Abstract
Background:
Uterine leiomyomas are the commonest benign tumors in women, with a higher preponderance amongst Africans. Several etiological factors have been suggested, with subtle variations in clinical presentation being reported in different studies. This may constitute a determinant for the management measures undertaken.
Aim:
To review the clinical presentation and management measures undertaken for uterine leiomyoma.
Subjects and Methods:
A retrospective study was conducted at Nnamdi Azikiwe University Teaching Hospital (NAUTH), Nnewi, from January 2002 to December 2006. A review of case records of patients with a diagnosis of uterine leiomyoma was done. The data were analyzed and presented in tables using comparative percentages.
Results:
Uterine leiomyoma constituted 117 of the 1094 gynecological admissions during this study period (10.7%, 117/1094). The mean (SD) age of presentation was 35.7 (6.1) years. Most of the patients were nulliparous (76.7%, 79/103) and 51.5% (53/103) were married. The commonest mode of presentation was lower abdominal mass (66.9%, 67/103) and the least was recurrent abortion (1%, 1/103). Surgery was employed in all cases, with myomectomy being the commonest modality used in 90.3% (93/103) of cases. The common postoperative complications were prolonged pain (49.5%, 51/103) and postoperative pyrexia (34.9%, 36/103).
Conclusion:
The symptom of lower abdominal mass correlates with late presentations in our setting. This makes the application of newer therapies like laparoscopic myomectomy difficult even when they are available. Other therapies which are independent of fibroid size (like uterine artery embolization) are not readily available in our environment. This further emphasizes the importance of myomectomy as the most important treatment modality in our environment.
Keywords: Myomectomy, Presentation, Uterine leiomyoma (fibroids)
Introduction
Uterine leiomyomas (fibroids) are the commonest benign tumors in women. Leiomyomas are the most common tumors in women of reproductive age. They are symptomatic in 50% of cases, with the peak incidence of symptoms occurring among women in their 30s and 40s.[1–3]
The exact etiology of the tumors is unknown; however, several interesting associations have been noted. There is a great predilection of fibroids for women of African descent.[4,5] Theories attribute this to genetic predisposition, diet, or environmental factors. However, few studies have addressed these relationships.
A history of pelvic infection seems to increase the risk for uterine fibroids, with the risk increasing with the number of infectious episodes.[6,7] Pelvic inflammatory disease as seen in chlamydial infection was implicated unlike those infections that affected mostly the external genitalia, e.g. herpes, which showed no association.[4]
Diet has also been implicated as a risk factor, and a higher intake of meat was associated with a higher incidence of fibroids while a diet comprising more green vegetables was protective.[8] Nulliparity and infertility have traditionally been associated with uterine fibroids,[7,9,10] with increasing parity decreasing the risk of fibroids up to fivefold.[4]
The strongest factor thought to have a causal relationship with fibroids is hormones. The absence of leiomyoma in the pre-pubertal years, its growth during reproductive life, and regression at menopause suggest some hormonal influence. Estrogens have been considered the major promoter of fibroid growth, although recent evidence suggests a role for progesterone,[11] particularly in its ability to stimulate a growth factor, transforming growth factor-beta (TGF-β), which seems to modulate cell development and proliferation.[12] High levels of TGF-β have been noted on the myometrium, particularly during the luteal phase of the cycle.
The presentation of uterine fibroids is quite variable, with most being asymptomatic. The commoner symptoms include an abdominal mass, menorrhagia, pain, dysmenorrhea, recurrent abortions, and pressure symptoms from the myoma.
Various treatment options exist depending on the size, location, number, and symptomatology. Options range from conservative therapy, where the fibroids have no obvious symptoms and simple monitoring is instituted, to medical and surgical where the myoma has caused significant symptoms. Medical management at present is only used for short-term therapy because of the significant risks with long-term treatment; also, there is a lack of evidence regarding the benefits and risks of long-term therapy with the newer medical agents. It is not a curative therapy but can be used in perimenopausal women to tide them over into the menopausal state or in women who need to buy time prior to surgery, e.g. in cases where the patient is unfit for surgery or when the fibroids are extremely large and shrinkage is desired before surgery. Thus, GnRH, alone or more commonly with “add-back therapy,” selective estrogen receptor modulators (SERMs), antiprogestins (RU486 and asnoprisinil), and aromatase inhibitors (carbegoline, danazol, and gestrinone) have all been utilized.[13] Surgical treatment could be by myomectomy or hysterectomy, with hysterectomy being the only option offering total cure.
The aim of this study is essentially to review the clinical features of patients with uterine fibroids at Nnamdi Azikiwe University Teaching Hospital (NAUTH), Nnewi, as well as the treatment modalities employed.
Subjects and Methods
This was a retrospective, hospital-based study carried out in NAUTH, Nnewi, from January 2002 to December 2006.
