietin-stimulating agent; FID, functional iron deficiency; CRP, C-reactive protein; ACD, anemia of chronic disease; AID, absolute iron deficiency; CHR, hypochromic reticulocytes.
AID is a common cause of anemia in cancer patients, most often provoked by bleeding or iatrogenic blood loss, less frequently for dietary reasons. Low values of the erythrocyte indices major histocompatibility complex (MHC), mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) indicate diminished iron incorporation. Most often serum iron (Fe) is low in AID, but strongly varies with inflammatory reactions; moreover, it might be low in FID too. The most important parameter to assess whole body iron is serum ferritin (SF) which is the intracellular storage protein of iron; 1 µg/l SF corresponds to about 8–10 mg of iron stored. AID is defined as SF <30 µg/l and decreased saturation of serum transferrin (TSAT) <15% [NCCN, 2012]. Unfortunately, SF is an acute phase protein. Consequently during an inflammatory reaction, serum levels increase and there is no longer a clear-cut SF threshold indicating AID; however, SF levels <100 ng/ml make AID very likely and are therefore predictive of a good response to Fe iv, without using ESA [Auerbach et al. 2010; Steinmetz et al. 2011]. Alternatively, intracellularly stored iron might be detected through staining of bone marrow smears which is not very practical though. However, the combination of SF, C-reactive protein (CRP), soluble transferrin receptor (sTfR), and low Hb-content of reticulocytes (Ret-Y, CHR) enables the detection of AID [Thomas and Thomas, 2002] even though SF levels might be >1000 µg/l [Steinmetz et al. 2010].
FID is a major contributor to ACD and accordingly also quite prevalent in cancer-associated anemia [Ludwig et al. 2011]. FID in cancer is most often provoked by tumor cells that interact with the immune system and by this cause a chronic state of inflammation along with the release of pro-inflammatory cytokines, such as interleukin-6 (IL-6) or tumor necrosis factor (TNF). In the clinical setting the extent of inflammation could be assessed through measurement of CRP, which is closely related to the serum IL-6 level. Cytokines foster the release of hepcidin, a peptide hormone and key regulator of iron homeostasis [Weiss and Goodnough, 2005]. Hepcidin blocks the membrane-tunnel protein ferroportin which normally transfers iron from the intracellular stores to transferrin, the transport protein in blood. Therefore, in the case of inflammatory reactions, increasing hepcidin levels lock iron in the cells; dietary iron is not released from the enterocytes in the small intestine, nor is storage iron released from the cells of the reticulo-endothelial system (e.g. macrophages, liver cells). As a consequence of the intracellular iron accumulation, the saturation of the iron transporting protein transferrin decreases. Therefore, despite high intracellular iron levels, a FID develops due to the restricted supply of iron to erythropoiesis (iron restricted erythropoiesis [IRE]). NCCN guidelines define FID if TSAT is <20% along with normal or elevated SF levels up to 800 µg/l [NCCN, 2012]. During inflammation SF and hepcidin increase whereas transferrin decreases. As a consequence TSAT might be within the normal range and thus is not a good indicator for intravenous iron [Auerbach et al. 2010; Steinmetz et al. 2011]. Hepcidin might be an alternative, but is unfortunately not yet available as a routine test [Goodnough et al. 2010; Thomas et al. 2011].
As iron is most important for the production of hemoglobin, IRE results in hypochromic anemia which is characterized initially by an increasing number of hypochromic reticulocytes (CHR, Ret-Y), and later by hypochromic erythrocytes (Hypo-Ery) [Brugnara, 2000; Thomas and Thomas, 2002; Goodnough et al. 2010]. In an attempt to compensate for this anemia the quantity of erythropoietic precursor cells (erythroblasts and proerythroblasts) and the number of transferrin receptors on the surface of these precursor cells increase. The transferrin receptor is shed into the plasma where the sTfR concentration correlates with the surface amount. Thus, an elevated level of sTfR reflects an increased number of erythropoietic precursor cells and an increasing iron need for erythropoiesis [Cazzola and Beguin, 1992; Pettersson et al. 1994; Suominen et al. 2000; Beguin, 2002; Lee et al. 2002]. The value of sTfR alone for the differential diagnosis of anemia and for the prediction of treatment response is rather low [Steinmetz et al. 2007], but the ratio of sTfR and the logarithm of serum ferritin (sTfR/log SF), the so-called ferritin index (FI), proved to discriminate FID and ACD [Punnonen et al. 1997]. However, cancer patients often have normal sTfR and FI despite a proven AID [Lee et al. 2002]. This may be explained by direct suppression of erythropoiesis through tumor cells and chemotherapy and a reduced production of endogenous erythropoeitin [Weiss and Goodnough, 2005]. The combination of the FI with the determination of hypochromic reticulocytes enables identification of patients with ACD, repleted iron stores (FI normal), but FID (CHR ≤28 pg) [Thomas and Thomas, 2002].
