Abstract
Introduction
Homologous recombination repair (HRR) is a critical pathway for the repair of DNA damage caused by cisplatin or PARP inhibitors. HRR may be impaired by multiple mechanisms in cancer, which complicates assessing the functional HRR status in cells. Here, we monitored the ability of non-small cell lung cancer (NSCLC) cells to form subnuclear foci of DNA repair proteins as a surrogate of HRR proficiency.
Methods
We assessed clonogenic survival of 16 NSCLC cell lines in response to cisplatin, mitomycin C (MMC), and the PARP inhibitor olaparib. Thirteen tumor explants from NSCLC patients were subjected to cisplatin ex-vivo. Cells were assayed for foci of repair-associated proteins such as BRCA1, FANCD2, RAD51, and γ-H2AX.
Results
Four cell lines (25%) showed an impaired RAD51 foci forming ability in response to cisplatin. Impaired foci formation correlated with cellular sensitivity to cisplatin and MMC as well as olaparib. Foci responses complemented or superseded genomic information suggesting alterations in the ATM/ATR and FA/BRCA pathways. Because baseline foci in untreated cells did not predict drug sensitivity we adapted an ex-vivo biomarker assay to monitor damage-induced RAD51 foci in NSCLC explants from patients. Ex-vivo cisplatin treatment of explants identified 2 tumors (15%) exhibiting compromised RAD51 foci induction.
Conclusions
A fraction of NSCLC harbors HRR defects that may sensitize the affected tumors to DNA damaging agents including PARP inhibitors. We propose that foci-based functional biomarker assays represent a powerful tool for prospective determination of treatment sensitivity, but will require ex-vivo techniques for induction of DNA damage to unmask the underlying HRR defect.
Keywords: lung cancer, homologous recombination, RAD51, biomarker
In patients with advanced or metastatic non-small cell lung cancer (NSCLC) treated with platinum-basedchemotherapy regimens, disease response rates are only in the range of 15-35%. While overexpression of the nucleotide excision repair protein ERCC1 or presence of mutant K-RAS in NSCLC have been associated with resistance to platinum-based chemotherapy (1), there is a paucity of biomarkers to predict which tumors are likely to be sensitive to platinum or targeted agents such as PARP inhibitors.
Homologous recombination repair (HRR) is a pathway critical for several cellular processes including the error-free repair of DNA double-strand breaks (DSB) and the recovery of stalled DNA replication forks (2). Deregulated HRR results in genomic instability which may cause or contribute to carcinogenesis. Importantly, such HRR-defective tumors may be more sensitive to DNA damaging chemotherapeutics such as cisplatin or PARP inhibitors. HRR may be altered by genetic, epigenetic, or other mechanisms, making it challenging to assess the functional HRR status in a given tumor. In addition, even though altered expression of genes involved in HRR, such as BRCA1 or FANCD2, has been described in NSCLC, it is unknown whether this is associated with a functionally relevant HRR defect (3-5). Interestingly, 7% of lung adenocarcinomas harbor mutations in the DNA damage response ATM kinase, which is involved in HRR as well as multiple other DNA repair and checkpoint functions (6), but the functional consequences of this alteration remain to be established.
Significantly, the activity of HRR as well as other DNA damage response/repair pathways is less dependent on protein expression levels than on the proper localization of these proteins to sites of damaged DNA, which ensures spatiotemporal coordination and execution of repair (2). Accordingly, in response to DNA damage, several of these proteins form microscopically visible foci that accumulate in the nuclei of cells. In contrast, in undamaged cells the frequency of these subnuclear foci is low. Thus, the ability of cells to form repair protein foci may be regarded as a functional biomarker of the integrity of the HRR network and associated sensitivity to cisplatin and other anti-cancer agents. The advantage of using these foci as biomarkers is that they can detect repair defects due to various mechanisms such as epigenetic events or gene mutations. Moreover, they potentially provide a global measurement of network function without needing to know the identities of all the components.
Here, we sought to discover functionally relevant HRR defects by interrogating a panel of NSCLC cell lines and tissues for their ability to form DNA damage-induced foci of RAD51 recombinase, which is the central effector of HRR in cells (2).
MATERIALS AND METHODS
Cell lines
Cell lines were selected from a published panel located in the Center for Molecular Therapeutics (CMT) at Massachusetts General Hospital, except for A549, Calu-6, and NCI-H2087, which were purchased directly from ATCC. Cell lines were obtained during 2008-10 and cultures passaged for < 3 months after thawing a given frozen vial. In the CMT, the identity of each of the cell lines in the panel was tested using a set of 16 short tandem repeats (STR) (AmpFLSTR Identifier KIT, ABI). In addition, single nucleotide polymorphism (SNP) profiles based on a panel of 63 SNPs assayed using the Sequenom Genetic Analyzer was used for in-house identity checking whenever a cell line was propagated and confirmed uniqueness of cell lines for the ones without available STR. A549 cells were maintained in DMEM, Calu-6 in α-MEM, ABC1 and NCI-H2126 in DMEM/F12, and HCC44, LU99B, NCI-H1299, NCI-H1563, NCI-H1703, NCI-H1792, NCI-H1915, NCI-H2087, NCI-H23 in NCI-H3122 in RPMI1640 (all Sigma-Aldrich). SV40-transformed human fibroblasts, ATM mutant AT5BIVA (GM5849A) and ATM wild-type NF (GM00637F), were provided by Simon Powell. Cells were grown in DMEM. Maintenance of PD20 with or without expression of wild-type FANCD2 has been described (7). All cell lines were maintained in a humidified incubator at 37°C and 5% CO2. All media was supplemented with 10% bovine growth serum (HyClone), 20 mM HEPES, 2 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml stretopmycin (all Sigma-Aldrich). No cell line was ever treated for mycoplasma and all lines tested mycoplasma free prior to the experiments (MycoAlert, Lonza).
