Abstract
Basal cell carcinoma (BCC) is the most common paraneoplastic disease among human neoplasms. The tumor affects mainly photoexposed areas, most often in the head and seldom appears on genitalia and perigenital region. BCC progresses slowly and metastases are found in less than 0.5% of the cases; however, a considerable local destruction and mutilation could be observed when treatment is neglected or inadequate. Different variants as nodular, cystic, micronodular, superficial, pigment BCC are described in literature and the differential diagnosis in some cases could be difficult. The staging of BCC is made according to Tumor, Node, Metastasis (TNM) classification and is essential for performing the adequate treatment. Numerous therapeutic methods established for treatment of BCC, having their advantages or disadvantages, do not absolutely dissolve the risk of relapses. The early diagnostics based on the good knowledge and timely organized and adequate treatment is a precondition for better prognosis. Despite the slow progress and numerous therapeutic methods, the basal cell carcinoma should not be underestimated.
Keywords: Basal cell carcinoma, clinical variants, stages, treatment
INTRODUCTION
Basal cell carcinoma (BCC) constitutes about 70% of keratinocyte tumors that comprise 90% of all malign skin diseases.[1,2] The incidence of BCC is about 2000 cases per 100 000 population, and the morbidity varies depending on the geographic width and the patients’ age, with growing tendency for individuals above 50 years of age. The risk for Caucasian race individual to develop BCC varies between 33 and 39% for men and 23 and 28% for women. The BCC affects mainly photoexposed areas, in about 80% of patients it appears in the head, and in half of them affects the skin of cheeks and the nose.[2] The other photoexposed areas such as the trunk and the limbs are less affected and in about 4% of patients lesions may appear on genitals and perianal area.[3] The tumor has slow progression and metastases are found in only 0.5% of the cases,[4] but it can result in considerable local destruction and disfigurement when treatment is neglected or inadequate. The high incidence of disease determines the big medical and social importance of this type of carcinomas.
The main etiological factor responsible for BCC is the chronic UV exposure at the expense mostly of UVB rays with length 290-320 mm.[5] This results in activating of proto-oncogenes and inactivation of tumor suppressive genes in the keratinocytes. The high doses of UV light induct free oxygen radicals, which combined with the reduced antioxidant protection system result in different degeneration processes inclusive carcinogenesis. The UV rays induct production of pyramidine dimers and loss of heterozygosity of both tumor suppressive (protective) genes-TP53 and PTCH, resulting in BCC as a sequence of microsatellite instability in selected tetra nucleotide combinations of the coding genes.
The genetic analysis of patients, not only with sporadic BCC, but also with autozome dominant Nevoid basal cell syndrome found mutations in PTCH1 gene located in 9q22.3. The tumor-suppressive gene P53 and melanocortin-1 reception gene[6] are also important for the development of neoplastic process.
Besides ultraviolet radiation there are other exogenous carcinogens such as exposure to the ionizing radiation, arsenic,[7] industrial chemical substances such as vinyl chloride,[8] polycyclic aromatic hydrocarbonates,[9] as well as alkalizing agents.
The role of the immune system suppression in the pathogenesis of skin carcinomas is also suspected since the incidence of BCC increased among immune suppressed patients and the lesions affect mainly the photo nonexposed skin of the body and the upper limbs.
CLINICAL VARIANTS OF BASAL CELL CARCINOMA
Nodular basal cell carcinoma
Nodular basal cell carcinoma comprises about 60-80% of the cases and occurs most often on the skin of the head. Clinically it is presented by elevated, exophytic pearl-shaped nodules with telangiectasie on the surface and periphery [Figure 1]. Subsequently, nodular BCC can extend into ulcerative or cystic pattern. The endophytic nodules are presented clinically as flat enduring plaques. The hemorrhagic lesions can resemble hemangioma or melanoma, especially if are pigmented. The lesions with big sizes and the central necrosis are defined as ulcus rodens. Histology revel nest-like infiltration from basaloid cells. [Figure 2]. Differential diagnosis can be made by traumatically changed dermal nevus and amelanotic melanoma.