Data for all the cases of histologically confirmed uterine fibroids were obtained from the records department. A total of 117 cases were seen, but 14 had some missing data; thus, only data from 103 of them were used for the study.
Information extracted includes age, parity, marital status, symptoms, type of treatment offered, and complications noted. The complications recorded were as follows:
Prolonged hospital stay was defined as stay in hospital after the 8th day of surgery as the mean stay in hospital for the majority of patients was 1 week. Prolonged postoperative pain was defined as pain requiring narcotic analgesics by the 4th postoperative day. Prolonged bleeding per vagina was defined as bleeding per vaginam (not menses) continuing up to 1 week post operation. Pyrexia was defined as temperatures of 38°C and above occurring on two separate occasions after 24 h of surgery.
Collation and analysis was done using SPSS version 11 (Chicago Illinois, USA), and displayed in figures and tables using comparative percentages
Results
Out of 117 cases of leiomyoma diagnosed in the study period, 103 case notes (88%, 103/117) were available for review and analysis.
The age distribution of patients with uterine fibroids at presentation as shown in Figure 1 indicates that the majority were between 30 and 44 years of age (75.6%, 78/103), while the least presentation (1.9%, 2/103) was seen in the age groups 20-24 and 50-54 years. The mean (SD) age at presentation was 35.7 (6.1) years.
Figure 1.
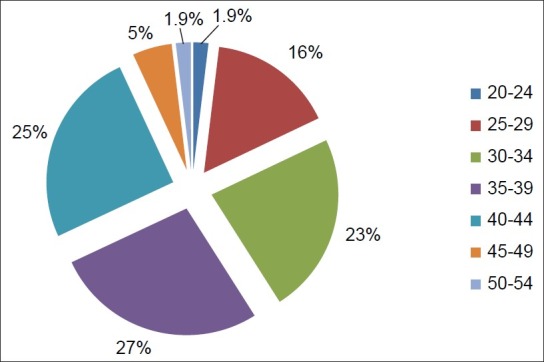
Pie chart of age distribution of patients presenting with uterine fibroids. Age range 24–51 years; mean age 35.7 years and SD ± 6.1
The parity of the patients at presentation as shown in Figure 2 ranged from 0 to 8. Majority of the patients were nulliparous (77.7% (80/103), n = 80), and the grand multiparous patients had the least prevalence (7.7% (8/103), n = 8). The multiparous patients (para 1-4) had a prevalence of 14.5% (15/103) (n = 15). Of the patients studied, 51.4% (53/103) (n = 53) were married while 45.6% (47/103) (n = 47) were single. Divorcees constituted 1.9 (2/103) (n = 2) and only 1 (1.0%) patient was a widow.
Figure 2.
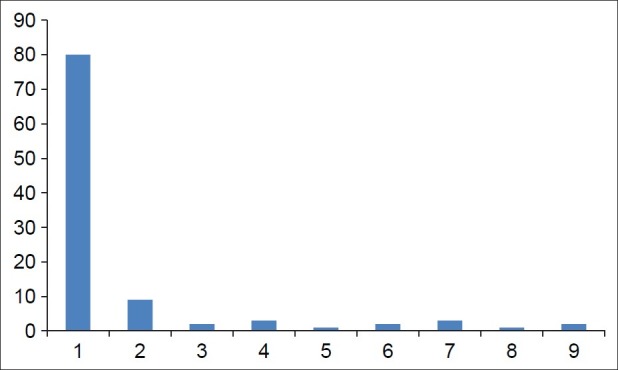
Bar chart of parity of fibroid patients
Table 1 shows the modes of presentation of patients with fibroids. The commonest modes of presentation include lower abdominal mass (67.7%, 69/103). Others were menorrhagia (41.7%, 43/103) and infertility (30.1%, 31/103). Several patients had more than one symptom.
Table 1.
Mode of presentation of patients presenting with fibroid

The mode of treatment is shown in Table 2 which indicates that the majority of patients had myomectomy (90.3%, 93/103). 8.7% (9/103) had hysterectomy. Only 1% (1/103) had a vaginal myomectomy. None of the patients had laporoscopic myomectomy.
Table 2.
Treatment modality of patients presenting with fibroid

Assessment of the uterine location of the fibroid nodules, as seen during myomectomy, shows that most patients had fibroids in multiple sites (56.3% (58/103), n = 58), 31% (32/103) (n = 32) had only intramural, 7.8% (8/103) (n = 8) had only submucous, and 5.8% (6/103) (n = 6) had only subserous location. There was a single case of intraligamentary fibroid. The cumulative frequency of the fibroids in their various locations reveals that 85.4% (88/103) included intamural, 53.4% (55/103) included subserous, and 32% (33/103) included submucous.
