Abstract
Background:
Chronic psychosocial stress and serum uric acid (SUA) level have been implicated in the etiology and cardiovascular events risk factors in hypertension. Studies have reported significant benefit of exercise in the overall management of hypertension. However, studies on the effect of exercise on psychosocial stress and SUA in the management of hypertension seem scanty.
Aim:
The aim of this study was to determine the effect of continuous training program on SUA and psychosocial status of black African (Nigerian) population with hypertension.
Subjects and Methods:
Age-matched randomized controlled trial was used; subjects with diagnosis of hypertension attending the hypertensive clinic of Murtala Muhammed Specialist Hospital (MMSH), Kano, Nigeria form the population for the study. Two hundred and seventeen subjects with mild to moderate (systolic blood pressure (SBP) between 140 and180 and diastolic blood pressure (DBP) between 90 and 109 mmHg) essential hypertension were grouped into continuous (112) and control groups (105). The continuous group involved in an 8 weeks continuous training (60%-79% HR max) of between 45 and 60 min, 3 times per week, while the controls group remain sedentary. SBP, DBP, SUA, VO2 max and psychosocial status were assessed. Student t-test and Pearson correlation test were used in data analysis.
Results:
The study revealed significant beneficial effect of continuous training programs on VO2 max, SBP, DBP, SUA, and psychosocial status (P < 0.05). Psychosocial status and SUA was significantly and positively and negatively correlated respectively with VO2 max at P < 0.01.
Conclusions:
This study concludes and supports the recommendations of moderate intensity (continuous) training program in blood pressure reduction, SUA and psychosocial stress management in hypertension.
Keywords: African, Blood pressure, Hypertension, Nigerian, Psychosocial stress, Serum uric acid
Introduction
Hypertension was particularly prevalent amongst African subjects with 59% being affected.[1] In the Heart of Soweto Study, 56% of predominantly black Africans, who attended the cardiac clinic at Chris Hani Baragwanath Hospital in Soweto, were diagnosed as hypertensive.[2] Hypertension is a major global health problem and public-health challenge, demanding a vast proportion of health care resources directly and indirectly because of its high and increasing prevalence and the concomitant risks of cardiovascular events such as stroke, kidney disease, decreased disability adjusted, and mortality.[3,4] Chronic psychosocial stress has been implicated in the etiology of hypertension.[5,6] In Africa-American, socio-environmental and psychosocial stress has been associated with higher blood pressure.[7] It has been reported that elevated serum uric acid (SUA) is an independent risk factor for hypertension and psychosocial stress.[3,4,8] Therefore, stress and or SUA reduction may be useful for treating hypertension in older African-American, yet there has been lack of controlled clinical trials of stress reducing for the management of hypertension.[9]
However, uric acid (UA) is the end-product of purine metabolism and has been associated with psychological features such as high energy/drive, positive effect, achievement, good performance, higher social status, and leadership. Externalized traits of temperament are associated with higher serum UA levels both in men and women.[8] Epidemiological studies suggest that psychosocial factors contribute significantly to the pathogenesis of cardiovascular disease (CVD).[10–13] Serum uric acid (SUA), a molecule which plays an important and complex role in oxidative and anti-oxidative processes has been reported to positively correlated with CVD and various measures of psychosocial stress,[12] especially in high risk patients.[13]
Epidemiological studies have shown that increased levels of physical activity reduce the incidence of all-cause mortality and cardiovascular-related deaths.[14] Although the mechanisms responsible for this benefit are not fully understood, exercise is known to have favorable effects on such traditional risk factors as elevated blood pressure (BP), hyperinsulinemia, and hyperlipidemia. Studies have also shown that exercise cannot only induce beneficial physiological adaptations, but can also improve psychological functioning.[15–19]
Cross-sectional studies have shown that individuals who are more active or physically fit have lower cardiovascular responses to stress.[20] However, longitudinal studies are less consistent but generally demonstrate that heart rate (HR) and BP levels are attenuated after exercise training in healthy normotensive men and women. To the best of our knowledge, there are few large randomized controlled trials investigating the association between exercise training, SUA and psychosocial status in hypertension, and of those few studies, none has investigated these effects on pure black African population. However, heredity[21] and genetical[22] factors have been implicated in the causative of hypertension; also there is possibility that apoliprotein (apo) E, angiotensin-converting enzyme (ACE), interleukin-6 (IL-6) genotype[23] and lipoprotein lipase (LPL) genotype effect in responses to exercise and physical activity in hypertension.[24,25] Also, SUA level is affected by age and by genetic and environmental factors.[26] These interpersonal and interracial differences clearly indicate the needs for study on pure older black African population. Therefore, the purpose of the present study was to investigate the effect of continuous training program on blood pressure, SUA, and psychosocial stress of pure black African subjects with hypertension.