Studies of iron as an adjunct to ESA for the treatment of anemia associated with chemotherapy
Approximately 30–50% of cancer patients with chemotherapy-related anemia do not respond to ESA alone and even in the remaining patients, response is often slow, not until 4–6 weeks after ESA initiation [Glaspy et al. 1997; Littlewood et al. 2001]. As response to ESA was shown to improve with intravenous iron supplementation in chronic kidney disease [Fishbane et al. 1995; Macdougall et al. 1996; Sepandj et al. 1996], Auerbach and colleagues performed the first randomized con-trolled-pilot trial testing combinations of ESAs and iron in cancer patients with chemotherapy-related anemia [Auerbach et al. 2004] (Table 1). Intravenous iron was superior to oral iron or ESA alone in increasing Hb and quality of life (QoL). Subsequently, five studies [Hedenus et al. 2007; Henry et al. 2007; Bastit et al. 2008; Pedrazzoli et al. 2008; Auerbach et al. 2010] confirmed a better response rate of intravenous iron along with ESAs as compared with ESA alone (Table 1). In three studies using Epoetin alpha or beta, adjunct treatment with intravenous iron increased the Hb response rate (Δ +0.8 to 1.2 g/dl) from 36–53% to 68–93% versus ESAs alone [Auerbach et al. 2004; Hedenus et al. 2007; Henry et al. 2007], i.e. an improvement of 28-40%. In three studies using darbepoetin alpha, the response rate to ESA alone was slightly higher at 62–73%. The addition of intravenous iron improved these rates by about 10–15% to an overall response rate of 77–86%. Moreover, there was a quicker onset of response in four [Hedenus et al. 2007; Bastit et al. 2008; Pedrazzoli et al. 2008; Auerbach et al. 2010] and an improvement of QoL in two [Auerbach et al. 2004; Bastit et al. 2008] out of six studies. The rate of transfusions in the ESA alone and the combined ESA and oral iron groups varied significantly among the six studies from 3% to 30% and in one only study intravenous iron significantly reduced the rate from 26% to 16% [Bastit et al. 2008]. An ESA dose saving effect was demonstrated in another study [Hedenus et al. 2007].
Table 1.
Studies examining iron as an adjunct to ESA.
| Study | Design | Inclusion criteria on iron status | Treatment | N | Baseline |
End of study |
Conclusion regarding Fe iv | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hb [g/dl] | SF [ng/ml] | TSAT [%] | +ΔHb | R rate* | TF rate | ||||||
| [Auerbach et al. 2004] | Multicenter, open label, randomized | SF ≤200 ng/ml or SF ≤300 ng/ml and TSAT <20% | ESA alone: Epoetin alpha 40,000U/we | 36 | 9.5±0.9 | 131± 106 | 15±8 | 0.9 | 25% | 19.5% | Better response and QoL improvement |
| ESA + ferrous sulphate 325 mg bid, p.o. | 43 | 9.7±0.7 | 129±71 | 18±14 | 1.5 | 36% | 7% | ||||
| ESA + Fe dextran 100 mg iv at each visit up to calc. dose, TD 1100–2400 mg | 37 | 9.7±0.8 | 92±68 | 19±17 | 2.5 | 68% | 11% | ||||
| ESA + total dose infusion (TDI) calc. Fe dextran; TD 1000-3000 mg | 41 | 9.4±1.0 | 107±78 | 14±10 | 2.4 | 68% | 12% | ||||
| [Henry et al. 2007] | Multicenter, open label, randomized | SF ≥100 and ≤900 ng/ml or TSAT ≥15 and ≤35% | ESA alone: Epoetin alpha 40,000U/we | 44 | 10.5±0.8 | 388±266 | 36±27 | 1.5 | 41% | 22% | Better response |
| ESA + ferrous sulphate 325 mg tid, p.o. | 44 | 10.3±0.7 | 374±270 | 29±21 | 1.6 | 45% | 10% | ||||
| ESA + ferric gluconate 125 mg qw iv x8; TD 990.9mg (7.9 doses) | 41 | 10.1±0.9 | 322±210 | 29±27 | 2.4 | 73% | 18% | ||||
| [Hedenus et al. 2007] | Multicenter, open label, randomized | SF <800 ng/ml and stainable Fe in bone marrow smear | ESA alone: Epoetin beta 30,000U/we | 34 | 10.3±0.5 | 130 (25–794) | 22 (5–39) | 1.6 | 53% | 3% | Better and quicker response; lower ESA dose |
| ESA + Fe sucrose 100 mg iv qw x7 + q2w x4; TD 1100mg | 33 | 10.3±0.5 | 128 (22–570) | 21 (6–45) | 2.8 | 93% | 6.5% | ||||
| [Bastit et al. 2008] | Multicenter, open label, randomized | SF >10 ng/ml and TSAT >15% and SF <800 ng/ml | ESA: Darbepoetin alpha 500µg q3w, no or oral Fe | 197 i 147 p | 10.0±0.9 | 279±270 | 30±24 | n.r. | 73% i | 26/20%** | Better and quicker response and QoL improvement; lower TF rate; similar safety |
| ESA + Sodium ferric gluconate in sucrose solution or iron sucrose 200 mg q3w iv x5; TD 1000mg | 200 I 134 p | 9.9±0.8 | 280±248 | 28±21 | n.r. | 86% i | 16/9% ** | ||||
| [Pedrazzoli et al. 2008] | Multicenter, open label, randomized | SF >100 + <800 ng/ml and TSAT >20% and < 40% | ESA alone: Darbepoetin alfa 150 µg qw | 76 I 50 p | 9.9±0.8 | 333±232 | 28±11 | n.r. | 61.8% i 70% p | 10% p | Better and quicker response, similar safety |
| ESA + Sodium ferric gluconat 125 mg qw x6 iv, TD 750 mg | 73 I 53 p | 9.9±0.8 | 351±238 | 31±15 | n.r. | 76.7% I 92.5% p | 3.8% p | ||||
| [Auerbach et al. 2010] | Multicenter, double blind, 2x2 factorial | SF >10 + <800 ng/ml and TSAT > 15% | ESA alone: Darbepoetin alfa 300/500 µg q3w | 122 I 88 p | 9.4±1.0 | 323±254 | 26±17 | 1.3 (0.9;1.6) | 72% # | 30% (23, 39) 22% ** (15, 31) | Better and quicker response; similar safety, no significant influence of ESA dose |
| ESA + 400 mg iv iron q3w as a dextran complex in ferric hydroxide TD 3.7 x 400 mg= 1480 mg, 110.3 mg/w | 116 I 76 p | 9.3±1.0 | 302±217 | 27±18 | 1.9 (1.5;2.2) | 82% # | 28% (20, 37) 20% ** (14, 29) | ||||
