Abstract
Acne inversa (AI) is a disabilitating chronic inflammatory disease with major negative impact on quality of life and significant co-morbidities. This is an important link to insights into immune dysfunction, which stimulated therapeutic approaches like tumor necrosis-α inhibitor therapy. This new off-label drug treatment is particularly beneficial when used in combination with wide excision of inflamed skin and subcutaneous tissue. Retinoids have been reported to be helpful in secondary prevention. The standard of therapy in advanced cases is surgery with wide excisions and healing by secondary intention. This treatment results in significant reduction of complaints and achieves satisfactory body contouring.
Keywords: Acne inversa, co-morbidities, drug therapy, hidradenitis suppurativa, immune dysfunctions, radical surgery, retinoids, tumor necrosis factor-α
INTRODUCTION
Acne inversa (AI) (syn.: Hidradenitis suppurativa, pyoderma fistulans significa, Verneuil's disease or smoker's boils) is a potential severe and disabilitating chronic inflammatory disorder of apocrine gland-bearing body areas, i.e., axillary and anogenital region.[1–3] Usually, the disease does not occur before puberty. Its incidence is 1:600 in Caucasians but higher in patients of African descent.[4] One year prevalence varies in different regions of the World between <1 % to 4%.[5]
In the following review, particular attention will be paid to open questions of our understanding of this disease and its treatment.
Pathogenesis
Current understanding of the AI pathogenesis suggests follicular occlusion of the pilosebaceous unit by infundibular hyperkeratosis plays a crucial role. Known risk factors for AI are smoking and obesity. Both are associated with more severe disease course.[1,5,6] Recently, more attention has been put into the understanding of the immunopathology of the skin.[1,5,7]
Histologic analysis demonstrated infundibular hyperkeratosis, hyperplasia of the follicular epithelium, and perifolliculitis as major characteristics of AI. These apparently precede rupture of the follicle. Hyperplasia of the follicular epithelium probably marks the beginning of sinus formation, which usually spreads horizontally.[8]
Histological investigations reported absence of or decreased volume of sebaceous gland in AI.[9] The inflammatory infiltrate is composed of tryptase-positive mast cells, CD3-positive T-lymphocytes, CD138-positive plasma cells, and factor XIIIa-positive dendritic cells in the perilesional area. The lesions show an additional neutrophil leukocyte and macrophage influx. Later, C20-positive/CD79a-positive cells become more frequent.[10]
Pro-inflammatory cytokines such as interleukin (IL)-1β, IL-10 and tumor necrosis factor-α (TNFα) are markedly increased in lesional and perilesional skin.[11,12] Elevated serum levels of TNFα have been measured in AI patients of Hurley stage II and III.[13]
A reduced expression of membranous IL-22 and IL-20 receptors and increased expression of the natural IL-22 inhibitor, IL-22 binding protein in AI lesions has been found.[14] Macrophages of lesional skin abundantly express IL-12 and IL-23, leading to the infiltration of IL-17 producing T helper lymphocytes.[15]
The γ-secretase component genes have been identified as the culprits for a subset of familial AI, which implicate the γ-secretase-Notch pathway in the molecular pathogenesis of this disease subset.[16] Nicastrin is a 150 - 160 kDa proteins. It is a component of the aspartyl protease γ-secretase complex and serves to stabilize and direct γ-secretase components to proper positions in the plasma membrane. It contains a 636 aa extracellular domain (aa 34 - 669) that shows a 58 aa sequence (aa 312-369), which interacts with γ-secretase substrates. Nicastin itself has no catalytic activity. Recently, nicastrin mutations have been detected in Chinese[16–18] and French families with AI.[19] Mutations of the sonic hedgehog pathway could not be identified in AI patients.[20]
Bacterial infection with germs like coagulase-negative staphylococci, Escherichia coli, and streptococci is considered as a secondary event. There role in exacerbations is unclear.[21] Bacteria can contribute to the ongoing inflammatory process producing biofilms.[22]
Controversies in pathogenesis
Lesional skin of AI patients shows altered innate and adaptive immunity, important for bacterial control. Wolk et al., (2011) observed a relative deficiency of anti-microbial proteins (AMPs) in these lesions and a positive correlation between lesional IL-22 and IL-20 versus AMP levels.[14] In contrast, Emilanov et al., (2011) detected, by immunohistochemistry, an excessive expression of AMPs-like cathelicidine LL37 in outer hair root sheaths and apocrine glands of lesional AI skin.[12] In addition, AMP human β-defensine 3 was increased in epidermis and dermis.[23] There is no explanation for the very different findings on AMP in AI patients. What is the role of clinical inflammation? Is there still an AMP deficiency when patients reach the fibrotic stage of disease? From chronic skin ulcers, we know that LL37 expression on wound edges is markedly decreased compared to acute wounds.[24] And last not least, which role play bacteria in this sense - are they initiators or aggravators? In conclusion, AMP deficiency is a variable feature of AI, and its role progression to more advanced disease stages remains unclear.
Several investigators described a significant downregulation of markers of innate immunity like Toll-like receptors 2, 3, 4, and 7 in both non-lesional and lesional AI.[25] The role of Toll-like receptors, however, is not completely clear since other investigators observed a highly increased expression of Toll-like receptor 2 by macrophages and dendritic cells in AI lesions at both the protein and the mRNA level.[26] The latter finding would be in a line with findings in acne vulgaris, where inflammatory lesions show an increased expression of Toll-like receptors.[27] One possible explanation could be that in early stages, Toll-like receptors are reduced and in later stages, with increased inflammation, they become over-expressed. The role of secondary bacterial infection needs to be considered as well. From wound healing in leg ulcers, we know that non-healing is associated with persistent Toll-like receptor 2 activities. Healing is accompanied by a decrease of receptor activity.[28] In this clinical situation, no association with bacterial colonization was found.[29] In conclusion, further studies are needed to clarify the role of Toll-like receptors in progression of AI.
Clinical features
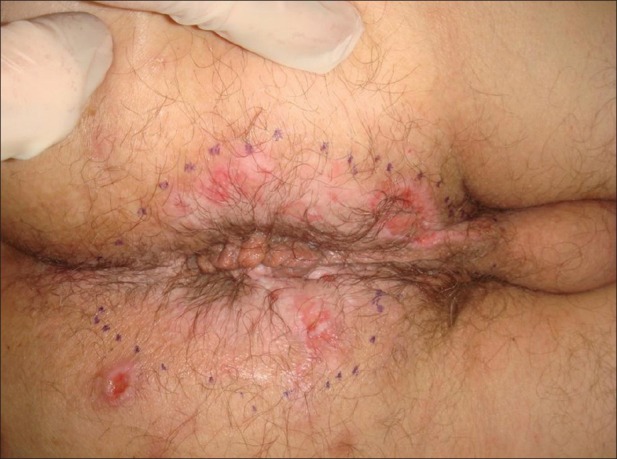
The disease starts with inflammatory, painful nodules and sterile abscesses followed by tissue fibrosis. Typical areas affected include axillae, groins, and anal fold [Figures 1–3]. The sub-mammary region and skin folds due to obesity can also be involved, but affection of these areas without axillary or groin involvement is rare.[30] Over time, sinus tracts and fistulas develop. Hypertrophic scarring can often be seen. This can be accompanied by malodorous putrid discharge.[31]
Figure 1.
