Abstract
Neuropeptides are crucial regulators of development and various physiological functions but little is known about their identity, expression and function in vectors of pathogens causing serious diseases, such as ticks. Therefore, we have used antibodies against multiple insect and crustacean neuropeptides to reveal the presence of these bioactive molecules in peptidergic neurons and cells of the ixodid tick Rhipicephalus appendiculatus. These antibodies have detected 15 different immunoreactive compounds expressed in specific central and peripheral neurons associated with the synganglion. Most central neurons arborize in distinct areas of the neuropile or the putative neurohaemal periganglionic sheath of the synganglion. Several large identified neurons in the synganglion project multiple processes through peripheral nerves to form elaborate axonal arborizations on the surface of salivary glands or to terminate in the lateral segmental organs (LSO). Additional neuropeptide immunoreactivity has been observed in intrinsic secretory cells of the LSO. We have also identified two novel clusters of peripheral neurons embedded in the cheliceral and paraspiracular nerves. These neurons project branching axons into the synganglion and into the periphery. Our study has thus revealed a complex network of central and peripheral peptidergic neurons, putative neurohaemal and neuromodulatory structures and endocrine cells in the tick comparable with those found in insect and crustacean neuroendocrine systems. Strong specific staining with a large variety of antibodies also indicates that the tick nervous system and adjacent secretory organs are rich sources of diverse neuropeptides related to those identified in insects, crustaceans or even vertebrates.
Keywords: Neuropeptides; Synganglion; Salivary glands; Lateral segmental organs; Tick, Rhipicephalus appendiculatus (Arachnida, Acari, Ixodidae)
Introduction
The general anatomy and structure of the central nervous system (CNS; synganglion) and associated peripheral nervous system and secretory organs of ticks have been extensively studied (for reviews, see Chow et al. 1972; Coons et al. 1974; Sonenshine 1991; Coons and Alberti 1999; Rees 2004; Chang and Kaufman 2005; Lees and Bowman 2007). However, little is known about peptidergic neuroendocrine and neuromodulatory systems in ticks. Classical paraldehyde-fuchsin or other staining techniques have revealed 18 neurosecretory centres in the synganglia of representative ixodid and argasid ticks (Obenchain 1974; Obenchain and Oliver 1975; Pound and Oliver 1982; Binnington 1987; Prullage et al. 1992; Szlendak and Oliver 1992). Although these studies have described a complex network of neurosecretory cells presumably producing and releasing some bioactive molecules, their structures and functions remain to be identified.
Several neurohaemal organs and release sites for these neurosecretory cells have been proposed. These include two layers of neural sheath surrounding the synganglion and major nerve roots, viz. the proximal neurilemma (neural lamella) and the more distal periganglionic sheath. A narrow space called the periganglionic sinus lies between the neurilemma and periganglionic sheath; this space is continuous with the heart and supplies the synganglion with fresh haemolymph (Fig. 1; Prullage et al. 1992; Szlendak and Oliver 1992). Ultrastructural studies have shown that nerve fibres with neurosecretory vesicles are common in the neural lamella and may discharge their granules into the periganglionic blood sinus (Coons and Alberti 1999). Another layer, the periganglionic sheath, contains branching axons and axon terminals and is regarded as a putative neurohaemal release site for specific neurosecretory cells (Sonenshine 1991; Neupert et al. 2005).