Figure 1.
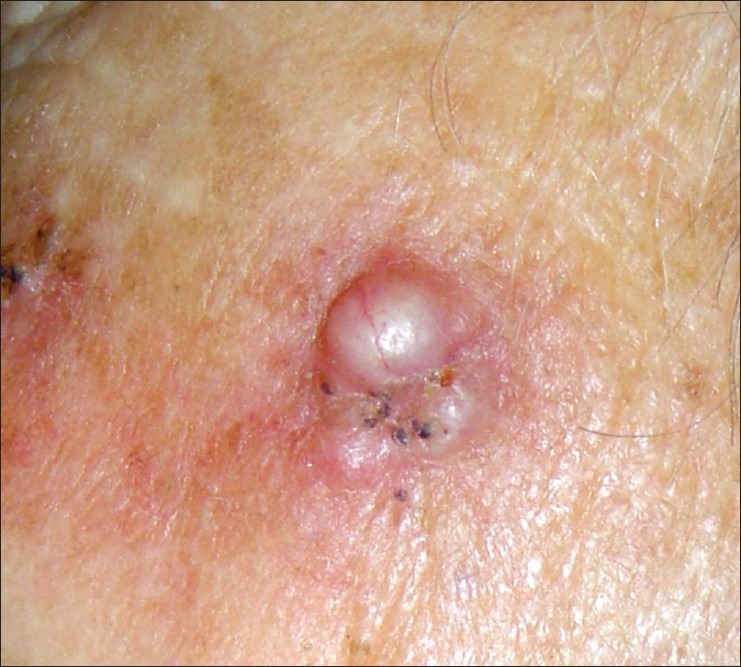
Nodular basal cell carcinoma at the left zygomatic area in an 86 years old woman
Figure 2.
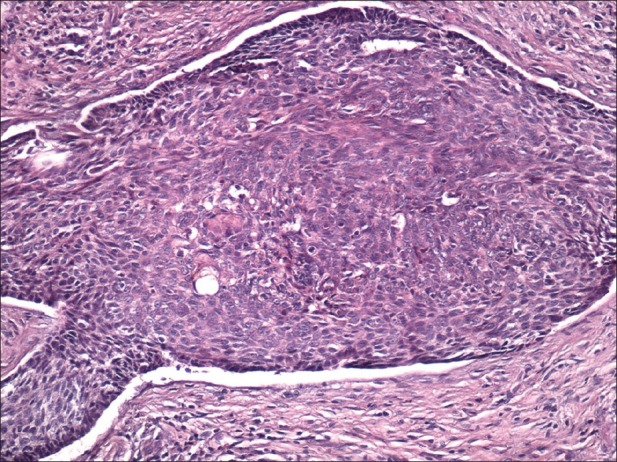
Nodular basal cell carcinoma. Peripheral palisading and retraction from surrounding stroma are clearly seen; this case also shows keratinization (H and E, ×200)
Cystic BCC
One or more cystic nodes with different sizes located peripherally to the centrally placed tumor nests.
Sclerodermiform (Morpheiform) BCC
The nests and clusters of tumor cells are surrounded by thick fibrotic stroma. Clinically, it is presented as infiltrated plaque with slightly shining surface and not well-defined borders. Immunochemistry shows expression of smooth muscle alpha-actin in tumor stroma.
Infiltrated basal cell carcinoma
This version of basal cell carcinoma is presented as thin bundles of basaloid cells with nest-like configuration located between the collagenous fibers on the dermis and infiltrating in the depth. Clinically, it is a whitish, compact, not-well defined plaque [Figure 3]. The most common localization is in the upper part of the trunk or the face. Seldom had the paresthesia or hyperesthesia as a symbol of perineural infiltration appeared, especially when the tumor is localized on face. This clinical version is often underestimated when the borders of surgical excision are estimated. Histologically this variant is presented as thin, nest-like bundles of basaloid cells infiltrating in the dermal collagenous fibers [Figure 4].