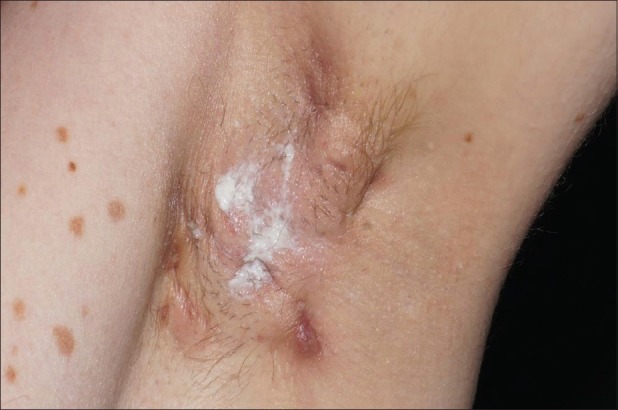
Acne inversa: Deep-seated nodules (boils) in the axillary region
Figure 3.
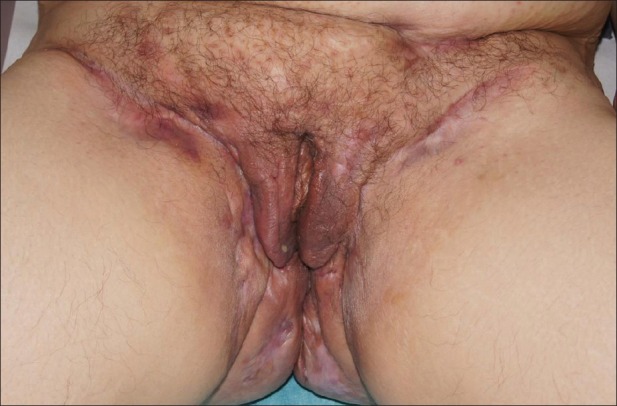
Anogenital acne inversa with secondary vulva edema and scarring
Figure 2.
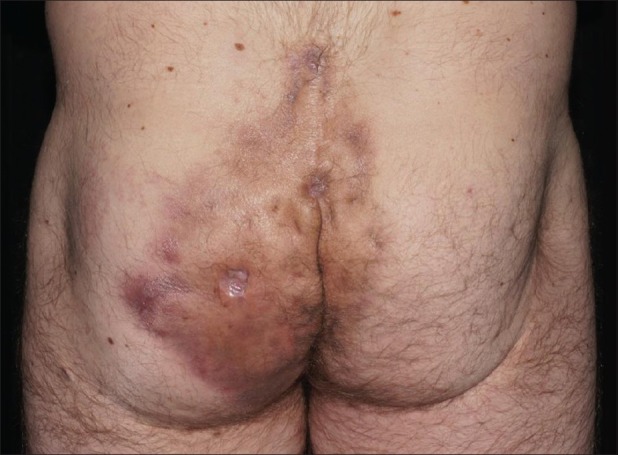
Fistulations and fibrosis in chronic acne inversa; post-inflammatory hyperpigmentation
Pain is a major symptom of AI. The quality of pain described by patients was hot, burning, pressure, cutting, sharp, taut, splitting, gnawing, sore, throbbing, and aching to sometime during their disease or as a chronic symptom in others.[32]
Pilonidal sinus is seen by some authors as a unilocalized type of AI.[8] The involvement of the head and neck region is a rare event. There is overlapping to acne keloidalis nuchae and dissecting folliculitis.[33,34]
AI is a chronic, recurrent, inflammatory disease with major negative impact on quality of life, with pain as just one of contributing factors. Stigmatization, depression, and anxiety are highest in patients with severe anogenital AI.[35–37]
Diagnosis and classification
AI diagnosis is primarily clinical. The three main features of the disease are summarized in Table 1. All criteria should be fulfilled for a definitive diagnosis.[1,3,26] Severity of AI is classified into three stages according to Hurley, which relies on subjective extent of the disease.[38] Another popular score for AI severity is the Sartorius scale, which highly correlates with pain intensity and duration and suppuration.[7] A further development is the Hidradenitis suppurativa score (HSS) including risk factors like smoking and obesity.[6,39]
Table 1.

Practical issue for staging
Because of its simplicity, Hurley score well suited for daily clinical practice, whereas more sophisticated scores may be of greater value for trials.
Differential diagnoses
Differential diagnostic includes Crohn's disease, nodular acne, tuberculosis, leprosy, and furunculosis. The treatment options are most appropriately chosen on the basis of disease severity and the existence of any associated risk factors or co-morbidities. Reactive arthritis is a differential diagnosis of AI since AI can develop articular symptoms.[40] Secondary lymphedema can develop as a consequence of longstanding AI; therefore, other types of lymphedema have to be considered in the differential diagnosis.[41]
Complications
The most common complications of AI are local, such as scarring and infection. Due to the chronic inflammatory process, however, systemic complications can develop like anemia, hypoproteinemia, nephritic syndrome, arthopathies, dactylitis, polyarthritis, secondary lymphedema (scrotal or vulvar), fistulae to rectum, vagina, urethra, peritoneum or bladder [Figures 3 and 4].[42–44] Lymphangioma circumscriptum of the vulva is a possible complication of AI.[45]
Figure 4.
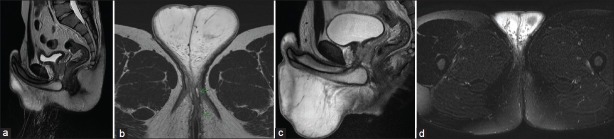
Fistulations and scrotal edema (Magnetic resonance imaging - MRI).(a) and (b) before treatment. Fistulations are marked by arrows. (c) and (d) Complete remission of fistulations and edema after treatment with remicade (2 infusions week o and week 2). All figures are made in spin spin relaxation technique (T2). No enhancer was used
Reactive arthritis can develop as a possible complication of AI.[40] Synovitis, acne pustulosis, hyperostosis, and osteitis (SAPHO)-syndrome has been described in AI patients.[46] Pyoderma gangrenosum is another complication in AI patients unresponsive to various treatments.[47] Secondary amyloid A amyloidosis has been observed occasionally with multi-systemic manifestations including renal and heart affection.[48]
Interstitial keratitis is a possible complication that responds to tumor necrosis factor-α inhibitors.[49] Corneal bilateral ulcers of Mooren's type have been described in a 47-year-old AI patient leading to corneal perforations.[50]
Squamous cell carcinoma developing on AI is a rare but life-threatening complication since these tumors are aggressive with early metastatic spread and a devastating mortality rate of 50% [Figure 5].[31,32,51,52]
Figure 5.
Anal squamous cell carcinoma as a complication of chronic severe anogenital acne inversa. Skin marks for proposed excision margins
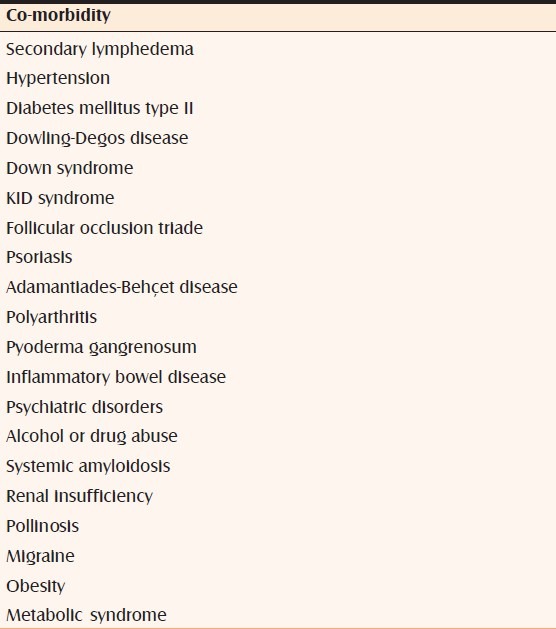
Co-morbidities [Table 2]
Table 2.
