Abstract
Self-injection of mercury can be life-threatening. We report a case of attempted suicide by self-intravenous injection of elemental mercury. The patient suffered from two side effects : membranous nephropathy and aplastic anemia. She was treated and the systemic effects of mercury were reversed after 4 years. The toxicology of mercury, mechanisms of renal and systemic toxicities, and the various therapeutic measures for mercury poisoning are discussed.
Keywords: Aplastic anemia, membranous nephropathy, mercury poisoning
Introduction
For centuries, mercury (Hg) was an essential part of many different medicines, such as diuretics, antibacterial agents, and laxatives. Presently, these drugs have been replaced and drug-induced signs of mercury toxicity are rare. Mercury toxicity as part of environmental pollution is a major concern because of increased usage of fossil fuels and agricultural products, both of which contain mercury.
Cases of nephrotic syndrome due to long-term contact with mercury compounds have been reported. So far, there is no reported case of membranous nephropathy as a result of intravenous elemental mercury injection. We report a case of aplastic anemia and nephrotic syndrome following intravenous mercury administration.
Case Report
A 19-year-old female was referred to our hospital with a history of intravenous injection of 5 ml of elemental mercury with suicidal inten. She had been initially treated with local excision and broncho-alveolar lavage (BAL) and penicillamine are used to remove the extravasated mercury at the site of injection. Two weeks later, she developed recurrent bouts of fever and pancytopenia for which she had been given multiple blood transfusions. She was referred to us for further evaluation. Clinical examination was unremarkable except for pallor.
Investigations revealed pancytopenia with a hemoglobin of 5.7 g/dl, total WBC count of 3600 cells/cu.mm, and a platelet count of 15,000/cu.mm. She had normal liver and renal functions and urine analysis showed 3+ proteinuria with 8-10 RBCs/HPF. X-ray chest revealed multiple opaque deposits throughout the lung fields [Figure 1]; similar deposits were seen in the pelvis on X-ray abdomen. Qualitative mercury analysis of her blood and urine for mercury was positive; quantitative analysis by atomic absorption spectrometry revealed a blood level of 58 mcg/l, and a urine level of 138 mcg/l (reference values in non-exposed individuals are below 8 mcg/l in blood and 4 mcg/l in urine).[1] Bone marrow biopsy revealed hypocellular bone marrow with organophilic heavy metal deposits [Figure 2].
Figure 1.
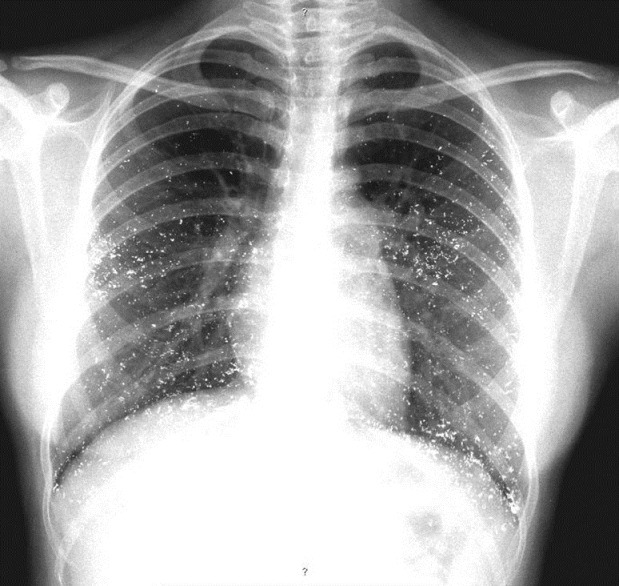
X-ray chest showing diffuse radio opaque mercurial deposits
Figure 2.