Tumor tissues
Tumor tissues were obtained from previously untreated patients undergoing surgical resection for clinical stage I NSCLC under a protocol approved by the Boston University Medical School and Partners Institutional Review Boards. Tumor samples were processed for ex-vivo foci analysis adapting a previously published protocol for breast cancer (8). Under this protocol, tumor tissues not needed for pathological diagnosis were placed into RPMI typically within 30 minutes of surgical resection and arrived in the laboratory 30-60 minutes later. Specimens were evenly divided into samples of < 5 mm size depending on the amount of tissue available. Samples were mock treated or exposed to 10 Gy radiation, followed by 5 hours incubation at 37°C and 5% CO2, and snap freezing, as described (8). In addition to the published protocol, 1-2 tumor samples were also exposed to cisplatin at 8 μM for 5 hours.
Treatment and clonogenic survival assay
Treatments with cisplatin, mitomycin C, or ionizing radiation were carried out as described (7, 9, 10). Olaparib (LC Laboratories and AstraZeneca) was dissolved in DMSO for a 20 mM stock solution and stored in aliquots at −20°C. Clonogenic cell survival was assessed as described (10).
Immunofluorescence microscopy
Exponentially growing cells were plated into 8-well chamber slides and treated with 8 μM cisplatin as described previously (10). Visualization of RAD51, FANCD2, and γ-H2AX foci in cell lines was performed as described (10, 11). For BRCA1, cells were incubated with an anti-BRCA1 mouse monoclonal antibody (OP92, Calbiochem) at a 1:200 dilution. For RAD51 and PCNA costaining, PD20 cells were permeabilized first with 0.5% Triton-X, 20mM HEPES, 50mM NaCl, 3mM KCl, 300mM Sucrose on ice for 5 minutes, followed by fixation with 2% paraformaldehyde at room temperature for 20 minutes and 100% methanol at −20°C for 10 minutes. Cells were exposed to primary antibodies against PCNA (rabbit polyclonal ab2426, Abcam) and RAD51 (mouse monoclonal ab213, Abcam) both at 1:200 dilution in 2% BSA, 0.1% Triton X-100, followed by incubation with secondary antibodies (Alexa Fluor-488 goat anti-mouse, Invitrogen A11029 and Alexa Fluor-568 goat anti-rabbit, Invitrogen A11011) at 1:1,000 dilution and DAPI counterstaining. Phospho-ATM foci were visualized with anti-phospho-ATM (serine1981) mouse monoclonal antibody (200-301-400, Rockland). Co-staining with PCNA was performed as above. For co-staining with 53BP1 foci, an anti-53BP1 rabbit polyclonal antibody (Abcam) was used. For all experiments, the number of foci per nucleus was determined by analyzing 100-300 nuclei per data point. For quantification of “induced foci”, the number of foci per nucleus in untreated cells was subtracted from the foci number in treated cells and appropriate cut-offs between 10 and 20 foci per nucleus were chosen to determine the fraction of cells positive for foci formation depending on the particular endpoint. Observers were generally blinded as to whether cells had been treated.
Detection of RAD51 foci in tumor tissues has been described (8). For visualization of γ-H2AX, cryosections were fixed with 2% paraformaldehyde for 15 minutes, permeabilized with 0.5% Triton X-100 for 5 minutes at room temperature, and stained with anti-γ-H2AX (phospho-S139) mouse monoclonal antibody (ab18311, Abcam) at 1:500 dilution at 4°C overnight, which was followed by incubation with secondary anti-mouse Alexa fluor-488 antibody (Invitrogen) at 1:1,000 dilution for 1 hour at room temperature. For PCNA staining, tissue sections were permeabilized first with 0.1% Tween-20 for 10 minutes and incubated with anti-PCNA rabbit polyclonal antibody (#ab2426, Abcam) at 1:200 dilution. For foci quantification, typically 7-10 random images per data point were captured and 200-400 nuclei scored. Nuclei with at least 2 foci were considered positive. For 3-dimensional rendering (Fig. 4C), a Z-series of images was taken for each treatment (SlideBook 5.0, IX81 Olympus confocal microscope). The step size was 0.1 μm, and the number of planes was 40. The Z-series was deconvolved using the Constrained Iterative Method. The deconvolved Z-series was assembled into 3D images by using the 3D Volume view option in SlideBook.
