Abstract
Rapamycin (sirolimus) is a fungal fermentation product that inhibits the proper functioning of a serine/threonine protein kinase in mammalian cells eponymously named mammalian target of rapamycin, or mTOR. Rapamycin is a novel class of anticancer and immunosuppressant drugs targeting the proteins at molecular level. Rapamycin (sirolimus) is routinely incorporated in drug-eluting stents used for cardiac angioplasty. In recent years, rapamycin was found to be efficacious in managing the symptom complex of tuberous sclerosis, i.e. renal angiomyolipoma, giant cell astrocytoma and pulmonary lymphangiomyomatosis. Various investigators have also proved that topically applied rapamycin causes regression of facial angiofibromas, giving better cosmetic results.
Keywords: Facial angiofibromas, Mechanism of action, Rapamycin, Tuberous sclerosis
Rapamycin is a lipophilic macrocyclic lactone which was first isolated from a soil bacterium Streptomyces hygroscopicus in Rapa Nui (Easter Island) in 1965,hence the name rapamycin.[1] Though rapamycin was shown to have antifungal properties, later on it was discovered to possess anti-T cell activity and was being used as immunosuppressant in prevention of graft rejection.[2] Rapamycin belongs to a novel class of anti-cancer drugs called as mTOR (mammalian target of rapamycin) inhibitors.
mTOR (mechanistic target of rapamycin) is a large atypical conserved serine-threonine kinase enzyme complex involved in cellular growth, stress, aging and vasculogenesis with a molecular weight of 290 kDa.[3,4] mTOR pathway is critical for normal cell function as it plays a pivotal role in integrating signals from nutrients, energy status and growth factors to regulate many homeostatic processes, including autophagy, ribosome biogenesis and metabolism modulated by phosphatidylinositol 3- kinases (PI3K)–Akt-dependent mechanisms. Although mammalian cells possess only single mTOR gene located on short arm of chromosome 1p36.2, mTOR pathway is composed of two distinct functional complex proteins- (i) mTORC1 consisting of mTOR, LST8/GβL (G protein beta subunit-like) and regulatory-associated protein of mTOR (raptor) and (ii) mTORC 2 consists of mTOR, GβL, and rapamycin insensitive component of mTOR (rictor).[5] It is to be noted that only mTORC 1 is inhibited by rapamycin and not mTORC2.
Functionally, mTORC1 is mainly responsible for the nutrient-sensitive functions of TOR, whereas TORC2 plays a chief role in cytoskeletal reorganization and cell survival. Under normal circumstances, mTOR signaling causes cell proliferation and is under tight regulation of proteins like tuberin and hamartin. Hamartin and tuberin are the protein products of the tuberous sclerosis genes (TSC1 and TSC2) located on chromosome 9 and 16 respectively.[6] Physiologically, the hamartin-tuberin complex activates the protein Ras homolog enriched in brain (Rheb) and exerts inhibitory control over mTOR.[7] Mutation in these two genes (TSC1 and TSC2) leads to defective functioning of these protein products and results in constitutive activation of mTOR pathway, leading to leading to phosphorylation of downstream targets including p70S6K (p70 S6 kinase) and 4E-BP1 (eukaryotic initiation factor 4E-binding protein 1) culminating in protein synthesis and abnormal cellular proliferation as evident in hamartomas of tuberous sclerosis.[8] Rapamycin can simulate the action of tuberin and hamartin protein and thus can prevent the procarcinogenic action of mTOR signaling.
Mechanism of Action of Rapamycin Figure 1
Figure 1.