Patients with AI show an increased prevalence of metabolic syndrome [Table 3].[53] AI can also be associated with chronic inflammatory bowel disease like Crohn's disease, what complicates the situation due to a stronger tendency to fistulas to the rectum, vagina etc.[54] Auto-inflammatory diseases are sometimes associated with AI.[55]
Table 3.
Odds ratio for metabolic disorders associated with acne inversa[44]

Treatment
There are three levels in the management of AI: Topical options, systemic options, and surgical methods, including laser therapy.[1,5,6]
Drug therapy
Targeting the biofilm formation, clindamycin-rifampicin, or rifampicin-moxifloxacin-metronidazole combinations seem to be effective in less severe AI.[56–58] Antibiosis is not effective in AI-associated SAPHO-syndrome.[46] Oral acitretin 0.5 mg/kg body weight has been used after surgery to prevent relapses.[59,60] Long-term acitretin monotherapy resulted in a complete remission in 9 of 12 patients with severe AI.[61] Dapsone was evaluated in a retrospective trial with 24 AI patients. Improvement was seen in 38% of patients while most patients did not respond. Eight percent of patients discontinued the treatment due to adverse effects. The relapse rate was high.[62] Drug therapy with the aforementioned compounds does not correct the primary immune dysfunction of the innate immune system.
In recent years, tumor necrosis-α inhibitors (TNFαI) like infliximab, adalimumab, and etanercept have been used to treat severe AI successfully in combination with surgery.[32,63–65] In rare cases, however, etanercept can induce AI.[66] In a meta-analysis, 65 studies including 459 patients on TNFαI were evaluated. A moderate to good response was seen in 82% of patients treated with infliximab, 76% treated with adalimumab, and 68% of patients treated with etanercept.[67]
More recently, ustekinumab - a human monoclonal antibody that binds to the p40 subunit of IL-12 and IL-23 - was used with success in a series of three AI patients, of whom one achieved a complete remission after 6 months.[68]
TNFαI are able to decrease elevated TNFα levels in serum and to reduce over-expression of LL-37 as shown for psoriasis.[69] Although TNFαI can improve the inflammatory signs and discharge in AI patients, they are not a cure for the disease. But, they could be used to decrease the amount of surgery necessary to cure AI. TNFαI also reduce inflammatory edema and are effective in inducing remissions of fistulations [Figure 4].
Controversies in drug therapy
Although drug therapy can lead to improvement in milder cases of AI, they are not curative. Patients with advanced AI treated by combinations of antibiotics need up to 12 months before some of them achieve a temporary remission. Adverse effects are common with gastrointestinal side effects in 64% of patients and vaginal candidiasis in 35% of female patients.[70] Treatment with TNFαI and ustekinumab is effective in more advanced cases of AI (Hurley grade III). Some patients with AI were treated with TNFαI for one year, but average recurrence-free interval was only 9.5 months for etanercept and 21.5 months for adalimumab.[71,72]
Are the high costs balanced against the treatment results [Table 4]? Relapses are common after continuous treatment.[82] And, is the treatment as save as for other diseases like psoriasis? Fatal pneumococcal sepsis and bilateral Candida chorioretinitis have been observed in single patients treated by infliximab or etanercept for AI.[86,87] Do benefits of long-term anti-TNFαI therapy outweigh the risks?
Table 4.
Complete remission with drug therapy with a focus on advanced acne inversa
In addition, there is no proof that drug therapy can improve the long-term course of AI. Can an early drug therapy prevent progression? We do not know. Can drug therapy cause a sustained remission? The answer is no.
On the other hand, drug therapy may be useful in conjunction with surgery, either to reduce to need of more extensive surgery (like biologicals) or to reduce the risk of relapses after limited surgery (i.e., retinoids). TNFαI seem to be successful in reactive arthritis associated with AI.
Laser and light therapy
Laser and light equipments have been evaluated to treat AI patients. Highton et al., (2011) used intense pulsed light (IPL) twice-weekly for four weeks. In their prospective trial, 18 patients were enrolled with lesions on both sides of the body. One side was treated, the other one served as control. A significant improvement was noted but no cure. Therefore, the authors concluded that IPL could be added to other AI treatments in particular for patients keen on avoiding surgery.[88]
Photodynamic therapy (PDT) with 20% 5-aminolevulinic acid was applied weekly for four weeks (n = 12) with either IPL or blue light. Blue light therapy was more comfortable for patients. Complete remission was obtained in 25% of patients[89] while earlier reports were unsuccessful.[90]
Long-pulsed Nd:YAG laser (1064 nm) was used in Hurley grade II patients (n = 19) twice a months. Improvement has been seen for axillary and groin lesions followed by fibrosis and scarring one and two months after treatment.[91] Two other prospective, randomized trials with long-pulsed Nd:YAG laser achieved a significant improvement over a 3- or 4-months period of treatment.[92,93] Carbon dioxide laser excision and marsupialization was successful in a series of 61 patients with long-standing AI. Recurrences were noted in only two of 185 sites treated.[94]
Controversies in laser and light therapy
Although laser and light seem to be attractive in controlling AI, the cure rate is low and the time needed to improvement is longer, i.e., several weeks to several months. Laser and light therapies may be used in patients with limited disease to achieve temporary improvement, but we do not have enough data about long-term effects. It seems to work better in the axillary region than in the anogenital area. At present, there is a combination of relative lack of data and relative abundance of treatment schedules and techniques. Does laser/light therapy reduce the risk of fibrosis, lymphedema, or scarring? Further studies are needed.
Surgery
Surgery is a cornerstone for acute abscess pain relief and for treatment of advanced AI.[32,95–97] Abscess pain relief is a symptomatic treatment. It has no effect on the further course of the disease. It causes scarring by itself.
In mild to moderate AI (Hurley grade I and II), surgery with primary closure is a treatment option. In a retrospective study, 66% of patients achieved a complete response.[98]
In more advanced disease, extensive surgery with second intention healing is the gold standard [Figure 6]. We reported recently of a series of 67 patients with ano-genital AI, Hurley grade III, treated by wide excision and secondary healing. This resulted in significantly reduced pain and a very low relapse rate of 2%. Granulation can be stimulated by the use of topical CO2 application.[32] In a study with 56 AI patients, wide surgical excision was performed. Healing by second intention was the choice in 32 (57.1%) patients, and 24 (42.9%) patients underwent delayed skin-grafting. Recurrence was observed in 1.8% of patients.[99]
Figure 6.
