Abstract
New Bedford Harbor (MA, U.S.A.; NBH) is a Superfund site inhabited by Atlantic killifish (Fundulus heteroclitus) with altered aryl hydrocarbon receptor (Ahr) signaling, leading to resistance to effects of polychlorinated biphenyls (PCBs) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). The Ahr is a transcription factor that regulates gene expression of many Phase I and II detoxifying enzymes and interacts with Nrf2, a transcription factor that regulates the response to oxidative stress. This study tested the hypothesis that PCB-resistant killifish exhibit altered sensitivity to oxidative stress. Killifish F1 embryos from NBH and a clean reference site (Scorton Creek, MA, U.S.A.; SC) were exposed to model pro-oxidant and Nrf2-activator, tert-butylhydroquinone (tBHQ). Embryos were exposed at specific embryonic developmental stages (5, 7, and 9 days post fertilization) and toxicity was assessed, using a deformity score, survival, heart rate, and gene expression to compare sensitivity between PCB-resistant and PCB-sensitive (reference) populations. Acute exposure to tBHQ resulted in transient reduction in heart rate in NBH and SC F1 embryos. However, embryos from NBH were more sensitive to tBHQ, with more frequent and severe deformities, including pericardial edema, tail deformities, small body size, and reduced pigment and erythrocytes. NBH embryos had lower basal expression of antioxidant genes catalase and glutathione-S-transferase alpha (gsta), and upon exposure to tBHQ, exhibited lower levels of expression of catalase, gsta, and superoxide dismutase compared to controls. This result suggests that adaptation to tolerate PCBs has altered the sensitivity of NBH fish to oxidative stress during embryonic development, demonstrating a cost of the PCB resistance adaptation.
Keywords: Fundulus heteroclitus, New Bedford Harbor, oxidative stress, deformities, ecotoxicology, adaptation
Introduction
The Atlantic killifish (Fundulus heteroclitus) has shown a remarkable ability to adapt to its environment. Killifish populations have evolved resistance to harmful levels of methylmercury (Weis et al. 1981), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Prince and Cooper 1995), polycyclic aromatic hydrocarbons (PAHs) (Meyer et al. 2002; Ownby et al. 2002; Meyer and Di Giulio 2003), and polychlorinated biphenyls (PCBs) (Nacci et al. 1999; Bello et al. 2001; Nacci et al. 2010). However, this adaptation can come at a fitness cost (Wirgin and Waldman 2004; Kinnison and Hairston 2007) and result in altered capability to combat additional stressors such as hypoxia (Meyer and Di Giulio 2003) and some pesticides (Clark and Di Giulio 2012).
The New Bedford Harbor, MA (NBH) Superfund site is highly contaminated by PCBs as well as heavy metals such as cadmium, lead, copper, and chromium (Nelson et al. 1996; Nacci et al. 2010). The NBH population of killifish has developed resistance to the PCBs and related compounds; NBH fish are 14- to >1,000-fold less sensitive to the biochemical and embryotoxic effects of dioxin-like halogenated aromatic hydrocarbons (HAHs) and polycyclic aromatic hydrocarbons (PAHs) as compared to fish from clean sites (Nacci et al. 1999; Bello et al. 2001; Oleksiak et al. 2011). The predominant teratogenic effects that occur in clean-site killifish when exposed to HAHs or PAHs during embryonic development are cardiac malformations (Matson et al. 2008; Arzuaga and Elskus 2010; Clark et al. 2010; Whitehead et al. 2010). The development of these deformities requires a functional aryl hydrocarbon receptor (Ahr), as demonstrated by experiments showing that killifish embryos in which Ahr2 protein has been knocked down are protected from the effects of PAHs or 3,3’,4,4’,5-pentachlorobiphenyl (PCB-126) (Clark et al. 2010). The resistance of NBH embryos to embryotoxic and teratogenic effects of PCBs suggests that Ahr signaling is down-regulated in NBH fish, a result confirmed by analysis of gene expression after exposure to PCB-126 (Oleksiak et al. 2011; Whitehead et al. 2012).
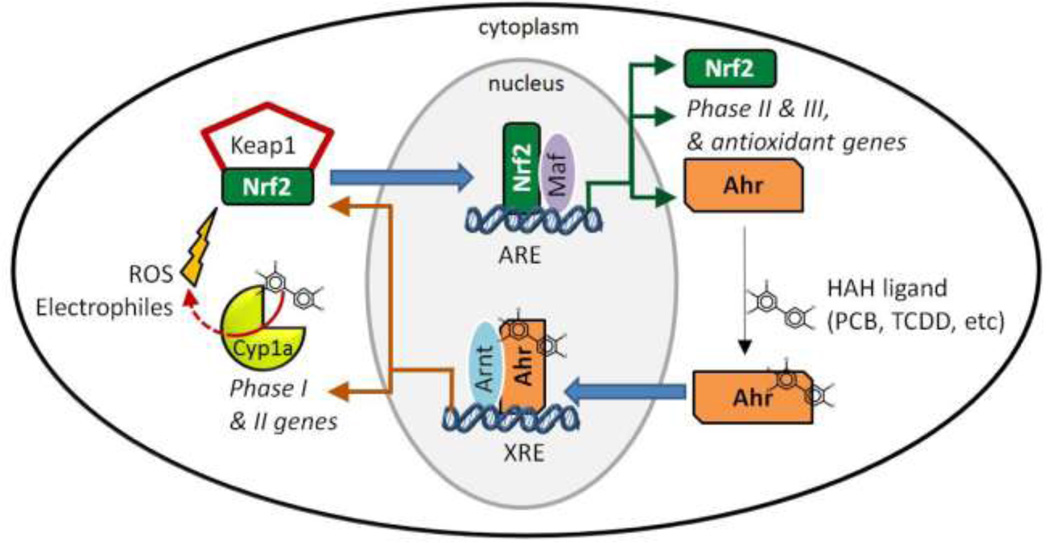
The Ahr mediates an adaptive and sometimes toxic response to numerous xenobiotics (Okey 2007). This cytosolic transcription factor is activated by a variety of ligands including HAHs, PAHs, co-planar PCBs, natural plant products, and tryptophan-based molecules (Denison and Nagy 2003; Denison et al. 2011). Ligand binding, such as by non-ortho PCBs contaminating the NBH Superfund site, initiates translocation of Ahr to the nucleus, where it dimerizes with the Ahr nuclear translocator (Arnt) (Schmidt and Bradfield 1996), both members of the basic helix loop helix (bHLH) / Per-Arnt-Sim (PAS) gene family (Crews 1998); Gu et al. 2000). The ligand-activated Ahr-Arnt heterodimer binds to xenobiotic response elements (XREs) in the promoter region of numerous target genes, including members of the cytochrome P450 family 1 (CYP1), genes encoding some phase II enzymes, and the oxidant-responsive transcription factor Nrf2 (nuclear factor erythroid-related factor-2) (Fig 1). The Ahr is required for the toxicity and other effects of non-ortho-substituted PCBs, TCDD, and PAHs. For example, loss of Ahr signaling through targeted inactivation of the Ahr locus protects mice from effects of TCDD, including acute toxicity (Fernandez-Salguero et al. 1996; Schmidt and Bradfield 1996) and teratogenicity (Peters et al. 1999; Mimura et al. 1997). Similarly, knockdown of Ahr (Ahr2) in fish embryos reduces or prevents the embryotoxic and teratogenic effects of TCDD (Prasch et al. 2003), PCB-126 (Jonsson et al. 2007; Clark et al. 2010) or PAHs (Clark et al. 2010; Billiard et al. 2006).
Figure 1. Diagram illustrating the Ahr- and Nrf2-dependent signaling pathways and interactions between them.
Ahr is constitutively expressed in the cytoplasm of cells, and binds a variety of ligands including HAHs such as non-ortho PCBs. Upon ligand binding, Ahr translocates to the nucleus where it dimerizes with the Arnt and binds to XREs in the promoter region of numerous target genes including Phase I enzymes such as Cyp1a, some phase II enzymes, and the oxidant-responsive transcription factor Nrf2. Some planar structures that are potent Ahr agonists are poor substrates for metabolism by CYP1 enzymes (White et al. 1997) and can uncouple the CYP1 reaction cycle, generating ROS (Schlezinger et al. 2006). ROS from this and other sources can activate Nrf2. Nrf2 is found constitutively in the cytoplasm bound to a repressor protein, Keap1. Under unstressed conditions, Keap1 sequesters Nrf2 in the cytoplasm, targeting it for proteasomal degradation. ROS and electrophiles cause release and nuclear accumulation of Nrf2. In the nucleus, Nrf2, associates with small MAF proteins and binds to antioxidant response elements (AREs) in the promoter regions of a set of cytoprotective genes encoding antioxidant and phase II enzymes (Kobayashi et al. 2006). Among the Nrf2 target genes is Ahr, closing the loop on Ahr-Nrf2 cross-talk. See text for additional details.
In addition to mediating the toxicity of PCBs and PAHs, the Ahr also participates in crosstalk with another transcription factor, Nrf2. Nrf2, a member of the Cap’n’collar, basic region-leucine zipper (CNC-bZIP) family of transcription factors, regulates the transcription of genes encoding many cytoprotective, antioxidant, and phase II enzymes in response to oxidative and electrophilic stress. Nrf2 is found constitutively in the cytoplasm bound to a repressor protein, Kelch-like ECH-associated protein 1 (Keap1). Under unstressed conditions, Keap1 sequesters Nrf2 in the cytoplasm (Fig. 1), targeting it for proteasomal degradation (Nguyen et al. 2004). ROS and electrophiles cause release and nuclear accumulation of Nrf2 (Fig 1). In the nucleus, Nrf2 associates with small Maf proteins and binds to antioxidant response elements (AREs) in the promoter regions of its target genes (Fig. 1) (Kobayashi et al. 2006).
There are many sources of oxidative stress in the environment that could impact Nrf2 signaling in fish. Some of these include hypoxia, hyperoxia, temperature-related changes in metabolism, and pollutants such as pesticides, PCBs, TCDD, PAHs, and metals (Lesser 2006); Lushchak 2011; Loro et al. 2012; Billiard et al. 2006).