RESULTS
A NSCLC cell line screen reveals putative HRR defects
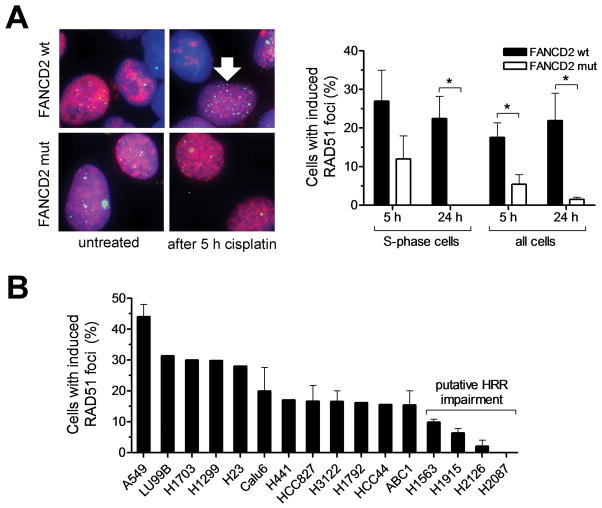
To identify NSCLC cell lines with impaired HRR, we sought to screen for the ability of cisplatin-treated cells to form subnuclear foci of RAD51, an established surrogate marker of HRR activity (12, 13). We first established that human cells with a known defect in the HRR pathway exhibit impaired RAD51 foci formation in the S-phase of the cell cycle, which is thought to reflect defective repair of replication forks that collide with drug-induced interstrand crosslinks (ICL) (2). Impaired RAD51 formation was not only seen in S-phase cells, but was also detectable in asynchronously growing cell populations at both 5 and 24 hours after start of drug treatment (p<0.05) (Fig. 1A, Fig. S1). Next, we screened a panel of 16 NSCLC cell lines for their proficiency to form RAD51 foci in response to cisplatin treatment. There was a range of foci forming ability, with 4/16 cell lines (25%) demonstrating induced foci in 10% or less of cells suggesting a putative HRR defect (Fig. 1B). Cell cycle analysis indicated that the reduction in RAD51 foci formation was not due to a lower fraction of cells in S-phase (Fig. S2).
FIGURE 1.
Monitoring the formation of subnuclear RAD51 foci in response to cisplatin treatment. A, FANCD2-mutant (mut) PD20 fibroblasts with or without exogenous expression of wild-type (wt) FANCD2 were exposed to cisplatin (8 μM) for 1 hour and subjected to immunofluorescence staining for the S-phase marker nuclear PCNA and RAD51 recombinase at 5 and 24 hours (h). Left panel shows representative images of co-staining for PCNA (red) and RAD51 (green), in conjunction with DAPI (blue) counterstaining. Right panel shows fraction of cells with induced RAD51 foci in PCNA-positive S-phase cells or all cells. Bars represent mean with standard error based on 3 independent repeat experiments. Arrow indicates representative nucleus with intact RAD51 foci formation in an S-phase cell. * indicates p<0.05 (t-test, two-tailed). B, Analogous to panel A, 16 NSCLC cell lines were exposed to cisplatin and screened for induced RAD51 foci at 5 h. Bars represent the fraction of cells with at least 15 induced RAD51 foci.
RAD51 foci defect correlates with crosslinker and PARP inhibitor sensitivity
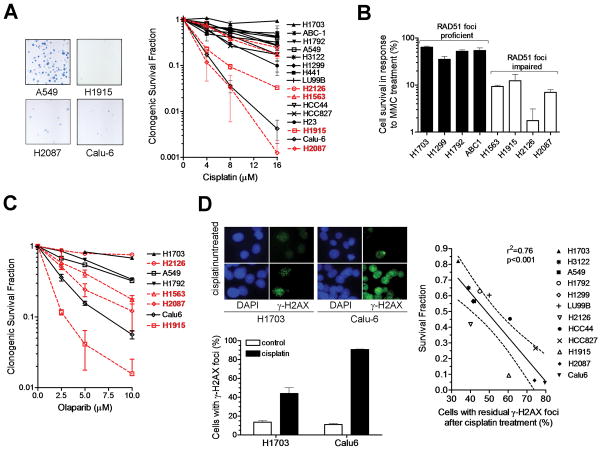
HRR-defective cells are expected to be sensitive to crosslinking agents, such as cisplatin or MMC, but also PARP inhibitors (14). We first determined the cisplatin sensitivity of our cell line panel in a colony formation assay. Two RAD51 foci impaired cell lines, H1915 and H2087, clearly demonstrated drug hypersensitivity (Fig. 2A), while the other two cell lines, H1563 and H2126, appeared to be relatively resistant to cisplatin. However, tumor cells could have acquired cisplatin resistance by other processes such as drug efflux mechanisms. We, therefore, also determined the MMC sensitivity of the 4 RAD51 foci-deficient cell lines and detected reduced clonogenic survival in all these cell lines compared to a set of RAD51 foci-proficient cell lines (Fig. 2B). Inhibition of PARP is known to cause synthetic lethality in HRR-deficient BRCA mutant cells (2). Consistent with this, 3 of the RAD51 foci-deficient cell lines demonstrated increased sensitivity to olaparib (Fig. 2C). It remained unclear why H2126 cells are resistant to olaparib, though it is possible that the very slow growth of this cell line may have reduced the number of drug-induced breaks encountered during a cell cycle.
FIGURE 2.