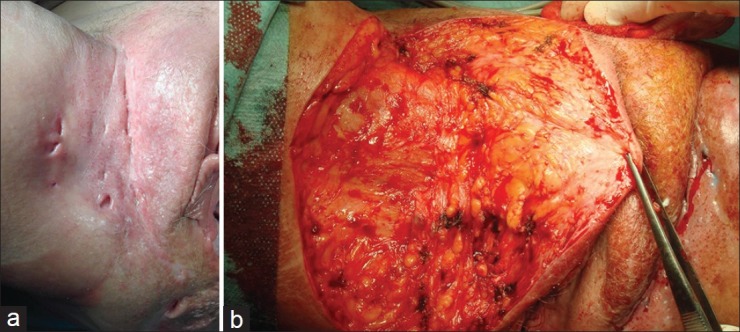
Wide excision surgery of chronic advanced acne inversa. (a) Before surgery. (b) Operation situs
In a study of 106 AI patients, the complication rate was 17.8% with suture dehiscence, postoperative bleeding, hematoma, and wound infection (3.7%). The relapse rate of 2.5% in this report was related to the severity of AI.[100] In conclusion, surgery remains the only curative treatment for AI.[101]
Surgery by second intention has the advantage of low relapse rates (0% to 5.8%) with good functional and esthetic outcome.[32] In larger series with second intention healing, the relapse rate was low, i.e., 0% to 5.8%.[100–103] The procedure needs adjuvant physiotherapy to avoid strictures and loss of mobility in particular for axillary AI. Time to heal, however, is delayed compared to the use of flaps and grafts. Wound bed granulation is supported by the use of transdermal CO2 application and negative topical pressure therapy.[32]
Combining extensive surgery with split skin mesh graft transplantation and topical negative pressure resulted in a graft take rate of 90% for the axillary region.[104] In another series of 11 patients with 24 sites, only three patients needed a re-grafting after split skin mesh grafts with topical negative pressure.[105]
A prospective study (n = 12) was conducted for AI patients with gluteal and anogenital manifestation. Superior and inferior gluteal artery perforator flaps were prepared. There was a single flap necrosis. These flaps are useful to cover large defects in the gluteal and perianal region.[106] For axillary AI transposition flaps or thoracodorsal artery perforator flaps have been used successfully.[107–109] The gluteal site is associated with a higher risk of complications after skin closure by flaps.[110]
When the lesions are limited to the groins, another surgical option is available, the combination with a medial thigh lift. In a Swiss study from Basel, a total of 8 patients with localized inguinal AI were identified, and 15 thigh lifts were performed. All wounds but one healed primarily with satisfactory functional and esthetic results. No major complications, no irritations of the genital area, and no recurrences were observed.[111]
In a retrospective analysis of 50 patients with AI, Hurley grade II to III wide surgical excision with surgical reconstruction by split-thickness skin grafts or fasciocutaneous flaps dramatically reduced the average stay in hospital to 5 days. The recurrence rate of 18.8% is about three-fold the recurrence rate by healing of second intent.[112] Flaps, in contrast to healing by secondary intention, can lead to localized secondary lymphedema. In extensive anogenital AI defect, closure is performed in many departments, but contour after skin grafting often is unsatisfactory.[108]
Controversies in surgery
Although higher numbers of patients have been analyzed for surgery than for any other treatment option, the vast majority of studies are retrospective. Only small prospective trials with less than 20 patients are available. Surgery is the only treatment option that achieves a high rate of stable remission or cure. Surgery is able to prevent devastating long-term risks such as lymphedema and squamous cell carcinoma. This treatment leads to a rapid improvement of QoL and pain relief. There is discussion about primary closure or secondary healing. No controlled trials have been performed to evaluate pros and cons of both. The use of vascular flaps reduced to time to healing versus healing by second intention. However, the functional and esthetic outcome is not necessarily better.
Can the outcome after surgery be further improved? Significant fewer infections have been observed when primary closure was combined with gentamicin sulfate antibiosis.[113] No such proof is available for patient with healing by second intention.
CONCLUSIONS
AI is a potentially severe disorder with negative impact on QoL and life-threatening complications. Conservative treatment plays a role in mild stages, but surgery remains the only treatment for cure, especially in advanced stages.
It is most remarkable that obesity is a major co-morbidity in AI but also a risk factor for severe AI. The adipose tissue in obesity is characterized by a chronic ongoing low-grade inflammation with macrophages invading its stroma, angiogenesis, and activation of the leukocyte adhesion cascade. Obese adipose tissue secretes a variety of inflammatory cytokines including TNFα.[114]
In advanced AI (Hurley grade III), the adipose tissue becomes directly affected by fistulation inflammation. And vice versa, one may speculate that adipose tissue itself contributes to severity and progress of AI in obese patients. If that is true, any treatment that does not normalize adipose tissue inflammation will fail to control AI.
Only extensive surgery is capable to restore the locally impaired innate immune system.[115] Further insight into the immune mechanisms, however, involved may lead to new drug therapies in the future.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779–93. doi: 10.1016/j.det.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Happle R, König A. Smoker's boils. Dermatology. 2011;222:282–4. doi: 10.1159/000327923. [DOI] [PubMed] [Google Scholar]
- 3.Yazdanyar S, Jemec GB. Hidradenitis suppurativa: A review of cause and treatment. Curr Opin Infect Dis. 2011;24:118–23. doi: 10.1097/QCO.0b013e3283428d07. [DOI] [PubMed] [Google Scholar]
- 4.Harrison BJ, Mudge M, Highes LE. The prevalence of hidradenitis in South Wales. In: Marks R, Plewig G, editors. Acne and Related Disorders. London: Martin Dunitz; 1991. pp. 365–6. [Google Scholar]
- 5.Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: A comprehensive review. J Am Acad Dermatol. 2009;60:539–61. doi: 10.1016/j.jaad.2008.11.911. [DOI] [PubMed] [Google Scholar]
- 6.Sartorius K, Emtestam L, Jenec GB, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831–9. doi: 10.1111/j.1365-2133.2009.09198.x. [DOI] [PubMed] [Google Scholar]
- 7.Canoui-Poitrine F, Revuz JE, Wolkenstein P, Viallette C, Gabison G, Pouget F, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51–7. doi: 10.1016/j.jaad.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Von Laffert M, Stadie V, Wohlrab J, Marsch WC. Hidradenitis suppurativa/acne inversa: Bilocated epithelial hyperplasia with very different sequelae. Br J Dermatol. 2011;164:367–371. doi: 10.1111/j.1365-2133.2010.10034.x. [DOI] [PubMed] [Google Scholar]