Subjects and Methods
Subjects
Population for the study was male essential hypertensive subjects attending the hypertensive clinic of Murtala Muhammed Specialist Hospital, Kano, Nigeria. Subject were fully informed about the experimental procedures, risk and protocol, after which they gave their informed consent in accordance with the American College of Sports Medicine[27] guidelines, regarding the use of human subjects as recommended by the human subject protocol. Ethical approval was granted by the Ethical Committee of Kano State Hospitals Management Board.
Inclusion criteria
Only those who volunteered to participate in the study were recruited. Subjects between the age range of 50 and 70 years with chronic mild to moderate and stable (>1 year duration) hypertension (SBP between 140 and 179 and DBP between 90 and 109 mmHg) were selected. Only those who had stopped taking antihypertensive drugs or on a single antihypertensive medication were recruited.[28] They were sedentary and have no history of psychiatry or psychological disorders or abnormalities.
Exclusion criteria
Obese or underweight (BMI between 20 and 30 kg/m2), smokers, alcoholic, diabetic, other cardiac, renal, respiratory disease patients were excluded. Those involved in vigorous physical activities and above averagely physically fit (VO2 max > 27 and > 33 ml/kg min for over 60 and 50 years old, respectively) were also excluded.
A total of 323 chronic and stable, essential mild to moderate male hypertensive patients satisfied the necessary study criteria. Subjects were aged matched and randomly grouped into experimental (162) and control (161) groups.
Procedure
Research design: In the present study, age matched randomized independent groups design was used to determine the influence of the continuous training program on cardiovascular parameters. All procedures were conducted at the physiotherapy department of MMSH, Kano, Nigeria.
Pretest procedure
Wash out period: All subjects on antihypertensive drugs were asked to stop all forms of medication and in replaced, were given placebo tablets (consisted of mainly lactose and inert substance).[28,29] Also subjects including those not on any antihypertensive medications were placed on placebo tablets for one week (7 days); this is known as “Wash out period.” The purpose of the wash out period was to get rid of the effects of previously taken antihypertensive drugs/medications. During the wash out period all subjects were on medical monitoring and were instructed to report to the hypertensive clinic for daily blood pressure monitoring and general observation. The Pretest and posttest procedures were conducted at the last day of the wash out period.
Physiological measurement
Subjects resting heart rate (HR), SBP, and DBP were monitored from the right arm as described by Musa et al.,[30] using an automated digital electronic BP monitor (Omron digital BP monitor, Model 11 EM 403c; Tokyo Japan).
Anthropometric measurement
Subjects’ physical characteristics (weight [kg] and height [m]) and body composition (body mass index [BMI] (kg/ m2)) assessment was done in accordance with standardized anthropometric protocol.[31,32]
Blood sample collection (venipuncture method): Both pre- and post-treatment venous blood samples were obtained after about 12 h overnight fast (fasting blood sample). Five milliliter syringe was used for blood sample collection, using the procedure described by Bachorik.[33] All samples were stored in a refrigerator at -80°C until analysis.[34]
Uric acid analysis: Uric acid analysis was determined using commercial enzymatic colorimetry method (PAP-Method) using the Human Kit (Human Gesellschaft Biochemical Diagnostic mbH, Germany) as recommended by the manufacturer.
Psychosocial assessment
Subjects were in a comfortable sitting position and were presented with a questionnaire tagged the General Well Being Schedule (GWBS). Subjects were instructed to respond to the subscales of the 18 items questions. Subjects were timed for a maximum of 20 min and the questionnaire collected immediately. High values indicate high psychosocial well-being or decrease psychosocial stress. The questionnaire was developed and validated by the National Centre for Health Statistics.[35]
Stress test
The Young Men Christian Association (YMCA) submaximal cycle ergometry test protocol was used to assess subject's aerobic power (VO2 max) as described by ACSM.[36] The stress test (pre and post training) was conducted under the supervision of experts in basic life support care and the emergency unit of the hospital was made ready to accommodate any incident that might occur during the stress test.
Test procedure
Training program
Following stress test and prior to the exercise training, all subjects in both control and continuous groups were reassessed by the physician and were prescribed with antihypertensive drug; methyldopa as necessary. Methyldopa was preferred because it does not alter normal hemodynamic responses to exercise[37] and it is a well-tolerated antihypertensive drug in the Africa[38] and mostly prescribed in the northern part (Kano) of Nigeria where the study was conducted and useful in the treatment of mild to moderately severe hypertension.[39] Subjects maintain these prescriptions with regular medical consultation and observation throughout the period of exercise training.
