Abstract
Background:
Chronic renal failure (CRF) is associated with a variety of cutaneous manifestations as a result of underlying etiology as well as the various treatment modalities.
Aim:
To evaluate the prevalence of various dermatoses in patients with CRF on hemodialysis and to study the effect of hemodialysis on the intensity of pruritus.
Materials and Methods:
A total of 35 patients of CRF on hemodialysis having at least one cutaneous manifestation were included in the study.
Results:
Twenty-four (68.71%) cases in our study belonged to the age group of 50-69 years, out of which 16 cases were in the sixth decade. Xerosis and pruritus occurred in 80% and 65.71% of cases, respectively. Other common findings included pallor (68.57%), dyspigmentation (34.29%), cutaneous infections (34.39%), acquired perforating dermatosis (17.4%), and nail changes (60%). Hemodialysis failed to improve pruritus in 17 (73.9%) of our patients. Twenty-six patients (74.28%) suffered from hypertension, 13 of them also were known cases of type II diabetes mellitus. Five patients suffered exclusively from type II diabetes mellitus.
Conclusions:
In our small study, xerosis was the commonest finding and pruritus, the commonest symptom. The intensity of pruritus was largely unaffected by hemodialysis.
Keywords: Acquired perforating disorder, chronic renal failure, hemodialysis, pruritus, xerosis
INTRODUCTION
The skin, most visible and accessible organ of the body, may function as an important diagnostic window to the diseases affecting the internal organs including the renal system.[1]
Some of the skin changes like pruritus, xerosis, hyperpigmentation, and acquired perforating dermatosis are said to be present regardless of hemodialysis. “Nephrogenic fibrosing dermopathy” and “bullous dermatoses of hemodialysis” develop only consequent to initiation of hemodialysis. Prolonged life-expectancy as a result of prompt treatment with hemodialysis enables newer cutaneous manifestations to appear.[2] The advent of hemodialysis as the treatment of chronic renal failure (CRF) has virtually made uremic frost and erythema papulatum uremicum, the most frequent skin findings encountered in the predialysis era, extinct.[2]
The frequently reported intraoral findings in CRF patients are xerostomia, macroglossia, and ulcerative stomatitis.[2] Hair are sparse and lustreless. Half-and-half nails, absent lunulae, and onychomycosis are common.[3]
MATERIALS AND METHODS
A total of 35 patients of CRF on hemodialysis, having at least one cutaneous manifestation, were selected for this cross-sectional study in a tertiary care hospital for a period of 2 years. Patients’ age, sex, primary and secondary diagnoses, medications, and present cutaneous status were noted. History included duration of CRF and hemodialysis [Table 1]. The effect of a single session of hemodialysis on the symptom of pruritus at that particular time was recorded as unchanged, worsened, or reduced. A complete clinical examination was done. Data were collected and recorded in a proforma. Photographs were taken. Skin biopsy and other investigations were done wherever required. Epidemiological analysis of the data was carried out.
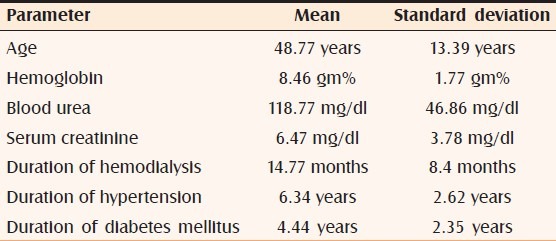
Table 1.
Mean and standard deviations of various parameters
RESULTS
There were 27 males and eight females in our study. The analysis of prevalence as per age [Table 1] revealed that the youngest patient was aged 23 years and the eldest, 65 (mean age, 48.77; standard deviation, 13.39 years). Maximum decadal prevalence of 45.71% occurred during the sixth decade followed by 22.85% during the seventh decade; these two decades (50-69 years) accounted for 24 (68.56%) cases.
Hemoglobin, blood urea, and serum creatinine were estimated in all patients [Table 1]. The means of hemoglobin, blood urea, and serum creatinine were 8.46 gm% (S.D. = 1.77 gm%), 118.77 mg (S.D. = 46.86 mg/dl), and 6.47 mg/dl (S.D. = 3.78 mg/dl), respectively.