- 9.Kamp S, Fiehn AM, Stenderup K, et al. Hidradenitis suppurativa: A disease of the absent sebaceous glands? Sebaceous gland number and volume are significantly reduced in uninvolved hair follicles from patients with hidradenitis suppurativa. Br J Dermatol. 2011;164:1017–22. doi: 10.1111/j.1365-2133.2011.10224.x. [DOI] [PubMed] [Google Scholar]
- 10.Van der Zee HH, de Ruiter L, Boer J, et al. Alterations in leukocyte subsets and histomorphology in normal-appearing perilesional skin and early and chronic hidradenitis suppurativa lesions. Br J Dermatol. 2012;166:98–106. doi: 10.1111/j.1365-2133.2011.10643.x. [DOI] [PubMed] [Google Scholar]
- 11.Van der Zee HH, de Ruiter L, van den Broecke DG, Dik WA, Laman JD, Prens EP. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: A rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292–8. doi: 10.1111/j.1365-2133.2011.10254.x. [DOI] [PubMed] [Google Scholar]
- 12.Emilanov VU, Bechara FG, Gläser R, Langan EA, Taungjaruwinai WM, Schröder JM, et al. Immunohistological pointers to a possible role for excessive cathelicidin (LL-37) expression by apocrine sweat glands in the pathogenesis of hidradenitis suppurativa/acne inversa. Br J Dermatol. 2012;166:1023–34. doi: 10.1111/j.1365-2133.2011.10765.x. [DOI] [PubMed] [Google Scholar]
- 13.Matusiak L, Bieniek A, Szepietowski JC. Increased serum tumour necrosis factor-alpha in hidradenitis suppurativa patients: Is there a basis for treatment with anti-tumour necrosis factor-alpha agents? Acta Derm Venereol. 2009;89:601–3. doi: 10.2340/00015555-0749. [DOI] [PubMed] [Google Scholar]
- 14.Wolk K, Warszawska K, Hoeflich C, Witte E, Schneider-Burrus S, Witte K, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: Pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228–39. doi: 10.4049/jimmunol.0903907. [DOI] [PubMed] [Google Scholar]
- 15.Schlapbach C, Hänni T, Yawalkar N, Hunger RE. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790–8. doi: 10.1016/j.jaad.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Wang B, Yang W, Wen W, Sun J, Su B, Liu B, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi: 10.1126/science.1196284. [DOI] [PubMed] [Google Scholar]
- 17.Li CR, Jiang MJ, Shen DB, Xu HX, Wang HS, Yao X, et al. Two novel mutations of the nicastrin gene in Chinese patients with acne inversa. Br J Dermatol. 2011;165:415–8. doi: 10.1111/j.1365-2133.2011.10372.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Gao M, Lv YM, Yang X, Ren YQ, Jiang T, et al. Confirmation by exome sequencing of the pathogenic role of NCSTN mutations in acne inverse (hidradentitis suppurativa) J Invest Dermatol. 2011;131:1570–2. doi: 10.1038/jid.2011.62. [DOI] [PubMed] [Google Scholar]
- 19.Miskinyte S, Nassif A, Merabtene F, Ungeheuer MN, Join-Lambert O, Jais JP, et al. Nicastrin mutations in French families with hidradenitis suppurativa. J Invest Dermatol. 2012;132:1728–30. doi: 10.1038/jid.2012.23. [DOI] [PubMed] [Google Scholar]
- 20.Mozeika E, Jemec GB, Nürnberg BM. Hedgehog pathway does not play a role in hidradenitis suppurativa pathogenesis. Exp Dermatol. 2011;20:841–2. doi: 10.1111/j.1600-0625.2011.01344.x. [DOI] [PubMed] [Google Scholar]
- 21.Sartorius K, Killasli H, Oprica C, Sullivan A, Lapins J. Bacteriology of hidradenitis suppurativa exacerbations. Deep tissue cultures obtained during carbon dioxide laser treatment. Br J Dermatol. 2012;166:879–83. doi: 10.1111/j.1365-2133.2011.10747.x. [DOI] [PubMed] [Google Scholar]
- 22.Kathju S, Lasko LA, Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65:385–9. doi: 10.1111/j.1574-695X.2012.00946.x. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann SC, Saborowski V, Lange S, Kern WV, Bruckner-Tuderman L, Rieg S. Expression of innate defense antimicrobial peptides in hidradenitis suppurativa. J Am Acad Dermatol. 2012;66:966–74. doi: 10.1016/j.jaad.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Grönberg A, Zettergren L, Agren MS. Stability of the cathelicidin peptide LL-37 in a non-healing wound environment. Acta Derm Venereol. 2011;91:511–5. doi: 10.2340/00015555-1102. [DOI] [PubMed] [Google Scholar]
- 25.Dréno B, Khammari A, Brocard A, Moyse D, Blouin E, Guillet G, et al. Hidradenitis suppurativa: The role of deficient cutaneous innate immunity. Arch Dermatol. 2012;148:182–6. doi: 10.1001/archdermatol.2011.315. [DOI] [PubMed] [Google Scholar]
- 26.Hunger RE, Surovy AM, Hassan AS, Braathen LR, Yawalkar N. Toll-like receptor 2 is highly expressed in lesions of acne inversa and colocalizes with C-type lectin receptor. Br J Dermatol. 2008;158:691–7. doi: 10.1111/j.1365-2133.2007.08425.x. [DOI] [PubMed] [Google Scholar]
- 27.Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari A, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105–13. doi: 10.1111/j.1365-2133.2005.06933.x. [DOI] [PubMed] [Google Scholar]
- 28.Pukstad BS, Ryan L, Flo TH, Stenvik J, Moseley R, Harding K, et al. Non-healing is associated with persistent stimulation of the innate immune response in chronic venous leg ulcers. J Dermatol Sci. 2010;59:115–22. doi: 10.1016/j.jdermsci.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Dressel S, Harder J, Cordes J, Wittersheim M, Meyer-Hoffert U, Sunderkötter C, et al. Differential expression of antimicrobial peptides in margins of chronic wounds. Exp Dermatol. 2010;19:628–32. doi: 10.1111/j.1600-0625.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 30.Vasconcelos BN, Fonseca JC, Obadia DL. Case for diagnosis. An Bras Dermatol. 2011;86:601–2. doi: 10.1590/s0365-05962011000300034. [DOI] [PubMed] [Google Scholar]
- 31.Jemec GB. Clinical practise. Hidradenitis suppurativa. N Engl J Med. 2012;366:158–64. doi: 10.1056/NEJMcp1014163. [DOI] [PubMed] [Google Scholar]
- 32.Wollina U, Meseg A, Tilp M, Schönlebe J, Heinig B, Nowak A. Management of severe anogenital acne inverse (hidradenitis suppurativa) Dermatol Surg. 2012;38:110–7. doi: 10.1111/j.1524-4725.2011.02157.x. [DOI] [PubMed] [Google Scholar]
- 33.Syed ZU, Hamzavi IH. Atypical hidradenitis suppurativa involving the posterior neck and occiput. Arch Dermatol Venereol. 2011;147:1343–4. doi: 10.1001/archdermatol.2011.329. [DOI] [PubMed] [Google Scholar]
- 34.Wollina U, Gemmeke A, Koch A. Dissecting cellulitis of the scalp responding to intravenous tumor necrosis factor-alfa antagonist. J Clin Aesthet Dermatol. 2012;5:36–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Onderdijk AJ, van der Zee HH, Esmann S, Lphaven S, Dufour DN, Jemec GB, et al. Depression in patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2012 doi: 10.1111/j.1468-3083.2012.04468.x. [In Press] [DOI] [PubMed] [Google Scholar]