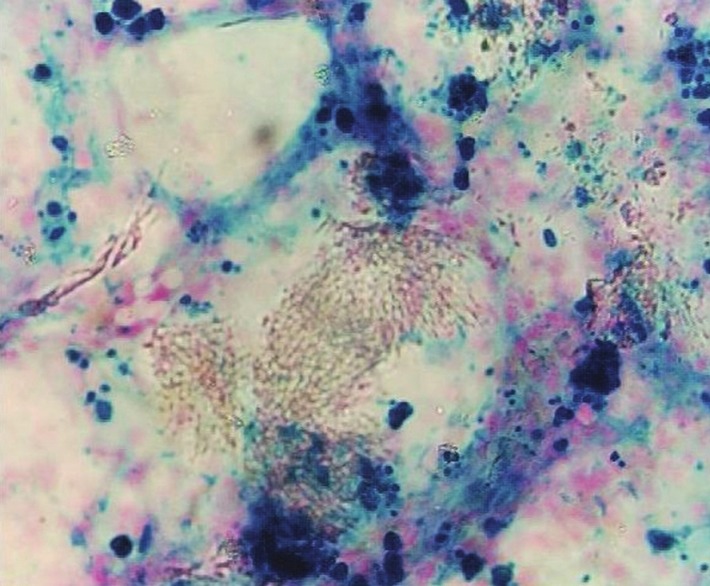
Bone marrow biopsy showing hypocellular bone marrow on H and E stain with organophilic deposits
She was treated symptomatically with packed RBC transfusion and was kept under close follow-up. Her counts gradually improved and she was asymptomatic. She developed progressively increasing edema with hypoalbuminemia (serum albumin 2.9 g/dl) and nephrotic range proteinuria (24-h urine protein 3792 mg) with a serum cholesterol of 278 mg/dl, normal renal functions, and blood pressure. Ultrasonogram of abdomen showed normal kidneys. Kidney biopsy revealed nine non-obsolescent glomeruli with patent capillary loops and thick capillary walls on H and E stain [Figure 3]; focal lucent holes and fuschinophilic specs were seen on Jones Silver stain and Masson Trichrome, respectively. Tubules, interstitium, and vasculature were normal. IF revealed 3+ granular positivity for IgG and 2+ for C3. IgA, IgM, and C1q were negative. The biopsy was consistent with membranous nephropathy (MN). Her follow-up after 4 months showed complete resolution of pancytopenia (hemoglobin 13.5 g/dl, WBC count 9190 cells/cu.mm, platelet count 210,000) and after 2 years, the proteinuria had resolved completely (serum albumin was 4.62 g/dl and serum cholesterol was 170 mg/dl). The radiographic opacities in the lungs and pelvis have shown almost complete clearance over 4 years [Figure 4]. After 4 years of follow-up, she has normal hemogram and renal function and urine does not show any proteinuria. She is currently asymptomatic and is on follow-up.
Figure 3.
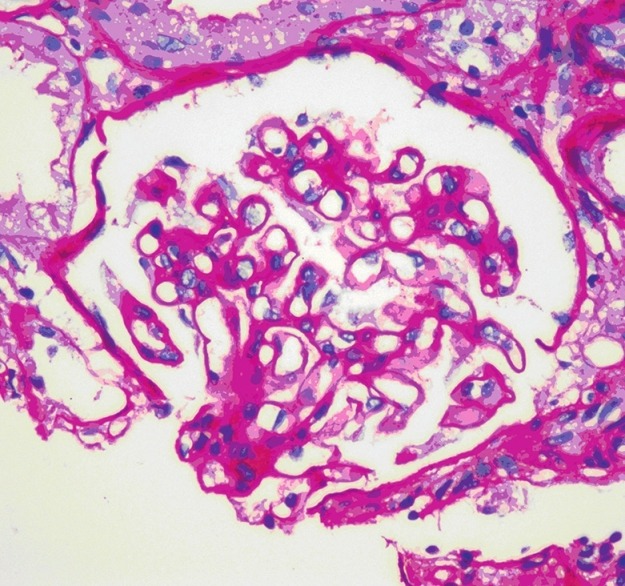
Kidney biopsy showing membranous nephropathy (H and E, ×400 magnification)
Figure 4.