Figure 3.
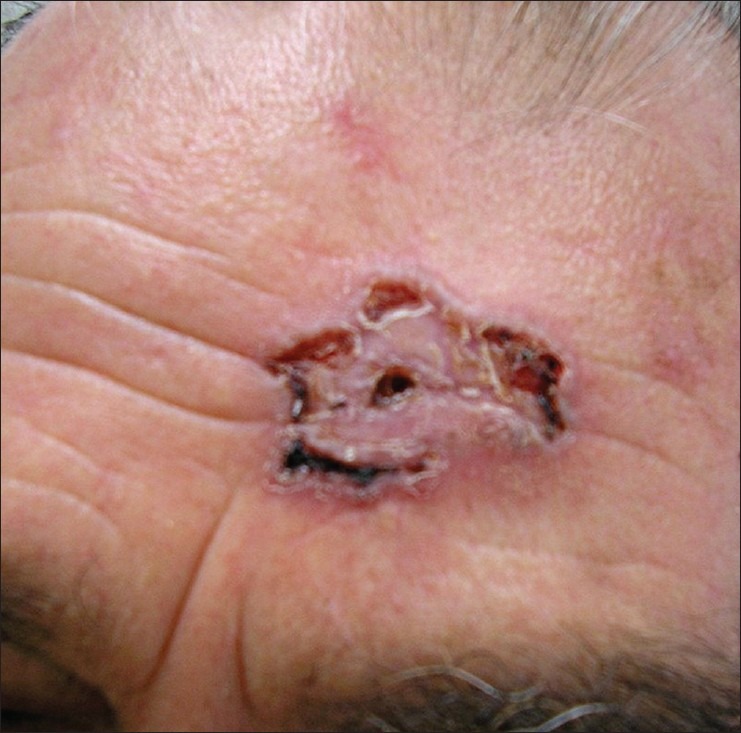
Infiltrated lesion with irregular outlines and size in the forehead area of a 76 years old man
Figure 4.
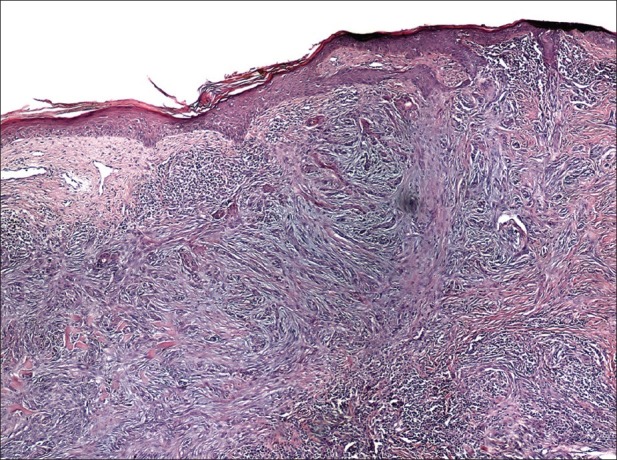
Infiltrated basal cell carcinoma. Thin bundles of basaloid cells invade the dermis (H and E,×100)
Micronodular basal cell carcinoma
Clinically found elevated or flat infiltrated tumors. They ulcerate seldom and have yellow-whitish color when they are flat, ostensibly clear outlines and thick at palpation. The most common localization is the skin of the back. On histology this tumor demonstrates small rounded nodules of basaloid cells and minimal palisading [Figure 5].
Figure 5.