- 36.Esmann S, Jemec GB. Psychosocial impact of hidradenitis suppurativa: A qualitative study. Acta Derm Venereol. 2011;91:328–32. doi: 10.2340/00015555-1082. [DOI] [PubMed] [Google Scholar]
- 37.Matusiak L, Bieniek A, Szepietowski JC. Psychophysiological aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264–8. doi: 10.2340/00015555-0866. [DOI] [PubMed] [Google Scholar]
- 38.Hurley HJ. In: Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus surgical approach. Roenigk PK, Roenigk HH, editors. New York: Dermatologic Surgery Marcel Dekker; 1989. pp. 729–39. [Google Scholar]
- 39.Sartorius K, Killasli H, Heilborn J, Jemec GB, Lapins J, Emtestam L. Interobserver variability of clinical scores in hidradenitis suppurativa is low. Br J Dermatol. 2010;162:1261–8. doi: 10.1111/j.1365-2133.2010.09715.x. [DOI] [PubMed] [Google Scholar]
- 40.Marquardt AL, Hackshaw KV. Reactive arthritis associated with hidradenitis suppurativa. J Natl Med Assoc. 2009;101:367–9. doi: 10.1016/s0027-9684(15)30886-5. [DOI] [PubMed] [Google Scholar]
- 41.Faye O, Petit F, Poli F, Petit T, Wechsler J, Gabison G, et al. Lymphedema as a complication of hidradenitis suppurativa in three patients. Ann Dermatol Venereol. 2007;134:567–9. doi: 10.1016/s0151-9638(07)89271-4. [DOI] [PubMed] [Google Scholar]
- 42.Amankwah Y, Haefner H. Vulvar edema. Dermatol Clin. 2010;28:765–77. doi: 10.1016/j.det.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Van Rappard DC, Mooij JE, Baeten DL, Mekkes JR. New-onset polyarthritis during successful treatment of hidradenitis suppurativa with infliximab. Br J Dermatol. 2011;165:194–8. doi: 10.1111/j.1365-2133.2011.10328.x. [DOI] [PubMed] [Google Scholar]
- 44.Fioravanti A, Lauraflori M, Guidelli GM, Giordano N. Dactylitis as a first manifestation of arthritis associated with hidradenitis suppurativa. Indian J Dermatol Venereol Leprol. 2011;77:74–6. doi: 10.4103/0378-6323.74999. [DOI] [PubMed] [Google Scholar]
- 45.Sims SM, McLean FW, Davis JD, Morgan LS, Wilkinson EJ. Vulvar lymphangioma circumscriptum: A report of 3 cases, 2 associated with vulvar carcinoma and 1 with hidradenitis suppurativa. J Low Genit Tract Dis. 2010;14:234–7. doi: 10.1097/LGT.0b013e3181dc1e5b. [DOI] [PubMed] [Google Scholar]
- 46.De Souza A, Solomon GE, Strober BE. SAPHO syndrome associated with hidradenitis suppurativa successfully treated with infliximab and methotrexate. Bull NYU Hosp J Dis. 2011;69:185–7. [PubMed] [Google Scholar]
- 47.Hsiao JL, Antaya RJ, Berger T, Maurer T, Shinkai K, Leslie KS. Hidradenitis suppurativa and concomitant pyoderma gangrenosum: A case series and literature review. Arch Dermatol. 2010;146:1265–70. doi: 10.1001/archdermatol.2010.328. [DOI] [PubMed] [Google Scholar]
- 48.Girouard SD, Falk RH, Rennke HG, Merola JF. Hidradenitis suppurativa resulting in systemic amyloid A amyloidosis: A case report and review of the literature. Dermatol Online J. 2012;18:2. [PubMed] [Google Scholar]
- 49.Blanco R, González-Vela MC, González-López MA, Fernández-Llaca H, Cañal J, González-Gay MA. Interstitial keratitis secondary to severe hidradenitis suppurativa responding to adalimumab. Cornea. 2012;31:206. doi: 10.1097/ICO.0b013e31820ce137. [DOI] [PubMed] [Google Scholar]
- 50.Meskin SW, Carlson EM. Mooren's-type ulceration associated with severe hidradenitis suppurativa: A case report and literature review. Ocul Immunol Inflamm. 2011;19:340–2. doi: 10.3109/09273948.2011.584653. [DOI] [PubMed] [Google Scholar]
- 51.Losanoff JE, Sochaki P, Khoury N, Levi E, Salwen WA, Basson MD. Squamous cell carcinoma complicating chronic suppurative hidradenitis. Am Surg. 2011;77:1449–53. [PubMed] [Google Scholar]
- 52.Pagliarello C, Paradisi A. The perils of a defective medical communication: Fatal neglected squamous cell carcinoma arising in perineal hidradenitis suppurativa. Case Rep Dermatol. 2011;3:5–7. doi: 10.1159/000323863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sabat R, Chanwangpong A, Schneider-Burrus S, Metternich D, Kokolakis G, Kurek A, et al. Increased prevalence of metabolic syndrome in patients with acne inverse. PLoS One. 2012;7:e31810. doi: 10.1371/journal.pone.0031810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.dos Santos CH, Netto PO, Kawaguchi KY, Parreira Alves JA, de Alencar Souza VP, Reverdito S. Association and management of Crohn's disease plus hidradenitis suppurativa. Inflamm Bowel Dis. 2012;18:E801–2. doi: 10.1002/ibd.21897. [DOI] [PubMed] [Google Scholar]
- 55.Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inverse) Dermatoendocrinol. 2010;2:9–16. doi: 10.4161/derm.2.1.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gener G, Canoui-Poitrine F, Revuz JE, Faye O, Poli F, Gabison G, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: A series of 116 consecutive patients. Dermatology. 2009;219:148–54. doi: 10.1159/000228334. [DOI] [PubMed] [Google Scholar]
- 57.Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439–46. doi: 10.1001/archdermatol.2011.1950. [DOI] [PubMed] [Google Scholar]
- 58.Join-Lambert O, Coignard H, Jais JP, Guet-Revillet H, Poirée S, Fraitag S, et al. Efficacy of rifampicin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology. 2011;222:49–58. doi: 10.1159/000321716. [DOI] [PubMed] [Google Scholar]
- 59.Puri N, Talwar A. A study on the management of hidradenitis suppurativa with retinoids and surgical excision. Indian J Dermatol. 2011;56:650–1. doi: 10.4103/0019-5154.91821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nazary M, van der Zee HH, Prens EP, Folkerts G, Boer J. Pathogenesis and pharmacotherapy of hidradenitis suppurativa. Eur J Pharmacol. 2011;672:1–8. doi: 10.1016/j.ejphar.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 61.Boer J, Nazary M. Long-term results of acitretin therapy for hidradenitis suppurativa.Is acne inverse also a misnomer? Br J Dermatol. 2011;164:170–5. doi: 10.1111/j.1365-2133.2010.10071.x. [DOI] [PubMed] [Google Scholar]
- 62.Yanzdanyar S, Boer J, Ingvarsson G, Szepietowski JC, Jemec GB. Dapsone therapy for hidradenitis suppurativa: A series of 24 patients. Dermatology. 2011;222:342–6. doi: 10.1159/000329023. [DOI] [PubMed] [Google Scholar]
- 63.Van Rappard DC, Mekkes JR. Treatment of severe hidradenitis suppurativa with infliximab in combination with surgical interventions. Br J Dermatol. 2012;167:206–8. doi: 10.1111/j.1365-2133.2012.10807.x. [DOI] [PubMed] [Google Scholar]