Cross-talk between the Ahr and Nrf2 signaling pathways has been demonstrated in mammals and fish (Wakabayashi et al. 2010; Kohle and Bock 2007). Nrf2 can directly affect transcription of Ahr genes and autoregulate its own expression, and Ahr regulates Nrf2 gene expression (Fig. 1) (Miao et al. 2005; Shin et al. 2007; Timme-Laragy et al. 2012b). In addition, several genes, such as Sod1, Nqo1, Ugt1a, and glutathione-S-transferase alpha (Gsta), have been shown to have both XRE and AREs and can be regulated by both the Ahr and Nrf2 (Nguyen et al. 2003).Yeager et al. (2009) showed that the Ahr-dependent induction of Nqo1, Ugt1a6, and Gsta1 by TCDD in mouse liver also required Nrf2. Despite the emerging recognition of Ahr-Nrf2 cross-talk, many questions regarding these interactions remain, in particular concerning the extent of Ahr-Nrf2 interactions in fish.
Insight into the interactions of Nrf2 and Ahr signaling pathways may be gained through investigation of the oxidative stress response of the PCB-resistant NBH killifish population, which has altered Ahr-dependent signaling (Oleksiak et al. 2011; Whitehead et al. 2012). NBH killifish have been extensively studied and this population has been shown repeatedly to have reduced sensitivity to HAHs and altered Ahr signaling, as epitomized by the lack of induction of Cyp1a (Bello 1999); Oleksiak et al. 2011; Arzuaga and Elskus 2010; Powell et al. 2000; Aluru et al. 2011; Nacci et al. 2010) and many other Ahrregulated genes (Oleksiak et al. 2011; Whitehead et al. 2012). The PCB-resistance of this population is also reflected in much higher LC20 values for embryotoxicity of PCB-126 (42,845 ng/L) as compared to that of the PCB-sensitive SC population (24 ng/L) (Nacci et al. 2010). The heritability of this tolerance to PCBs has also been well documented in both F1 and F2 generations (Nacci et al. 2010; Nacci et al. 1999; Nacci et al. 2002; Bello 1999).
In this study, we hypothesized that genetic adaptation to PCBs and the resulting down-regulation of the Ahr pathway in NBH fish (Oleksiak et al. 2011; Whitehead et al. 2012) would also result in altered sensitivity to oxidant exposure. We exposed embryos from NBH killifish (i.e. F1 generation) and reference site embryos from Scorton Creek, MA (SC) to a model pro-oxidant and Nrf2-activator, tert-butylhydroquinone (tBHQ) (Nguyen et al. 2004). While the effects of tBHQ have not yet been examined in killifish, this compound has been shown to be an effective oxidant in other fish, including zebrafish (Kobayashi et al. 2002; Yang et al. 2007; Timme-Laragy et al. 2012b) and rainbow trout (Samson et al. 2001). Acute exposures of killifish embryos to tBHQ were conducted at specific developmental times corresponding to different stages of liver development, and the resulting deformities, survival, heart rate, and expression of several antioxidant genes were analyzed. Both killifish populations showed comparable responses in heart rate, but NBH F1 embryos were more sensitive to tBHQ teratogenesis compared to reference site F1 embryos. Acute exposure to tBHQ during development resulted in decreased expression of several antioxidant genes in the NBH population, which may provide a mechanistic explanation for the difference in sensitivity to oxidative stress.
Materials and Methods
Animals: collection, care, and breeding
Adult killifish were collected using minnow traps, as described earlier (Powell et al. 2000; Bello et al. 2001; Oleksiak et al. 2011), from the PCB-contaminated Superfund site, NBH, and reference site, SC, during the summers of 2010 and 2011. The SC site is considered clean as determined by its location away from urban sources of pollution and by the low PCB concentrations measured in sediments and fish. The sediment PCB concentration at SC is 1 ng PCB/g dry weight, whereas the sediment PCB concentration at NBH (near where our fish were collected) is 22,666 ng/g (Nacci et al. 2010). These differences in levels of contamination are reflected in adult fish captured from these sites: the PCB concentration in SC fish (0.177 µg/g dry weight) is much less than that of NBH fish (272 µg/g dry weight) (Bello 1999). Fish were determined to be sexually mature and healthy and approximately 2–4 years of age. Fish were maintained in flow-through glass aquaria with filtered seawater.
In this paper we refer to the fish obtained from NBH as “PCB-resistant.” Although the embryos used in this study were not examined for resistance to PCBs, embryos from this population have been shown repeatedly and over many years to have reduced sensitivity to non-ortho PCBs and related dioxin-like compounds, as demonstrated by resistance to embryotoxicity (Nacci et al. 2010; Nacci et al. 1999; Whitehead et al. 2012), lack of induction of Cyp1a by PCB-126, PCDD, TCDF, and PAHs (Bello 1999); Oleksiak et al. 2011; Arzuaga and Elskus 2010; Powell et al. 2000; Aluru et al. 2011; Nacci et al. 2010), and loss of induction of many other Ahr-regulated genes (Oleksiak et al. 2011; Whitehead et al. 2012). Although the NBH Superfund site has undergone some remediation, high levels of PCBs remain (Diane Nacci, US EPA, personal communication) and the killifish have maintained their resistance to PCBs, as assessed most recently in 2008 and 2009 (our unpublished results).
The day following collection of adults from NBH and SC, embryos were obtained by in vitro fertilization (IVF) (Trinkaus 1967). For IVF, 3–6 adult males were utilized and 20–50 adult females. The embryos were maintained in Petri dishes with 25 ppm filtered sterile seawater changed daily. The incubator was maintained at a 14-hour light and 10-hour dark cycle.
Chemical and dosing
To compare the sensitivity to oxidative stress in killifish embryos from a PCB-resistant population vs. embryos from a clean environment, we conducted exposures to a model pro-oxidant, tBHQ, which was obtained from Sigma (St. Louis, MO, U.S.A) and dissolved in DMSO (dimethyl sulfoxide, ACROS Organics, NJ). Prior to chemical exposure, killifish developmental stage was determined as described by Armstrong and Child (1965) and Bozinovic et al. (2011).
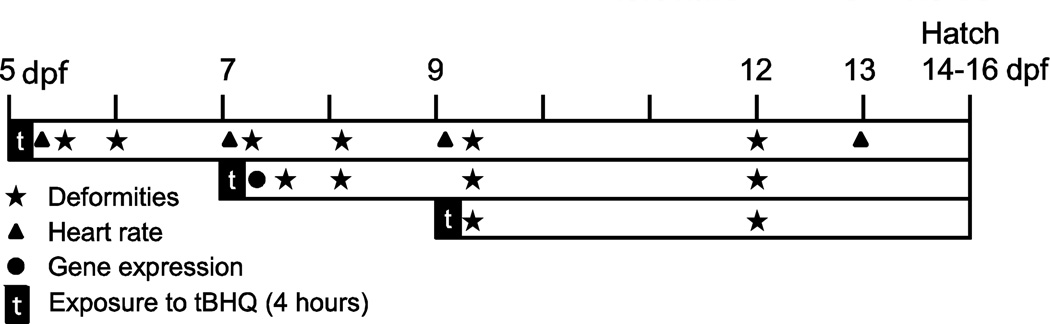
Exposures for deformity analysis and heart rate measurements were designed to compare both population and stage-specific sensitivity to tBHQ, and employed exposure protocols used previously for zebrafish (Timme-Laragy et al. 2012b). Embryos were exposed to tBHQ once for four hours at 5, 7, or 9 dpf (Fig. 2). These ages reflect various stages of liver development (Bozinovic et al. 2011; Armstrong and Child 1965) and correspond to developmental stages 28 (first appearance of the liver rudiment), 32 (functional liver), and 34 (liver growth), respectively. Five pools of ten embryos were exposed in 100 mm diameter glass Petri dishes to either 5, 10, or 20 µM tBHQ or solvent control (DMSO, 0.01% v/v) for four hours, then were washed three times in fresh 25 ppm filtered sterile seawater and placed in standard Costar 6 well dishes with 10 mL fresh 25 ppm filtered sterile seawater and held in an incubator at 23°C. Each experiment thus examined a total of 200 embryos from each population, with N = five pools of ten embryos per tBHQ concentration. Deformities and heart rate were monitored throughout development at the indicated time points until hatch (Fig. 2). These experiments occurred over two breeding cycles (Summer 2010 and 2011) with three experiments conducted during each cycle.
Figure 2. Timeline of chemical exposures and data collected from each time point dosed.
The acute four-hour duration of tBHQ exposure is indicated by the black square labeled “t” and the white portions of the line represent time spent in clean water. Days post fertilization (dpf) are provided as a reference when specific endpoints were assessed in each of the three exposure regimes.
Exposures for gene expression were conducted at 7 dpf, the timepoint when a functioning liver has developed, and that is within the window of sensitivity. Three pools of five embryos (N =3 pools) were exposed in glass scintillation vials to 10 mL sterile filtered 25 ppm seawater containing one of four treatments: water only (no treatment), 0.01% DMSO, 5 µM tBHQ, or 10 µM tBHQ. Following the acute four-hour exposure, embryos were immediately snap-frozen in liquid nitrogen and stored at −80°C until isolation of total RNA.
An additional set of experiments was conducted using another pro-oxidant, tert-butylhydroperoxide (tBOOH), which also has been used previously to generate oxidative stress in fish embryos (Timme-Laragy et al. 2009). Embryos were exposed for six hours at stage 32 (7 dpf) to 20 mM or 40 mM tBOOH or solvent control (water), followed immediately by sampling for gene expression analysis as described for tBHQ-treated embryos.
Toxicity assessment
Several developmental deformities were observed: pericardial edema (fluid retention in the membrane surrounding the heart), cardiac elongation (lengthening of the heart), decreased body girth (overall thickness of the body), decreased tail length (how long the tail extends from the body), decreased density of erythrocytes (number of red blood cells flowing through the bloodstream), decreased pigmentation (density of dark pigment on the eyes and body), and hemorrhage (discharge of blood from the vessels at any location throughout the body). Each deformity was scored qualitatively on a scale of 0 to 3 as normal (0), mild deformities (1), moderate deformities (2) and severe deformities (3). A deformity index was calculated as the sum of scores for individuals in that dose group divided by the maximum score possible, and multiplied by 100. This approach is in concordance with other deformity assessments in this species (Wassenberg and Di Giulio 2004; Whitehead et al. 2010).