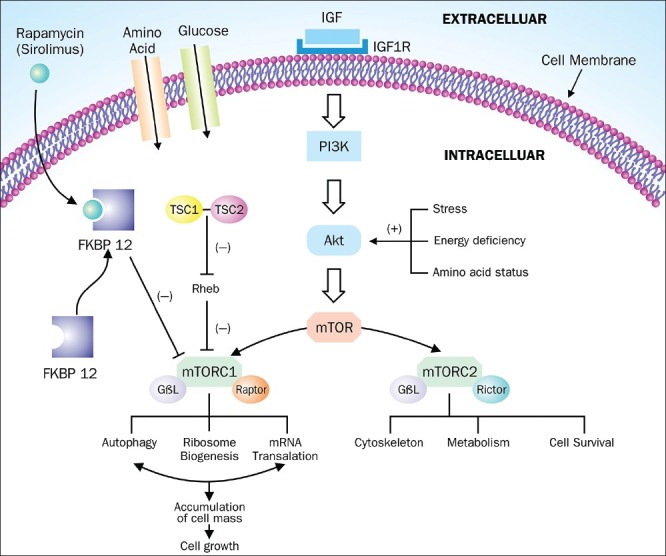
Mechanism of action of rapamycin: Intracellularly rapamycin binds to FKBP12 protein and binds to mTORC1 thereby inhibiting its downstream pathway. Protein products of TSC 1 and TSC 2 gene i.e. hamartin and tuberin inhibits the functioning of mTORC1 pathway via Rheb protein and thus mutation of these TSC proteins causes constitutive activation of mTOR pathway leading to cellular proliferation
Rapamycin belongs to the class of macrocyclic immunosuppressive drugs that are active only when bound to immunophilins. Cyclosporine and tacrolimus (FK506) are other members who also act via binding to immunophilins.[9] Intracellularly, rapamycin binds to FKBP12 (FK binding protein 12 kDa), an immunophilin and forms a complex FKBP12-rapamycin. mTOR possess a binding domain portion called FKBP12-rapamycin binding domain (FRB). After binding to FRB domain of mTOR protein, FKBP12-rapamycin complex potently inhibits the activity of mTORC1 complex via autophosphorylation and dissociation of mTORC1 complex and thus blocking the binding of mTOR to its substrates.[10] Inhibition of mTOR pathway blocks cytokine-driven T-cell proliferation by inhibiting the progression from the G1 to the S phase of the cell cycle,thus explaining its role in immunosuppression.
Indications
Currently, the only FDA approved indication for rapamycin is to prevent organ rejection after transplant surgery.[11,12] Off-label indications include topical treatment of facial angiofibromas[13,14] systemic treatment for renal angiomyolipoma[15] lymphangioleiomyomatosis.[16,17] brain tumors associated with tuberous sclerosis[18,19] and for chemotherapy of various malignancies (renal and hepatocellular cancer and mantle cell lymphomas).[20,21] Other conditions where rapamycin has been used are Kaposi sarcoma,[22] psoriasis[23] and lichen planus.[24]
Pharmacodynamics/Pharmacokinetics
Rapamycin is very poorly water soluble, severely limiting its bioavailability. Congeners of rapamycin have been developed with better pharmacokinetic properties i.e temsirolimus (CCI-779), everolimus (RAD001) and ridaforolimus (AP23573) and are collectively known as rapalogs. Currently rapamycin (sirolimus) is available in the market in two formulations: Rapamune® oral solution (60mg per 60ml in an amber colored bottle) and Rapamune® tablet available in 1mg (white triangular-shaped tablet) and 2 mg (yellow-to-beige triangular-shaped tablet) strength.[25] Oral solution needs to be kept at cold temperature of 2-8° centigrade.
Topical Rapamycin for Angiofibromas Associated with Tuberous Sclerosis
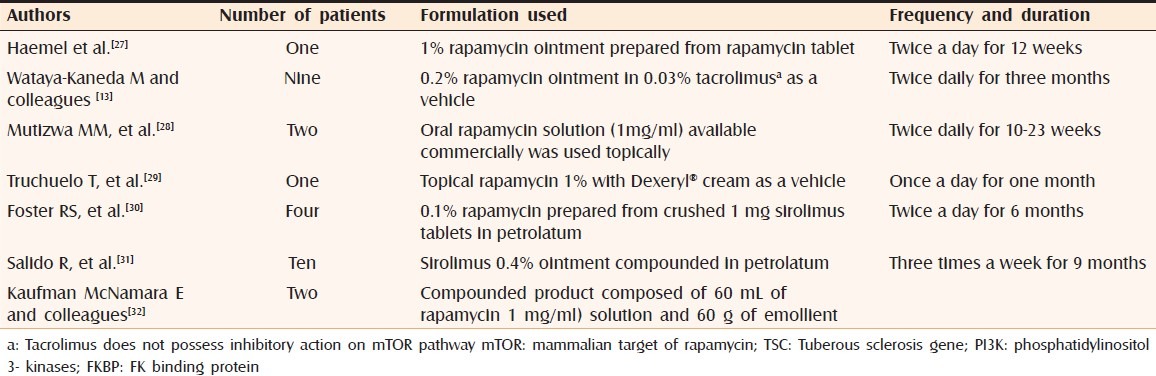
Angiofibromas shows prominent vascular component owing to increased expression of angiogenic factors like vascular endothelial growth factor (VEGF) and mTOR overactivation that promotes angiogenesis as discussed earlier. Inhibition of mTOR pathway decreases the output of VEGF by inhibiting hypoxia-inducible factor (HIF) expression and by directly repressing VEGF-stimulated endothelial cell proliferation.[26] Facial angiofibromas are a chief cause of concern among the patients having TSC owing to unsightly appearance of facial papules. Rapamycin is a large molecule, difficult to formulate in the ointment form.[13] Various investigators have used different concentrations of topical rapamycin for the management of facial angiofibromas [Table 1].