- 64.Van der Zee HH, Laman JD, de Ruiter L, Dik WA, Prens EP. Adalimumab (antitumour necrosis factor-α) treatment of hidradenitis suppurativa ameliorates skin inflammation: An in situ and ex vivo study. Br J Dermatol. 2012;166:298–305. doi: 10.1111/j.1365-2133.2011.10698.x. [DOI] [PubMed] [Google Scholar]
- 65.Sharon VR, Garcia MS, Bagheri S, Goodarzi H, Yang C, Ono Y, et al. management of recalcitrant hidradenitis suppurativa with ustekinumab. Acta Derm Venereol. 2012;92:320–1. doi: 10.2340/00015555-1229. [DOI] [PubMed] [Google Scholar]
- 66.Pellegrino M, Taddeucci P, Peccianti C, Mei S, Fioravanti A, Fimiani M. Etanercept induced hidradenitis suppurativa. G Ital Dermatol Venereol. 2011;146:503–4. [PubMed] [Google Scholar]
- 67.Van Rappard DC, Limpens J, Mekkes JR. The off-label treatment of severe hidradenitis suppurativa with TNF-alpha inhibitors: A systematic review. J Dermatolog Treat. 2012 doi: 10.3109/09546634.2012.674193. [In Press] [DOI] [PubMed] [Google Scholar]
- 68.Gulliver WP, Jemec GB, Baker KA. Experience with ustekinumab for the treatment of moderate to severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2012;26:911–4. doi: 10.1111/j.1468-3083.2011.04123.x. [DOI] [PubMed] [Google Scholar]
- 69.Gambichler T, Kobus S, Kobus A, Tigges C, Scola N, Altmeyer P, et al. Expression of antimicrobial peptides and proteins in etanercept-treated psoriasis patients. Regul Pept. 2011;167:163–6. doi: 10.1016/j.regpep.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 70.Join-Lambert O, Coignard H, Jais JP, Guet-Revillet H, Poirée S, Fraitag S, et al. Efficacy of rifampicin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology. 2011;222:49–58. doi: 10.1159/000321716. [DOI] [PubMed] [Google Scholar]
- 71.Arenbergova M, Gkalpakiotis S, Arenberger P. Effective long-term control of refractory hidradenitis suppurativa with adalimumab after failure of conventional therapy. Int J Dermatol. 2010;49:1445–9. doi: 10.1111/j.1365-4632.2010.04638.x. [DOI] [PubMed] [Google Scholar]
- 72.Blanco R, Martínez-Taboada VM, Villa I, González-Vela MC, Fernández-Llaca H, Agudo M, et al. Long-term successful adalimumab therapy in severe hidradenitis suppurativa. Arch Dermatol. 2009;145:580–4. doi: 10.1001/archdermatol.2009.49. [DOI] [PubMed] [Google Scholar]
- 73.Sotiriou E, Goussi C, Lallas A, Chovarda E, Apalla Z, Lazaridou E, et al. A prospective open-label clinical trial of efficacy of the every week administration of adalimumab in the treatment of hidradenitis suppurativa. J Drugs Dermatol. 2012;11(Suppl):s15–20. [PubMed] [Google Scholar]
- 74.Miller I, Lynggaard CD, Lophaven S, Zachariae C, Dufour DN, Jemec GB. A double-blind placebo-controlled randomized trial of adalimumab in the treatment of hidradenitis suppurativa. Br J Dermatol. 2011;165:391–8. doi: 10.1111/j.1365-2133.2011.10339.x. [DOI] [PubMed] [Google Scholar]
- 75.Lee RA, Dommasch E, Treat J, Sciacca-Kirby J, Chachkin S, Williams J, et al. A prospective clinical trial of open-label etanercept for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2009;60:565–73. doi: 10.1016/j.jaad.2008.11.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adams DR, Yankura JA, Fogelberg AC, Anderson BE. Treatment of hidradenitis suppurativa with etanercept injection. Arch Dermatol. 2010;146:501–4. doi: 10.1001/archdermatol.2010.72. [DOI] [PubMed] [Google Scholar]
- 77.Pelekanou A, Kanni T, Savva A, Mouktaroudi M, Raftogiannis M, Kotsaki A, et al. Long-term efficacy of etanercept in hidradenitis suppurativa: Results from an open-label phase II prospective trial. Exp Dermatol. 2010;19:538–40. doi: 10.1111/j.1600-0625.2009.00967.x. [DOI] [PubMed] [Google Scholar]
- 78.Giamarellos-Bourboulis EJ, Pelekanou E, Antonopoulou A, Petropoulou H, Baziaka F, Karagianni V, et al. An open-label phase II study of the safety and efficacy of etanercept for the therapy of hidradenitis suppurativa. Br J Dermatol. 2008;158:567–72. doi: 10.1111/j.1365-2133.2007.08372.x. [DOI] [PubMed] [Google Scholar]
- 79.Grant A, Gonzalez T, Montgomery MO, Cardenas V, Kerdel FA. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: A randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205–17. doi: 10.1016/j.jaad.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 80.Mekkes JR, Bos JD. Long-term efficacy of a single course of infliximab in hidradenitis suppurativa. Br J Dermatol. 2008;158:370–4. doi: 10.1111/j.1365-2133.2007.08332.x. [DOI] [PubMed] [Google Scholar]
- 81.Lesage C, Adnot-Desanlis L, Perceau G, Bonnet M, Palot JP, Bernard P, et al. Efficacy and tolerance of prolonged infliximab treatment of moderate-to-severe forms of hidradenitis suppurativa. Eur J Dermatol. 2012 doi: 10.1684/ejd.2012.1795. [In Press] [DOI] [PubMed] [Google Scholar]
- 82.Paradela S, Rodríguez-Lojo R, Fernández-Torres R, Arévalo P, Fonseca E. Long-term efficacy of infliximab in hidradenitis suppurativa. J Dermatolog Treat. 2012;23:278–83. doi: 10.3109/09546634.2012.683767. [DOI] [PubMed] [Google Scholar]
- 83.Delage M, Samimi M, Atlan M, Machet L, Lorette G, Maruani A. Efficacy of infliximab for hidradenitits suppurativa: Assessment of clinical and biological inflammatory markers. Acta Derm Venereol. 2011;91:169–71. doi: 10.2340/00015555-1025. [DOI] [PubMed] [Google Scholar]
- 84.Boer J, van Gemert MJ. Long-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad Dermatol. 1999;40:73–6. doi: 10.1016/s0190-9622(99)70530-x. [DOI] [PubMed] [Google Scholar]
- 85.Soria A, Canoui-Poitrine F, Wolkenstein P, Poli F, Gabison G, Pouget F, et al. Absence of efficacy of oral isotretinoin in hidradenitis suppurativa: A retrospective study based on patients’ outcome assessment. Dermatology. 2009;218:134–5. doi: 10.1159/000182261. [DOI] [PubMed] [Google Scholar]
- 86.Benítez-Macías JF, García-Gil D, Brun-Romero FM. Fatal pneumococcal sepsis in patients with hidradenitis suppurativa treated with infliximab. Med Clin (Barc) 2008;131:799. doi: 10.1016/s0025-7753(08)75512-x. [DOI] [PubMed] [Google Scholar]
- 87.Arriola-Villabos P, Díaz-Valle D, Alejandre-Alba N, López-Abad C, Méndez-Fernández R, Benítez-del-Castillo JM. Bilateral Candida chorioretinitis following etanercept treatment for hidradenitis suppurativa. Eye (Lond) 2008;22:599–600. doi: 10.1038/sj.eye.6703100. [DOI] [PubMed] [Google Scholar]