Twenty-six patients (74.28%) suffered from hypertension, 13 of them were also known cases of type II diabetes mellitus (refer Table 1 for the mean durations since the onset of these diseases). Five patients suffered exclusively from type II diabetes mellitus. Four cases of adult polycystic kidney disease too figured in our study. We had two patients having uremic fetor and their blood urea levels were more than 200.
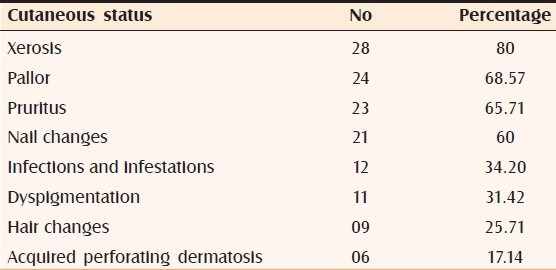
Various cutaneous manifestations observed in our study are tabulated [Table 2].
Table 2.
Prevalence of cutaneous manifestations encountered in our study
DISCUSSION
Pruritus
Pruritus was the commonest and most bothersome symptom observed in our study, affecting 23 (65.71%) patients. Its prevalence in previous studies has varied widely from 19% to 90%.[2,4] A recent Indian study[2] quoted its prevalence as 53%. In our study pruritus remained unchanged in 73.9% (17 out of 23), worsened in 17.39% (4 out of 23), and diminished in 8.7% (2 out of 23) patients on hemodialysis. Jamal and Subramanian[5] also found similar results in their study. This nonamelioration of pruritus has been postulated to be due to the inability of hemodialysis to clear the blood off the pruritogenic middle molecular weight substances (molecular weight range 300-1200), like β2 microglobulin, advanced glycosylation end products, and parathyroid hormones[6] Various modalities of treatment tried for uremic pruritus have been unsatisfactory; even renal transplantation has given relief in only a few cases.[7] The efficacy of oral cholestyramine, opioid naltrexone, oral ondansetron and topical capsaicin cream, and UVB phototherapy has been documented.[8]
Pallor
Pallor, the second common finding after xerosis, was found in 24 (68.57%) of our patients. The mean of hemoglobin in these 24 patients (8.25 gm%, S.D. = 1.10) was lower than the mean of the entire sample population (8.47 gm%, S.D. = 1.10). Udayakumar et al.[2] also detected pallor in 60% of their cases. Deficient erythropoietin production by the failing kidneys[9] and dietary deficiencies of iron, folic acid, and vitamin B12 contribute to anemia.
Xerosis
Xerosis, the commonest finding in our study, seen in 28 patients (80%) predominantly involved extensor aspect of legs, thighs, and forearms [Figure 1]. Data from several studies record prevalence of xerosis ranging from 46% to 90%.[2,10–12] Eighteen (64.26%) of our xerotic patients were known cases of diabetes mellitus and the ichthyosis involved large areas of the body as compared to other CRF patients. The pathogenesis of xerosis is unknown; various causes implicated include reduced sweat[13] secondary to high dose diuretic regimens used to treat CRF, elevated plasma vitamin A, elevated retinol binding protein,[14] alkalinity of skin,[15] and protein-calorie malnutrition[16] owing to dietary restrictions. The therapy remains nonspecific and challenging. Emollients reduce scaling, and creams containing endocannabinoids (N-acetylethanolamine and N-palmitoylethanolamine) are known to be effective.[15]
Figure 1.