| [Steensma et al. 2011] | Multicenter, open label, randomized | SF > 20 ng/ml and TSAT < 60% | ESA alone: Darbepoetin alfa 500µg q3w | 163 I 106 p | 10.0±0.7 | 456±479 | 22±13 | 2.4 (1.5) | 65% | 13% | No benefit in response or QoL, but more SAE |
| ESA + oral iron, ferrous sulfate 325 mg | 163 I 113 p | 9.9±0.7 | 480±484 | 20±12 | 2.4 (1.5) | 67% | 13% | ||||
| ESA + Sodium ferric gluconate 187.5 mg q3w x5 iv, TD 937.5mg | 164 I 105 p | 9.9±0.7 | 461±527 | 23±13 | 2.6 (1.5) | 70% | 12% | ||||
| [Steinmetz et al. 2010] | Multicenter, open label, diagnostic-based, not randomized | CHR>28 pg and FI <3.2/2.0 | ESA alone: Epoitin beta 30,000U/we | 204 I 194 p | 9.8±1.1 | 433 (32–8360) | 34±22 | 1.3 (1.5)§ | 53%100% | 41/22%** | subgroups of patients responding to Fe alone or the combination of ESA + iv iron |
| CHR <28 pg and FI <3.2/2.0 | ESA + Fe saccharate 200 mg qw x5-10, TD 1000-2000 mg | 22 I 21 p | 9.4±1.0 | 432 (96–3911) | 19±8 | 2.3 (2.0)§ | 100% | 33/19%** | |||
| CHR>28 pg and FI > 3.2/2.0 | No ESA; oral ferrous sulphate 300 mg/d or Fe saccharate 200 mg qw iv | 40 I 31 p | 10.2±1.0 | 223 (11–1423) | 21±16 | 1.6 (2.4)§ | 48% | 19/3% ** | |||
| CHR <28pg and FI > 3.2/2.0 | 20 I 19 p | 9.8±1.0 | 83 (13–1440) | 17±14 | 1.5 (1.5)§ | 74% | 16/5% ** | ||||
Values are given as means ± standard deviation, as means (lower, higher limit of 95% confidence Interval), or as median (minimum–maximum)
Responder: patients who had achieved a maximum Hb level > 12 (# ≥ 11 g/dl) or an increase in Hb of >2 g/dL during the study (§ until week 8);
Week 5 to end of treatment period.
ITT: intent to treat.
PP: per protocol.
TD: Total iv iron dose given (mean) or per protocol; bid: twice/day; tid: thrice/day; qw: once weekly; q2w: every other week; q3w: every third week; iv: intravenous; n.d.: not determined; calc.: calculation of iron dose, formula to reach a desired Hb level of 140 g/l: dose (ml) = 0.0442 (desired Hb – observed Hb) x LBW + (0.26 x LBW), where LBW is the patient’s lean body weight in kg; QoL: quality of life; (S)AE: (serious) adverse event, n.r.: not reported; CHR: Hb content of reticulocytes in pg; FI: ferritin index (soluble transferrin receptor (sTfR) in mg/l/log ferritin in μg/l, CRP values higher than 5 mg/L, the cutoff for the FI is 2.0 instead of 3.2); ESA: erythropoietin-stimulating agent; TSAT: transferrin saturation; SF: serum ferritin; Hb: hemoglobin.
There was only one study that despite its large size failed to demonstrate significant clinical benefit of adjunct intravenous iron [Steensma et al. 2011]. A higher Hb response in the intravenous iron group was not significant, improvement of QoL was comparable and rates of transfusion were the same in all three study arms. Moreover, the study reported a higher rate of serious adverse events (SAEs) in the intravenous iron group as compared with the oral or no iron groups (55% versus 45% and 46%, respectively). Aapro and colleagues argued that the study might have failed due to the relatively low total (937.5 mg iron) and weekly iron dose (62.5 mg) and the high dropout rate due to which many patients did not receive the planned dose [Aapro et al. 2011]. Indeed, patients who received at least 750 mg of iron had a higher response rate than those who received less than 750 mg intravenous, oral or no iron (80% versus 56%, 67% and 65%, respectively) [Steensma, 2011].
Studies using iron alone for treatment of anemia in cancer patients
The first study investigating intravenous iron alone in chemotherapy-associated anemia was performed in women with cervical cancer treated with chemoradiotherapy [Kim et al. 2007] (Table 2). The primary objective was to prevent exacerbation of anemia and to reduce the transfusion volume through intravenous iron sucrose. In the setting of this single-center trial the transfusion rate dropped from 64% to 40%. Another single-center, prospective, open-label, randomized study explored whether intravenous iron reduces red blood cell (RBC) transfusions in anemic gynecologic cancer patients receiving platinum-based chemotherapy [Dangsuwan and Manchana, 2010]. Again, this was a small study enrolling 22 patients per arm only, but intravenous iron resulted in a significant Hb increase of 0.9 g/dl and a significant reduction of the transfusion rate from 63.6% to 22.7%. While in both of these studies [Kim et al. 2007; Dangsuwan and Manchana, 2010] all patients received intravenous iron regardless of patients’ actual iron status, in the German TANDEM trial patients were assigned to different treatment regimens based on the results of the diagnostic plot [Thomas and Thomas, 2002]. This diagnostic tool revealed in 207 (68%) out of 303 patients with a baseline ferritin ≥20 ng/ml no iron deficiency and were treated with ESAs alone. A total of 23 patients (8%) had ACD with FID and received ESA + intravenous iron, while 46 (15%) and 27 (9%) patients had mild and distinct AID, respectively, and received either oral or intravenous iron alone. Response rates were comparable in all groups, but the need for transfusions was slightly lower in groups receiving iron only [Steinmetz et al. 2010] (Table 1).
Table 2.