Sensitivity of NSCLC cell lines to DNA damaging anti-cancer drugs. A, Left panel shows representative examples of colony forming ability after treating A549 cells (200 cells seeded) or cisplatin-sensitive cell lines (2,000-5,000) with 16 μM cisplatin. Right panel, clonogenic survival fraction is plotted against cisplatin concentration. Data points represent mean with standard error based on at least 2 independent repeat experiments. Cell lines with putative RAD51 foci formation defects are highlighted in red. B, Clonogenic survival fraction after treatment with 0.25 mg/ml mitomycin C (MMC) for 1 hour is plotted for a subset of cell lines. C, Clonogenic survival fraction after treatment with 10 μM of olaparib for 72 hours. Cell lines with putative RAD51 foci formation defects are highlighted in red. Bars represent mean with standard error based on at least 2 independent repeat experiments. D, Left panels illustrate the difference in the formation of γ-H2AX foci at 24 hours following treatment of cisplatin-resistant H1703 and cisplatin-sensitive Calu-6 cells with 8 μM cisplatin. Right panel shows the correlation between clonogenic cell survival versus residual γ-H2AX foci present at 24 hours after cisplatin treatment with 8 μM. Dotted line reflects 95% confidence limits of the linear regression line.
Interestingly, one seemingly RAD51-proficient cell line, Calu-6, was sensitive to cisplatin as well as olaparib (Fig. 2A,C). Because there could be a defect parallel to or downstream of RAD51 foci formation, we also assessed the levels of residual γ-H2AX foci as a marker of DSB (15). Following cisplatin treatment, we found high levels of DSB in Calu-6 cells, pointing towards an underlying recombinational repair defect (Fig. 2D). For all cell lines, there was an excellent correlation between the levels of residual DSB and cisplatin sensitivity (Fig. 2D). Thus, both RAD51 and γ-H2AX foci may be used to identify functionally relevant recombinational repair defects that are associated with cisplatin sensitivity.
ERCC1 is also involved in the repair and restart of replication forks, and is used as a biomarker of cisplatin resistance in the clinic (1). We did not find a correlation between RAD51 foci induction and ERCC1 gene expression, suggesting that ERCC1 is not a surrogate for RAD51 (Fig. S3).
Multiple mechanisms underlying crosslinker sensitivity in NSCLC cells
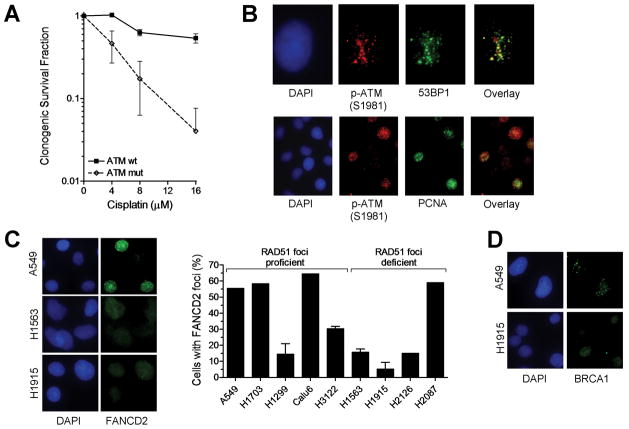
Of the 4 cell lines with impaired RAD51 function, one cell line, H2126, has a genomic alteration in a pathway known to control HRR in response to replication stress (16), i.e., a heterozygous mutation in the ATR kinase. H2087 cells harbor a mutation in the ATM kinase impairing phosphorylation at the S1981 site (Fig. S4A). ATM is known to be involved in non-homologous end-joining and HRR following the induction of DSB by ionizing radiation but has not been previously implicated in cisplatin resistance (17). Follow-up analysis in several cell systems established that ATM is required for cellular cisplatin resistance (Fig. 3A, Fig. S4B). The kinetics of ATM activation followed the time course of DSB induction (Fig. S4C). Phosphorylated ATM co-localized with DSB (Fig. 3B), was detected during DNA replication within 6 hours following cisplatin treatment (Fig. 3B, Fig. S4D), and formed in a manner that appeared at least partially dependent on ATR (data not shown), consistent with a role of ATM at stalled replication forks (18).
FIGURE 3.
Pathway correlates of cisplatin sensitivity. A, Clonogenic survival of human fibroblasts with wild-type (wt) or mutant (mut) ATM treated with varying doses of cisplatin for 1 hour. Data points represent mean with standard error based on 3 independent repeat experiments. B, Representative images demonstrating co-localization of phospho-ATM foci with 53BP1 or PCNA at 24 hours following cisplatin treatment of A549 cells. C, Analysis of FANCD2 foci formation at 5 hours in a subset of cell lines treated with 8 μM cisplatin. Bars represent the fraction of cells with 20 or more foci per nucleus. D, Representative images illustrating the reduced ability of H1915 cells to form BRCA1 foci in response to cisplatin.
For RAD51 foci-impaired cell lines H1563 and H1915, no pre-existing genomic alteration in a putative HRR pathway was known. We thus screened for reduced expression of components of the FA/BRCA pathway and detected decreased FANCD2 and BRCA1 expression, respectively (data not shown), consistent with prior reports showing altered expression of these genes in lung cancer (3, 4). Significantly, we found a reduced ability to form FANCD2 foci in both cell lines (Fig. 3C). In addition, in H1915 cells there was reduced foci formation of BRCA1 (Fig. 3D), which is upstream of FANCD2 foci formation (19).
Unexpectedly, our analysis also revealed that H1299 cells exhibit reduced expression of FANCF (Fig. S5A), which was associated with impaired FANCD2 foci formation (Fig. 3C) and mono-ubiquitination of FANCD2 (Fig. S5B). However, these cells were not crosslinker sensitive (Fig. 2A, 2B), likely due to unusually high levels of RAD51 protein (Fig. S5C) that may act in a compensatory manner (20, 21).