Heart rate and hatching rate were measured as physiological endpoints. Heart rate was only examined in the set of embryos exposed at 5 dpf (Fig. 2). Heart rate was calculated by measuring the heart rate of 1 randomly selected embryo from each pool of embryos, counting the number of heartbeats for 15 seconds and multiplying this value by 4 to obtain the number of beats per minute. Heart rate readings were conducted immediately following the exposure at 5 dpf, and subsequently on 7, 9, and 13 dpf. Hatching was measured at 15 dpf, and percentages calculated based on the total number of embryos per tBHQ concentration (N = 50).
Gene expression
Total RNA was extracted using RNA STAT-60 (Tel-Test B, Inc., Friendswood, TX) according to the manufacturer’s directions. cDNA was synthesized from 1 µg RNA using iScript (Bio-Rad Laboratories, Hercules, CA, U.S.A). Quantitative PCR was performed using iQ SYBER Green Supermix (Bio-Rad Laboratories) in an iCycler iQ Real-Time PCR Detection System (Bio-Rad Laboratories). The PCR conditions were: 95°C, 3:30 min; 95°C, 15s and a primer-optimized Tm of 60–66°C, 30 s (40 cycles); 95°C, 1:00 min. Tm values for each primer set are provided in Supplemental Table 1, along with primer sequences and description of gene function. At the end of the PCR run, the products were subject to a melt curve analysis. Primer pairs were also validated to amplify only one product by visualizing QPCR product on an acrylamide gel, and primer efficiencies were measured by serial dilutions of the QPCR product. There were three or four biological replicates, with two technical replicates of each sample. Expression data were quantified based on threshold cycle values where values for each sample were averaged and normalized to two housekeeping genes: β-actin and ef1α. Because there was no statistical difference between data analyzed using the different housekeeping genes, only the β-actin-normalized data are presented here. Changes in RNA expression are reported as mean fold change and SEM, calculated according to the following equation, where the control is defined as untreated SC embryos:
Relative mRNA expression=2−ΔΔCt where ΔΔCt=[Ct(target gene)- Ct(actin)]variable-[Ct(target gene)- Ct(actin)]control
Statistical analysis
Statistical comparisons were analyzed using a two-factor analysis of variance (ANOVA) using Statview (SAS, Cary, NC). Gene expression data were log transformed prior to analysis, and Fisher’s PLSD was used as a post hoc test (p < 0.05). To account for the number of comparisons among treatments in the QPCR data, a Bonferroni correction was applied (p < 0.0018). Data are presented as mean and SEM.
Results
Deformity incidence and severity
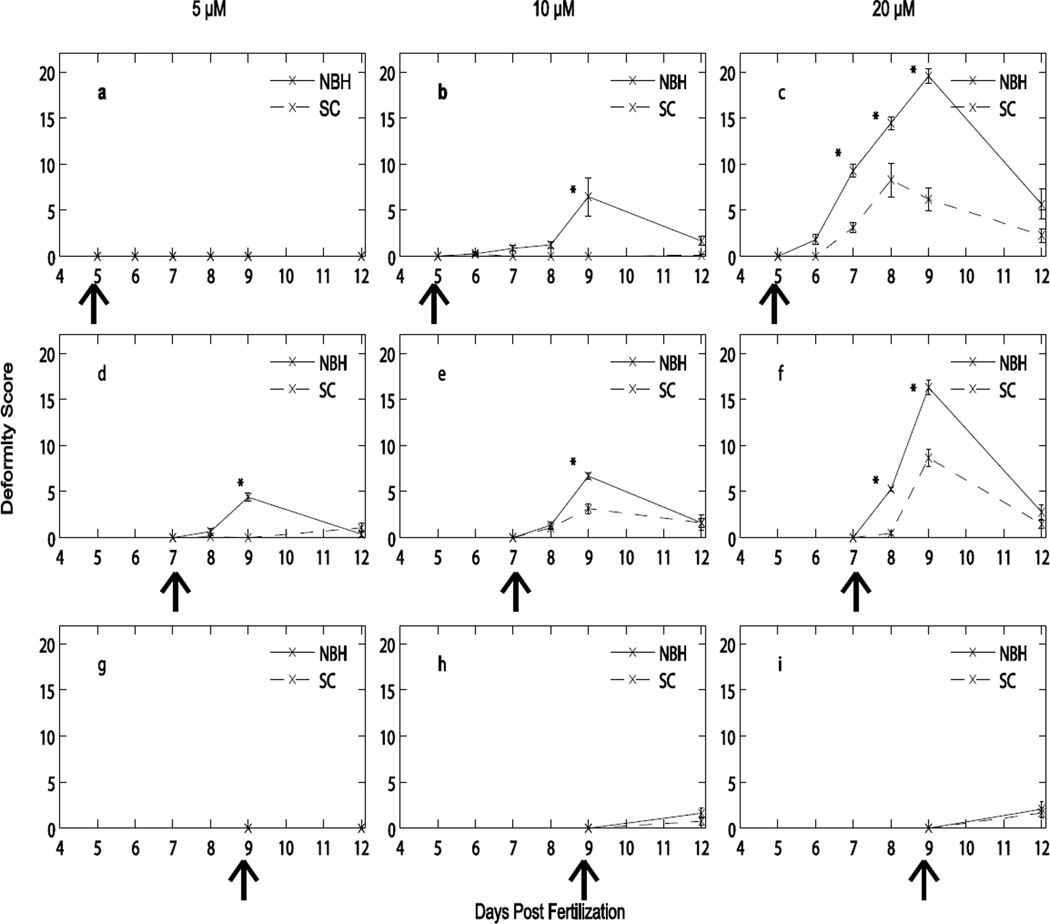
A population- and time-dependent response to tBHQ was evident with both populations (Fig. 3). Both PCB-resistant (NBH) and PCB-sensitive (SC) embryos exhibited deformities in response to an acute exposure to tBHQ, but embryos from NBH had higher rates of deformities and the deformities were more severe (Fig. 3). It is important to note that some individual embryos expressed more than one deformity on a specific day and over the course of observation.
Figure 3. Developmental deformities after acute exposure to tBHQ.
Mean ± SEM of 5 pools of 10 embryos exposed for four hours at 5 dpf, 7 dpf, or 9 dpf and monitored through 12 dpf. Each row presents data from embryos exposed at a specific stage (Stage 28, row 1, panels a, b, c; Stage 32, row 2, panels d, e, f; Stage 34, row 3, panels g, h, i). Each column of graphs examines exposures of 5 µM (a, d, g), 10 µM (b, e, h) and 20 µM (c, f, i) tBHQ. Control embryos did not develop deformities (data not shown). Arrows indicate when the embryos were dosed and data collection began. * denote significance within that exposure day between populations (Scorton Creek, SC, and New Bedford Harbor, NBH).
Embryos dosed at 5 dpf did not show any deformities as a result of the lowest tBHQ concentration, 5 µM (Fig. 3a). At 10 µM, only embryos from the NBH population developed deformities, the majority of which were observed at 9 dpf, or 4 days post treatment (Fig. 3b). At the highest concentration of tBHQ, 20 µM, embryos from NBH had a significantly higher incidence and more severe deformities than embryos from SC (Fig. 3c).
In contrast to the embryos exposed at 5 dpf to the lowest concentration of tBHQ, embryos from NBH exposed to this concentration at 7 dpf developed deformities, the majority of which were observed at 9 dpf (Fig. 3d). A similar result was obtained following exposures to 10 µM and 20 µM. Deformities peaked at 9 dpf (2 days post treatment). With these higher concentrations, both populations developed deformities, but deformity scores were significantly higher in the NBH embryos (Fig. 3e, f).
Regardless of whether embryos were dosed at 5 or 7 dpf, the most severe deformities occurred at 9 dpf (Fig. 3). However, if exposures began at 9 dpf, very few deformities occurred (Fig. 3g, h, i). More embryos at this time point suffered mortality compared to embryos exposed to the same tBHQ concentration at earlier stages, at which very few mortalities occurred (Table 1). The NBH embryos exhibited 2 deaths from the 5 dpf exposure and no mortality from the 7 dpf exposure. SC embryos exhibited 1 death each from the 5 and 7 dpf exposures. The 9 dpf exposure resulted in 7 NBH mortalities and 9 SC mortalities. We have thus identified a window of sensitivity to deformities from exposure to tBHQ that includes stages 28 and 32 and a window of sensitivity to mortality at stage 34.
Table 1.
Mortality and hatching success of killifish embryos from New Bedford Harbor (NBH) and Scorton Creek (SC) following an acute exposure to tBHQ at different days post fertilization (dpf). For each day, 200 embryos per population were exposed, with 50 embryos per tBHQ concentration. For each concentration, there were 5 pools of 10 embryos. Percentages are cumulative within a concentration
| NBH | ||||
|---|---|---|---|---|
| Exposure Day | tBHQ concentration (µM) |
% Mortality | Time of mortality (dpf) |
% Hatching @ 15 dpf |
| 5 dpf | DMSO | 0 | 60 | |
| 5 | 0 | 64 | ||
| 10 | 0.5 | 12 | 66 | |
| 20 | 0.5 | 7 | 66 | |
| 7 dpf | DMSO | 0 | 64 | |
| 5 | 0 | 56 | ||
| 10 | 0 | 74 | ||
| 20 | 0 | 70 | ||
| 9 dpf | DMSO | 0 | 36 | |
| 5 | 0 | 52 | ||
| 10 | 0 | 60 | ||
| 20 | 3.5 | 12 | 52 | |
| SC | ||||
| Exposure Day | tBHQ concentration (µM) |
% Mortality | Time of mortality (dpf) |
% Hatching @ 15 dpf |
| 5 dpf | DMSO | 0 | 66 | |
| 5 | 0 | 62 | ||
| 10 | 0 | 74 | ||
| 20 | 0.5 | 6 | 72 | |
| 7 dpf | DMSO | 0 | 60 | |
| 5 | 0 | 66 | ||
| 10 | 0 | 56 | ||
| 20 | 0.5 | 8 | 68 | |
| 9 dpf | DMSO | 0 | 36 | |
| 5 | 0 | 62 | ||
| 10 | 0 | 52 | ||
| 20 | 4.5 | 12 | 88 | |
Interestingly, the deformities we observed (pericardial edema, reduced pigmentation, reduced erythrocytes, truncation of the tail, and a small body size) were largely of a transient nature. Recovery from these deformities occurred by 12 dpf, a few days before hatching, for both populations. This occurred irrespective of whether embryos were dosed at 5 or 7 dpf (Fig. 3). The percentage of embryos that hatched by 15 dpf varied between 36–74% and did not demonstrate any significant treatment effect (Table 1). These hatching rates are within the normal expected range for killifish hatching at this timepoint (Dimichele and Taylor 1980; Timme-Laragy et al. 2006; Tingaud-Sequeira et al. 2009).