Studies examining intravenous iron alone
| Study | [Kim et al. 2007] | [Dangsuwan and Manchana, 2010] | [Steinmetz et al. 2011] | |||
|---|---|---|---|---|---|---|
| Design | single center, prospective, open label, randomized | single center, prospective, open label, randomized | multi-center, prospective, non-interventional | |||
| Objective | To prevent exacerbation of anemia and reduce the transfusion volume in cervical cancer patients treated with chemoradiotherapy | To reduce RBC transfusions in anemic gynecologic cancer patients receiving platinum-based chemotherapy | To evaluate the effectiveness and tolerability of FCM in routine treatment of anemia in cancer patients | |||
| Fe treatment | - | Iv iron sucrose 200 mg in 200 ml NaCl if 10 < Hb < 12 g/dl | 200 mg of oral ferrous tid | Iv iron sucrose 200 mg in 200 ml NaCl if Hb <10 g/dl | FCM dose and frequency at discretion of the oncologist | FCM dose and frequency and ESA treatment at discretion of the oncologist |
| Fe inclusion criteria | None | none | Anemia and need of iron at discretion of the oncologist | |||
| N of patients | 45 | 30 | 22 | 22 | 347 | 73 |
| TD Fe iv [mg] | - | n.r. | n.r. | n.r. | 1000 (600, 1500) | |
| Hb [g/dl] | 11.33 (2.14) | 11.27 (1.94) | 9.0 (0.6) | 8.9 (0.6) | 10.1 (1.0) | 9.6 (1.1) |
| SF [ng/ml] | n.r. | n.r. | n.r. | n.r. | 334 (500) | 461 (491) |
| TSAT [%] | n.r. | n.r. | n.r. | n.r. | 15 (16) | 27 (30) |
| +ΔHb [g/dl] | n.r. | n.r. | 0.4 (−2.1 to 3.0) | 0.9 (−0.9 to 2.6) | 1.4 (0.2, 2.3) | 1.6 (0.7, 2.4 |
| TF rate | 64% | 40% | 63.6% | 22.7% | Week 1-4 12% Week >5 7% | Week 1-4 23% Week >5 11% |
| Conclusion regarding Fe iv | Reduced TF rate; similar rate of AE and SAE | Reduced TF rate; superior Hb increase; similar rate of AE and SAE | High efficacy of FCM, even without ESA; good tolerability and safety | |||
Baseline levels given as means (SD) or median (min to max) or median (25%, 75%ile). For abbreviations see Table 1.
The so far largest cohort of cancer patients treated for anemia with iron alone was in an observational study in Germany enrolling a total of 420 patients [Steinmetz et al. 2011]. Of these 347 (82.6%) received ferric carboxymaltose (FCM) as intravenous iron preparation only, the remaining 73 (17.3%) patients received FCM as an adjunct to ESA. About three out of four patients (74.3%) received cytotoxic chemotherapy concomitantly. At baseline, median Hb was 10.0 g/dl (95% confidence interval [CI] 9.1–10.6), ferritin was ≤100 ng/ml in 37.5%, and transferrin saturation <20% in 75.6% of patients. Average increase in Hb levels was 1.4 g/dL (median; 25%ile, 75%ile: 0.2, 2.3) in the overall population, 1.4 g/dl (0.3, 2.3) in patients censored for transfusions during the study, 1.4 g/dl (0.2, 2.3) in patients who received FCM only, and 1.6 g/dl (0.7, 2.4) in patients who received FCM plus ESA. The transfusion rate between week 5 and end of observation amounted to 7% and 11% of patients treated with FCM and FCM + ESA, respectively.
Safety of intravenous iron
There are many oral iron formulations, but in Europe only six different preparations are available for intravenous use (Table 3). Most randomized studies (Tables 1 and 2) made use of iron natrium–gluconate–sucrose complex (in Europe: Ferrlecit®, Sanofi-Aventis) as an adjunct to ESAs. All but one of these studies showed similar rates of adverse events (AEs) in patients receiving ESAs alone, ESAs plus oral iron or ESAs plus intravenous iron. One study [Steensma et al. 2011] revealed a substantially higher rate of SAEs in the intravenous iron arm (darbepoetin plus ferric natrium–gluconate–sucrose complex). It was conjectured that these may be due to the three single doses of ferric–gluconate given concomitantly with ESAs, instead of two doses as per the drug label [Aapro et al. 2011]. However, sometimes life-threatening anaphylactic reactions as with older iron-dextran solutions have never been observed in cancer trials. In particular, the current high molecular weight sequestrants such as sucrose, isomaltosid and carboxymaltose have an excellent compatibility, not requiring a test dose at therapy initiation. In over 600 cancer patients treated with ferric carboxymaltose the rate of possibly or probably drug-related AEs, mainly nausea and diarrhea, were reported for 2.3% (n = 14) of patients only. Three SAEs comprised one death after a possibly related respiratory insufficiency in a 74-year-old man with advanced, pulmonary-metastasized head and neck cancer and two unlikely related cases of tachycardia and dyspnea. However, in none of the trials published so far has there been any signal for initially discussed serious adverse drug reactions that might occur with intravenous iron in cancer patients such as increased risk of infection, tumor progression and problems associated with an iatrogenic hemochromatosis, e.g. cardiovascular or thromboembolic events [Marx, 2002; Bailie et al. 2005; Chertow et al. 2006; Auerbach et al. 2007].
Table 3.