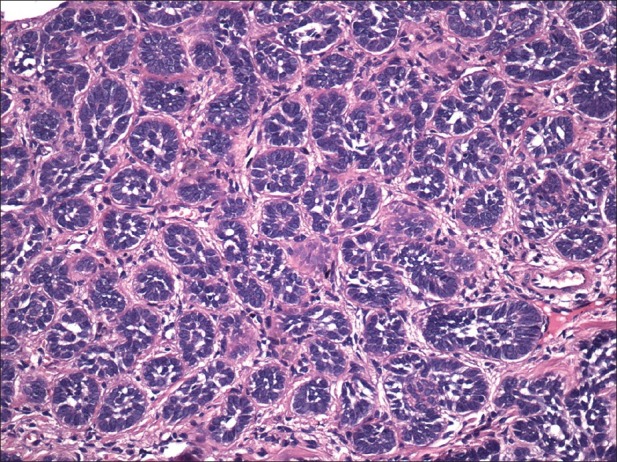
Micronodular basal cell carcinoma. Small rounded nodules of basaloid cells with approximately the size of hair bulb (H and E, ×200)
Superficial basal cell carcinoma
This version occurs as erythematous plaque with different sizes (from several millimeters to more than 10 cm). It is about 10-30% of basal cell carcinoma and occurs on the body skin. There is an erythematous squamous plaque with clear borders, pearl-shape edge, superficial erosion, without tendencies for invasive growth [Figure 6]. The regression areas are presented as pale sections with fibrosis. The differential diagnosis includes Bowen disease, psoriasis, or eczema. The numerous superficial BCC are met often in case of arsenic exposure. Histology showed nests of basaloid cells located subepidermally, with clear connection with the basal layer of the epidermis and no infiltration of tumor cells in the reticular dermis [Figure 7].
Figure 6.
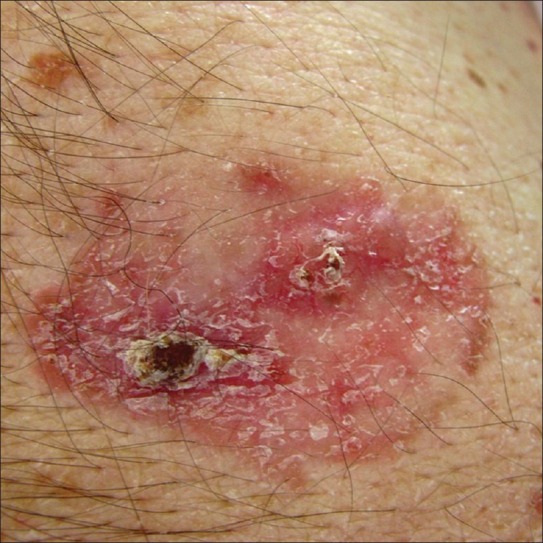
Erythematous squamous plaque in abdominal area of a 74 years old man
Figure 7.
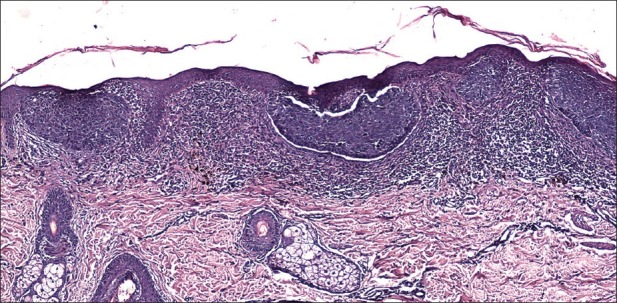
Superficial basal cell carcinoma. Several nests of basaloid cells are located subepidermally with clear connection with the basal layer of the epidermis (H and E, ×100)
Pigment basal cell carcinoma
The pigmentation can be found in different clinical versions of basal cell carcinoma including nodular, micronodular, multifocal and superficial BCC, and the color varies from dark brown to black [Figure 8]. Histology showed nests of basaloid cells, abundance of melanin and melanophages, and moderate inflammatory infiltrate. The melanocytes are located among tumor nests, while the melanophages are present in the stroma. The differential diagnosis has to be made with malignant melanoma.