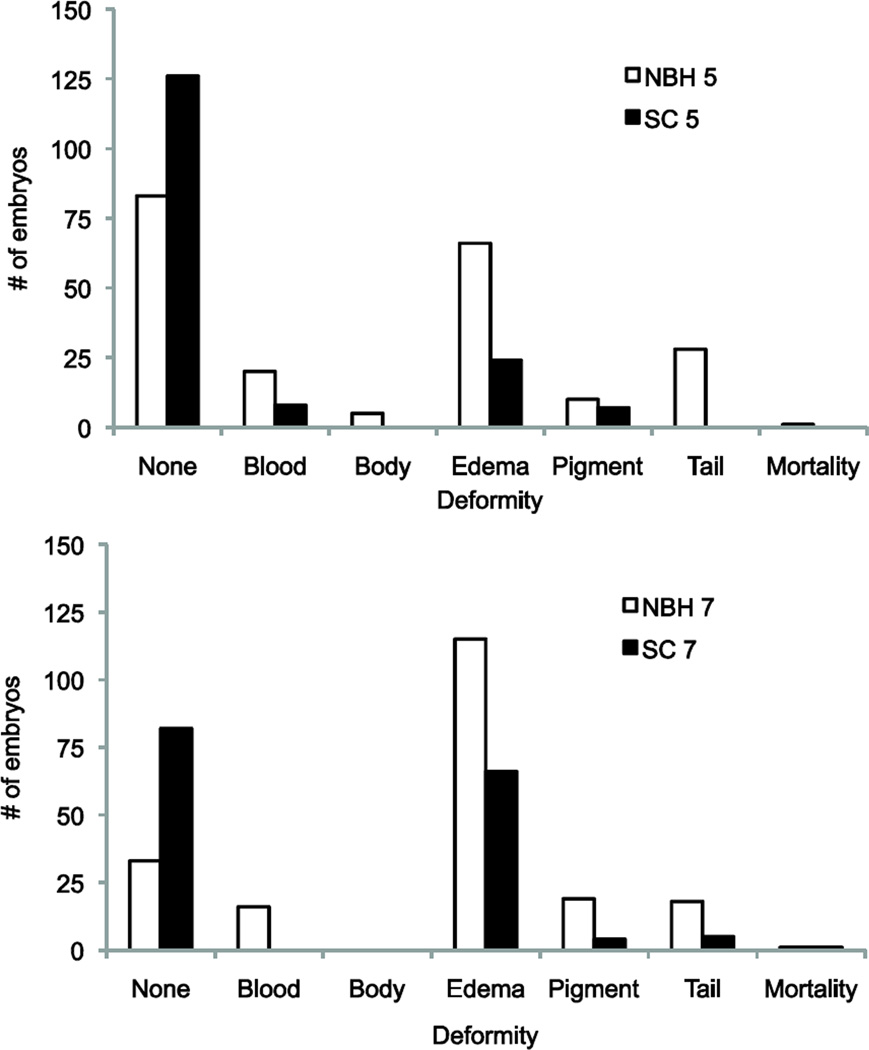
Closer examination of the range of deformities revealed another difference in response to tBHQ between the embryos from these two populations (Fig. 4). While both populations exposed at 5 dpf displayed pericardial edema, decreased pigmentation, and reduced blood cells, NBH embryos had a greater variety of deformities present, including truncation of the tail and small body size. The number of affected embryos displaying these deformities also differed, with greater numbers of embryos affected in the NBH population for all deformity types. A similar result was found in embryos exposed at 7 dpf, where both populations showed pericardial edema, decreased pigmentation, and shortened tail, but the NBH embryos also displayed reduced erythrocyte density. Again, incidence of these deformities was much higher in the NBH embryos (Fig. 4). Consistent with these differences, the number of embryos showing no deformities (normal development) was greater in SC as compared to NBH.
Figure 4. The number of embryos from a) New Bedford Harbor (NBH), and b) the reference site Scorton Creek (SC) exhibiting specific types of deformities at 9 dpf.
Embryos were exposed to tBHQ for 4 hours at 5 dpf or 7 dpf. These numbers are the cumulative incidence of each deformity type observed from exposure to 5 µM, 10 µM or 20 µM tBHQ (50 embryos per concentration), and contribute to the overall deformity score in Figure 3. Many individual embryos exhibited multiple deformities, while some embryos had no deformities. Abbreviations of deformities: no deformities (none), shortened tail (tail), pericardial edema (edema), reduced body girth (body), decreased number of red blood cells (blood), decreased pigmentation (pigment).
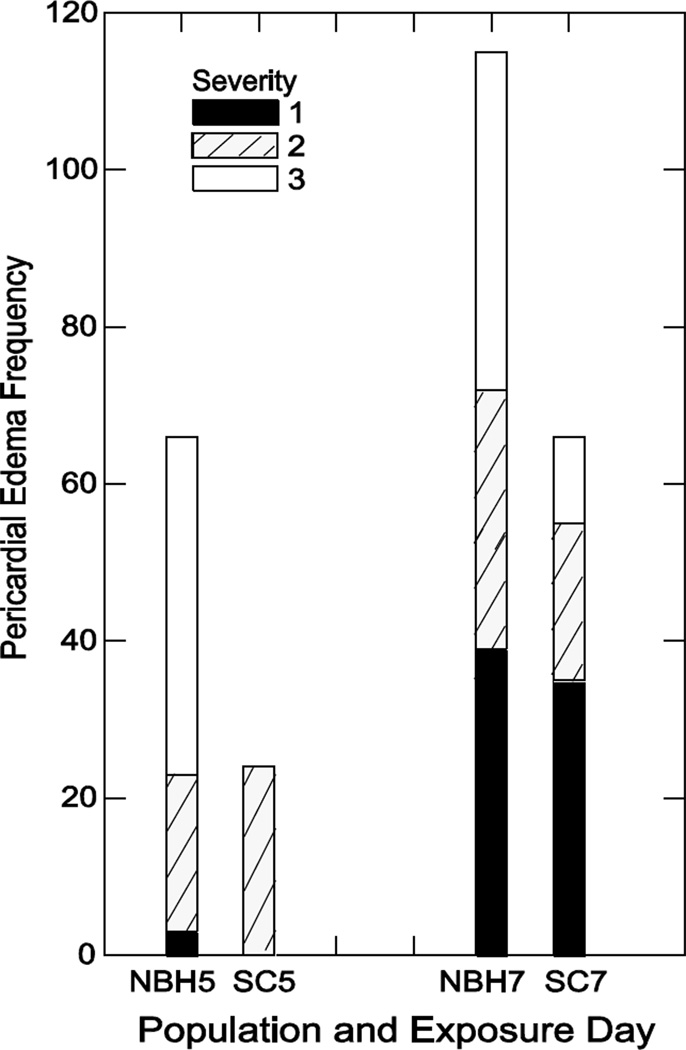
Pericardial edema was the most prevalent deformity in both populations. This deformity was given a relative score between 0 (normal) and 3 (severe). We conducted further analysis, and found that NBH embryos exposed at stages 28 and 32 (5 and 7 dpf) exhibited a greater incidence of--and more severe-- pericardial edema (measured at stage 34) as compared to SC embryos (Fig. 5).
Figure 5. Frequency of pericardial edema at 9 dpf.
Each bar is the total number of embryos exhibiting pericardial edema at 9 dpf, with each segment representing the number of fish exhibiting each severity score of 1, 2, or 3. Shown are embryos monitored after acute exposure to tBHQ on 5 or 7 dpf. These numbers combine deformities from exposure to 5 µM, 10 µM and 20 µM tBHQ and contribute to the overall frequency of pericardial edema in Figure 3.
Embryonic heart rate
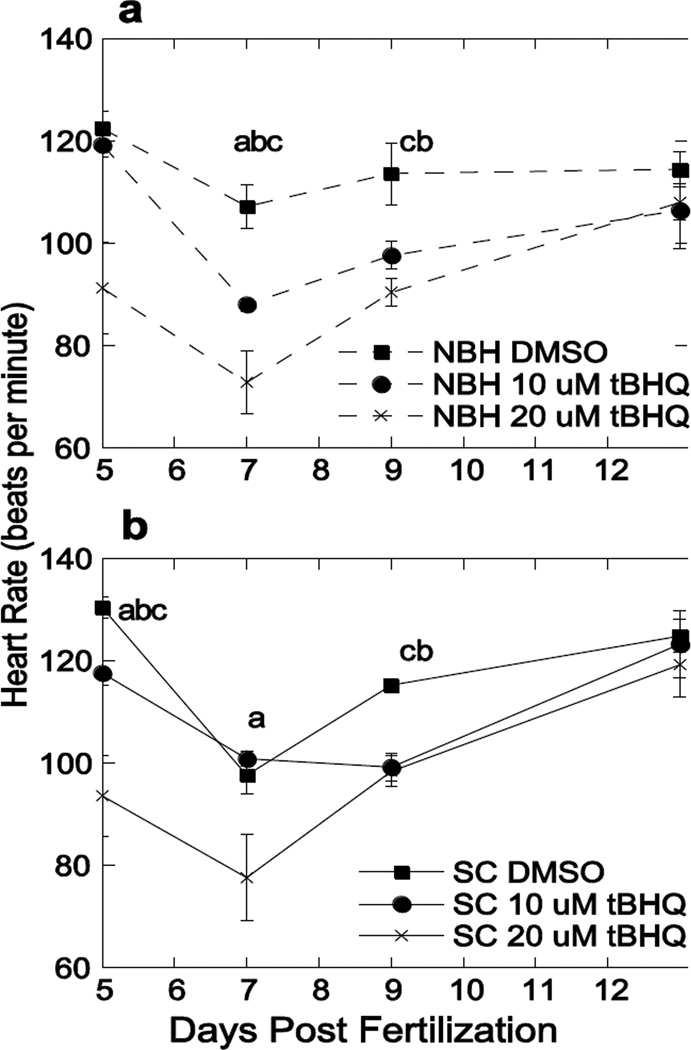
The frequent occurrence of heart deformities warranted the investigation of heart rate. A tBHQ-independent decrease in heart rate occurred between 5 and 7 dpf for both populations (Fig. 6). There was no change in heart rate with acute exposure to the lowest concentration of 5 µM tBHQ (data not shown), but heart rates decreased in a concentration-dependent manner in both killifish populations exposed to or 20 µM tBHQ. This decrease in heart rate persisted through 9 dpf, after which heart rates recovered to control levels (Fig. 6). The lowered heart rate preceded the appearance of pericardial edema and heart deformities, and the restoration of heart rate to control values mirrored the recovery from those deformities that occurred prior to hatching (Fig. 3). At 13 dpf, average heart rates of NBH embryos at all concentrations were 114.4, 106.4, and 108 beats/min, while those of SC embryos were 124.8, 123.2, and 119.2 beats/min. Interestingly, there was no statistically significant difference in the heart rates between these two embryo populations, neither basally nor in response to tBHQ. The resulting deformities showed a strong disparity in both incidence and severity (Figs. 3–5) whereas heart rate showed no population-based difference (Fig. 6).