The continuous group (group 1)
Subjects in the continuous group exercised on a bicycle ergometer at a low intensity of between 60% and 79% of their HR max as recommended by ACSM.[27] The starting workload was 100 kg (17 W) which was increased at a pedal speed of 50 r/min to obtain a HR max reserve 35% was increased in the first two weeks to and level up at 59% HR max reserve throughout the remaining part of the training period. The initial of exercise session was increased from 45 minutes in the first two weeks of training to and leveled up at 60 min throughout the remaining part of the training. Exercise session of three times per week was maintained throughout the 8 weeks training period for continuous group.
The control group (group 2)
Subjects in the control group were instructed not to undertake any vigorous physical activity during the 8 weeks period of study.
Posttest procedure
Wash out Period: At the end of the 8 weeks training period, all subjects were asked to stop methyldopa and subjects were prescribed with placebo tablets for one week as in pretest procedure.
Post training SBP, DBP, psychosocial status assessment and stress test were conducted as earlier described in the pretest procedures using standardized protocols, techniques and methods.
All pre- and post-test measurements were recorded on a data sheet. Two hundred and seventeen subjects (112 from continuous, and 105 from control group) completed the eight weeks training program. One hundred and six subjects (50 from continuous, and 56 from control group) had dropped out because of noncompliance, unfavorable responses to methyldopa, and exercise training or had incomplete data; therefore, the data of 217 subjects were used in the statistical analysis [Figure 1].
Figure 1.
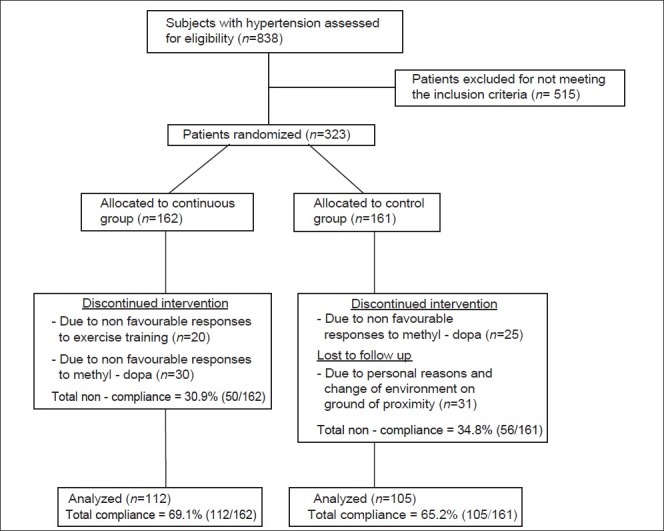
Study design flow chart. Psychosocial r = 0.399** SUA r = -0.266** significant at 0.01**
Statistical analysis
Following data collection, the measured and derived variables were statistically analyzed. The descriptive statistics (means and standard deviations) of the subjects physical characteristics, estimated VO2 max, psychosocial status, and cardiovascular parameters were determined. Student's t-test and Pearson product moment correlation test were computed for the variables of interest. In the t and correlation tests, the difference between subjects post-training and pre-training measurements (changed score) were used as dependent measures. The score changed was the difference between the posttest and pretest values. All statistical analysis was performed on a Toshiba compatible microcomputer using the statistical package for the social science (SPSS) Version 16.0, (Chicago IL, USA). The probability level for all the above tests was set at 0.05 to indicate significance.
Results
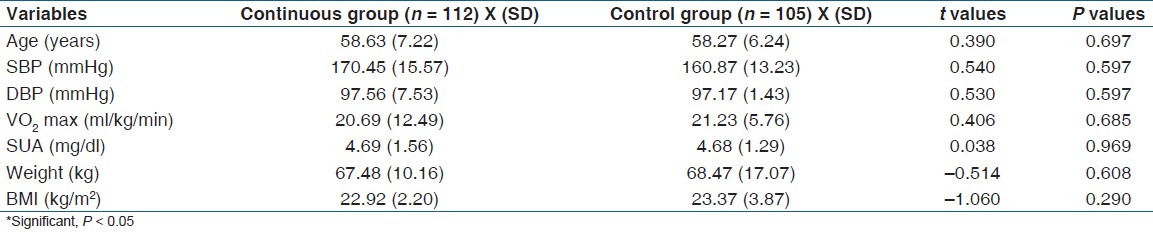
The subject's age ranged between 50 and 70 years. Mean age, height, weight, and BMI of subjects in continuous exercise group were (58.63 (7.22) years, 1.73 (6.97) m, 67.49 (10.16) kg, 22.48 (2.89) kg m-2), respectively, while for the control group mean age, height, weight and BMI were (58.27 (6.24) years, 1.68 (5.31) m, 68.47 (17.07) kg, 23.37 (5.31) kg m-2, respectively). There was no significant difference in age (P =.697), baseline SBP (P = 0.597), baseline DBP (P = 0.597), baseline SUA (P = 0.969) and baseline VO2 max (P = 0.685) between groups. The physical characteristics of the subjects are comparable [Table 1].