Figure 8.
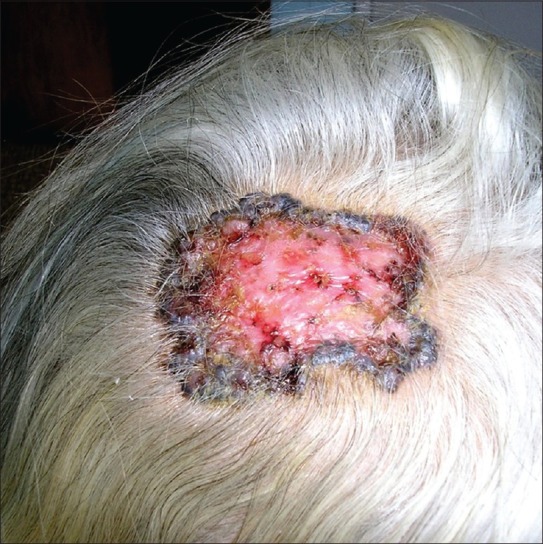
An irregular, periphery spreading erosive pigmented plaque on head of a 78 years old woman
Fibroepithelioma of Pinkus
These tumors usually originate as elevated pink or erythematous nodules that can resemble seborrheic keratoses or acrochordon. The lesions are solitary, seldom numerous, the most common location is on the skin of the back and affect especially women. Histology is typical [Figure 9]. The radiotherapy is considered a prepossessing factor for tumor formation. The differential diagnosis includes actinic keratosis, keratoacanthoma, seborrheic keratosis, squamous cell carcinoma, etc.
Figure 9.
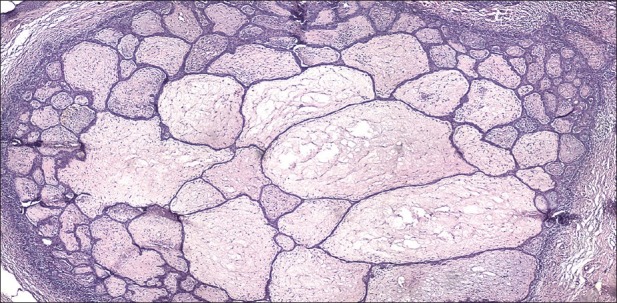
Fibroepithelioma of Pinkus. Trabecular, elongated and branched thin strands of basaloid cells extend into the dermis (H and E, ×100)
DISEASE STAGING
The staging of BCC is made according to TNM classification, established by the American Joint Committee of Cancer.[10] The same classification is used for staging of squamous-cell carcinoma [Table 1].
Table 1.
TNM classification of basal cell carcinoma[10]
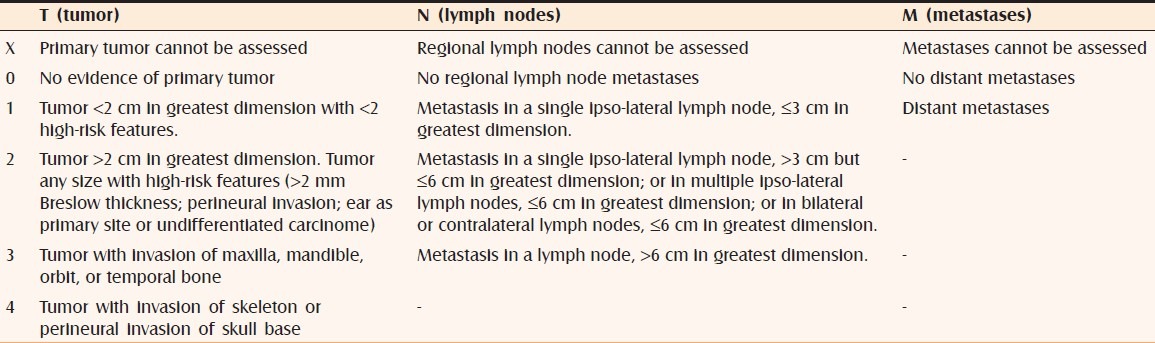
Patients with a primary cutaneous BCC or other cutaneous carcinoma with or without the clinical, radiological, or pathological evidence of regional or distant metastases are divided into the following stages:
Stage I : Tumor is 2 cm or less in size, no metastases;
Stage II : Tumor is than 2 cm in size, no metastases;
Stage III : Any tumor size, one involved lymph node measuring 3 cm or less in size or tumor extension into the maxilla, mandible, orbit, or temporal bone;
Stage IV : Patients with tumor with direct or perineural invasion of skull base or those with two or more involved lymph nodes or multiple and distant metastases.