Iron preparations for intravenous use.
| Formulation (brand, company) | Iron [mg/ml] | Volume and Fe content / vial | Dosing instructions |
|---|---|---|---|
| Ferric natrium-gluconat-sucrose complex (EU: Ferrlecit®, Sanofi-Aventis; US: Ferrlecit®; Watson Pharma, Inc) | 12.5 | 3.2 ml = 40.0 mg 5.0 ml = 62.5 mg | Very slow injection or infusion with 62.5 mg in 100–250 ml over 30 min |
| Ferric hydroxyd-dextran complex (EU: Cosmofer®, Pharmacosmos, Holbek, Denmark) | 50 | 2.0 ml = 100 mg 5.0 ml = 250 mg 10.0 ml = 500 mg | 25 mg iron as a test-dose in 15 min.; 60 min later: Infusion maximal 200 mg iron in 100 ml in at least 30 min. |
| Maximum dose: 20 mg/kg body weight in 4–6 h | |||
| Ferric hydroxid-saccharose complex (EU: FerMed®, Medice Arzneimittel Pütter, Iserlohn, Germany) | 20 | 5.0 ml = 100 mg | Not exceeding 200 mg iron in 200 ml in at least 30 min. |
| Maximum dose: 200 mg per day | |||
| Ferric hydroxid-saccharose complex (EU: Venofer®, Vifor Pharma, Bern, Switzerland; US: Venofer®; American Regent, Inc) | 20 | 2.5ml = 50 mg 5.0ml = 100 mg | 25 mg iron as a test dose in 15 min. |
| Not exceeding 200 mg iron in 200 ml in at least 30 min. | |||
| Maximum dose: 200 mg per day | |||
| Ferric carboxymaltose (EU: Ferinject®, Vifor Pharma, Bern, Switzerland) | 50 | 2.0 ml = 100 mg 10.0 ml = 500 mg | Test dose not recommended; not exceeding 1000 mg iron in 250 ml in at least 15 min. |
| Maximum dose: 1000 mg per day | |||
| Iron isomaltosid (EU: Monofer®, Pharmacosmos, Holbek, Denmark) | 100 | 1.0 ml = 100 mg 2.0 ml = 200 mg 5.0 ml = 500 mg 10.0 ml = 1000 mg | Test dose not recommended; bolus injection of maximal 200 mg in 4 min; not exceeding 20 mg iron per kg body weight in 500 ml in at least 60 min. |
| Maximum dose: 20 mg/kg body weight per day |
Abbreviations: min: minutes; h: hour; EU: Europe; US: United States of America
How should we manage anemia in cancer patients?
When and by which diagnostic parameters should we assess iron metabolism?
In patients being treated for cancer the cause of anemia should be investigated if Hb rapidly decreases by >2 g/dl or falls below 11 g/dl (Figure 1). Based on current practice guidelines (Table 4) at least SF and TSAT should be measured in addition to careful anamnesis. The assumption of FID should not be restricted to patients with SF <800–1000 ng/ml only, but additional parameters such as FI and hypochromic reticulocytes should be considered [Cazzola and Beguin, 1992; Pettersson et al. 1994; Suominen et al. 2000; Beguin, 2002; Lee et al. 2002; Thomas and Thomas, 2002].
Table 4.
Practice guidelines recommendations of iron management.
| Canadian guidelines [Mikhael et al. 2007] | EORTC [Bokemeyer et al. 2007; Aapro and Link, 2008] | ASH/ASCO [Rizzo et al. 2010] | NCCN [NCCN, 2012] | |
|---|---|---|---|---|
| Iron monitoring | Baseline and periodic monitoring | Baseline (exclude ID) | Baseline and periodic monitoring | Hb <11 g/dl or >2 g/dl below baseline. |
| Patients considered for ESA therapy | ||||
| Iron parameter | Ferritin, TSAT | Not defined | TSAT or ferritin | Reticulocytes, MCV, ferritin, TSAT |
| Definition of ID | SF <100ng/mL, TSAT <15% | Not defined | Not defined | AID: SF <30 ng/mL, TSAT <15% |
| FID: SF ≤800 ng/mL, TSAT <20% | ||||
| Iron supplement | ID: iron iv first + ESA (SF <1000 ng/mL, TSAT <35%) | ID correction | ID correction iron iv to be considered to reduce ESA need, but not as standard of care | AID: iron iv or oral |
| AID and FID: iron iv | FID: iron iv as adjunct to ESA | |||
| Iron iv dosing regimen | IS: 100 mg QW, 200 mg Q2–3W | No dosing recommendation | No dosing recommendation | IS: 200 mg Q2–3W |
| SFG: 125 mg QW | SFG: 8x 125 mg QW or 5x 200 mg (3-4h) Q3W | |||
| LWID: 100 mg QW or TDI | Max 1000 mg total dose LWID: 100 mg |
EORTC, European Organisation for Research and Treatment of Cancer; ID, iron deficiency; SF, serum ferritin ; AID, absolute iron deficiency; FID, functional iron deficiency; IS, iron sucrose; SFG, sodium ferric gluconate; LWID, low molecular weight iron dextran; ESA: erythropoietin-stimulating agent; TSAT: transferrin saturation; SF: serum ferritin; Hb: hemoglobin.
Which patients should be treated with iron?
Patients with AID should always be treated with iron. In patients with FID iron should be considered as an adjunct to ESAs (Figure 1). There is some evidence that the response to ESAs might be enhanced even in patients not meeting the definition of AID and FID, e.g. patients with TSAT >15% and SF >10 ng/ml [Bastit et al. 2008] (Table 2). The noninterventional study with ferric carboxymaltose described an effective intravenous iron-only therapy even in patients with SF >500 ng/ml [Steinmetz et al. 2011] (Table 2). Moreover, patients without any evidence of FID at baseline may also develop FID under ESA therapy as indicated by an increase of sTfR and FI [Steinmetz et al. 2010]. There is still an urgent need for prospective trials optimizing anemia treatment based on baseline assessment and monitoring of iron status and erythropoiesis.
Should we treat patients with oral or intravenous iron?
Three studies prospectively tested oral versus intravenous iron and none showed a significant benefit of oral iron [Auerbach et al. 2004; Henry et al. 2007; Steensma et al. 2011]. This might be due to hindered oral intake during chemotherapy and of course due to the common inflammation reaction giving rise to the release of hepcidin preventing enterocytes from iron discharge into the circulation. In patients with AID without inflammation oral iron will be effective. On the other hand, intravenous iron always entails a quicker response and lacks the risk of incomplete absorption and of side effects such as gastrointestinal discomfort and constipation.