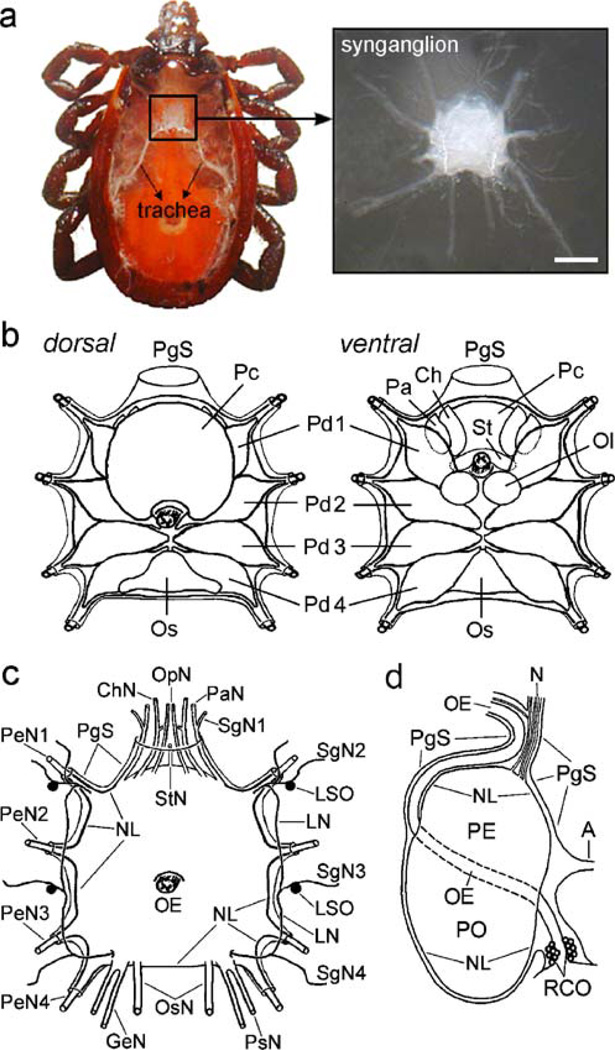
Fig. 1.
Anatomy of the synganglion and associated peripheral nerves and organs. a Left Tick showing the position of the synganglion. Right Dorsal view of the tick synganglion and its general morphology. Bar 200 µm. b Representations of various lobes in the synganglion (Ch cheliceral lobe, Ol olfactory lobe, Os opisthosomal lobe, Pa palpal lobe, Pd1–4 pedal lobes 1–4, Pc prothocerebral lobe, St stomodeal lobe, PgS periganglionic sheath). c Representation of all peripheral nerves and lateral segmental organs (LSO); dorsal view (ChN cheliceral nerve, GeN genital nerve, LN lateral nerve, OsN opistosomal nerve, OpN optical nerve, PaN palpal nerve, PsN paraspiracular nerve, PeN1–4 pedal nerves 1–4, StN stomodeal nerve, SgN1–4 salivary gland nerves 1–4, E oesophagus, NL neurilemma [neural lamella]). d Lateral view of the synganglion depicting positions of associated structures and organs (adapted from Obenchain 1974; A aorta, OE, N nerves, NL neurilemma, PgS periganglionic sheath, RCO retrocerebral organ, PE preoesophageal part of the synganglion, PO postoesophageal part of the synganglion)
The lateral segmental organs (LSO) are additional structures with a proposed neurohaemal role. They were first described in the ixodid tick Boophilus microplus (Binnington and Tatchell 1973) and later were observed in many other species (Chow and Wang 1974; Obenchain and Oliver 1975; Panfilova 1978; Binnington 1981; Marzouk et al. 1985; El Shoura 1989; Coons and Alberti 1999). Most species contain two to four pairs of the LSO interconnected by the lateral nerves along the synganglion (Sonenshine 1991). However, the true physiological function of the LSO remains unclear and no bioactive compounds confirming their secretory activity have been identified.
The retrocerebral organ complex (ROC) is also considered to be a neurohaemal organ in ticks (Sonenshine 1991; Coons and Alberti 1999). It is attached to the oesophagus close to the synganglion and some authors have proposed its analogous role to the corpora cardiaca/corpora allata complex of insects (Roshdy et al. 1973; Binnington 1981; Marzouk et al. 1985; Zhu and Oliver 1991). Although the ROC is a putative candidate for the production of juvenile hormone (JH) and/or neuropeptides in ticks, conclusive evidence for its neuroendocrine function(s) has not been reported (Chang and Kaufman 2005).
Numerous studies have provided evidence that tick salivary glands are innervated by several nerves from the synganglion (for a review, see Kaufman 1983). However, no neurons projecting their axons to the salivary gland surface have been identified. These observations indicate that the tick neuroendocrine system is composed of neurosecretory/modulatory neurons in the synganglion and various neuronal projections extending into the neurilemma, periganglionic sheath, RCO, LSO and salivary glands. However, the true physiological roles of these putative neurohormonal and secretory organs await confirmation.