Table 1.
Groups mean (SD) base line characteristics and independent t-test (n = 217)
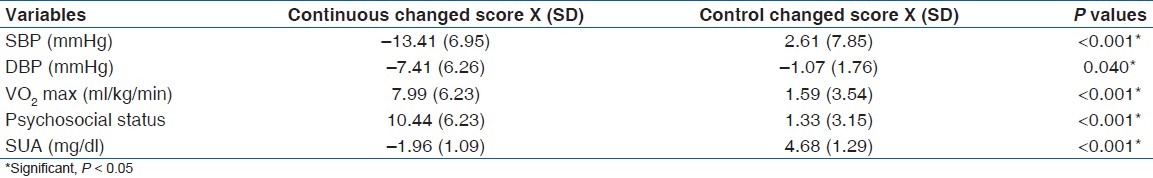
Groups pre and post treatment mean BP (SD) mmHg; psychosocial status, SUA (mg/dl) and VO2 max ml kg-1 min-1 are depicted in Table 2. Changed score values and Student's t-test results [Table 3] indicated a significant effect in the exercise groups over control in SBP (P <0.001), DBP (P = 0.040), psychosocial status (P <0.001), SUA (P <0.001) and VO2 max (P <0.001) at P < 0.05.
Table 2.
Groups mean (SD) pretest and posttest values (n = 217)
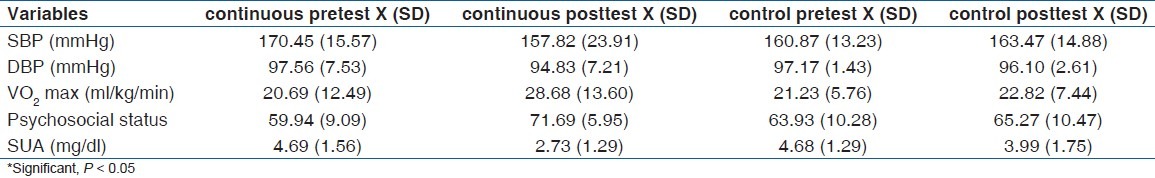
Table 3.
Independent t-test between groups changed score (pretest–post-test values) values (n= 217)
There was a significant positive and negative correlation between changes between VO2 max and psychosocial status (r = 0.399) and SUA (–0.266), respectively, at P < 0.01 [Figure 2].
Figure 2.
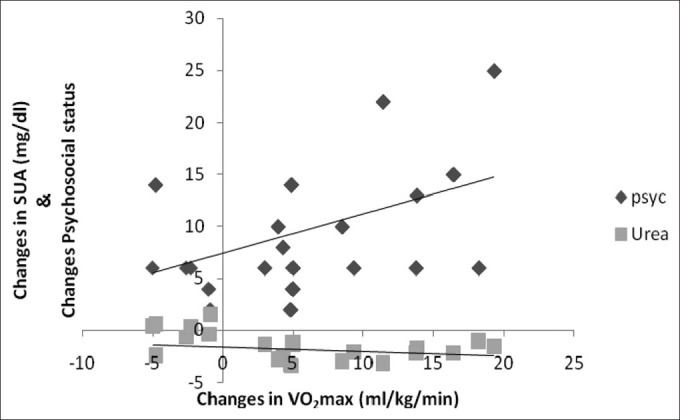
Correlation between training changes in VO2 max, SUA, and psychosocial status (n = 112)
Discussion
Findings from the present study revealed a significant decrease in SBP, DBP, SUA and increase in VO2 max in the continuous group over control group. The favorable changes resulting from aerobic training in both SBP and DBP demonstrated in the present study is consistent with several other studies.[40–43] Also, result of the present study indicated a significant increase in psychosocial wellbeing (reduction in psychosocial stress) and significant decrease in SUA in the continuous group over control group.
Psychosocial status
The favorable changes resulting from aerobic training on psychosocial status as demonstrated in the present study is consistent with the study of Smith et al.,[44] they investigated the effect of aerobic exercise on 133 sedentary hypertensive (SBP:130-180 mmHg; DBP:85-110 mmHg) males and females. Participants were grouped into aerobic group, aerobic with weight reduction group and control group, participants engaged in 6 months treatment period. They reported a significant decreased in self-reported depressive syndrome in the treatment groups compare to the placebo group.
The observation in the present study is also in line with a study by Ulrik et al.,[45] and Klocek et al.,[46] though on heart failure and CHD patients, respectively. Both studies demonstrated significant improvement in the quality of life in the interval training over continuous. The mechanism of the superior effect of intensive physical training on the quality of life is not presently known, but it is reasonable to suggest that it is due to greater physiological adaptation and thereby improved capacity for activity in interval group.