Figure 6. Concentration-dependent response of embryonic heart rate (beats per minute) after acute exposure to DMSO, 10 µM or 20 µM tBHQ.
Mean ± SEM, where N = 5 representative embryos, each representing a pool of ten embryos exposed at 5 dpf and monitored through 13 dpf for a) New Bedford Harbor (NBH) and b) Scorton Creek (SC). Letters denote significance between concentrations at the indicated time point: (a) 10 µM and 20 µM tBHQ, (b) DMSO and 10 µM tBHQ, and (c) DMSO and 20 µM tBHQ based on a two-factor ANOVA.
Heart rates were measured only in embryos that had survived the initial exposure. Of the 50 SC embryos exposed to 20 µM tBHQ, six (12%) died between 7 and 9 dpf. NBH embryos had greater mortalities, with18 deaths (36%) from the 20 µM tBHQ exposure spread out from 6 to 13 dpf, with the highest mortality (11 embryos, 22%) at 7 dpf. These percentages vary somewhat from Table 1 because the heart rate data are from a different clutch of embryos examined during the same summer.
Gene expression
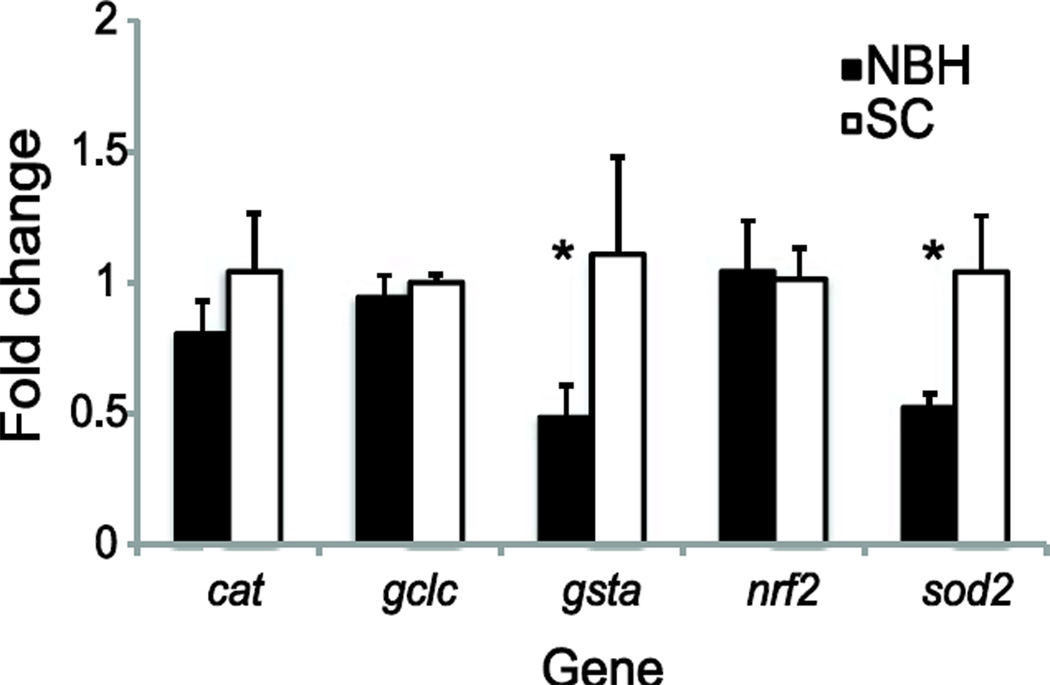
To examine whether expression or regulation of antioxidant genes played a role in the differential sensitivity to tBHQ, we measured expression of the transcription factor nrf2 and several antioxidant genes known to be regulated at least in part by nrf2 in response to oxidative stress: glutamate cysteine ligase catalytic subunit (gclc), glutathione-S-transferase-alpha (gsta) (Sharma et al. 2004), Mn-superoxide dismutase (sod2), and catalase (cat) (Lee and Johnson 2004).
Embryos from NBH had lower basal expression of gsta and sod2 than embryos from SC (Fig. 7). However, no significant differences were found in expression levels of any of the genes in DMSO-(vehicle control)-treated embryos from the two sites (Fig. 8). The response of the two populations to tBHQ treatment differed significantly for expression of catalase, gsta, and sod2. Expression of all three of these genes decreased significantly following acute tBHQ treatment in the NBH population but not in embryos from SC. We also observed a significant decrease in nrf2 expression in response to tBHQ in the NBH population, but these differences were not statistically different from expression levels in treatment-matched SC fish (Fig. 8).
Figure 7. Basal expression of nrf2 and genes known to be regulated by nrf2.
Data presented are the mean ± SEM (β-actin-corrected) where N = 3 pools of five unexposed control embryos at 7 dpf. Values are normalized to SC embryos. Data were analyzed using a two-factor ANOVA followed by Fisher’s PLSD; * indicates a significant difference in basal gene expression between New Bedford Harbor (NBH) and Scorton Creek (SC) embryos, p < 0.05.
Figure 8. Treatment and population effects on antioxidant gene expression in embryos from New Bedford Harbor (NBH) and Scorton Creek (SC).
The expression of catalase, gclc, gsta, nrf2, and sod2 were examined following acute exposure to DMSO (vehicle control), 5 µM or 10 µM tBHQ, or untreated water, at 7 dpf. Data presented are the mean ± SEM (β-actin-corrected) where N = 3 pools of five embryos. Values are normalized to those of untreated SC embryos. * denotes a significant effect of population in the two-factor ANOVA, and # indicates a significant treatment effect assessed by Fisher’s PLSD and Bonferroni-corrected, p < 0.0018
Evidence of oxidative stress is commonly provided by a transcriptional response of antioxidant genes, usually an increase in expression. The decrease in expression of antioxidant genes following prooxidant exposure observed in this study was confirmed using another pro-oxidant, tBOOH. No deformities or mortalities were observed at these concentrations (20 mM and 40 mM). For all genes measured, a trend towards down-regulation of antioxidant gene expression occurred in response to tBOOH (Supplemental Fig. 1).
Discussion
This study demonstrates differential sensitivity to a pro-oxidant Nrf2-activator, tBHQ, in embryos from two populations of killifish (Fundulus heteroclitus) with different pollutant-exposure histories. We show an increase in deformities following acute exposure to tBHQ that are more severe and more frequent in F1 embryos from a population with altered Ahr signaling adapted to tolerate PCBs (NBH Superfund Site). Interestingly, many of the embryos were able to recover from these deformities and go on to hatch. We also found significant population differences in expression of several antioxidant genes, both basally and in response to tBHQ, which may provide some insight into the mechanism(s) underlying the differential sensitivity.
Oxidative stress, defined as a disruption of intracellular redox balance and signaling (Jones 2006), is a common challenge faced by aquatic organisms, particularly those in the estuarine environment. Oxidative stress can result from changes in temperature, salinity, hyperoxia in over-saturated waters, hypoxia, reperfusion following hypoxic or anoxic events, and exposure to aquatic ozone (Lesser 2006); Lushchak 2011). Oxidative stress may also result from UV-light or from pollutant exposure (Meyer and Di Giulio 2003; Bacanskas et al. 2004; Arzuaga and Elskus 2010; Lushchak 2011; Billiard et al. 2008). Here, we used a chemical previously characterized as a pro-oxidant, tBHQ, to initiate oxidative stress during specific stages of embryonic development in order to investigate the response to oxidative stress between PCB-adapted and control populations of killifish.
tBHQ is a prototypical mono-functional inducer of the oxidative stress response mediated by Nrf2 (Nguyen et al. 2003; Gharavi et al. 2007; Kensler et al. 2007). It undergoes autoxidation to the quinone (Kahl et al. 1989), which then undergoes redox cycling through one-electron reduction to the semiquinone radical, followed by reaction with O2 to generate superoxide. Electrophilic metabolites may also play a role in its activation of Nrf2 (van Ommen et al. 1992; Nakamura et al. 2003).
For early life-stages, maintenance of redox balance is critical because of its role in the control of cell division and differentiation, key events during embryonic development. Early embryos have not yet developed a robust antioxidant defense system compared with adults; because of this, they are also extremely susceptible to developmental disruptions and abnormalities following oxidative stress (Wells et al. 2005).
The ability of pollutant-adapted killifish to respond to oxidative stress has been investigated both in the NBH population as well as in several others; the results of these studies demonstrate a strong dependence on age during exposure and on the chemical mode of action, particularly in relation to the aryl hydrocarbon receptor (Ahr) (Table 2). Arzuaga and Elskus (2010) reported that PCB-resistant embryos from Newark Bay and NBH exhibited reduced ROS production following exposure to PCB-126, likely due to the lack of Ahr activation and thus a lack of Cyp1a induction, the presumed source of ROS.Meyer et al. (2003) found that larvae (F1 and F2 generations) from the PAH-contaminated Elizabeth River (ER; Virginia, U.S.A.) had higher basal levels of some antioxidant defenses and were less sensitive to oxidative stress from model pro-oxidant tBOOH, but this study did not include embryonic stages. However, the ER larvae demonstrated an increase in sensitivity to phototoxicity when exposed to the PAH fluoranthene and ambient UV (Meyer and Di Giulio 2003). Wild-caught adults from the ER population had higher total glutathione and increased levels of lipid peroxidation and glutathione peroxidase activity in the liver, although some of these parameters changed with seasonal breeding status (Bacanskas et al. 2004).