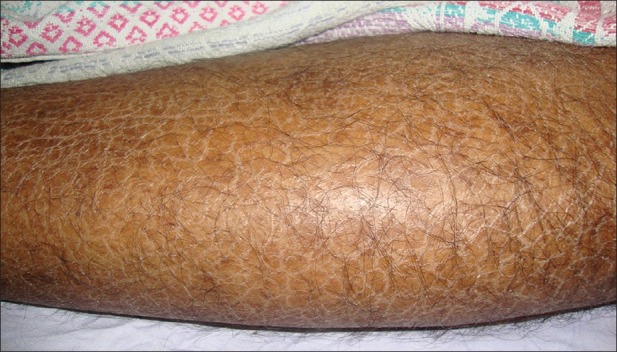
Xerosis involving extensor aspects of lower limb
Dyspigmentation
Seven of our patients had diffuse brown pigmentation involving face and sun exposed parts of upper and lower limbs [Figure 2], and four had yellow tinge on their faces [Figure 3]. Thus, the overall prevalence of the two types of dyspigmentation described in CRF patients was 31.42% in our study. Udayakumar et al.[2] reported this prevalence as 43% in their study. Diffuse hyperpigmentation happens due to increased levels of β-melanocyte stimulating hormone as a result of its inadequate excretion through kidney and dialysis.[17] Yellowish tinge is due to excess deposition of two major pigments namely carotenoids and lipochromes in the epidermis and subcutaneous tissue.[18] The higher clearance of the all the above-mentioned substances through high-flux hemodialysis or hemodiafiltration may improve the dyspigmentation.[19]
Figure 2.
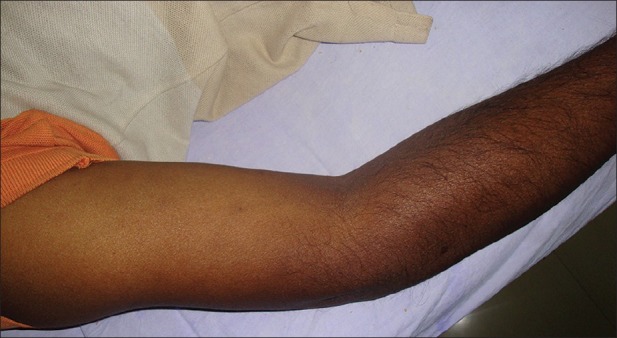
Brown dyspigmentation seen on sunexposed part of upper limb
Figure 3.

Yellow tinge over face
Hair changes
Hair changes were seen in nine (25.71%) patients, of which seven patients showed sparse hair on scalp. Two patients had dry lustreless hair, which is postulated to occur due to decreased secretion of sebum in CRF patients.[20]
Nail changes
Nail changes were seen in 21 (60%) of our patients. The more common findings were Beau's lines seen in six (28.57%) patients and subungual hyperkeratosis in five (23.8%) patients. Half-and-half nails [Figure 4] and platynychia were seen in four (19.04%) patients each. We found that patients having nail changes and mean duration of hemodialysis as 22.3 months as compared to 14.77 overall mean. Dyachenko et al.,[3] in their exclusive study of nail changes in patients of CRF, found half-and-half nails (16.9%) as the commonest finding followed by absent lunulae (13%) and onychomycosis (10.4%). The prevalence of half-and-half nail, a characteristic finding of CRF, ranges from 16% to 50.6%[3,11,21] as compared to 1.4%[21] in the general population.
Figure 4.

Half-and-half nails involving finger nails
Acquired perforating dermatosis
Six (17.14%) cases in our study had “acquired perforating dermatosis” (APD), four of whom had lesions mainly over extensor aspect of legs, the commonest site for this disorder. All the affected patients complained of severe itching. The lesions were hyperkeratotic papules ranging in size from 2 to 10 mm in diameter often with a hyperkeratotic plug [Figure 5]. All these patients were diabetic. Udayakumar et al.[2] and Sultan et al.[21] reported the prevalence of APD in their studies as 21% and 10%, respectively.
Figure 5.