Table 1.
Topical rapamycin used for treatment of facial angiofibromas
Irritation and burning sensation is the most common side effect seen after topical rapamycin. Patients should be prescribed topical hydrocortisone 0.1% cream or desonide 0.05% lotion along with liberal emollients to counteract any irritation and ensure compliance. It is practical to use commercially available oral solution of rapamycin (1 mg/ml) as a topical formulation since compounding pharmacies are not always readily accessible and the stability and efficacy of compounded preparation cannot be ensured. The major limiting factor in prescribing topical rapamycin is the high cost of the medication. Haemel et al. compounded topical rapamycin from crushed rapamycin tablet into a 30 ml of 1% ointment and it priced about $3000.[28] Topical rapamycin can be safely prescribed in children in whom angiofibromas are still in the growing phase.[27,30] Patients receiving rapamycin therapy should avoid taking grapefruit juice as it inhibits the metabolism of rapamycin akin to cyclosporine.
Systemic Side Effects
Topically applied rapamycin has minimal systemic absorption, precluding any adverse systemic effects. If facilities are available, trough drug levels should be monitored by chromatographic and immunoassay methodologies. However, whether this applies to topical rapamycin therapy needs to evaluated. More robust studies are required to evaluate the extent of systemic absorption of topically applied rapamycin and to determine the safety of topically administered rapamycin.
CONCLUSION
Topical rapamycin appears to be a promising and effective way of treating facial angiofibromas which are cosmetically disfiguring in patients with TSC. Topical rapamycin needs to be studied in a larger cohort of subjects to determine the duration and frequency of application. The major disadvantage is the cost of therapy which is prohibitively expensive at the present date in our resource poor setting.[32]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886–918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arns W, Budde K, Eitner F, Gwinner W, Hugo C, Pressmar K, et al. [Conversion from a calcineurin inhibitor to a sirolimus-based therapy after renal transplantation - An update of existing recommendations] Dtsch Med Wochenschr. 2011;136:2554–9. doi: 10.1055/s-0031-1292822. [DOI] [PubMed] [Google Scholar]
- 3.Dobashi Y, Watanabe Y, Miwa C, Suzuki S, Koyama S. Mammalian target of rapamycin: A central node of complex signaling cascades. Int J Clin Exp Pathol. 2011;4:76–95. [PMC free article] [PubMed] [Google Scholar]
- 4.Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–83. doi: 10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]
- 5.Russell RC, Fang C, Guan KL. An emerging role for TOR signaling in mammalian tissue and stem cell physiology. Development. 2011;138:3343–56. doi: 10.1242/dev.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim WS. Mammalian target of rapamycin inhibitors for treatment in tuberous sclerosis. Korean J Pediatr. 2011;54:241–5. doi: 10.3345/kjp.2011.54.6.241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59:490–8. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 8.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–51. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sehgal SN. Rapamune (Sirolimus, rapamycin): An overview and mechanism of action. Ther Drug Monit. 1995;17:660–5. doi: 10.1097/00007691-199512000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Gabardi S, Baroletti SA. Everolimus: A proliferation signal inhibitor with clinical applications in organ transplantation, oncology, and cardiology. Pharmacotherapy. 2010;30:1044–56. doi: 10.1592/phco.30.10.1044. [DOI] [PubMed] [Google Scholar]
- 12.Cravedi P, Ruggenenti P, Remuzzi G. Sirolimus for calcineurin inhibitors in organ transplantation: Contra. Kidney Int. 2010;78:1068–74. doi: 10.1038/ki.2010.268. [DOI] [PubMed] [Google Scholar]
- 13.Wataya-Kaneda M, Tanaka M, Nakamura A, Matsumoto S, Katayama I. A topical combination of rapamycin and tacrolimus for the treatment of angiofibroma due to tuberous sclerosis complex (TSC): A pilot study of nine Japanese patients with TSC of different disease severity. Br J Dermatol. 2011;165:912–6. doi: 10.1111/j.1365-2133.2011.10471.x. [DOI] [PubMed] [Google Scholar]