- 88.Highton L, Chan WY, Khawa N, Laitung JK. Treatment of hidradenitis suppurativa with intense pulsed light: A prospective study. Plast Recosntr Surg. 2011;128:459–65. doi: 10.1097/PRS.0b013e31821e6fb5. [DOI] [PubMed] [Google Scholar]
- 89.Schweiger ES, Riddle CC, Aires DJ. Treatment of hidradenitis suppurativa by photodynamic therapy with aminolevulinic acid: Preliminary results. J Drugs Dermatol. 2011;10:381–6. [PubMed] [Google Scholar]
- 90.Passeron T, Khemis A, Ortonne JP. Pulsed dye laser-mediated photodynamic therapy for acne inversa is not successful: A pilot study on four cases. J Dermatolog Treat. 2009;20:297–8. doi: 10.1080/09546630902882063. [DOI] [PubMed] [Google Scholar]
- 91.Xu LY, Wright DR, Mahmoud BH, Ozog DM, Mehregan DA, Hamzavi IH. Histopathologic study of hidradenitis suppurativa following long-pulsed 1064-nm Nd: YAG laser treatment. Arch Dermatol. 2011;147:21–8. doi: 10.1001/archdermatol.2010.245. [DOI] [PubMed] [Google Scholar]
- 92.Tierney E, Mahmoud BH, Hexsel C, Ozog D, Hamzavi IH. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol Surg. 2009;35:118–98. doi: 10.1111/j.1524-4725.2009.01214.x. [DOI] [PubMed] [Google Scholar]
- 93.Mahmoud BH, Tierney E, Hexsel CL, Pui J, Ozog DM, Hamzavi IH. Prospective controlled clinical and histopathologic study of hidradenitis suppurativa treated with the long-pulsed neodymium:yttrium-aluminium-garnet laser. J Am Acad Dermatol. 2010;62:637–45. doi: 10.1016/j.jaad.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 94.Hazen PG, Hazen BP. Hidradenitis suppurativa: Successful treatment using carbon dioxide laser excision and marsupialization. Dermatol Surg. 2010;36:208–13. doi: 10.1111/j.1524-4725.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 95.Bieniek A, Matusiak L, Okulewicz-Gojlik D, Szepietowski JC. Surgical treatment of hidradenitis suppurativa: Experiences and recommendations. Dermatol Surg. 2010;36:1998–2004. doi: 10.1111/j.1524-4725.2010.01763.x. [DOI] [PubMed] [Google Scholar]
- 96.Menderes A, Sunay O, Vayvada H, Yilmaz M. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;7:240–7. doi: 10.7150/ijms.7.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Balik E, Eren T, Bulut T, Büyükuncu Y, Bugra D, Yamaner S, et al. Surgical approach to extensive hidradenitis suppurativa in the perineal/perianal and gluteal regions. World J Surg. 2009;33:481–7. doi: 10.1007/s00268-008-9845-9. [DOI] [PubMed] [Google Scholar]
- 98.Van Rappard DC, Mooij JE, Mekkes JR. Mild to moderate hidradenitis suppurativa treated with local excision and primary closure. J Eur Acad Dermatol Venereol. 2012;26:898–902. doi: 10.1111/j.1468-3083.2011.04203.x. [DOI] [PubMed] [Google Scholar]
- 99.Bocchini SF, Habr-Gama A, Kiss DR, et al. Gluteal and perianal hidradenitis suppurativa: Surgical treatment by wide excision. Dis Colon Rectum. 2003;46:944–9. doi: 10.1007/s10350-004-6691-1. [DOI] [PubMed] [Google Scholar]
- 100.Rompel R, Petres J. Long-term results of wide surgical excision in 106 patients with hidradenitis suppurativa. Dermatol Surg. 2000;26:638–43. doi: 10.1046/j.1524-4725.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- 101.Ellis LZ. Hidradenitis suppurativa: Surgical and other management techniques. Dermatol Surg. 2011 doi: 10.1111/j.1524-4725.2011.02186.x. [In Press] [DOI] [PubMed] [Google Scholar]
- 102.Harrison BJ, Mudge E, Hughes LE. Recurrence after surgical treatment of hidradenitis suppurativa. Br Med J. 1987;294:487–9. doi: 10.1136/bmj.294.6570.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kurzen H, Schönfelder-Funcke S, Hartschuh W. Surgical treatment of acne inversa at the university of Heidelberg. Coloproctology. 2000;22:76–80. [Google Scholar]
- 104.Calibre C, Bouhanna A, Salmin JP, Bodin F, Benaïssa-Beck M, Bruano-Rodier C. Axillary hidradenitis suppurativa: A single-stage surgical treatment. Ann Chir Plast Esthet. 2011 doi: 10.1016/j.anplas.2011.05.004. [In Press] [DOI] [PubMed] [Google Scholar]
- 105.Chen E, Friedman HI. Management of regional hidradenitis suppurativa with vacuum-assisted closure and split thickness skin grafts. Ann Plas Surg. 2011;67:397–401. doi: 10.1097/SAP.0b013e3181f77bd6. [DOI] [PubMed] [Google Scholar]
- 106.Unal C, Yirmibesoglu OA, Ozdemir J, Hasdemir M. Superior and inferior gluteal artery perforator flaps in reconstruction of gluteal and perianal/perineal hidradenitis suppurativa lesions. Microsurgery. 2011;31:539–44. doi: 10.1002/micr.20918. [DOI] [PubMed] [Google Scholar]
- 107.Varkarakis G, Daniels J, Coker K, Oswald T, Akdemir O, Lineaweaver WC. Treatment of axillary hidradenitis with transposition flaps: A 6-year experience. Ann Plast Surg. 2010;64:592–4. doi: 10.1097/SAP.0b013e3181da1c4f. [DOI] [PubMed] [Google Scholar]
- 108.Ortiz CL, Castillo VL, Pilarte FS, Barraquer EL. Experience using the thoracodorsal artery perforator flap in axillary hidradenitis suppurativa cases. Aesthetic Plast Surg. 2010;34:785–92. doi: 10.1007/s00266-010-9544-4. [DOI] [PubMed] [Google Scholar]
- 109.Busnardo FF, Coltro PS, Olivan MV, Busnardo AP, Ferreira MC. The thoracodorsal artery perforator flap in the treatment of axillary hidradenitis suppurativa: Effect on preservation of arm abduction. Plast Reconstr Surg. 2011;128:949–53. doi: 10.1097/PRS.0b013e3182268c38. [DOI] [PubMed] [Google Scholar]
- 110.Buyukasik O, Hasdemir AO, Kahramansoy N, Col C, Erkol H. Surgical approach to extensive hidradenitis suppurativa. Dermatol Surg. 2011;37:835–42. doi: 10.1111/j.1524-4725.2011.01961..x. [DOI] [PubMed] [Google Scholar]
- 111.Rieger UM, Erba P, Pierer G, Kalbermatten DF. Hidradenitis suppurativa of the groin treated by radical excision and defect closure by medial thigh lift: Aesthetic surgery meets reconstructive surgery. J Plast Reconstr Aesthet Surg. 2009;62:1355–60. doi: 10.1016/j.bjps.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 112.Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol. 2012;12:9. doi: 10.1186/1471-5945-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Biumer MG, Ankersmit MF, Wobbes T, Klinkenbijl JH. Surgical treatment of hidradenitis suppurativa with gentamicin sulfate: A prospective randomized study. Dermatol Surg. 2008;34:224–7. doi: 10.1111/j.1524-4725.2007.34041.x. [DOI] [PubMed] [Google Scholar]
- 114.Nishimura S, Manabe I, Nagai R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov Med. 2009;8:55–60. [PubMed] [Google Scholar]
- 115.Siemionow M, Nasir S. Immunologic responses in vascularized and nonvascularized skin allografts. J Reconstr Microsurg. 2008;24:497–505. doi: 10.1055/s-0028-1088232. [DOI] [PubMed] [Google Scholar]