An ex-vivo biomarker assay to monitor the effects of anti-cancer agents in live tumor explants
Our cell line work indicated that chemosensitivity is correlated with an impaired ability to induce RAD51 foci following drug treatment (Fig. 1, S6A), but not with the baseline number of foci in untreated cells (Fig. S6A). Thus, assaying RAD51 foci on archived tumor samples (from pre-treatment biopsies) was not expected to be informative.
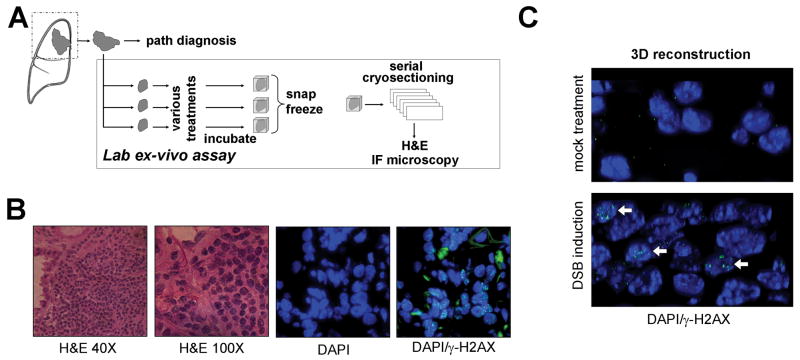
We, therefore, adapted an ex-vivo biomarker assay previously tested in breast cancer to determine if impaired HRR activity can be detected in NSCLC (8). Fresh tumor tissues from patients with untreated NSCLC were collected and within 90 minutes of removal from the patient exposed to cisplatin or radiation in the laboratory (Fig. 4A). Tissues were incubated in parallel for 5 hours before snap freezing. Serial cryosections were analyzed for viable tumor by H&E staining and nuclear protein foci using immunofluorescence microscopy (Fig. 4B). Damage-induced γ-H2AX foci were readily visualized following irradiation or cisplatin treatment (Fig. 4B,C, Fig. S6B).
FIGURE 4.
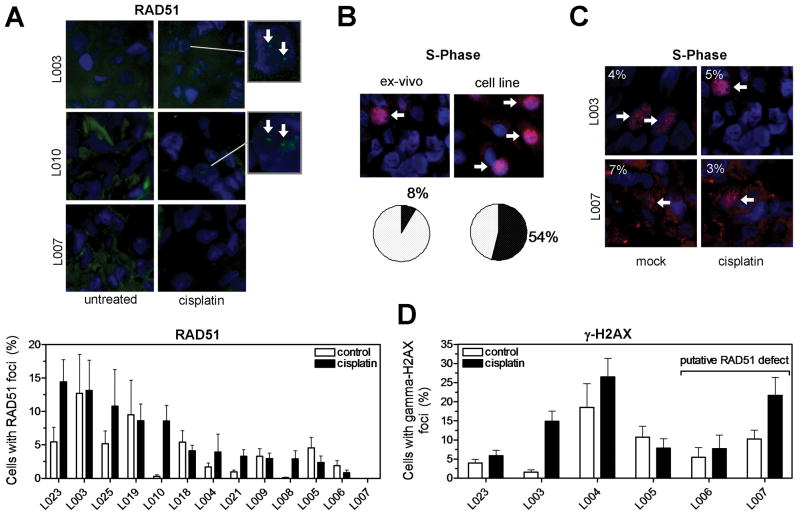
Ex-vivo foci assay in NSCLC explants. A, Surgical specimens from untreated patients were incubated under standard cell culture conditions and exposed to 8 μM cisplatin, 10 Gy radiation, or mock treatment ex-vivo. Specimens were snap frozen after 5 or 24 hours and subjected to cryosectioning at a later time. B, Serial cryoslides were analyzed by H&E staining to identify viable tumor and immunofluorescence (IF) microscopy to visualize repair protein foci. C, 3-dimensional rendering of tissue nuclei counterstained with γ-H2AX 5 hours after mock treatment or irradiation ex-vivo.
We assessed a total of 13 NSCLC explants for cellular proficiency to form RAD51 foci in response to cisplatin treatment (Fig. 5A). Similar to the cell line data, there was a range of foci induction with levels overall lower than in cell lines, i.e., on average only 5.8% of tumor cells (95% CL, 3.0-8.7%) exhibited RAD51 foci. In 2/13 tumors (15%), i.e., explants L006 and L007, very few RAD51 foci (in < 1% of cells) were seen in the cisplatin-treated specimens, which is indicative of impaired HRR activity. The overall lower fraction of cells with RAD51 foci in explants compared to cell lines was explained by a several fold fraction of cells in S-phase (Fig. 5B, S6C). Importantly, tumors with putative HRR defects displayed an S-phase fraction comparable to RAD51 foci-proficient tumors, as illustrated in Fig. 5C. In addition, low RAD51 foci levels did not result from a lack of DNA damage induction, as shown by the ability of cisplatin to generate γ-H2AX foci in these explants (Fig. 5D, S6B). Taken together, these data demonstrate the feasibility of monitoring a drug-induced cellular DNA damage response in live tumor explants ex-vivo.
FIGURE 5.