Neuropeptides are important active messengers in the neuroendocrine systems of most animals. Immunohistochemical staining with antibody to insulin suggested, for the first time, that the tick synganglion produced neuropeptides (Zhu and Oliver 1991; Davis et al. 1994), with insulin-like immunoreactivity (IR) being detected in the synganglion of Ornithodoros parkeri and Dermacentor variabilis. Subsequent immunohistochemical studies with antibodies to FMRFamide and cockroach allatostatin I indicated the presence of specific neuropeptides in various neurons and axonal arborizations in the synganglion of argasid and ixodid ticks (Zhu et al. 1995; Zhu and Oliver 2001). Whole-mount immunohistochemistry was used to detect insect kinin-like IR and periviscerokinin-like IR in specific neurons and axons of the ticks Ixodes ricinus and B. microplus. Periviscerokinin was the first neuropeptide identified in ticks by mass spectrometric analysis of single neurons (Neupert et al. 2005). Molecular cloning and functional analysis of the G-protein-coupled receptor for kinin-like peptide in the southern cattle tick B. microplus further indicated that ticks produced multiple neuropeptides and receptors (Holmes et al. 2000, 2003; Taneja-Bageshwar et al. 2006). Bioassays revealed the presence of ecdysteroidotropic neurohormone in the ixodid tick Amblyomma hebraeum (Lomas et al. 1997), and an opioid peptide sharing high sequence similarity with mammalian haemorphins was identified in synganglia extracts of Amblyomma testudinarium (Liang et al. 2005). However, the origin of this opioid peptide needs confirmation, because of possible contamination with the mammalian blood in the tick oesophagus, which cannot be separated from the synganglion (Neupert et al. 2005).
In this paper, we have used immunohistochemical techniques to detect the expression of multiple neuropeptide-like compounds in the tick synganglion and associated peripheral neuroendocrine organs. This is the first report describing the peptidergic nature of numerous previously unknown central and peripheral neurons, the elaborate innervation of salivary glands and the axon terminals and intrinsic cells in the LSO.
Materials and methods
Animals
A colony of Rhipicephalus appendiculatus was reared under the same laboratory conditions as those described for Dermacentor reticulatus (Slovák et al. 2002). The synganglia with attached peripheral nerves, LSO, RCO and salivary glands were investigated in 1– to 2-month-old unfed females. Representations were made from microphotographs of fixed ganglia by using Adobe Photoshop or were adapted from the published literature as indicated.
Whole-mount immunohistochemical staining
For the whole-mount immunohistochemical staining of tick neuroendocrine organs, we followed a procedure successfully used for the staining of insect endocrine cells (Žitňanová et al. 2001). Ticks were dissected in icecold phosphate-buffered saline (PBS: 137 mM NaCl, 1.45 mM NaH2PO4.H2O, 20.5 mM Na2HPO4, pH 7.2). The dorsal cuticle and gut were removed with sharp forceps and the synganglion with attached LSO were cleaned of remaining blood and fixed overnight in 4% paraformaldehyde in PBS (pH 7.2). Following washes in PBS containing 1% Triton X-100 (PBST), the periganglionic sheath was carefully removed from selected synganglia. The tissues were then pre-absorbed with 5% normal goat serum (Sigma, St. Louis, Mo., USA) in PBST for 10 min and incubated in monoclonal antibody or polyclonal antiserum for 2 days. All the antibodies and the dilutions used in this study are listed in Table 1. After several washes with PBST, the tissues were incubated overnight with Alexa 488- or Alexa 555-labelled goat anti-rabbit IgG or goat anti-mouse IgG (Molecular Probes, Carlsbad, Calif., USA). Alternatively, we used horseradish peroxidase (HRP)-labelled secondary antibodies (Jackson Immunoresearch Lab., West Grove, Pa., USA). The HRP was visualized with 0.05% 3,3′diaminobenzidine tetrahydrochloride (Sigma) in 0.1 M TRIS-HCl buffer (pH 7.6) and 0.01% H2O2. The end of the reaction was determined visually to avoid excessive backround staining. Stained tissues were washed in PBS and mounted in glycerol.
Table 1.