What is the optimal intravenous dose of iron?
In AID the need for iron might be calculated based on the formula of Ganzoni:
Total Fe dose [mg] = body weight [kg] × (target-Hb − actual Hb [g/dl]) × 2.4 + (500–1000 mg Fe).
However, this approach was used in one trial only [Auerbach et al. 2004] which was based on a modified formula calculated a need of 1000–3000 mg iron. The other studies combining ESAs with iron used a total dose between 750 and 2000 mg at a fixed dose schedule (Table 1). Only in the first one [Auerbach et al. 2004] did a small group of patients (n = 41) receive the total dose as one infusion. The response was the same as in patients receiving the weekly schedule. One-week infusion intervals with single doses of 100–200 mg were used in five out of eight combined studies whereas in the remaining three studies there were 3-week intervals with single doses of 187–400 mg, corresponding to weekly doses of 62.5–133 mg. A weekly dose of 62.5 mg was discussed to be too low as the study failed to show a significant benefit of intravenous iron [Aapro et al. 2011; Steensma et al. 2011]. In the observational study with ferric carboxymaltose patients received iron only at single doses of 500 or 1000 mg and on average a total dose of 1000 mg (median). Thus, the total dose might be 1000 mg or more, but future dose optimization studies will be helpful.
Which intravenous preparation should be used?
Former reluctance in using intravenous iron was mostly driven by safety concerns, including life-threatening anaphylactic reactions. All of today’s preparations show good or excellent tolerability (Table 3) and in particular macromolecular sequestrants such as carboxymaltose or isomaltosid allow for fast infusion of high total iron doses at low rates of side effects. Most experience in the cancer patient population is available for ferric carboxymaltose.
In summary, iron proved efficacious and effective for the treatment of cancer-associated anemia. Most studies confirmed an increased efficacy of the combination of ESAs with intravenous iron, but iron alone may be a useful option too. However, there is still an urgent need for trials investigating diagnostic approaches for the optimal tailoring of iron and/or ESA therapy in cancer patients with anemia.
Acknowledgments
Thanks are due to Uwe Totzke for reviewing and revising the draft version of the manuscript.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr. Steinmetz was engaged in clinical studies for Amgen, Roche, Ortho Biotec - Janssen Cilag, Vifor, as a consultant in ad-boards for Amgen, Roche, Ortho Biotec - Janssen Cilag, Vifor, Medice/Pharmacosmos, Holbek, and received grants for clinical studies for Amgen, Roche, Vifor.
References
- Aapro M., Beguin Y., Birgegard G., Gascon P., Hedenus M., Osterborg A. (2011) Too-low iron doses and too many dropouts in negative iron trial? J Clin Oncol 29: e525-e526; author reply e527–e528 [DOI] [PubMed] [Google Scholar]
- Aapro M.S., Link H. (2008) September 2007 update on EORTC guidelines and anemia management with erythropoiesis-stimulating agents. Oncologist 13(Suppl. 3): 33–36 [DOI] [PubMed] [Google Scholar]
- Auerbach M., Ballard H., Glaspy J. (2007) Clinical update: intravenous iron for anaemia. Lancet 369: 1502–1504 [DOI] [PubMed] [Google Scholar]
- Auerbach M., Ballard H., Trout J.R., McIlwain M., Ackerman A., Bahrain H., et al. (2004) Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol 22: 1301–1307 [DOI] [PubMed] [Google Scholar]
- Auerbach M., Silberstein P.T., Webb R.T., Averyanova S., Ciuleanu T.E., Shao J., et al. (2010) Darbepoetin alfa 300 or 500 mug once every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. Am J Hematol 85: 655–663 [DOI] [PubMed] [Google Scholar]
- Bailie G.R., Clark J.A., Lane C.E., Lane P.L. (2005) Hypersensitivity reactions and deaths associated with intravenous iron preparations. Nephrol Dial Transplant 20: 1443–1449 [DOI] [PubMed] [Google Scholar]
- Barrett-Lee P., Bokemeyer C., Gascon P., Nortier J.W., Schneider M., Schrijvers D., et al. (2005) Management of cancer-related anemia in patients with breast or gynecologic cancer: new insights based on results from the European Cancer Anemia Survey. Oncologist 10: 743–757 [DOI] [PubMed] [Google Scholar]
- Bastit L., Vandebroek A., Altintas S., Gaede B., Pinter T., Suto T.S., et al. (2008) Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. J Clin Oncol 26: 1611–1618 [DOI] [PubMed] [Google Scholar]
- Beguin Y. (2002) Prediction of response and other improvements on the limitations of recombinant human erythropoietin therapy in anemic cancer patients. Haematologica 87: 1209–1221 [PubMed] [Google Scholar]
- Birgegard G., Gascon P., Ludwig H. (2006) Evaluation of anaemia in patients with multiple myeloma and lymphoma: findings of the European CANCER ANAEMIA SURVEY. Eur J Haematol 77: 378–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlius J., Schmidlin K., Brillant C., Schwarzer G., Trelle S., Seidenfeld J., et al. (2009) Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet 373: 1532–1542 [DOI] [PubMed] [Google Scholar]
- Bokemeyer C., Aapro M.S., Courdi A., Foubert J., Link H., Osterborg A., et al. (2007) EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer 43: 258–270 [DOI] [PubMed] [Google Scholar]
- Brownlie T.t., Utermohlen V., Hinton P.S., Haas J.D. (2004) Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr 79: 437–443 [DOI] [PubMed] [Google Scholar]