Detection of cisplatin-induced RAD51 foci in live NSCLC explants. A, Upper panel shows representative images demonstrating subnuclear RAD51 foci following 5 hour incubation after cisplatin. Lower panel displays the fraction of nuclei with RAD51 foci in a panel of 13 tumor explants. Bars represent mean with standard error. B, Illustration of S-phase fraction in representative tumor tissue versus A549 cells using PCNA/DAPI counterstaining (see also Fig. S6C). C, Illustration of PCNA-positive S-phase fraction in RAD51 foci-proficient versus -deficient tumors. D, Quantification of fraction of cells with cisplatin-induced γ-H2AX foci at 5 hours. Bars represent average fraction of nuclei with at least 2 foci +/- standard error based on 8-10 random images and 200-400 nuclei per data point.
DISCUSSION
The identification of HRR-deficient tumors is a major challenge in cancer research, in particular when taking into account the complexity of the DNA damage response network (2). In addition, assessing the expression of individual network components is unlikely to reveal the overall incidence of defects that can occur anywhere in the network. Lastly, it is not established whether reduced expression of genes involved in HRR translates into functional repair impairment.
There has been increasing interest in determining HRR activities in cancers by assaying for the subnuclear location of central pathway components, such as RAD51, BRCA1, and FANCD2, as well as unrepaired DSB marked by γ-H2AX (8, 22, 23). The advantage of using foci as biomarkers is that they can capture repair defects due to various mechanisms such as gene mutations, epigenetic changes, or alterations in signal transduction pathways (2). Moreover, they provide a global measurement of network function without needing to know the identities of all the components, many of which remain unknown.
Here, we report for the first time the presence of impaired RAD51 foci formation in 4/16 (25%) NSCLC cell lines. All 4 cell lines were MMC hypersensitive (Fig. 2B) while the sensitivity profile for cisplatin and olaparib was more heterogeneous (Fig. 2A,C). Though the exact reason for this differential effect remains to be elucidated, it is conceivable that residual HRR function in some cell lines is sufficient to cope with the consequences of intra-strand DNA damage associated with olaparib and/or cisplatin treatment. In contrast, a higher percentage of the DNA damage generated by MMC consists of damage affecting both DNA strands, i.e., ICLs, which places a much greater burden on the cellular DNA repair response than the damage resulting from the other two compounds (2). Thus, assaying for tumors with defective RAD51 formation should at least enrich for those that may respond to cisplatin or PARP inhibitors.
In addition, we detected a strong correlation between persisting γ-H2AX foci and cisplatin sensitivity. These data and the identification of one RAD51 proficient cell line, Calu-6, which demonstrates high γ-H2AX levels and cisplatin sensitivity, support the notion that joint assessment of RAD51 and γ-H2AX foci may be sufficient to reliably identify platinum- and perhaps also olaparib-sensitive tumors. Importantly, RAD51 foci form independently of ERCC1 (24), and we do not find a correlation between foci induction and ERCC1 expression (Fig. S3). Whether ERCC1 in conjunction with RAD51 and γ-H2AX foci constitutes a useful biomarker set requires further study.
Our data indicate that foci responses yield information that can complement or supersede genomic information. For example, ATM-mutant H2087 cells were sensitive to cisplatin and displayed impaired RAD51 foci (Fig. 3) even though ATM has not been previously implicated in cellular cisplatin resistance. As a fraction of lung adenocarcinoma (7%) harbor non-recurrent mutations throughout the ATM gene (6), the assessment of foci responses in these tumors may provide information on the functional significance of these alterations. As another example, FANCF hypermethylation was reported in 14% of NSCLC, but the functional impact of these epigenetic events has remained unknown (4). Here, we found that the commonly studied H1299 cell line displays suppressed FANCF expression and impaired downstream FANCD2 function (Fig. 3, S5), yet retains RAD51 function and drug resistance (Fig. 2), which highlights the usefulness of RAD51 and γ-H2AX foci as biomarkers of functionally relevant repair defects and drug sensitivity.
How can foci assays in cell lines be translated into patients? It is important to appreciate that the functional status of HRR is typically revealed only when cells are exposed to DNA damage (8). Assessing foci responses in live tumors would require a repeat biopsy following initial administration of treatment, which is a challenging undertaking. Alternatively, pre-treatment biopsies can be interrogated for their functional HRR status in order to select the appropriate treatment regimen for a given patient (Fig. 4A). We report here for the first time that cisplatin induces a damage response in NSCLC explants from untreated patients (Fig. 5D, S6B). At least 2 out of 13 tumors analyzed in this fashion demonstrated low RAD51 foci levels consistent with a functional HRR defect (Fig. 5A). Whether patients with these tumors indeed derive benefit from treatment with cisplatin or a PARP inhibitor remains to be determined.
While this type of functional ex-vivo foci assay represents a potentially powerful tool for the detection of pre-existing and clinically relevant defects within the complex HRR pathway, several technical challenges remain, including: (1) potential intra-tumoral heterogeneity in foci responses, (2) low fraction of cells in S-phase, compared to cell lines, necessitating co-staining techniques to detect replication-associated RAD51 foci, (3) need for quantification and automation of foci scoring, and (4) potential changes in hypoxia/reoxygenation upon removal of the tumor tissue from the patient and incubation in the laboratory at 20% oxygen. Data from our laboratory and by Vaira et al. (25) indicate that adequately cultured tumor explants are viable for at least 24 hours and up to 5 days, offering a promising avenue for assessing not only foci responses but also surrogate endpoints of cell fate such as apoptosis or senescence, thereby allowing pharmacodynamic profiling of human tumors.