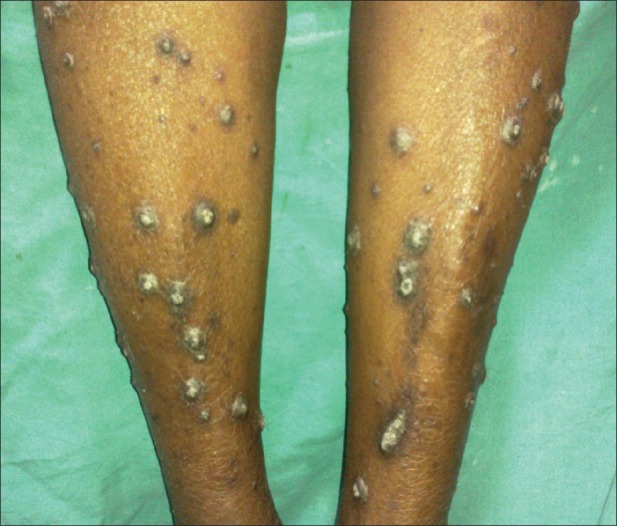
Acquired perforating dermatosis affecting bilateral shins
Cutaneous infections
Cutaneous infections were seen in 12 (34.2%) patients in our study; eight of whom had dermatophytosis (seven, tinea cruris; one, tinea faciei). Two of our patients had scabies and one each, herpes zoster and pyoderma. The percentages of cutaneous infections in an Indian study[2] and an Egyptian study[21] were 55% and 40%, respectively. Increased susceptibility to infection can either be due to inflammation caused by the use of nonsterile dialysate and nonbiocompatible membranes[4] during hemodialysis or CRF per se due to diminished T and B lymphocyte function and count, and reduced natural killer cell activity.[22]
Other dermatoses
Various other dermatoses like calcinosis cutis, bullous dermatoses of hemodialysis, drug reactions, arteriovenous shunt dermatitis, and nephrogenic fibrosing dermopathy have been frequently reported in other studies.[22–26] Absence of above findings in our study could be due its small sample size.
CONCLUSIONS
In our small study, xerosis, pallor, dyspigmentation, and pruritus were the commonest cutaneous manifestations observed in patients of CRF on hemodialysis. Of these, xerosis was extensive in diabetes mellitus patients and acquired perforating dermatosis too affected them. Uremic fetor affected patients having blood urea levels more than 200 mg/dl. Correlation of other manifestations with their etiologies is difficult due to small sample size. Nail changes were more prevalent in patients who received hemodialysis for longer period of time. Hemodialysis after a single session failed to ameliorate the intensity of pruritus in majority of our patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Mazyryk HA, Brodkin RH. Cutaneous clues to renal disease. Cutis. 1991;47:241–8. [PubMed] [Google Scholar]
- 2.Udayakumar P, Balasubramanian S, Ramalingam KS, Srinivas CR, Lakshmi C, Mathew AC. Cutaneous manifestations in patients with chronic renal failure on hemodialysis. Indian J Dermatol Venereol Leprol. 2006;72:119–25. doi: 10.4103/0378-6323.25636. [DOI] [PubMed] [Google Scholar]
- 3.Dyachenko P, Monselise A, Shustak A, Ziv M, Rozenman D. Nail disorders in patients with chronic renal failure and undergoing haemodialysis treatment: A case control study. J Eur Acad Dermatol Venereol. 2007;21:340–4. doi: 10.1111/j.1468-3083.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 4.Pico MR, Lugo-Somolinos A, Sanchez JL, Burgos-Calderon R. Cutaneous alterations in patients with chronic renal failure. Int J Dermatol. 1992;31:860–3. doi: 10.1111/j.1365-4362.1992.tb03543.x. [DOI] [PubMed] [Google Scholar]
- 5.Jamal A, Subramanian PT. Pruritus among End-Stage renal failure patients on Hemodialysis. Saudi J Kidney Dis Tranpl. 2000;11:181–5. [PubMed] [Google Scholar]
- 6.Kato A, Hamada M, Maruyama Y, Hishida A. Pruritus and hydration state of stratum corneum in hemodialysis patients. Am J Nephrol. 2000;20:437–42. doi: 10.1159/000046196. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen T, Andersen KE, Kristiansen J. Pruritus and xerosis in patients with chronic renal failure. Dan Med Bull. 1980;27:269–71. [PubMed] [Google Scholar]
- 8.Etter L, Myers SA. Pruritus in systemic disease: Mechanisms and management. Dermatol Clin. 2002;20:459–72. doi: 10.1016/s0733-8635(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 9.Graham RM, Cox NH. Rook's textbook of dermatology. 7th ed. Oxford: Wiley-Blackwell; 2004. Systemic disease and the skin; pp. 59–75. [Google Scholar]