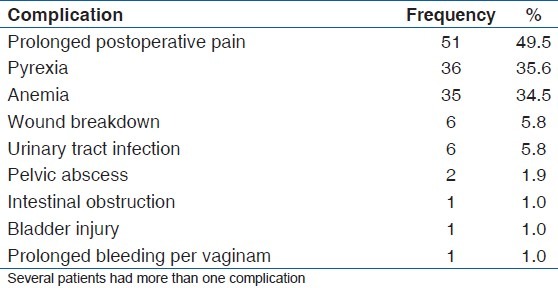
Complications seen postoperatively are demonstrated in Table 3. Prolonged postoperative pain was seen in 49.5% (51/103) (n = 51) of the patients. 34.9% (36/103) (n = 36) had pyrexia and anemia was seen in 33.9% (35/103) (n = 35) of the patients.
Table 3.
Postoperative complications of patients who underwent myomectomy
Excised tissue weight was not available.
Discussion
This study revealed a prevalence of 10.7% for fibroids of all gynecological admissions within the study period. This cannot be generalized to the population due to the asymptomatic nature of most fibroid cases and the fact that some symptomatic patients may not seek medical attention, or may seek help from non-orthodox centers, the so called “Iceberg phenomenon.” This prevalence however, is only slightly more than that seen at Ilesha (8.35%),[13] but a little less than the incidence of 13.4% recorded by Aboyeji and Ijaiya at Ilorin.[14]
Peak age of occurrence was noted in this study in the 4th decade of life, specifically 30-39 years 50.5%, and is in agreement with other reports.[15]
Nulliparity had a strong link with the development of fibroids, and in this series, 76.7% of the patients were nulliparous. For parity range 0-1, the number increased to 86.4%. Adinma in his study noticed a similar trend,[7] and other studies[16] have shown the protective effect of pregnancy with respect to the development of leiomyoma.
Analysis of clinical presentations showed that lower abdominal mass (67%) was the commonest symptom at presentation. This is likely due to the late presentation typical of our women. Infertility as a presenting symptom was seen mostly in the married patients who made up 51.5% of the study population as against singles who made up 45.6%. Abdominal mass as the foremost symptom was similarly noted by Oguniyi and Fasuba in Ilesha, southwestern Nigeria.[13] This is in contradistinction to the report published by Adinma, who documented menorrhagia as the commonest presenting symptom.[7]
Fibroids are generally associated with an increased risk of heavy or prolonged menstrual flow.[15,16] Proposed reasons for this include increase in endometrial surface area, distortion and congestion of surrounding vessels, abnormal endometrial development, increase in blood flow to the uterus, and poor uterine contractility.[4,15] These are, however, not universally accepted.[17]
Menorrhagia and infertility were seen in 41.7% and 30.1% of the patients, respectively. 20.4% of the patients experienced lower abdominal pain, and this could be due to the large size of the tumorscausing pressure effects. Complicated fibroids may also present with abdominal pain.
Dysmenorrhea was evident in 15.5% of the population unlike in Adinma's series where it was present in a third of the population studied.[7] Irregular vaginal bleeding (9.7%), urinary symptoms (8.7%), and recurrent abortion (1%) were all rare modes of presentation. With respect to recurrent abortions, a more significant effect of fibroids might have been seen on comparing with women with non-fibroid uteri used as controls.[18]
Most of the myomata were in multiple locations (56.3%), and on subcategorization of location, the most common site was intramural (85.4), followed by subserous (53.4%), and submucous (32%). This trend was also recorded by Akinyemi et al.,[15] who reported location frequencies of 70%, 20%, and 10% for intramural, subserous, and submucous, respectively. These figures reflect the fact that all myomata start intramurally before migrating outward or inward as the case may be.
No patient received medical treatment, and of the surgical options, myomectomy was the most commonly employed (90.3%). Hysterectomy (8.7%) and polypectomy (1%) were the other modalities used. This was not surprising since most of the patients were nulliparous and still wanted to keep their reproductive ability. Furthermore, the lack of menses associated with hysterectomy was not acceptable to the older patients in whom it may have been a better option. Laparoscopic myomectomy was not done in any of the patients.
Postoperative pain (49.5%), pyrexia (34.9%), and anemia (33.9%) were the common complications seen in our cohort of patients. The difficulty in assessing pain in individuals is quite well known, given the subjectivity of the symptom. Pyrexia occurring longer than 24 h after surgery has been thought to be due to hematoma formation within the cavities created during the surgery. Use of newer techniques, e.g. uterine artery embolization, may reduce the occurrence of this complication. Anemia is due to the blood loss at surgery and this may be reduced by the use of the myomectomy clamp, vasopressin, or the use of a tourniquet at surgery, which is the method commonly employed in this center.