- Brugnara C. (2000) Reticulocyte cellular indices: a new approach in the diagnosis of anemias and monitoring of erythropoietic function. Crit Rev Clin Lab Sci 37: 93–130 [DOI] [PubMed] [Google Scholar]
- Cash J.M., Sears D.A. (1989) The anemia of chronic disease: spectrum of associated diseases in a series of unselected hospitalized patients. Am J Med 87: 638–644 [DOI] [PubMed] [Google Scholar]
- Cazzola M., Beguin Y. (1992) New tools for clinical evaluation of erythron function in man. Br J Haematol 80: 278–284 [DOI] [PubMed] [Google Scholar]
- Chertow G.M., Mason P.D., Vaage-Nilsen O., Ahlmen J. (2006) Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant 21: 378–382 [DOI] [PubMed] [Google Scholar]
- Dangsuwan P., Manchana T. (2010) Blood transfusion reduction with intravenous iron in gynecologic cancer patients receiving chemotherapy. Gynecol Oncol 116: 522–525 [DOI] [PubMed] [Google Scholar]
- de Benoist B., McLean E., Egli I., Cogswell M. (2008) Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. Geneva, Switzerland: World Health Organization [Google Scholar]
- Dubray B., Mosseri V., Brunin F., Jaulerry C., Poncet P., Rodriguez J., et al. (1996) Anemia is associated with lower local-regional control and survival after radiation therapy for head and neck cancer: a prospective study. Radiology 201: 553–558 [DOI] [PubMed] [Google Scholar]
- Fein D.A., Lee W.R., Hanlon A.L., Ridge J.A., Langer C.J., Curran W.J., Jr, et al. (1995) Pretreatment hemoglobin level influences local control and survival of T1-T2 squamous cell carcinomas of the glottic larynx. J Clin Oncol 13: 2077–2083 [DOI] [PubMed] [Google Scholar]
- Fishbane S., Frei G.L., Maesaka J. (1995) Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am J Kidney Dis 26: 41–46 [DOI] [PubMed] [Google Scholar]
- Glaser C.M., Millesi W., Kornek G.V., Lang S., Schull B., Watzinger F., et al. (2001) Impact of hemoglobin level and use of recombinant erythropoietin on efficacy of preoperative chemoradiation therapy for squamous cell carcinoma of the oral cavity and oropharynx. Int J Radiat Oncol Biol Phys 50: 705–715 [DOI] [PubMed] [Google Scholar]
- Glaspy J., Bukowski R., Steinberg D., Taylor C., Tchekmedyian S., Vadhan-Raj S. (1997) Impact of therapy with epoetin alfa on clinical outcomes in patients with nonmyeloid malignancies during cancer chemotherapy in community oncology practice. Procrit Study Group. J Clin Oncol 15: 1218–1234 [DOI] [PubMed] [Google Scholar]
- Glaspy J., Crawford J., Vansteenkiste J., Henry D., Rao S., Bowers P., et al. (2010) Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer 102: 301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnough L.T., Nemeth E., Ganz T. (2010) Detection, evaluation, and management of iron-restricted erythropoiesis. Blood 116: 4754–4761 [DOI] [PubMed] [Google Scholar]
- Grotto H.Z. (2008) Anaemia of cancer: an overview of mechanisms involved in its pathogenesis. Med Oncol 25: 12–21 [DOI] [PubMed] [Google Scholar]
- Hedenus M., Birgegard G., Nasman P., Ahlberg L., Karlsson T., Lauri B., et al. (2007) Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: a randomized multicenter study. Leukemia 21: 627–632 [DOI] [PubMed] [Google Scholar]
- Henry D.H., Dahl N.V., Auerbach M., Tchekmedyian S., Laufman L.R. (2007) Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 12: 231–242 [DOI] [PubMed] [Google Scholar]
- Kim Y.T., Kim S.W., Yoon B.S., Cho H.J., Nahm E.J., Kim S.H., et al. (2007) Effect of intravenously administered iron sucrose on the prevention of anemia in the cervical cancer patients treated with concurrent chemoradiotherapy. Gynecol Oncol 105: 199–204 [DOI] [PubMed] [Google Scholar]
- Krause A., Neitz S., Magert H.J., Schulz A., Forssmann W.G., Schulz-Knappe P., et al. (2000) LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett 480: 147–150 [DOI] [PubMed] [Google Scholar]
- Lee E.J., Oh E.J., Park Y.J., Lee H.K., Kim B.K. (2002) Soluble transferrin receptor (sTfR), ferritin, and sTfR/log ferritin index in anemic patients with nonhematologic malignancy and chronic inflammation. Clin Chem 48: 1118–1121 [PubMed] [Google Scholar]
- Littlewood T.J., Bajetta E., Nortier J.W., Vercammen E., Rapoport B. (2001) Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol 19: 2865–2874 [DOI] [PubMed] [Google Scholar]
- Ludwig H., Müldür E., Endler G., Hübl W., Moneuse P., Klement B. (2011) High prevalence of iron deficiency across different tumors correlates with anemia, increases during cancer treatment and is associated with poor performance status. Haematologica 96: Abstract; 982 [Google Scholar]
- Ludwig H., Van Belle S., Barrett-Lee P., Birgegard G., Bokemeyer C., Gascon P., et al. (2004) The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 40: 2293–2306 [DOI] [PubMed] [Google Scholar]
- Macdougall I.C., Tucker B., Thompson J., Tomson C.R., Baker L.R., Raine A.E. (1996) A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int 50: 1694–1699 [DOI] [PubMed] [Google Scholar]
- Marx J.J. (2002) Iron and infection: competition between host and microbes for a precious element. Best Pract Res Clin Haematol 15: 411–426 [PubMed] [Google Scholar]