Table 2.
Literature survey of oxidative stress studies in pollutant-resistant killifish (Meyer and Di Giulio 2003; Meyer et al. 2003; Bacanskas et al. 2004; Arzuaga and Elskus 2010)
| Toxicant | Mode of action | Endpoint | Life stage | Resistant population study site |
Sensitivity compared to reference fish |
Ref |
|---|---|---|---|---|---|---|
| PCB-126 | Ahr-dependent, ROS generated by CYP1 |
Superoxide detected by in vivo dihydroethidium staining (7 dpf) and heart deformities (10 dpf) |
Embryo (exposed 2–5 dpf) |
New Bedford Harbor (MA), Newark Bay (NJ) |
Less sensitive (Beaufort, NC) |
Arzuaga and Elskus, 2010 |
| 3-MC | Ahr-dependent | Superoxide detected by in vivo dihydroethidium staining (5 dpf) |
||||
| tBOOH | Oxidative stress | Survival time, TOSC, total glutathione |
Larvae F1 and F2generations 1 week post hatch and 5 months post hatch |
Elizabeth River (VA) |
Less sensitive (Kings Creek, VA), higher TOSC and glutathione |
Meyer et al. 2003 |
| PAH- contaminated sediment extract |
Ahr-dependent | Total glutathione, GCL activity, GR activity, GPx activity, lipid peroxidation |
Adult ,F1 and F2livers |
Elizabeth River (VA) |
Higher levels of glutathione, GPx, and LPO |
Bacanskas et al. 2004 |
| Fluoranthene, hypoxia, UV |
phototoxicity | Survival | Larvae, F1 and F2 |
Elizabeth River (VA) |
More sensitive (Kings Creek) |
Meyer and Di Giulio 2003 |
| tBHQ | Nrf2 activation, quinone redox cycling, electrophilic metabolite |
Deformities, gene expression |
Embryos (5, 7, 9 dpf) |
New Bedford Harbor (MA) |
More sensitive (Scorton Creek) to deformities and gene expression of antioxidant genes (gsta, sod2, cat) |
This study |
| tBOOH | Lipid peroxidation |
Gene expression | Embryos 7 dpf |
New Bedford Harbor (MA) |
No difference | This study |
The results of the present study demonstrate increased sensitivity of killifish embryos from the contaminated NBH Superfund site to chemical-induced oxidative stress as indicated by deformities, physiological responses, and gene expression. This is in direct contrast to what has been found in this population in response to PCBs and other Ahr agonists, where embryos from the contaminated site exhibit resistance compared with reference populations (Nacci et al. 1999; Bello et al. 2001; Arzuaga and Elskus 2010; Oleksiak et al. 2011; Nacci et al. 2010; Whitehead et al. 2012). The difference in response between the NBH and reference populations supports the idea that the adaptation to contaminants such as TCDD and PCB has reduced the ability of contaminated site embryos to effectively respond to the additional stressor of pro-oxidant exposure, but the mechanism by which this occurs remains unknown.
Although we interpret the population difference in sensitivity to chemical-generated oxidative stress as reflecting the genetic adaptation to PCBs that has occurred in the NBH fish, an alternative hypothesis is that the maternal PCB exposure or other factors experienced by the F1 embryos from NBH may have contributed to the results of this study. At present, it is not possible to distinguish between these two explanations; additional studies using F2 embryos (eliminating differences in PCB exposure while retaining the genetic adaptation) or co-exposure to PCBs and tBHQ will be needed.
Strikingly, many of the killifish embryos, from both populations, were able to recover from these deformities and go on to hatch successfully. Recovery from deformities occurred after a delay of a few days after cessation of exposure. This ability to recover from morphological abnormalities has been previously observed in fish. For example, Chinese sturgeon (Acipenser sinesis) embryos develop spinal curvature, or lordosis, after lead exposure (Hou et al. 2011). These embryos were able to regain normal morphology over time with recovery accompanying elimination of lead from the body after transfer to lead-free water. The killifish embryos for this study were also maintained in clean water after the exposure period and were able to recover. This observation of recovery from chemical-induced deformities warrants further investigation into the underlying mechanisms, and whether it may be related to the maturation of antioxidant defenses as embryos develop. It will also be important to determine whether there are any later-life consequences for these fish, such as functional deficits experienced as adults. Such deficits, is they were to occur, could have population-level consequences.
Gene expression was examined in this study as a biomarker of oxidative stress as well as to gain insight into the mechanism of the differential sensitivity to tBHQ between NBH and reference site killifish. One of the key regulators of the antioxidant defense system and response to oxidative stress is the transcription factor Nrf2. Nrf2 has been shown to participate in crosstalk with the Ahr pathway through several mechanisms (Ma et al. 2004; Miao et al. 2005; Shin et al. 2007; Yeager et al. 2009; Kalthoff et al. 2010; Wakabayashi et al. 2010), making it of particular interest given the resistance to Ahr-mediated teratogenesis in this population. This is the first investigation of Nrf2 expression in the Atlantic killifish. We demonstrate that it is expressed during embryonic development and at similar levels in both populations (Fig. 7), but the ability of Nrf2 to respond to an activator such as tBHQ (Nguyen et al. 2004) and its role in gene regulation requires further study. We found significant decreases in expression of nrf2 in response to tBHQ treatment in the NBH population, but the mechanism by which this occurs remains unclear.
We found a significant difference in how antioxidant gene expression in the two populations responded to tBHQ exposure, with NBH embryos showing significant changes in gene expression of gsta, sod2, and catalase. We expected to see an increase in expression levels of antioxidant genes following acute exposure to tBHQ; instead, we found a reduction in gene expression in the NBH population. We confirmed this directional response with a second model pro-oxidant, tBOOH, which causes oxidative stress via a different mechanism than tBHQ. With tBOOH, both populations showed a trend towards decreased antioxidant gene expression (Supplemental Fig. 1). This directional response is perplexing, but not unprecedented.Wu et al. (2011) also showed a trend towards a decrease in expression of sod3, glutathione reductase, and catalase in medaka embryos treated with ethanol.Pierron et al. (2007) showed decreases in expressions of sod2 and catalase in response to cadmium or hypoxia in the gills of glass eels (Anguilla anguilla).
While both populations of fish embryos exposed to tBHQ and tBOOH exhibited down-regulation of antioxidant genes, only responses to tBHQ differed between the two populations. We hypothesize that this may be due to the altered Ahr-pathway signaling associated with the genetic adaption in the NBH population. This hypothesis is supported in the literature. For instance, in AHR-knockout mice, hematopoietic cell progenitor populations have been shown to be more sensitive to hydrogen peroxide, and liver tissue has reduced expression levels of the antioxidant genes sod1, sod2, and trx compared to wild type mice (Hirabayashi and Inoue 2010). Future experiments will directly examine the crosstalk between Ahr and Nrf2 in the killifish using morpholino knock down of Ahr to recapitulate the unresponsive Ahr signaling pathway found in the NBH population, followed by exposures to prooxidant chemicals.
Our current understanding of oxidative stress and antioxidant defenses during embryonic development is still in its infancy. Ontological fluctuations of antioxidant defenses and redox conditions may be part of the normal developmental program (Arzuaga and Elskus 2010; Timme-Laragy et al. 2012a; Timme-Laragy et al. 2012b). There are also important species-specific differences in response to oxidative stress that are beginning to emerge. For instance, zebrafish (Danio rerio) that are exposed to tBHQ do not develop the suite of malformations seen in tBHQ-exposed killifish; the only morphologic response is a temporary reduction in pigmentation (Timme-Laragy & Hahn, unpublished). This difference in deformity response is perhaps due to normal developmental timing, with the slower developmental program of the killifish allowing a greater opportunity for malformations to occur. Also, expression of antioxidant genes in the zebrafish is increased with exposure to tBHQ and other pro-oxidants (Timme-Laragy et al. 2012b; Timme-Laragy et al. 2009; Mukaigasa et al. 2012; Kobayashi et al. 2002; Yang et al. 2007), while tBHQ and tBOOH exposures in the killifish embryo result in a reduced expression of these same genes. The mechanisms responsible for this difference are currently unknown, but some studies have suggested that populations whose natural ecology involves exposure to large changes in oxygen concentration may be better equipped to combat oxidative stress (Lesser 2006). In embryos that normally inhabit an environment that experiences fluctuating oxygen levels, like the estuarine environment inhabited by the killifish, expression of antioxidant genes is perhaps already at its physiological maximum.
The concentrations of tBHQ used in this study are very likely to have caused oxidative stress, based on results of studies using similar concentrations in zebrafish (Timme-Laragy et al. 2012b), and as evidenced in the present study by the reduction in heart rates and development of deformities. We also found changes in antioxidant gene expression, but not in the direction expected. In future studies, it will be helpful to include additional biochemical assessments of oxidative stress as well as direct measurement of ROS using fluorescent dyes.
Our data support the conclusion that changes in gene expression could contribute to the differential sensitivity to tBHQ. However, sensitivity to oxidative stress can also be regulated by post-transcriptional mechanisms such as those involving microRNAs, and post-translational mechanisms such as changes of enzyme activity. While these were not investigated here, they would be interesting to examine in future research. The use of DMSO could be another confounding factor, as it has some properties as a hydroxyl radical scavenger. However, the concentration of DMSO used in these experiments (0.01% v/v) was lower than the concentration at which the radical-scavenging activity is significant (Kahler 2000). DMSO has been widely used previously in studies of oxidative stress in zebrafish embryos without adverse effects on antioxidant gene expression (Kobayashi et al. 2002; Timme-Laragy et al. 2009) but at least one study reported changes in gene expression with low levels of DMSO (Turner et al. 2012).
Assessing the effect of multiple stressors on fish inhabiting contaminated sites is a valuable way to determine the costs associated with adapting to a polluted environment. Many studies have been conducted on the evolved resistance of Superfund site resident fish and their progeny to the contaminants to which they are exposed. In addition to studies of oxidative stress, factors such as hypoxia, UV light (Table 2), and pesticides (Clark and Di Giulio 2012) have also been investigated in various resistant killifish populations. The current study contributes to the understanding of how fish adapted to one stressor may exhibit altered responses to other stressors, and demonstrates the complexity of responses to multiple stressors.
In conclusion, we observed an increase in sensitivity of contaminated site NBH embryos to pro-oxidant exposure at the physiological and molecular levels. Future studies examining antioxidant defenses in New Bedford Harbor killifish will better elucidate the mechanism for enhanced sensitivity to pro-oxidant exposure.