Conclusions
This study compares favorably with others in our environment and has not shown any huge difference with respect to incidence, clinical presentation, and complications of myomata. The preference for myomectomy is largely due to the strong reproductive desire of this group of patients and also the psychological premium placed on their menstrual cycle, even for those who have completed their families, and is quite different from that seen in the western world where hysterectomy is preferred. This brings to fore the importance of proper training to reduce the occurrence of complications from the surgery.
The absence of more recent methods of therapy was quite glaring. The relative absence of uterine artery embolization in our environment is clear to see. Also, laparoscopic myomectomy, though available, was not an option for several of the women either due to the size of the myoma or financial constraints.
Sensitisation of women to report early for assessment of such illnesses is advocated. Also, training of staff to enable centers offer the newest techniques to the patients is encouraged.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Wise LA, Palmer JR, Stewart EA, Rosenberg L. Age-specific incidence rates for self-reported uterine leiomyomata in the black women's health study. Obstet Gynecol. 2005;105:563–8. doi: 10.1097/01.AOG.0000154161.03418.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeWaay DJ, Syrop CH, Nygaard IE, Davis WA, Van Voorhis BJ. Natural history of uterine polyps and leiomyomata. Obstet Gynecol. 2002;100:3–7. doi: 10.1016/s0029-7844(02)02007-0. [DOI] [PubMed] [Google Scholar]
- 3.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma black and white women: Ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–7. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 4.Payson M, Leppert P, Segar J. Epidemiology of myomas. Obstet Gynecol Clin North Am. 2006;33:1–11. doi: 10.1016/j.ogc.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinemann K, Thiel C, Mohner S, Lewis MA, Raff T, Kuh-Habich D, et al. Benign gynaecological tumours: Estimated incidence. Results of the German Cohort Study on Womens health. Eur J Obstet Gynecol Reprod Biol. 2003;107:78–80. doi: 10.1016/s0301-2115(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 6.Stewart EA, Nowak RA. New concepts in the treatment of uterine leiomyoma. Obstet Gynecol. 1998;92:624–7. doi: 10.1016/s0029-7844(98)00243-9. [DOI] [PubMed] [Google Scholar]
- 7.Adinma JIB. Uterine fibroid and fertility in Enugu. Nigeria medical journal. 1994;5:3–5. [Google Scholar]
- 8.Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: A review. Environ Health Perspect. 2003;111:1037–49. doi: 10.1289/ehp.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato F, Miyake H, Nishi M, Kudo R. Fertility and uterine size among Asian women undergoing hysterectomy for leiomyomas. Int J Fertil Womens Med. 2000;45:34–7. [PubMed] [Google Scholar]
- 10.Ikechebelu JI, Adinma JIB, Ikegwuonu SO, Orie EF. Clinical Correlates of unexplained infertility in south eastern Nigeria. Trop J Obstet Gynaecol. 2002;19:8–11. [Google Scholar]
- 11.Mauro T, Matuso H, Samoto T, Shimomura Y, Kurachi O, Gao Z, et al. Effects of progesterone on uterine leiomyoma growth and apoptosis. Steroids. 2000;65:585–92. doi: 10.1016/s0039-128x(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 12.Arici A, Sozen I. Transforming growth factor–beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73:1006–11. doi: 10.1016/s0015-0282(00)00418-0. [DOI] [PubMed] [Google Scholar]
- 13.Sankaran S, Manyonda IT. Medical management of fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:655–76. doi: 10.1016/j.bpobgyn.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Aboyeji AP, Ijaiya MA. Uterine fibroids: A ten year clinical review in llorin, Nigeria. Niger J Med. 2002;11:16–9. [PubMed] [Google Scholar]
- 15.Akinyemi BO, Adewoye BR, Fakoya TA. Uterine fibroids. A review. Niger J Med. 2004;13:318–29. [PubMed] [Google Scholar]
- 16.Chen CR, Buck GM, Courey NG, Perez KM, Wactawski-Wende J. Risk factors for uterine fibroids among women undergoing tubal sterilisation. Am J Epidemiol. 2001;153:20–6. doi: 10.1093/aje/153.1.20. [DOI] [PubMed] [Google Scholar]
- 17.Marinho JL, Eskenazi B, Warner M, Samuels S, Vercellini P, Gavoni N, et al. Uterine leiomyoma and menstrual cycle characteristics in a population based cohort study. Hum Reprod. 2004;19:2350–5. doi: 10.1093/humrep/deh407. [DOI] [PubMed] [Google Scholar]
- 18.Check JH, Choe JK, Lee G, Dietterichl C. The effects of IVF outcome on small intramural fibroids not compressing the uterine cavity as determined by a prospective matched control study. Hum Reprod. 2002;17:1244–8. doi: 10.1093/humrep/17.5.1244. [DOI] [PubMed] [Google Scholar]