THERAPEUTIC APPROACHES AT TREATMENT OF BCC
Surgical treatment
It is the first line therapeutic method. The excision performs with 3-10 mm margins outside the tumor, depending on the size and localization of the carcinoma.[11] The results of 5-year follow-up after the surgical treatment of BCC with up to 1.5 cm diameter size of the primary tumor show the recurrences in 12% of the cases, while in primary carcinomas with sizes above 3 cm diameter the recurrence rate is 23%.[12] It is considered that the recurrences originate from the periphery nests of the tumor that could not always be found by routine histology. The primary tumors of cochlea, eyelids, scalp, and nose relapse in 12-25%.
Mohs surgery
The microscope-controlled surgery consists of intraoperative of the wound edges for presence of tumor cells. The aim is to spare the tissues and to have small percentage of local relapses. It is applied in primary BCCs with localization requiring maximal sparing of tissues (nose, lips, eyelids), or with high risk of relapse (nose, lips, temporal bones, mucous membranes, penis). Tumors with diameter above 2 cm as well as tumors with unclear borders or with aggressive histological signs (infiltrative, morphologic, perineural infiltrating) are highly indicated for microscope-controlled surgery.[13] Mohs surgery is resource-demanding, but lowers the risk of recurrence and can be cost-effective if is considered in BCC with an aggressive growth pattern on the face.[14]
Electric cauterization and curettage
Quick and easy applicable methods for treatment of tumors with diameter sizes from 2 to 5 cm. The relapse rate varies between 1 and 15% in small tumors[15] and increases to 50% in tumors above 3 cm in diameter because it is difficult to determine the tumor filtration depth.
Cryotherapy
This method is appliable in small-sized and well-defined BCC with clear borders and mainly when the patients have contraindications for surgical excision. As an easy, simple, and no time-consuming method, cryotherapy has certain advantages over surgical techniques in cases with multiple BCC tumors. The recurrence rate after treatment of small-sized (<8 mm) primary BCC is about 8%[16] however, these patients need careful follow-up. Specific contraindications are localizations in naso-labial fold, ala nasi, tragus, eyelid edge.
Roentgen therapy
As well as sugary is one of the initial historically method for BCC treatment. We used fractional superficial X-ray therapy with total dose of 60-70 Gy with excellent therapeutic result and relapse rate of 6.3%.[2] Roentgen therapy is particularly useful in tumors up to 5 cm (T2N0M0) on nose, naso-labial, and retroauricular folds and in elder patients who reject or in whom surgery is relatively contraindicated.[2] Advanced or neglected cases can be put to therapy with intensified electrons or needling with radioactive isotopes. The contraindications of superficial X-ray therapy include Basal cell nevus syndrome, Bazex syndrome, Xeroderma pigmentosum, Epidermodysplasia verruciformis, since the ionizing radiation can lead to new tumors in the area of radiation.