In conclusion, it should be possible to use the ability of cells to form repair foci as a functional biomarker of the integrity of the HRR pathway. The absence of repair foci induction, coupled with a persistence of DSB markers such as γ-H2AX, would be indicative of chemosensitivity or PARP inhibitor sensitivity. One can envision developing mechanism-based “HRR foci signatures” that reflect nodal points in the HRR pathway or network of associated DDR proteins. Such a foci signature likely will include at a minimum BRCA1, FANCD2, RAD51, and γ-H2AX to capture the multiple deficiencies that appear to underlie crosslinker and PARP inhibitor sensitivity in NSCLC.
Alterations in HRR in tumors can be therapeutically targeted by novel approaches such as PARP inhibitors as well as traditional DNA damaging chemotherapeutics or radiation. The efforts to exploit pre-existing HRR defects or disrupt proficient or hyperactive HRR in malignancies are in their infancy, but hold great promise to broadly impact cancer therapies in the very near future (2). As our understanding of the regulation of HRR pathways in normal and malignant cells deepens, various rational treatment strategies are likely to materialize (26).
Supplementary Material
Acknowledgments
The authors wish to thank Simon Powell and Markus Grompe for their generous contribution of materials and Nectaria Vassilakis for help with collection of patient tissues. The excellent technical assistance of Chake Tokadjian is acknowledged. AstraZeneca supported this research by providing olaparib.
GRANT SUPPORT
This work was supported by the Department of Defense W81XWH-06-1-0309 (L.A.K., H.W.), the Dana-Farber/Harvard Cancer Center SPORE in Lung Cancer, NCI P50 CA090578 (L.Z., H.W.), NCI R01 GM097360 (J.W.), the Federal Share of program income earned by Massachusetts General Hospital on C06 CA059267, Proton Therapy Research and Treatment Center (J.W., L.Z., L.S., H.W.), the Ellison Medical Foundation (J.W.), and by the German Cancer Aid (Deutsche Krebshilfe) 108903 (J.D.D.).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest or relating to the work reported in this manuscript.
References
- 1.Shepherd FA, Rosell R. Weighing tumor biology in treatment decisions for patients with non-small cell lung cancer. J Thorac Oncol. 2007;2 (Suppl 2):S68–76. doi: 10.1097/01.JTO.0000269737.05962.a0. [DOI] [PubMed] [Google Scholar]
- 2.Willers H, Pfäffle HN, Zou L. Targeting Homologous Recombination Repair in Cancer. Academic Press, Elsevier; 2012. pp. 119–160. [Google Scholar]
- 3.Ferrer M, Span SW, Vischioni B, Oudejans JJ, van Diest PJ, de Winter JP, Giaccone G, Kruyt FA. FANCD2 expression in advanced non-small-cell lung cancer and response to platinum-based chemotherapy. Clin Lung Cancer. 2005;6:250–254. doi: 10.3816/CLC.2005.n.005. [DOI] [PubMed] [Google Scholar]
- 4.Marsit CJ, Liu M, Nelson HH, Posner M, Suzuki M, Kelsey KT. Inactivation of the Fanconi anemia/BRCA pathway in lung and oral cancers: implications for treatment and survival. Oncogene. 2004;23:1000–1004. doi: 10.1038/sj.onc.1207256. [DOI] [PubMed] [Google Scholar]
- 5.Rosell R, Skrzypski M, Jassem E, Taron M, Bartolucci R, Sanchez JJ, Mendez P, Chaib I, Perez-Roca L, Szymanowska A, Rzyman W, Puma F, Kobierska-Gulida G, Farabi R, Jassem J. BRCA1: A Novel Prognostic Factor in Resected Non-Small-Cell Lung Cancer. PLoS ONE. 2007;2:e1129. doi: 10.1371/journal.pone.0001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, Fulton L, Fulton RS, Zhang Q, Wendl MC, Lawrence MS, Larson DE, Chen K, Dooling DJ, Sabo A, Hawes AC, Shen H, Jhangiani SN, Lewis LR, Hall O, Zhu Y, Mathew T, Ren Y, Yao J, Scherer SE, Clerc K, Metcalf GA, Ng B, Milosavljevic A, Gonzalez-Garay ML, Osborne JR, Meyer R, Shi X, Tang Y, Koboldt DC, Lin L, Abbott R, Miner TL, Pohl C, Fewell G, Haipek C, Schmidt H, Dunford-Shore BH, Kraja A, Crosby SD, Sawyer CS, Vickery T, Sander S, Robinson J, Winckler W, Baldwin J, Chirieac LR, Dutt A, Fennell T, Hanna M, Johnson BE, Onofrio RC, Thomas RK, Tonon G, Weir BA, Zhao X, Ziaugra L, Zody MC, Giordano T, Orringer MB, Roth JA, Spitz MR, Wistuba II, Ozenberger B, Good PJ, Chang AC, Beer DG, Watson MA, Ladanyi M, Broderick S, Yoshizawa A, Travis WD, Pao W, Province MA, Weinstock GM, Varmus HE, Gabriel SB, Lander ES, Gibbs RA, Meyerson M, Wilson RK. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhnert VM, Kachnic LA, Li L, Purschke M, Gheorghiu L, Lee R, Held KD, Willers H. FANCD2-deficient human fibroblasts are hypersensitive to ionising radiation at oxygen concentrations of 0% and 3% but not under normoxic conditions. Int J Radiat Biol. 2009;85:523–531. doi: 10.1080/09553000902883810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willers H, Taghian AG, Luo CM, Treszezamsky AD, Sgroi D, Powell SN. Utility of DNA repair protein foci for the detection of putative BRCA1-pathway defects in breast cancer biopsies. Mol Cancer Res. 2009;7:1304–1309. doi: 10.1158/1541-7786.MCR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kachnic LA, Fournier L, Gheorghiu L, Rosenberg C, Powell SN, Willers H. Utility of chemotherapy-induced Rad51 foci for the identification of homologous recombination defects in breast cancer cell lines. Proceedings of the 55th Annual Meeting of the Radiatation Research Society; Savannah, GA. 2009. p. 99. [Google Scholar]