- 14.DeKlotz CM, Ogram AE, Singh S, Dronavalli S, MacGregor JL. Dramatic improvement of facial angiofibromas in tuberous sclerosis with topical rapamycin: Optimizing a treatment protocol. Arch Dermatol. 2011;147:1116–7. doi: 10.1001/archdermatol.2011.254. [DOI] [PubMed] [Google Scholar]
- 15.Cabrera López C, Martí T, Catalá V, Torres F, Mateu S, Ballarín Castán J, et al. Effects of rapamycin on angiomyolipomas in patients with tuberous sclerosis. Nefrologia. 2011;31:292–8. doi: 10.3265/Nefrologia.pre2011.Apr.10812. [DOI] [PubMed] [Google Scholar]
- 16.Casanova A, María Girón R, Acosta O, Barrón M, Valenzuela C, Ancochea J. Lymphangioleiomyomatosis treatment with sirolimus. Arch Bronconeumol. 2011;47:470–2. doi: 10.1016/j.arbres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 17.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, et al. National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Major P. Potential of mTOR inhibitors for the treatment of subependymal giant cell astrocytomas in tuberous sclerosis complex. Aging (Albany NY) 2011;3:189–91. doi: 10.18632/aging.100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koenig MK, Butler IJ, Northrup H. Regression of subependymal giant cell astrocytoma with rapamycin in tuberous sclerosis complex. J Child Neurol. 2008;23:1238–9. doi: 10.1177/0883073808321764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voss MH, Molina AM, Motzer RJ. mTOR inhibitors in advanced renal cell carcinoma. Hematol Oncol Clin North Am. 2011;25:835–52. doi: 10.1016/j.hoc.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riaz H, Riaz T, Hussain SA. mTOR inhibitors: A novel class of anti-cancer agents. Infect Agent Cancer. 2012;7:1. doi: 10.1186/1750-9378-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saggar S, Zeichner JA, Brown TT, Phelps RG, Cohen SR. Kaposi's sarcoma resolves after sirolimus therapy in a patient with pemphigus vulgaris. Arch Dermatol. 2008;144:654–7. doi: 10.1001/archderm.144.5.654. [DOI] [PubMed] [Google Scholar]
- 23.Ormerod AD, Shah SA, Copeland P, Omar G, Winfield A. Treatment of psoriasis with topical sirolimus: Preclinical development and a randomized, double-blind trial. Br J Dermatol. 2005;152:758–64. doi: 10.1111/j.1365-2133.2005.06438.x. [DOI] [PubMed] [Google Scholar]
- 24.Soria A, Agbo-Godeau S, Taïeb A, Francès C. Treatment of refractory oral erosive lichen planus with topical rapamycin: 7 cases. Dermatology. 2009;218:22–5. doi: 10.1159/000172830. [DOI] [PubMed] [Google Scholar]
- 25.Official website of US FDA. [Last accessed on 2012 Mar 22]. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021083s033,021110s043lbl.pdf .
- 26.Del Bufalo D, Ciuffreda L, Trisciuoglio D, Desideri M, Cognetti F, Zupi G, et al. Antiangiogenic potential of the Mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 2006;66:5549–54. doi: 10.1158/0008-5472.CAN-05-2825. [DOI] [PubMed] [Google Scholar]
- 27.Haemel AK, O’Brian AL, Teng JM. A novel approach to facial angiofibromas in tuberous sclerosis. Arch Dermatol. 2010;146:715–18. doi: 10.1001/archdermatol.2010.125. [DOI] [PubMed] [Google Scholar]
- 28.Mutizwa MM, Berk DR, Anadkat MJ. Treatment of facial angiofibromas with topical application of oral rapamycin solution (1 mgmL(-1)) in two patients with tuberous sclerosis. Br J Dermatol. 2011;165:922–3. doi: 10.1111/j.1365-2133.2011.10476.x. [DOI] [PubMed] [Google Scholar]
- 29.Truchuelo T, Díaz-Ley B, Ríos L, Alcántara J, Jaén P. Facial angiofibromas treated with topical rapamycin: An excellent choice with fast response. Dermatol Online J. 2012;18:15. [PubMed] [Google Scholar]
- 30.Foster RS, Bint LJ, Halbert AR. Topical 0.1% rapamycin for angiofibromas in paediatric patients with tuberous sclerosis: A pilot study of four patients. Australas J Dermatol. 2012;53:52–6. doi: 10.1111/j.1440-0960.2011.00837.x. [DOI] [PubMed] [Google Scholar]
- 31.Salido R, Garnacho-Saucedo G, Cuevas-Asencio I, Ruano J, Galán-Gutierrez M, Vélez A, et al. Sustained clinical effectiveness and favorable safety profile of topical sirolimus for tuberous sclerosis - associated facial. J Eur Acad Dermatol Venereol. 2011 Aug 11; doi: 10.1111/j.1468-3083.2011.04212.x. doi: 10.1111/j.1468-3083.2011.04212.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman McNamara E, Curtis AR, Fleischer AB., Jr Successful treatment of angiofibromata of tuberous sclerosis complex with rapamycin. J Dermatolog Treat. 2012;23:46–8. doi: 10.3109/09546634.2010.489598. [DOI] [PubMed] [Google Scholar]