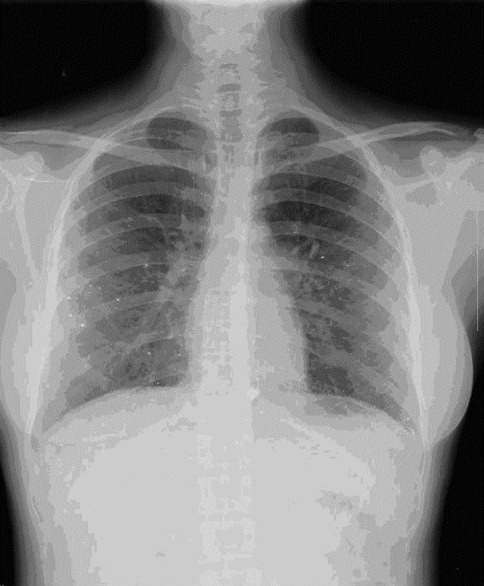
X-ray chest after 4 years, showing almost complete clearance of radio opaque deposits
Discussion
Mercury (Hg) is a transitional metal and the only metal found naturally in a liquid state. It exists as a variety of compounds and in different oxidation states, including elemental, organic, and inorganic states. Mercury is usually found in only insignificant quantities in the human body, as it has no biological function. The normal mercury level in the blood in unexposed individuals is <8 mcg/l and in the urine, it is <4 mcg/l. Mercury in any form is toxic. Mercury can be absorbed into the body through numerous routes, including inhalation, ingestion, absorption through skin, and injection—both subcutaneous and intravenous. Intravenous mercury injection is very rare. It is usually seen in suicidal attempts. Recently, some cases of injected mercury have been reported to be used as an aphrodisiac.[2]
Common sources of elemental mercury include barometers, batteries, and dental amalgams. It is also seen in many mercury and neon lamps. Inorganic mercury is usually found in cosmetics, disinfectants, and tattooing inks. Recently, there have been case reports of mercury toxicity from facial cosmetic creams.[3] Many antiseptics, germicidal agents, and laundry products have been found to contain organic mercury. A very controversial source of organic mercury is thimerosal, an additive preservative used in vaccines to prevent bacterial contamination. Mercury is often used in many alternative forms of medicine, such as Ayurveda, Siddha, and Unani, in the form of Bhasma. Bhasma is obtained by incinerating mercury along with herbs.[2]
Neurological, gastrointestinal, and renal systems are the most commonly affected organ systems in cases of mercury exposure. Organic mercury is most toxic to the central nervous system, especially the short-chained forms as they can easily cross the blood–brain barrier. Chronic organic mercury toxicity leads to the classic triad of tremors, gingivitis, and erethism (i.e., a constellation of neuropsychiatric symptoms that include insomnia, memory loss, emotional instability, and vasomotor disturbance). Acute toxicity can lead to metabolic encephalopathy and peripheral neuropathy.
Inorganic mercury, on the other hand, is more toxic to the gastrointestinal tract. Acute toxicity is due to the inactivation of vital enzymes by the heavy metal.[1] Acute inorganic mercury poisoning can lead to severe corrosive gastroenteritis with hemorrhagic diarrhea. It can also lead to acute renal failure due to acute tubular necrosis.[4] Elemental mercury toxicity usually occurs due to inhalation of vapors. Recovery is usually without sequela, but pulmonary complications of inhaled toxicity may include interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, and interstitial fibrosis.
There are no data available regarding the metabolism of mercury in humans. However, metabolism of mercury after intravenous or intramuscular injection in the rat has been studied. Hg203 was used as a tracer. By the intravenous route, mercury was rapidly distributed to all organs. In the first few hours, a large fraction was taken up by the liver and was cleared via fecal excretion within a few days. The kidney is the major site of deposition. As the mercury content of other tissues decreases after the first day, that of the kidney increases for about 2 weeks to about 85-90% of the total body burden. The mercury in the kidney is excreted via the urine at a rate of about 1% of the body burden per day. By the intramuscular route, the clearance from the site of injection takes about 2 weeks. Significant amounts of mercury do not appear in the liver. By the end of 2 weeks, as in the intravenous studies, most of the mercury is localized in the kidney.[5]
Data regarding the effects of mercury toxicity on the bone marrow are quite sparse. A case report published in BMJ in 1970 records a case of aplastic anemia in a patient who suffered from mercury toxicity due to occupational exposure.[6] There is only one other case report since then.[7] A study was done, exposing egrets to methyl mercury. They were found to have developed hypocellular bone marrow and decline in PCV.[8] Our patient had developed aplastic anemia following intravenous mercury administration; the bone marrow recovered over 4 months and the cell counts became normal.