- 10.Kachnic LA, Li L, Fournier L, Willers H. Fanconi anemia pathway heterogeneity revealed by cisplatin and oxaliplatin treatments. Cancer Lett. 2010;292:73–79. doi: 10.1016/j.canlet.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Willers H, Kachnic LA, Luo C-M, Li L, Purschke M, Borgmann K, Held KD, Powell SN. Biomarkers and Mechanisms of FANCD2 Function. Journal of Biomedicine and Biotechnology. 2008:821529. doi: 10.1155/2008/821529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan SS, Lee SY, Chen G, Song M, Tomlinson GE, Lee EY. BRCA2 is required for ionizing radiation-induced assembly of Rad51 complex in vivo. Cancer Res. 1999;59:3547–3551. [PubMed] [Google Scholar]
- 13.Zhang J, Willers H, Feng Z, Ghosh JC, Kim S, Weaver DT, Chung JH, Powell SN, Xia F. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Molecular & Cellular Biology. 2004;24:708–718. doi: 10.1128/MCB.24.2.708-718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, Giavara S, O’Connor MJ, Tutt AN, Zdzienicka MZ, Smith GC, Ashworth A. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–8115. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 15.Olive PL, Banath JP. Kinetics of H2AX phosphorylation after exposure to cisplatin. Cytometry B Clin Cytom. 2009;76:79–90. doi: 10.1002/cyto.b.20450. [DOI] [PubMed] [Google Scholar]
- 16.Sorensen CS, Hansen LT, Dziegielewski J, Syljuasen RG, Lundin C, Bartek J, Helleday T. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol. 2005;7:195–201. doi: 10.1038/ncb1212. [DOI] [PubMed] [Google Scholar]
- 17.Beucher A, Birraux J, Tchouandong L, Barton O, Shibata A, Conrad S, Goodarzi AA, Krempler A, Jeggo PA, Lobrich M. ATM and Artemis promote homologous recombination of radiation-induced DNA double-strand breaks in G2. Embo J. 2009;28:3413–3427. doi: 10.1038/emboj.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stiff T, Walker SA, Cerosaletti K, Goodarzi AA, Petermann E, Concannon P, O’Driscoll M, Jeggo PA. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. Embo J. 2006;25:5775–5782. doi: 10.1038/sj.emboj.7601446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Higuera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, Grompe M, D’Andrea AD. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Molecular Cell. 2001;7:249–262. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 20.Schild D, Wiese C. Overexpression of RAD51 suppresses recombination defects: a possible mechanism to reverse genomic instability. Nucleic Acids Res. 2010;38:1061–1070. doi: 10.1093/nar/gkp1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlacher K, Wu H, Jasin M. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell. 2012;22:106–116. doi: 10.1016/j.ccr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graeser M, McCarthy A, Lord CJ, Savage K, Hills M, Salter J, Orr N, Parton M, Smith IE, Reis-Filho JS, Dowsett M, Ashworth A, Turner NC. A marker of homologous recombination predicts pathologic complete response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2010;16:6159–6168. doi: 10.1158/1078-0432.CCR-10-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banuelos CA, Banath JP, Kim JY, Aquino-Parsons C, Olive PL. gammaH2AX expression in tumors exposed to cisplatin and fractionated irradiation. Clin Cancer Res. 2009;15:3344–3353. doi: 10.1158/1078-0432.CCR-08-3114. [DOI] [PubMed] [Google Scholar]
- 24.Al-Minawi AZ, Lee YF, Hakansson D, Johansson F, Lundin C, Saleh-Gohari N, Schultz N, Jenssen D, Bryant HE, Meuth M, Hinz JM, Helleday T. The ERCC1/XPF endonuclease is required for completion of homologous recombination at DNA replication forks stalled by inter-strand cross-links. Nucleic Acids Res. 2009;37:6400–6413. doi: 10.1093/nar/gkp705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaira V, Fedele G, Pyne S, Fasoli E, Zadra G, Bailey D, Snyder E, Faversani A, Coggi G, Flavin R, Bosari S, Loda M. Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proc Natl Acad Sci U S A. 107:8352–8356. doi: 10.1073/pnas.0907676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahim YH, Garcia-Garcia C, Serra V, He L, Torres-Lockhart K, Prat A, Anton P, Cozar P, Guzman M, Grueso J, Rodriguez O, Calvo MT, Aura C, Diez O, Rubio IT, Perez J, Rodon J, Cortes J, Ellisen LW, Scaltriti M, Baselga J. PI3K Inhibition Impairs BRCA1/2 Expression and Sensitizes BRCA-Proficient Triple-Negative Breast Cancer to PARP Inhibition. Cancer Discov. 2012 Oct 25; doi: 10.1158/2159-8290.CD-11-0348. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.