- 10.Morton CA, Lafferty M, Hau C, Henderson I, Jones M, Lowe JG. Pruritus and skin hydration during dialysis. Nephron Dial Transplant. 1996;11:2031–6. doi: 10.1093/oxfordjournals.ndt.a027092. [DOI] [PubMed] [Google Scholar]
- 11.Tawade N, Gokhale BB. Dermatologic manifestation of chronic failure. Indian J Dermatol Venereol Leprol. 1996;62:155–6. [PubMed] [Google Scholar]
- 12.Sidappa K, Nair BK, Ravindra K, Siddhesh ER. Skin in systemic disease. In: Vali RG, Valia AR, editors. IADVL Textbook and atlas of dermatology. 2nd ed. Mumbai: Bhalani Publishing House; 2000. pp. 938–84. [Google Scholar]
- 13.Cawley EP, Hoch-Bigeti C, Bondy GM. The eccrine sweat glands of patients in uraemia. Arch Dermatol. 1961;884:9. doi: 10.1001/archderm.1961.01580180005001. [DOI] [PubMed] [Google Scholar]
- 14.Vahlquist A, Berne B, Berne C. Skin content and plasma transport of Vitamin A and β-carotene in Chronic Renal Failure. Eur J Clin Invest. 1982;12:63–7. doi: 10.1111/j.1365-2362.1982.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 15.Szepietowski JC, Reich A, Schwartz RA. Uraemic Xerosis. Nephrol Dial Transplant. 2044;19:2709–12. doi: 10.1093/ndt/gfh480. [DOI] [PubMed] [Google Scholar]
- 16.Bencini PL, Montagnino G, Citterio A, Graziani G, Crosti C, Ponticelli C. Cutaneous abnormalities in uremic patients. Nephron. 1985;50:316–21. doi: 10.1159/000183485. [DOI] [PubMed] [Google Scholar]
- 17.Smith AG, Shuster S, Comaish JS, Plummer NA, Thody AJ, Alvarez-ude F. Plasma immunoreactive β-Melanocyte stimulating hormone and skin pigmentation in chronic renal failure. Br Med J. 1975;1:658–9. doi: 10.1136/bmj.1.5959.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsaltas TT. Studies of lipochromes in uremic patients and normal controls. II. Isolation and identification of carotenoids and lipochromes and their oxidation products in plasma. Trans Am Soc Artif Intern Organs. 1970;16:272–8. [PubMed] [Google Scholar]
- 19.Moon SJ, Kim DK, Chang JH, Kim CH, Kim HW, Park SY. The impact of dialysis modality on skin hyperpigmentation in hemodialysis patients. Nephron Dial Transplant. 2009;24:2803–9. doi: 10.1093/ndt/gfp143. [DOI] [PubMed] [Google Scholar]
- 20.Kint A, Bussels L, Fernandez M, Ringoir S. Skin and nail disorders in relation to chronic renal failure. Acta Dermatovener. 1974;54:137–40. [PubMed] [Google Scholar]
- 21.Sultan MM, Mansour HH, Wahby IM, Houdery AS. Cutaneous manifestations in egyptians patients with chronic renal failure on regular hemodialysis. J Egypt Women Dermatol Soc. 2010;7:49–55. [Google Scholar]
- 22.Gupta AK, Gupta MA, Cardella CJ, Haberman HF. Cutaneous associations of chronic renal failure and dialysis. Int J Dermatol. 1986;25:498–504. doi: 10.1111/j.1365-4362.1986.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 23.Raymond GG, Saenz RV, Chandler C. Drug induced skin manifestations. US Pharmacist. 1978;3:44–8. [Google Scholar]
- 24.Gilchrest BA, Rowe JW, Mihm MC. Bullous dermatosis of hemodialysis. Ann Intern Med. 1975;83:480–3. doi: 10.7326/0003-4819-83-4-480. [DOI] [PubMed] [Google Scholar]
- 25.Goh GL, Phay KL. Arterio-venous shunt dermatitis in chronic renal failure patients on hemodialysis. Clin Exp Dermatol. 1988;13:1038–40. doi: 10.1111/j.1365-2230.1988.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 26.Levine JM, Taylor RA, Elman LB, Bird SJ, Lavi E, Stolzenberg ED, et al. Involvement of skeletal muscle in dialysis-associated systemic fibrosis (nephrogenic fibrosing dermopathy) Muscle Nerve. 2004;30:569–77. doi: 10.1002/mus.20153. [DOI] [PubMed] [Google Scholar]