Another similar result was reported by Georgiades et al,[47] in their study, they investigated the effects of exercise and weight loss on cardiovascular responses during mental stress in mildly to moderately overweight patients with elevated blood pressure. Ninety-nine men and women with high normal or unmedicated stage 1 to stage 2 hypertension; (systolic blood pressure 130-179 mm×Hg, diastolic blood pressure 85-109 mm Hg), underwent a battery of mental stress tests, including simulated public speaking, anger recall interview, mirror trace, and cold pressor, before and after a 6-month treatment program. Subjects were randomly assigned to 1 of 3 treatments: (1) aerobic exercise, (2) weight management combining aerobic exercise with a behavioral weight loss program, or (3) waiting list control group. Their results demonstrated that exercise, particularly when combined with a weight loss program, can lower both resting and stress-induced blood pressure levels and hemodynamic pattern resembling that targeted for antihypertensive therapy.
However, a contradictory finding to the present study was reported by Pierce et al.,[48] they investigated the effects of 16 weeks of physical exercise training on the psychological functioning of patients with mild hypertension. Ninety patients were examined at baseline and after 16 weeks of training, patients completed a psychometric test battery that included objective measures of neuropsychological performance and standardized self-report measures of psychosocial functioning. Patients were randomly assigned to 1 of 3 groups: aerobic exercise, strength training and flexibility exercise, or a waiting list control group. After training, there were no group differences on any of the psychological measures. Another contradictory finding, though, on healthy subjects was reported by Kohut et al.[49] They investigated the effect of aerobic exercise on psychosocial scores (depression, optimism, sense of coherence), sixty four years healthy old adults, also subgroup of subjects treated with non selective beta (1) beta (2) adrenergic antagonist were assigned to either aerobic exercise or control for 3 days /week, 45 min for 10 months. They reported non-significant effect of aerobic exercise compared to control on psychosocial scores.
The reasons for discrepancies in findings between the present study and others might not be unconnected to the type, mode, frequency, duration of intervention, condition of subjects that varied across studies. The mechanisms responsible for exercise-related improvements in psychosocial status are not known. However, a number of psychological factors have been proposed to explain the effect that exercise has on depressed mood including increased self-efficacy, a sense of mastery, positive thoughts, distraction from negative thoughts, and enhanced self-concept. Also a number of biologic pathways also have been suggested including increased central norepinephrine neurotransmission,[50,51] alterations in the hypothalamo-pituitary-adrenocortical axis,[52] and increased secretion of amine metabolites as well as serotonin synthesis and metabolism.[53–55]
Serum uric acid
The present study also demonstrated a significant reduction in exercise group serum uric acid level over control. This finding is in line with the report of Filipovsky et al.,[56] who investigated the effect of 5 weeks aerobic physical training course on uricaemia levels of 77 sedentary subjects with hypertension. They reported significant decrease in uric acid level at P < 0.001. This significant change persisted up to between 3 and 7 months after the intervention of exercise training. They concluded that 5-week intensive physical training had a favorable on both short and long-term effect on uricaemia levels in hypertension. Langlois et al.,[57] reported a contrary notion; they investigated whether uric acid (UA) status is related to lower limb function in hypertensive with peripheral arterial disease (PAD). One hundred and forty five nonhypertensive subjects with PAD and 166 subjects with hypertension and PAD participated. Subjects involved in aerobic exercise on treadmill. They reported a significant increased in serum uric acid concentration in PAD hypertensive (404 (101) versus 347 (80) μmol/l, P < 0.001).
Leyver et al.,[58] investigated the relationship between SUA concentrations and the measures of functional capacity. Fifty nine patients with a diagnosis of chronic heart failure due to coronary heart disease (n = 34) or idiopathic dilated cardiomyopathy (n = 25) and 20 healthy controls underwent assessment of functional capacity. Maximal oxygen uptake (VO2 max) and SUA were measured during a maximal treadmill exercise test. They reported an inverse relationship between SUA concentrations and measures of functional capacity in patients with cardiac failure. They concluded that the strong correlation between SUA and VO2 max suggests that in chronic heart failure increased SUA concentrations reflect an impairment of oxidative metabolism.
Conclusion
The present study supports the recommendations of moderate intensity (continuous) training program in blood pressure reduction and dual therapeutic effects on psychosocial stress reduction and SUA in the management of hypertension.
Clinical rehabilitation impact
The study demonstrated a rationale for the adjunct therapeutic role of moderate intensity training in the down regulation of blood pressure, SUA and psychosocial stress management. Therefore, exercise specialists and other professionals in rehabilitation should feel confident in the use of this mode of training in the non-pharmacological adjunct multipurpose management of hypertension.