Supplementary Material
Acknowledgements
We thank Ms. Diana Franks, Drs. Sibel Karchner, Larissa Williams, Neel Aluru, and Jared Goldstone for assistance and helpful discussions. This research was funded by a WHOI Summer Student Fellowship to R. Harbeitner supported by WHOI Academic Programs Office funds and National Science Foundation grant OCE 0649139, by National Institutes of Health grants P42ES007381 (Superfund Basic Research Program at Boston University; Hahn), F32ES017585 (Timme-Laragy), and R01ES016366 (Hahn), and by Walter A. and Hope Noyes Smith.
Abbreviations
- Ahr
aryl hydrocarbon receptor
- PCBs
polychlorinated biphenyls
- TCDD
2, 3, 7, 8-tetrachlorodibenzo-p-dioxin
- NBH
New Bedford Harbor
- SC
Scorton Creek
- tBHQ
tertbutylhydroquinone
- HAHs
halogenated aromatic hydrocarbons
- PAHs
polycyclic aromatic hydrocarbons
- PCB-126
3,3’,4,4’,5-pentachlorobiphenyl
- Arnt
aryl hydrocarbon receptor nuclear translocator
- XRE
xenobiotic response element
- Cyp1a
cytochrome P4501a
- Nrf2
nuclear factor erythroid-related factor-2
- ROS
Reactive oxygen species
- Keap1
Kelch-like ECH-associated protein 1
- ARE
antioxidant response element
- tBOOH
tert-butylhydroperoxide
- IVF
in vitro fertilization
- ER
Elizabeth River
Footnotes
The authors declare that they have no conflict of interest.
References
- Aluru N, Karchner SI, Hahn ME. Role of DNA methylation of AHR1 and AHR2 promoters in differential sensitivity to PCBs in Atlantic Killifish, Fundulus heteroclitus. Aquat Toxicol. 2011;101(1):288–294. doi: 10.1016/j.aquatox.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong PB, Child JS. Stages in the normal development of Fundulus heteroclitus. Biol Bull. 1965;128(2):143–168. [Google Scholar]
- Arzuaga X, Elskus A. Polluted-site killifish (Fundulus heteroclitus) embryos are resistant to organic pollutant-mediated induction of CYP1A activity, reactive oxygen species, and heart deformities. Environ Toxicol Chem. 2010;29(3):676–682. doi: 10.1002/etc.68. [DOI] [PubMed] [Google Scholar]
- Bacanskas LR, Whitaker J, Di Giulio RT. Oxidative stress in two populations of killifish (Fundulus heteroclitus) with differing contaminant exposure histories. Mar Environ Res. 2004;58(2–5):597–601. doi: 10.1016/j.marenvres.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Bello SM. Ph.D Thesis. >Woods Hole Oceanographic Institution / Massachusetts Institute of Technology Joint Program in Oceanography; 1999. Characterization of resistance to halogenated aromatic hydrocarbons in a population of Fundulus heteroclitusfrom a marine superfund site. [Google Scholar]
- Bello SM, Franks DG, Stegeman JJ, Hahn ME. Acquired resistance to Ah receptor agonists in a population of Atlantic killifish (Fundulus heteroclitus) inhabiting a marine superfund site: in vivo and in vitro studies on the inducibility of xenobiotic metabolizing enzymes. Toxicol Sci. 2001;60(1):77–91. doi: 10.1093/toxsci/60.1.77. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Meyer JN, Wassenberg DM, Hodson PV, Di Giulio RT. Nonadditive effects of PAHs on Early Vertebrate Development: mechanisms and implications for risk assessment. Toxicol Sci. 2008;105(1):5–23. doi: 10.1093/toxsci/kfm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol Sci. 2006;92(2):526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- Bozinovic G, Sit TL, Hinton DE, Oleksiak MF. Gene expression throughout a vertebrate's embryogenesis. BMC Genomics. 2011;12:132. doi: 10.1186/1471-2164-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BW, Di Giulio RT. Fundulus heteroclitusadapted to PAHs are cross-resistant to multiple insecticides. Ecotoxicology. 2012;21(2):465–474. doi: 10.1007/s10646-011-0807-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BW, Matson CW, Jung D, Di Giulio RT. AHR2 mediates cardiac teratogenesis of polycyclic aromatic hydrocarbons and PCB-126 in Atlantic killifish (Fundulus heteroclitus) Aquat Toxicol. 2010;99(2):232–240. doi: 10.1016/j.aquatox.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews ST. Control of cell lineage-specific development and transcription by bHLH-PAS proteins. Genes Dev. 1998;12(5):607–620. doi: 10.1101/gad.12.5.607. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124(1):1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimichele L, Taylor MH. The environmental control of hatching in Fundulus heteroclitus. J of Exp Zool. 1980;214(2):181–187. [Google Scholar]
- Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140(1):173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- Gharavi N, Haggarty S, El-Kadi AO. Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Curr Drug Metab. 2007;8(1):1–7. doi: 10.2174/138920007779315035. [DOI] [PubMed] [Google Scholar]
- Gu Y-Z, Hogenesch JB, Bradfield CA. The PAS Superfamily: Sensors of Environmental and Developmental Signals. Annu Rev Pharmacol Toxicol. 2000;40(1):519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y, Inoue T. Benzene-induced bone-marrow toxicity: a hematopoietic stem-cell-specific, aryl hydrocarbon receptor-mediated adverse effect. Chem Biol Interact. 2010;184(1–2):252–258. doi: 10.1016/j.cbi.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Hou JL, Zhuang P, Zhang LZ, Feng L, Zhang T, Liu JY, Feng GP. Morphological deformities and recovery, accumulation and elimination of lead in body tissues of Chinese sturgeon, Acipenser sinensis early life stages: a laboratory study. J Appl Ichthyol. 2011;27(2):514–519. [Google Scholar]
- Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8(9– 10):1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Jonsson ME, Jenny MJ, Woodin BR, Hahn ME, Stegeman JJ. Role of AHR2 in the expression of novel cytochrome P450 1 family genes, cell cycle genes, and morphological defects in developing zebra fish exposed to 3,3',4,4',5-pentachlorobiphenyl or 2,3,7,8-tetrachlorodibenzo-pdioxin. Toxicol Sci. 2007;100(1):180–193. doi: 10.1093/toxsci/kfm207. [DOI] [PubMed] [Google Scholar]
- Kahl R, Weinke S, Kappus H. Production of reactive oxygen species due to metabolic activation of butylated hydroxyanisole. Toxicology. 1989;59(2):179–194. doi: 10.1016/0300-483x(89)90056-5. [DOI] [PubMed] [Google Scholar]
- Kahler CP. Evaluation of the use of the solvent dimethyl sulfoxide in chemiluminescent studies. Blood Cells Mol Dis. 2000;26(6):626–633. doi: 10.1006/bcmd.2000.0340. [DOI] [PubMed] [Google Scholar]
- Kalthoff S, Ehmer U, Freiberg N, Manns MP, Strassburg CP. Interaction between oxidative stress sensor Nrf2 and xenobiotic-activated aryl hydrocarbon receptor in the regulation of the human phase II detoxifying UDP-glucuronosyltransferase 1A10. J Biol Chem. 2010;285(9):5993–6002. doi: 10.1074/jbc.M109.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kinnison MT, Hairston NG. Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Functional Ecol. 2007;21(3):444–454. [Google Scholar]
- Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26(1):221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Itoh K, Suzuki T, Osanai H, Nishikawa K, Katoh Y, Takagi Y, Yamamoto M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;7(8):807–820. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- Kohle C, Bock KW. Coordinate regulation of Phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochem Pharmacol. 2007;73(12):1853–1862. doi: 10.1016/j.bcp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Lee JM, Johnson JA. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol. 2004;37(2):139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- Lesser MP. Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol. 2006;68:253–278. doi: 10.1146/annurev.physiol.68.040104.110001. [DOI] [PubMed] [Google Scholar]
- Loro VL, Jorge MB, Silva KR, Wood CM. Oxidative stress parameters and antioxidant response to sublethal waterborne zinc in a euryhaline teleost Fundulus heteroclitus : protective effects of salinity. Aquat Toxicol. 2012;110– 111:187–193. doi: 10.1016/j.aquatox.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Lushchak VI. Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol. 2011;101(1):13–30. doi: 10.1016/j.aquatox.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Ma Q, Kinneer K, Yongyi B, Chan J, Kan Y. Induction of murine NAD(P)H:quinone oxidoreductase by 2,3,7,8-tetrachlorodibenzo-p-dioxin requires the CNC (cap 'n' collar) basic leucine zipper transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2): cross-interaction between AhR (aryl hydrocarbon receptor) and Nrf2 signal transduction. Biochem J. 2004;377(1):205–213. doi: 10.1042/BJ20031123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CW, Clark BW, Jenny MJ, Fleming CR, Hahn ME, Di Giulio RT. Development of the morpholino gene knockdown technique in Fundulus heteroclitus : a tool for studying molecular mechanisms in an established environmental model. Aquat Toxicol. 2008;87(4):289–295. doi: 10.1016/j.aquatox.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Di Giulio RT. Heritable Adaptation and Fitness Costs in Killifish (Fundulus Heteroclitus) Inhabiting a Polluted Estuary. Ecol Appl. 2003;13(2):490–503. [Google Scholar]