Laser treatment
It is suitable for treatment of superficial BCC. CO2 laser succeeded complete clearance after one treatment in more than 80% of BCC patients. According to recent data the recurrence rate after ablative laser therapy varies between 3.7 and 15.5%.[17,18]
5-Fluoruracil
This method is based on the chemical destruction of the tumor. It has limited use in nodular-ulcerative and superficial BCC, but there is a high degree of relapses because of underestimating of the lesion depth.
Imiquimod
Imidazoquinoline amineis is a synthetic immune modulator, stimulating excretion of cytokines such as IFN-alpha, IL-6, and TNF-alpha.[19] Imiquimod is indicated for single primary superficial BCC, sized from 0.5 to 2 cm diameter. It is not applied in the area of the eyes, nose, lips, and cochlea. The efficacy of 6 weeks imiquimod treatment of BCC varies from 70 to 94%.[20]
Interferon alpha (IFN)
Intralesional application of IFN is an effective alternative or surgery with clearance rates up to 96%.[21]
Photodynamic therapy
Photodynamic therapy is a relatively new method in which the tumor photosensitizes with methyl-aminolevulinate and irradiates with red light with wavelength of approximately 630 nm[21] for treatment of single or numerous superficial or morpheiform BCC. The radical influence of this method varies between 78 and 88%.[22]
In conclusion, BCC is a relatively frequent disease which is regularly diagnosed at the outpatients’ practice. The early diagnostics based on the good knowledge and timely organized and adequate treatment is a precondition for better prognosis. Despite the slow progress and numerous therapeutic methods, the BCC should not be underestimated. Numerous methods of treatment do not absolutely dissolve the risk of relapses and if the case is neglected, let without further control or received no adequate therapy, BCC may destroys the underlying tissues and spread metastases.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Urbach F. Incidence of non melanoma skin cancer. Dermatol Clin. 1991;9:751–5. [PubMed] [Google Scholar]
- 2.Dourmishev A, Popova L, Dourmishev L. Basal-cell carcinoma and squamous-cell carcinomas: Epidemiology, location and radiotherapy. Skin Cancer. 1996;11:195–200. [Google Scholar]
- 3.Mulvany NJ, Allen DG. Differentiated intraepithelial neoplasia of the vulva. Int J Gynecol Pathol. 2008;27:125–35. doi: 10.1097/pgp.0b0318134ea34. [DOI] [PubMed] [Google Scholar]
- 4.Lo JS, Snow SN, Reizner GT, Mohs FE, Larson PO, Hruza GJ. Metastatic basal cell carcinoma: Report of twelve cases with a review of the literature. J Am Acad Dermatol. 1991;24:715–9. doi: 10.1016/0190-9622(91)70108-e. [DOI] [PubMed] [Google Scholar]
- 5.Dessinioti C, Antoniou C, Katsambas A, Stratigos AJ. Basal cell carcinoma: What's new under the sun. Photochem Photobiol. 2010;86:481–91. doi: 10.1111/j.1751-1097.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- 6.de Zwaan SE, Haass NK. Genetics of basal cell carcinoma. Australas J Dermatol. 2010;51:81–92. doi: 10.1111/j.1440-0960.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- 7.Cabrera HN, Gómez ML. Skin cancer induced by arsenic in the water. J Cutan Med Surg. 2003;7:106–11. doi: 10.1177/120347540300700202. [DOI] [PubMed] [Google Scholar]
- 8.Chan PC, Haseman JK, Boorman GA, Huff J, Manus AG, Cardy RH. Forestomach lesions in rats and mice administered 3-chloro-2-methylpropene by gavage for two years. Cancer Res. 1986;46:6349–52. [PubMed] [Google Scholar]