Limitation
The present study demonstrated a rationale bases for the role of continuous exercise training in the down regulation of the blood pressure, SUA and psychosocial stress. However, the limitation of the study includes failure to perform the intension-to-treat analysis (ITTA) in the randomized controlled trial. Though, it has been reported that ITTA provides unbiased assessments of treatment efficacy;[59] however, on-treatment analysis (per protocol analysis) has the advantage that it provides for a new treatment to show additional efficacy and it most closely reflects the scientific model underlined in the protocol.[60] These limitation however, warrants attention in future studies.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Derman EW, Whitesman S, Dreyer M, Patel DN, Nossel C, Schwellnus MP. South African Family Practice. 2009;51:382–6. [Google Scholar]
- 2.Sliwa K, Wilkinson D, Hansen C, Ntyintyane L, Tibazarwa K, Becker A, et al. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): A cohort study. Lancet. 2008;371:915–22. doi: 10.1016/S0140-6736(08)60417-1. [DOI] [PubMed] [Google Scholar]
- 3.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 4.Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, et al. Guidelines for management of hypertension: Report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens. 2004;18:139–85. doi: 10.1038/sj.jhh.1001683. [DOI] [PubMed] [Google Scholar]
- 5.Joint National Committee on prevention, evaluation and treatment of high blood pressure. The fifth report of the JNC on detection, evaluation and treatment of high blood pressure (JNC) Arch Intern Med. 1993;153:154–83. [PubMed] [Google Scholar]
- 6.Markovitz JH, Mathews KA, Kannel WB, Cobb JL, D’Agostino RB. Psychological predictors of hypertension in the Framingham study: Is there tension in hypertension? JAMA. 1993;270:2439–43. [PubMed] [Google Scholar]
- 7.Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–83. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzi TM, Borba DL, Dutra G, Lara DR. Association of serum uric acid levels with emotional and affective temperaments. J Affect Disord. 2010;121:161–4. doi: 10.1016/j.jad.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Magnus MH. Cardiovascular health among African-Americans: A review of the health status, risk reduction, and intervention strategies. Am J Health Promot. 1991;5:282–90. doi: 10.4278/0890-1171-5.4.282. [DOI] [PubMed] [Google Scholar]
- 10.Strodl E, Kenardy J, Aroney C. Perceived stress as a predictor of the self-reported new diagnosis of symptomatic CHD in older women. Int J Behav Med. 2003;3:205–20. doi: 10.1207/s15327558ijbm1003_02. [DOI] [PubMed] [Google Scholar]
- 11.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364:953–62. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 12.Bove M, Carnevali L, Cicero AF, Grandi E, Gaddoni M, Noera G, et al. Psychosocial factors and metabolic parameters: Is there any association in elderly people? The Massa Lombarda Project. Aging Ment Health. 2010;14:801–6. doi: 10.1080/13607861003713299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsouli SG, Liberopoulos EN, Mikhailidis DP, Athyros VG, Elisaf MS. Elevated serum uric acid levels in metabolic syndrome: An active component or an innocent bystander? Metabolism. 2006;55:1293–301. doi: 10.1016/j.metabol.2006.05.013. Review. [DOI] [PubMed] [Google Scholar]
- 14.Hakim AA, Petrovitch H, Burchfiel CM, Ross GW, Rodriguez BL, White LR, et al. Effects of walking on mortality among nonsmoking retired men. N Engl J Med. 1998;338:94–9. doi: 10.1056/NEJM199801083380204. [DOI] [PubMed] [Google Scholar]
- 15.Siegel W, Blumenthal J. The role of exercise in the prevention and treatment of hypertension. Ann Behav Med. 1991;13:23–30. [Google Scholar]
- 16.Kriska AM, Blair SN, Pereira MA. The potential role of physical activity in the prevention of non-insulin dependent diabetes mellitus: The epidemiological evidence. Exerc Sport Sci Rev. 1994;22:121–43. [PubMed] [Google Scholar]
- 17.Wood PD, Stefanick ML, Dreon DM, Frey-Hewitt B, Garay SC, Williams PT, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319:1173–9. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- 18.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 19.Blumenthal JA, Emery CF, Walsh MA, Cox DR, Kuhn CM, Williams RB, et al. Exercise training in healthy type A middle-aged men: Effects on behavioral and cardiovascular responses. Psychosom Med. 1988;50:418–33. doi: 10.1097/00006842-198807000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Crews DJ, Landers DM. A meta-analytic review of aerobic fitness and reactivity to psychosocial stressors. Med Sci Sports Exerc. 1987;19:S114–20. [PubMed] [Google Scholar]