Injection of elemental mercury can lead to life-threatening pulmonary embolism. Abscess formation is a common local manifestation. Often, the mercury deposits in various tissues, usually in the adipose tissue, lungs, and around the pelvis and is slowly absorbed into the circulation where it has long-term effects on the kidneys. Acute mercury poisoning results in acute tubular necrosis, especially with involvement of the proximal tubules, whereas chronic mercury exposure mainly causes immune complex glomerulonephritis and membranous nephropathy.[9]
In humans, the mechanism of mercury-induced membranous nephropathy has not been elucidated. Experimental studies in rats point to a genetic susceptibility and to polyclonal stimulation of the immune system in mercury-induced membranous nephropathy. Brown-Norway (BN) rats, Dorus-Zadel black rats, and MAXX rats were developed membranous nephropathy after administration of HgCl2.[10,11] In addition, the exposure of BN rats to Hg vapor induced a membranous nephropathy.[12] The underlying pathogenic mechanism is characterized by a T-lymphocyte-dependent polyclonal B-cell activation, with subsequent production of autoantibodies against proteins of the glomerular basement membrane such as laminin and fibronectin and also against heparin sulfate proteoglycans.[13,14] It is not known whether similar mechanisms operate in humans.
The diagnosis rests mainly on a history of exposure. Supporting evidence may be provided by a raised urinary concentration of mercury. The concentration usually falls to normal within weeks of removal from exposure, although the renal abnormalities may persist for much longer. Under these circumstances, raised concentrations of the metal may be found in the hair for up to 6 months.[15]
Studies have shown that it may be possible to differentiate idiopathic from heavy metal-induced membranous nephropathy (MN). Light microscopic findings are identical in both but immunofluorescence shows predominantly IgG1 deposits in mercury-induced membranous nephropathy which differentiates it from idiopathic membranous nephropathy where IgG4 deposits are seen. This finding of IgG1 deposition is seen in MN secondary to malignancies.[16]
The treatment of intravenous mercury poisoning consists of extensive exposure of the injected site and removal of the metal from the vessels. This should be followed by chelation therapy as deposits of mercury in the soft tissues will continue releasing mercury into the blood stream. The complications of retained mercury include microscopic inflammation of the lung, axonopathy of various nerves, asthenozoospermia, liver toxicity, and even cardiac granulomas, the exact mechanism of which is not fully understood. At least 2 years of close follow-up is recommended following mercury poisoning.[17]
The treatment of mercury-induced MN due to chronic intoxication consists of removal of the patient from the area of exposure. With this, the prognosis is good : In a previous series of nephrotic syndrome, largely secondary to skin lightening creams, 50% of the patients attained remission of proteinuria, 77% of these doing so spontaneously when the use of the cream was stopped.[18]
Conclusion
Case reports on intravenous injection of mercury are extremely rare. So far, there is no report of intravenous mercury injection leading to aplastic anemia and membranous nephropathy. The patient has been closely followed up with us over the past 4 years and we have documented the complications and have been able to document that the effects on bone marrow and kidneys are reversible.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Aymaz S, Gross O, Krakamp B, Ortmann M, Dienes HP, Weber M. Membranous nephropathy from exposure to mercury in the fluorescent-tube-recycling industry. Nephrol Dial Transplant. 2001;16:2253–5. doi: 10.1093/ndt/16.11.2253. [DOI] [PubMed] [Google Scholar]