- Meyer JN, Nacci DE, Di Giulio RT. Cytochrome P4501A (CYP1A) in Killifish (Fundulus heteroclitus ): Heritability of Altered Expression and Relationship to Survival in Contaminated Sediments. Toxicol Sci. 2002;68(1):69–81. doi: 10.1093/toxsci/68.1.69. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Smith JD, Winston GW, Di Giulio RT. Antioxidant defenses in killifish (Fundulus heteroclitus) exposed to contaminated sediments and model prooxidants: short-term and heritable responses. Aquat Toxicol. 2003;65(4):377–395. doi: 10.1016/j.aquatox.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Miao W, Hu L, Scrivens PJ, Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J Biol Chem. 2005;280(21):20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- Mimura J, Yamashita K, Nakamura K, Morita M, Takagi T, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M, Fujii-Kuriyama Y. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzop- dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2(10):645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- Mukaigasa K, Nguyen LT, Li L, Nakajima H, Yamamoto M, Kobayashi M. Genetic Evidence of an Evolutionarily Conserved Role for Nrf2 in the Protection against Oxidative Stress. Mol Cell Biol. 2012;32(21):4455–4461. doi: 10.1128/MCB.00481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacci D, Champlin D, Jayaraman S. Adaptation of the Estuarine Fish Fundulus heteroclitus (Atlantic Killifish) to Polychlorinated Biphenyls (PCBs) Estuar Coast. 2010;33(4):853–864. [Google Scholar]
- Nacci D, Coiro L, Champlin D, Jayaraman S, McKinney R, Gleason TR, Munns WR, Jr, Specker JL, Cooper KR. Adaptations of wild populations of the estuarine fish Fundulus heteroclitus to persistent environmental contaminants. Mar Biol. 1999;134(1):9–17. [Google Scholar]
- Nacci DE, Champlin D, Coiro L, McKinney R, Jayaraman S. Predicting the occurrence of genetic adaptation to dioxinlike compounds in populations of the estuarine fish Fundulus heteroclitus. Environ Toxicol Chem. 2002;21(7):1525–1532. [PubMed] [Google Scholar]
- Nakamura Y, Kumagai T, Yoshida C, Naito Y, Miyamoto M, Ohigashi H, Osawa T, Uchida K. Pivotal role of electrophilicity in glutathione S-transferase induction by tert-butylhydroquinone. Biochemistry. 2003;42(14):4300–4309. doi: 10.1021/bi0340090. [DOI] [PubMed] [Google Scholar]
- Nelson W, Bergen B, SJ B, Morrison G, Voyer R, Strobel C, Rego S, Thursby G, Pesch C. National Health and Environmental Effects Research Laboratory, Atlantic Ecology Division; Narragansett, RI: 1996. New Bedford Harbor long-term monitoring assessment report: baseline sampling US Environmental Protection Agency. [Google Scholar]
- Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic Biol Med. 2004;37(4):433–441. doi: 10.1016/j.freeradbiomed.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Okey AB. An aryl hydrocarbon receptor odyssey to the shores of toxicology: the Deichmann Lecture, International Congress of Toxicology-XI. Toxicol Sci. 2007;98(1):5–38. doi: 10.1093/toxsci/kfm096. [DOI] [PubMed] [Google Scholar]
- Oleksiak MF, Karchner SI, Jenny MJ, Franks DG, Welch DB, Hahn ME. Transcriptomic assessment of resistance to effects of an aryl hydrocarbon receptor (AHR) agonist in embryos of Atlantic killifish (Fundulus heteroclitus) from a marine Superfund site. BMC Genomics. 2011;12:263. doi: 10.1186/1471-2164-12-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby DR, Newman MC, Mulvey M, Vogelbein WK, Unger MA, Arzayus LF. Fish (Fundulus heteroclitus) populations with different exposure histories differ in tolerance of creosote-contaminated sediments. Environ Toxicol Chem. 2002;21(9):1897–1902. [PubMed] [Google Scholar]
- Peters JM, Narotsky MG, Elizondo G, Fernandez-Salguero PM, Gonzalez FJ, Abbott BD. Amelioration of TCDD-induced teratogenesis in aryl hydrocarbon receptor (AhR)-null mice. Toxicol Sci. 1999;47(1):86–92. doi: 10.1093/toxsci/47.1.86. [DOI] [PubMed] [Google Scholar]
- Pierron F, Baudrimont M, Gonzalez P, Bourdineaud JP, Elie P, Massabuau JC. Common pattern of gene expression in response to hypoxia or cadmium in the gills of the European glass eel (Anguilla anguilla ) Environ Sci Technol. 2007;41(8):3005–3011. doi: 10.1021/es062415b. [DOI] [PubMed] [Google Scholar]
- Powell WH, Bright R, Bello SM, Hahn ME. Developmental and tissue-specific expression of AHR1, AHR2, and ARNT2 in dioxin-sensitive and -resistant populations of the marine fish Fundulus heteroclitus. Toxicol Sci. 2000;57(2):229–239. doi: 10.1093/toxsci/57.2.229. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl Hydrocarbon Receptor 2 Mediates 2,3,7,8-Tetrachlorodibenzo-p-dioxin Developmental Toxicity in Zebrafish. Toxicol Sci. 2003;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Prince R, Cooper KR. Comparisons of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on chemically impacted and nonimpacted subpopulations of Fundulus heteroclitus : ITCDD toxicity. Environ Toxicol Chem. 1995;14(4):579–587. [Google Scholar]
- Samson SL, Paramchuk WJ, Gedamu L. The rainbow trout metallothionein-B gene promoter: contributions of distal promoter elements to metal and oxidant regulation. Biochim Biophys Acta. 2001;1517(2):202–211. doi: 10.1016/s0167-4781(00)00273-6. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Struntz WD, Goldstone JV, Stegeman JJ. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquat Toxicol. 2006;77(4):422–432. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal. 2004;6(2):289–300. doi: 10.1089/152308604322899350. [DOI] [PubMed] [Google Scholar]
- Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler TW. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol Cell Biol. 2007;27(20):7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme-Laragy AR, Goldstone J, Hansen J, Stegeman J, Hahn M. Glutathione dynamics and differential sensitivity to pro-oxidants during zebrafish development. Abstract 1183. Toxicologist. 2012a;126(1) [Google Scholar]
- Timme-Laragy AR, Karchner SI, Franks DG, Jenny MJ, Harbeitner RC, Goldstone JV, McArthur AG, Hahn ME. Nrf2b, novel zebrafish paralog of oxidant-responsive transcription factor NFE2-related factor 2 (NRF2) J Biol Chem. 2012b;287(7):4609–4627. doi: 10.1074/jbc.M111.260125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme-Laragy AR, Levin ED, Di Giulio RT. Developmental and behavioral effects of embryonic exposure to the polybrominated diphenylether mixture DE-71 in the killifish (Fundulus heteroclitus. Chemosphere. 2006;62(7):1097–1104. doi: 10.1016/j.chemosphere.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Timme-Laragy AR, Van Tiem LA, Linney EA, Di Giulio RT. Antioxidant responses and NRF2 in synergistic developmental toxicity of PAHs in zebrafish. Toxicol Sci. 2009;109(2):217–227. doi: 10.1093/toxsci/kfp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingaud-Sequeira A, Zapater C, Chauvigne F, Otero D, Cerda J. Adaptive plasticity of killifish (Fundulus heteroclitus) embryos: dehydration-stimulated development and differential aquaporin-3 expression. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R1041–R1052. doi: 10.1152/ajpregu.91002.2008. [DOI] [PubMed] [Google Scholar]
- Trinkaus J. Methods in Developmental Biology. In: FW Hilt NW, editor. Fundulus. New York: Thomas Y Crowell Company; 1967. pp. 113–122. [Google Scholar]
- Turner C, Sawle A, Fenske M, Cossins A. Implications of the solvent vehicles dimethylformamide and dimethylsulfoxide for establishing transcriptomic endpoints in the zebrafish embryo toxicity test. Environ Toxicol Chem. 2012;31(3):593–604. doi: 10.1002/etc.1718. [DOI] [PubMed] [Google Scholar]
- van Ommen B, Koster A, Verhagen H, van Bladeren PJ. The glutathione conjugates of tert-butyl hydroquinone as potent redox cycling agents and possible reactive agents underlying the toxicity of butylated hydroxyanisole. Biochem Biophys Res Commun. 1992;189(1):309–314. doi: 10.1016/0006-291x(92)91559-9. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who's listening? Antioxid Redox Signal. 2010;13(11):1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ Health Perspect. 2004;112(17):1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis JS, Weis P, Heber M, Vaidya S. Methylmercury tolerance of killifish (Fundulus heteroclitus) embryos from a polluted vs non-polluted environment. Mar Biol. 1981;65(3):283–287. [Google Scholar]
- Wells PG, Bhuller Y, Chen CS, Jeng W, Kasapinovic S, Kennedy JC, Kim PM, Laposa RR, McCallum GP, Nicol CJ, Parman T, Wiley MJ, Wong AW. Molecular and biochemical mechanisms in teratogenesis involving reactive oxygen species. Toxicol Appl Pharmacol. 2005;207(2 Suppl):354–366. doi: 10.1016/j.taap.2005.01.061. [DOI] [PubMed] [Google Scholar]
- White RD, Shea D, Stegeman JJ. Metabolism of the aryl hydrocarbon receptor agonist 3,3',4,4'-tetrachlorobiphenyl by the marine fish scup (Stenotomus chrysops) in vivo and in vitro. Drug Metab Dispos. 1997;25(5):564–572. [PubMed] [Google Scholar]
- Whitehead A, Pilcher W, Champlin D, Nacci D. Common mechanism underlies repeated evolution of extreme pollution tolerance. Proc Biol Sci. 2012;279(1728):427–433. doi: 10.1098/rspb.2011.0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead A, Triant DA, Champlin D, Nacci D. Comparative transcriptomics implicates mechanisms of evolved pollution tolerance in a killifish population. Mol Ecol. 2010;19(23):5186–5203. doi: 10.1111/j.1365-294X.2010.04829.x. [DOI] [PubMed] [Google Scholar]
- Wirgin I, Waldman JR. Resistance to contaminants in North American fish populations. Mutat Res. 2004;552(1–2):73–100. doi: 10.1016/j.mrfmmm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Wu M, Shariat-Madar B, Haron MH, Wu M, Khan IA, Dasmahapatra AK. Ethanol-induced attenuation of oxidative stress is unable to alter mRNA expression pattern of catalase, glutathione reductase, glutathione-S-transferase (GST1A), and superoxide dismutase (SOD3) enzymes in Japanese rice fish (Oryzias latipes) embryogenesis. Comp Biochem Physiol C Toxicol Pharmacol. 2011;153(1):159–167. doi: 10.1016/j.cbpc.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Kemadjou JR, Zinsmeister C, Bauer M, Legradi J, Muller F, Pankratz M, Jakel J, Strahle U. Transcriptional profiling reveals barcode-like toxicogenomic responses in the zebrafish embryo. Genome Biol. 2007;8(10):R227. doi: 10.1186/gb-2007-8-10-r227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager RL, Reisman SA, Aleksunes LM, Klaassen CD. Introducing the "TCDD-inducible AhRNrf2 gene battery". Toxicol Sci. 2009;111(2):238–246. doi: 10.1093/toxsci/kfp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.