- 9.Kubasiewicz M, Starzyñski Z. Case-referent study on skin cancer and its relation to occupational exposure to polycyclic aromatic hydrocarbons. I. Study design. Pol J Occup Med. 1989;2:221–8. [PubMed] [Google Scholar]
- 10.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 6th Printing. 6th ed. Berlin: Springer; 2010. [Google Scholar]
- 11.Gulleth Y, Goldberg N, Silverman RP, Gastman BR. What is the best surgical margin for a Basal cell carcinoma: A meta-analysis of the literature. Plast Reconstr Surg. 2010;126:1222–31. doi: 10.1097/PRS.0b013e3181ea450d. [DOI] [PubMed] [Google Scholar]
- 12.Silverman MK, Kopf AW, Bart RS, Grin CM, Levenstein MS. Recurrence rates of treated basal cell carcinomas.Part 3: Surgical excision. J Dermatol Surg Oncol. 1992;18:471–6. doi: 10.1111/j.1524-4725.1992.tb03307.x. [DOI] [PubMed] [Google Scholar]
- 13.Smeets NW, Kuijpers DI, Nelemans P, Ostertag JU, Verhaegh ME, Krekels GA, et al. Mohs’ micrographic surgery for treatment of basal cell carcinoma of the face–results of a retrospective study and review of the literature. Br J Dermatol. 2004;151:141–7. doi: 10.1111/j.1365-2133.2004.06047.x. [DOI] [PubMed] [Google Scholar]
- 14.Roscher I, Brevig T, Mørk G, Helsing P, Gjersvik P. [Mohs surgery in basal cell carcinoma on the face] Tidsskr Nor Laegeforen. 2011;131:2475–9. doi: 10.4045/tidsskr.11.0343. [DOI] [PubMed] [Google Scholar]
- 15.Peikert JM. Prospective trial of curettage and cryosurgery in the management of non-facial, superficial, and minimally invasive basal and squamous cell carcinoma. Int J Dermatol. 2011;50:1135–8. doi: 10.1111/j.1365-4632.2011.04969.x. [DOI] [PubMed] [Google Scholar]
- 16.Moesen I, Duncan M, Cates C, Taylor A, Wintle RV, Ismail A, et al. Nitrous oxide cryotherapy for primary periocular basal cell carcinoma: Outcome at 5 years follow-up. Br J Ophthalmol. 2011;95:1679–81. doi: 10.1136/bjo.2009.173021. [DOI] [PubMed] [Google Scholar]
- 17.Tucker SB, Polasek JW, Perri AJ, Goldsmith EA. Long-term follow-up of basal cell carcinomas treated with perilesional interferon alfa 2b as monotherapy. J Am Acad Dermatol. 2006;54:1033–8. doi: 10.1016/j.jaad.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 18.Jung DS, Cho HH, Ko HC, Bae YC, Oh CK, Kim MB, et al. Recurrent basal cell carcinoma following ablative laser procedures. J Am Acad Dermatol. 2011;64:723–9. doi: 10.1016/j.jaad.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 19.Geisse JK, Rich P, Pandya A, Gross K, Andres K, Ginkel A, et al. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: A double-blind, randomized, vehicle-controlled study. J Am Acad Dermatol. 2002;47:390–8. doi: 10.1067/mjd.2002.126215. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Villaverde R, Sanchez-Cano D, Burkhardt-Perez P, Sintes RN. Has imiquimod 5% cream a role in the management of recurrent basal cell carcinoma? Eur J Dermatol. 2009;19:481–3. doi: 10.1684/ejd.2009.0741. [DOI] [PubMed] [Google Scholar]
- 21.Torres T, Fernandes I, Costa V, Selores M. Photodynamic therapy as adjunctive therapy for morpheaform basal cell carcinoma. Acta Dermatovenerol Alp Panonica Adriat. 2011;20:23–5. [PubMed] [Google Scholar]
- 22.Baas P, Saarnak AE, Oppelaar H, Neering H, Stewart FA. Photodynamic therapy with meta-tetrahydroxyphenylchlorin for basal cell carcinoma: A phase I/II study. Br J Dermatol. 2001;145:75–8. doi: 10.1046/j.1365-2133.2001.04284.x. [DOI] [PubMed] [Google Scholar]