- 2.Gopalakrishna A, Pavan Kumar TV. Intravenous injection of elemental mercury : A report of two cases. Indian J Plast Surg. 2008;41:214–8. doi: 10.4103/0970-0358.44942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McRill C, Boyer LV, Flood TJ, Ortega L. Mercury toxicity due to use of a cosmetic cream. J Occup Environ Med. 2000;42:4–7. doi: 10.1097/00043764-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira DB, Foster G, Savill J, Syme PD, Taylor A. Membranous nephropathy caused by mercury-containing skin lightening cream. Postgrad Med J. 1987;63:303–4. doi: 10.1136/pgmj.63.738.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aser R, Hayes AD. The metabolism of mercury in the rat studied by isotope techniques. JPET. 1960;130:166–76. [Google Scholar]
- 6.Ryrie DR, Toghill PJ, Ranna MK, Galan GN. Marrow suppression from mercury poisoning? Br Med J. 1970;1:499. doi: 10.1136/bmj.1.5694.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavithran K. Mercury and aplastic anaemia. Natl Med J India. 1994;7:252. [PubMed] [Google Scholar]
- 8.Spalding MG, Frederick PC, McGill HC, Bouton SN, Richey LJ, Schumacher IM, et al. Histologic, neurologic and immunologic effects of methyl mercury in captive great egrets. J Wildl Dis. 2000;36:423–35. doi: 10.7589/0090-3558-36.3.423. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Shi JZ, Yu LM, Goyer RA, Waalkes MP. Mercury in traditional medicines : Is cinnabar toxicologically similar to common mercurials? Exp Biol Med (Maywood) 2008;233:810–7. doi: 10.3181/0712-MR-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sapin C, Druet E, Druet P. Induction of anti-glomerular basement membrane antibodies in the Brown-Norway rat by mercuric chloride. Clin Exp Immunol. 1977;28:173–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Aten J, Veninga A, Bruijn JA, Prins FA, de Heer E, Weening JJ. Antigenic specificities of glomerular-bound autoantibodies in membranous glomerulopathy induced by mercuric chloride. Clin Immunol Immunopathol. 1992;63:89–102. doi: 10.1016/0090-1229(92)90098-9. [DOI] [PubMed] [Google Scholar]
- 12.Hua J, Pelletier L, Berlin M, Druet P. Autoimmune glomerulonephritis induced by mercury vapour exposure in the Brown Norway rat. Toxicology. 1993;79:119–29. doi: 10.1016/0300-483x(93)90125-c. [DOI] [PubMed] [Google Scholar]
- 13.Icard P, Pelletier L, Vial MC, Mandet C, Pasquier R, Michel A, et al. Evidence for a role of antilaminin-producing B cell clones that escape tolerance in the pathogenesis of HgCl 2 -induced membranous glomerulopathy. Nephrol Dial Transplant. 1993;8:122–7. [PubMed] [Google Scholar]
- 14.Fukatsu A, Brentjens JR, Killen PD, Kleinman HK, Martin GR, Andres GA. Studies on the formation of glomerular immune deposits in brown Norway rats injected with mercuric chloride. Clin Immunol Immunopathol. 1987;45:35–47. doi: 10.1016/0090-1229(87)90109-7. [DOI] [PubMed] [Google Scholar]
- 15.Barr RD, Smith H, Cameron HM. Tissue mercury levels in the mercury-induced nephrotic syndrome. Am J Clin Pathol. 1973;59:515–7. doi: 10.1093/ajcp/59.4.515. [DOI] [PubMed] [Google Scholar]
- 16.Li SJ, Zhang SH, Chen HP, Zeng CH, Zheng CX, Li LS, et al. Mercury-induced membranous nephropathy : Clinical and pathological features. Clin J Am Soc Nephrol. 2010;5:439–44. doi: 10.2215/CJN.07571009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellabban MG, Ali R, Hart NB. Subcutaneous metallic mercury injection of the hand. Br J Plast Surg. 2003;56:47–9. doi: 10.1016/s0007-1226(03)00021-3. [DOI] [PubMed] [Google Scholar]
- 18.Barr RD, Rees PH, Cordy PE, Kungu A, Woodger BA, Cameron HM. Nephrotic syndrome in adult Africans in Nairobi. Br Med J. 1972;2:131–4. doi: 10.1136/bmj.2.5806.131. [DOI] [PMC free article] [PubMed] [Google Scholar]