- 21.Myers RH, Kiely DK, Cupples LA, Kannel WB. Parental history is an independent risk factor for coronary artery disease: The Framingham Study. Am Heart J. 1990;120:963–9. doi: 10.1016/0002-8703(90)90216-k. [DOI] [PubMed] [Google Scholar]
- 22.Hypertension Control. Geneva: WHO; 1996. World Health Organization Expert Committee on hypertension control; pp. 24–31. [PubMed] [Google Scholar]
- 23.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcription regulation. J BiolChem. 2000;275:18138–44. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 24.Groove ML, Morrison A, Folsom AR, Boerwinkle E, Hoelscher DM, Bray MS. Gene-environment interaction and the GNB3 gene in the Atherosclerosis Risk in Communities study. Int J Obes (Lond) 2007;31:913–26. doi: 10.1038/sj.ijo.0803545. [DOI] [PubMed] [Google Scholar]
- 25.Hagber JM, Farrell RE, Dengel DR, Wilund KR. Exercise training-induced blood pressure and plasma lipid improvements in hypertensives may be genotype dependent. Hypertension. 1999;34:18–23. doi: 10.1161/01.hyp.34.1.18. [DOI] [PubMed] [Google Scholar]
- 26.Kuzuya M, Ando F, Iguchi A, Shimokata H. Effect of aging on serum uric acid levels: Longitudinal changes in a large Japanese population group. J Gerontol A BiolSci Med Sci. 2002;57:M660–4. doi: 10.1093/gerona/57.10.m660. [DOI] [PubMed] [Google Scholar]
- 27.Guide lines for exercise testing and Prescription. 4th ed. Philadelphia: Lea and Febiger; 1991. American College of Sport Medicine. [Google Scholar]
- 28.Townsend RR, Mcfadden CB, Ford V, Cadee JA. A randomized double-blind, placebo-controlled trial of casein protein hydrolysnte (C12 peptide) in human essential hypertension. Am J Hypertens. 2004;17:1056–8. doi: 10.1016/j.amjhyper.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Akinpelu AO. Beneficial effects of exercise training on human hypertension. Journal of the NigSoc of Physio. 1990;10:28–30. [Google Scholar]
- 30.Musa DI, Ibrahim DM, Toriola AL. Cardiorespiratory fitness and risk factors of CHD in pre-adolescent Nigerian girls. J Hum Mov Stud. 2002;42:455–5. [Google Scholar]
- 31.International standards for anthropometric assessment. PatcheFstroom. South Africa: ISAK; 2001. International Society for the Advancement of Kinanthropometry (ISAK) [Google Scholar]
- 32.Ross WD, Marfell-Jones MJ. Physiological testing of the high performance athletes. In: MacDugall JD, Wenger A, Green HJ, editors. Kinanthropometry. Champaign IL: Human Kinetics Books; 1991. pp. 223–308. [Google Scholar]
- 33.Bachorik PS. Collection of blood samples for lipoprotein analysis. ClinChem. 1982;28:1375–8. [PubMed] [Google Scholar]
- 34.Barbieri M, Ferrucci L, Corsi AM, Macchi C, Lauretani F, Bonafe M, et al. Is chronic inflammation a determinant of blood pressure in the elderly? Am J Hypertens. 2003;16:537–43. doi: 10.1016/s0895-7061(03)00861-6. [DOI] [PubMed] [Google Scholar]
- 35.Stephens T, Graig CL. The well being of Canadian: Highlights of the 1988 Cambell SurveyOttawa Canadian fitness and lifestyle Research Institute. Ottawa: 1990. [Google Scholar]
- 36.ASCM's guidelines for exercise testing and prescription. 5th ed. Baltimore: Williams and Wilkins; 1995. American College of Sports Medicine. [Google Scholar]
- 37.Katzung BG. Basic and clinical pharmacology. 7th ed. New York: Lange Medical Books/Craw Hill; 1998. [Google Scholar]
- 38.Mancia G, Ferari L, Gregorini L, Leonett L, Terzoli L, Biachini C, et al. Effects of treatment with methyldopia on basal haemodynamic and on rural control. In: Robertson JS, Pickering GW, Goldwell ADS, editors. The therapeutics of hypertension. London: Royal Society of Medicine and Academic Press Inc. Ltd; 1980. pp. 70–8. [Google Scholar]
- 39.Salako LA. Treatment of hypertension: Cardiovascular disease in Africa. Ibadan: Ciba Geigy Ltd; 1976. [Google Scholar]
- 40.American College of Sport Medicine. Position Stand. Physical activity, physical fitness and hypertension. Med Sci Sports Exerc. 1993;25:1–10. [PubMed] [Google Scholar]