- Mikhael J., Melosky B., Cripps C., Rayson D., Kouroukis C.T. (2007) Canadian supportive care recommendations for the management of anemia in patients with cancer. Curr Oncol 14: 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. (2010) A European Patient Record Study on Diagnosis and Treatment of Chemotherapy-induced Anaemia. Milan, Italy: ESMO; [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCN (2012) Practice Guidelines in Oncology; Cancer and Chemotherapy-Induced Anemia. Available at: http://www.nccn.org/professionals/physician_gls/pdf/anemia.pdf
- Park C.H., Valore E.V., Waring A.J., Ganz T. (2001) Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276: 7806–7810 [DOI] [PubMed] [Google Scholar]
- Pedrazzoli P., Farris A., Del Prete S., Del Gaizo F., Ferrari D., Bianchessi C., et al. (2008) Randomized trial of intravenous iron supplementation in patients with chemotherapy-related anemia without iron deficiency treated with darbepoetin alpha. J Clin Oncol 26: 1619–1625 [DOI] [PubMed] [Google Scholar]
- Pettersson T., Kivivuori S.M., Siimes M.A. (1994) Is serum transferrin receptor useful for detecting iron-deficiency in anaemic patients with chronic inflammatory diseases? Br J Rheumatol 33: 740–744 [DOI] [PubMed] [Google Scholar]
- Punnonen K., Irjala K., Rajamaki A. (1997) Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood 89: 1052–1057 [PubMed] [Google Scholar]
- Rizzo J.D., Brouwers M., Hurley P., Seidenfeld J., Arcasoy M.O., Spivak J.L., et al. (2010a) American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol 28: 4996–5010 [DOI] [PubMed] [Google Scholar]
- Rizzo J.D., Brouwers M., Hurley P., Seidenfeld J., Arcasoy M.O., Spivak J.L., et al. (2010b) American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood 116: 4045–4059 [DOI] [PubMed] [Google Scholar]
- Rizzo J.D., Lichtin A.E., Woolf S.H., Seidenfeld J., Bennett C.L., Cella D., et al. (2002) Use of epoetin in patients with cancer: evidence-based clinical practice guidelines of the American Society of Clinical Oncology and the American Society of Hematology. Blood 100: 2303–2320 [DOI] [PubMed] [Google Scholar]
- Sepandj F., Jindal K., West M., Hirsch D. (1996) Economic appraisal of maintenance parenteral iron administration in treatment of anaemia in chronic haemodialysis patients. Nephrol Dial Transplant 11: 319–322 [DOI] [PubMed] [Google Scholar]
- Spahn D.R., Moch H., Hofmann A., Isbister J.P. (2008) Patient blood management: the pragmatic solution for the problems with blood transfusions. Anesthesiology 109: 951–953 [DOI] [PubMed] [Google Scholar]
- Steensma D.P. (2011) Art of oncology: new voices wanted. J Clin Oncol 29: 3343–3344 [DOI] [PubMed] [Google Scholar]
- Steensma D.P., Sloan J.A., Dakhil S.R., Dalton R., Kahanic S.P., Prager D.J., et al. (2011) Phase III, randomized study of the effects of parenteral iron, oral iron, or no iron supplementation on the erythropoietic response to darbepoetin alfa for patients with chemotherapy-associated anemia. J Clin Oncol 29: 97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz H.T., Tsamaloukas A., Schmitz S., Wiegand J., Rohrberg R., Eggert J., et al. (2010) A new concept for the differential diagnosis and therapy of anaemia in cancer patients. Support Care Cancer 19: 261–269 [DOI] [PubMed] [Google Scholar]
- Steinmetz T., Hellmich M., Neise M., Aldaud A., Lerchenmuller C., Tsamaloukas A., et al. (2007) Prediction of the responsiveness to treatment with erythropoiesis-stimulating factors: a prospective clinical study in patients with solid tumors. Oncologist 12: 748–755 [DOI] [PubMed] [Google Scholar]
- Steinmetz T., Totzke U., Schweigert M., Mittermuller J., Nawka S., Tesch H., et al. (2011) A prospective observational study of anaemia management in cancer patients - results from the German Cancer Anaemia Registry. Eur J Cancer Care (Engl) 20: 493–502 [DOI] [PubMed] [Google Scholar]
- Steinmetz T., Totzke U., Soling U., Groschek M., Mittermuller J., Schweigert M., et al. (2008) Hemoglobin levels that trigger erythropoiesis-stimulating agent treatment decisions for cancer-associated anemia–examination of practice in Germany. Curr Med Res Opin 24: 2751–2756 [DOI] [PubMed] [Google Scholar]
- Steinmetz T., Tschechne B., Virgin G., Klement B., Franzem M., Wamhoff J., et al. (2011) Ferric Carboxymaltose for the Correction of Cancer- and Chemotherapy-associated Anemia in Clinical Practice. London: EHA [Google Scholar]
- Suominen P., Mottonen T., Rajamaki A., Irjala K. (2000) Single values of serum transferrin receptor and transferrin receptor ferritin index can be used to detect true and functional iron deficiency in rheumatoid arthritis patients with anemia. Arthritis Rheum 43: 1016–1020 [DOI] [PubMed] [Google Scholar]
- Thomas C., Kobold U., Balan S., Roeddiger R., Thomas L. (2011) Serum hepcidin-25 may replace the ferritin index in the Thomas plot in assessing iron status in anemic patients. Int J Lab Hematol 33: 187–193 [DOI] [PubMed] [Google Scholar]
- Thomas C., Thomas L. (2002) Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem 48: 1066–1076 [PubMed] [Google Scholar]
- Verdon F., Burnand B., Stubi C.L., Bonard C., Graff M., Michaud A., et al. (2003) Iron supplementation for unexplained fatigue in non-anaemic women: double blind randomised placebo controlled trial. BMJ 326: 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J.S., O’Brien M.E., Ashley S. (2002) Management of anemia in patients receiving chemotherapy. J Clin Oncol 20: 601–603 [DOI] [PubMed] [Google Scholar]
- Weiss G., Goodnough L.T. (2005) Anemia of chronic disease. N Engl J Med 352: 1011–1023 [DOI] [PubMed] [Google Scholar]