- 41.Lamina S. Effects of continuous and interval training programs in the management of hypertension: A randomized controlled trial. J ClinHypertens (Greenwich) 2010;12:841–9. doi: 10.1111/j.1751-7176.2010.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westhoff TH, Franke N, Schmidt S, Vallbracht-Israng K, Meissner R, Yildirim H, et al. Too old to benefit from sports? The cardiovascular effects of exercise training in elderly subjects treated for isolated systolic hypertension. Kidney Blood Press Res. 2007;30:240–7. doi: 10.1159/000104093. [DOI] [PubMed] [Google Scholar]
- 43.Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, et al. Exercise training restores baroreflex sensitivity in never trained hypertensive patients. Hypertension. 2007;49:1298–306. doi: 10.1161/HYPERTENSIONAHA.106.085548. [DOI] [PubMed] [Google Scholar]
- 44.Smith PJ, Blumenthal JA, Babyak MA, Georgiades A, Hinderliter A, Sherwood A. Effects of exercise and weight loss on depressive symptoms among men and women with hypertensive. J Psychosom Res. 2007;63:463–9. doi: 10.1016/j.jpsychores.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation. 2007;115:3086–94. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 46.Klocek M, Kubinyi A, Bacior B, Kawecka-Jaszcz K. Effect of physical training on quality of life and oxygen consumption in patients with congestive heart failure. Int J Cardiol. 2005;103:323–9. doi: 10.1016/j.ijcard.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 47.Georgiades A, Sherwood A, Gullette EC, Babyak MA, Hinderliter A, Waugh R, et al. Effects of exercise and weight loss on mental stress-induced cardiovascular responses in individuals with high blood pressure. Hypertension. 2000;36:171–6. doi: 10.1161/01.hyp.36.2.171. [DOI] [PubMed] [Google Scholar]
- 48.Pierce TW, Madden DJ, Siegel WC, Blumenthal JA. Effects of aerobic exercise on cognitive and psychosocial functioning in patients with mild hypertension. Health Psychol. 1993;12:286–91. doi: 10.1037//0278-6133.12.4.286. [DOI] [PubMed] [Google Scholar]
- 49.Kohut ML, McCann DA, Russell DW, Konopka DN, Cunnick JE, Frank WD, et al. Aerobic exercise, but not flexibility/resistance, exercise, reduces serum IL-18, CRP and IL-6, independent of beta-blockers, BMI and psychosocial factors in older adults. Brain BehavImmun. 2006;20:201–9. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Rubin RT. Pharmacoendocrinology of major depression. Eur Arch Psychiatry NeurolSci. 1989;238:259–67. doi: 10.1007/BF00449807. [DOI] [PubMed] [Google Scholar]
- 51.Sothman MS, Ismail AH. Relationships between urinary catecholamine metabolites, particularly MHPG, and selected personality and physical fitness characteristics in normal subjects. Psychosom Med. 1984;46:523–33. doi: 10.1097/00006842-198411000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Droste SK, Gesing A, Ulbricht S, Muller MB, Linthorst AC, Reul JM. Effects of long-term voluntary exercise on the mouse hypothalmic-pituitary-adrenocortical axis. Endocrinology. 2003;114:3012–23. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- 53.Dishman RK, Renner KJ, Youngstedt SD, Reigle TG, Bunnell BN, Burke KA, et al. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Res Bull. 1997;42:399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- 54.Dunn AL, Dishman RK. Exercise and the neurobiology of depression. Exerc Sport Sci Rev. 1991;19:41–98. [PubMed] [Google Scholar]
- 55.Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: Source, fate, and functions. Physiol Rev. 1990;70:963–85. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- 56.Filipovsky J, Simon J, Chrástek J, Rosolova H, Haman P, Petrikova V. Changes of blood pressure and lipid pattern during a physical training course in hypertensive subjects. Cardiology. 1991;78:31–8. doi: 10.1159/000174762. [DOI] [PubMed] [Google Scholar]
- 57.Longlois M, De Bacquer D, Duprez D, De Buyzere M, Delanghe J, Blaton V. Serum uric acid in hypertensive patients with and without peripheral arterial disease. Atherosclerosis. 2003;168:163–8. doi: 10.1016/s0021-9150(03)00093-5. [DOI] [PubMed] [Google Scholar]
- 58.Leyva F, Anker S, Swan JW, Godsland IF, Wingrove CS, Chua TP, et al. Serum uric acid as an index of impaired oxidative metabolism in chronic heart failure. Eur Heart J. 1997;18:858–65. doi: 10.1093/oxfordjournals.eurheartj.a015352. [DOI] [PubMed] [Google Scholar]
- 59.Montori VM, Guyatt GH. Intention-to-treat principle. CMAJ. 2001;165:1339–41. [PMC free article] [PubMed] [Google Scholar]
- 60.Stewart WC, Jackson AL, Jenkins JN. Dropout rates for intent-to-treat and per protocol analyses. Am J Ophthalmol. 2004;137:639–45. doi: 10.1016/j.ajo.2003.11.028. [DOI] [PubMed] [Google Scholar]