Mouse monoclonal antibodies (M) and rabbit polyclonal antisera (R) used
| Antibody to | Type | Dilution | Source/first characterization |
|---|---|---|---|
| Adipokinetic hormone (AKH) | R | 1:1,000 | D. Kodrík, unpublished |
| Arg-Phe-NH2(RFa) | R | 1:1,000 | Grimmelikhuijzen and Spencer 1984 |
| Bommo-bombyxin (insulin-like) | M | 1:300 | Mizoguchi et al. 1987 |
| Bommo-prothoracicotropic hormone (PTTH) | M | 1:1,000 | Mizoguchi et al. 1990 |
| Bommo-myosupressin (BMS) | M | 1:1,000 | Yamanaka et al. 2006 |
| Crustacean cardioactive peptide (CCAP) | R | 1:500 | Stangier et al. 1988 |
| Drome-pigment dispersing factor (PDF) | R | 1:1,000 | Persson et al. 2001 |
| Dippu-allatostatin | M | 1:100 | Stay et al. 1992 |
| Leuma-kinin | R | 1:1,000 | Nässel et al. 1992 |
| Locmi-tachykinin | R | 1:2,000 | Nässel et al. 1993 |
| Locmi-tachykinin | R | 1:1,000 | Schoofs et al. 1993 |
| Manse-allatostatin | R | 1:1,000 | Žitňan et al. 1995 |
| Manse-allatotropin | R | 1:1,000 | Žitňan et al. 1995 |
| Manse-diuretic hormone (DH) | R | 1:2,000 | Žitňan et al. 1995 |
| Manse-eclosion hormone (EH) | R | 1:1,000 | Žitňan et al. 2002 |
| Manse-ion transport peptide (ITP) | R | 1:10,000 | Dai et al. 2007 |
| Manse-myoinhibitory peptide I (MIP-I) | M | 1:1,000 | Kim et al. 2006 |
| Manse-pre-ecdysis triggering hormone (PETH; PRVa) | R | 1:1,000 | Žitňan et al. 1999 |
| Peram-corazonin | R | 1:5,000 | Roller et al. 2003 |
For whole-mount double-staining, we incubated fixed synganglia in a mixture of monoclonal antibodies and polyclonal rabbit antisera. After being washed with PBST, the tissues were incubated with a mixture of Alexa 488- and Alexa 555-labelled secondary antibodies, washed in PBST and mounted in glycerol.
Stained tissues were observed under a Nikon microscope (Eclipse 600) by using fluorescein and rhodamine filters for fluorescent microscopy, and Nomarski differential interference optics for light microscopy. Double-stained synganglia were analysed under a confocal microscope (Zeiss LSM510).
Negative controls included preabsorption of each antibody with its respective antigen. Each diluted antibody (see Table 1) was incubated with 1 µM of the corresponding antigen for 24 h and used for immunohistochemistry as described above. The bombyxin antibody was pre-absorbed with 0.1 µM synthetic N-terminal bombyxin. This treatment invariably abolished all immunohistochemical staining.
Nomenclature of peptidergic neurons
The synganglion of both argasid and ixodid ticks is composed of 18 neurosecretory centres containing a total of ~130–150 putative neurosecretory cells (Binnington 1987; Balashov 1998). Our antibodies reacted only with a limited number of these cells, whereas numerous previously unknown neurons were observed. To characterize these peptidergic neurons, we modified the established nomenclature ofWhite et al. (1986) and named each neuron with letters. The first two letters refer to the position of each neuron in a specific lobe of the synganglion: cheliceral (Ch), palpal (Pa), stomodeal (St), prothocerebral (Pc), pedal 1–4 (Pd1–4), opisthosomal (Os), preoesophageal (Pe) or postoesophageal (Po), whereas the following letters refer to its dorsal (D), ventral (V), anterior (A), posterior (P), medial (M) or lateral (L) location. The names of the peripheral neurons (PN) are indicated by their location in a specific nerve trunk: cheliceral (Ch) and paraspiracular (Ps). Neurons projecting axons onto the surface of the putative neurohaemal periganglionic sheath or lateral segmental organs are considered to be neurosecretory cells (NC); interneurons (IN) arborize within the synganglion, whereas neurons innervating ducts and/or acini of salivary glands are denoted as SG.
Results
In this paper, we provide a comprehensive review of the peptidergic neurosecretory/modulatory systems by using neuropeptide antibodies and immunohistochemical staining of the synganglion and associated peripheral structures/organs of the tick R. appendiculatus. Since most tick neuropeptides have not been identified, we have tested the hypothesis that most arthropod neuropeptides are conserved and used antibodies generated against multiple insect and crustacean neuropeptides (Table 1).
Synganglion
The tick CNS contains a single synganglion that lies in the anterior-ventral part of the body and that is composed of several lobes or ganglia (Fig. 1; Obenchain 1974). Fifteen out of 18 antibodies showed strong specific reaction in various peptidergic cells and axonal projections in the synganglion, LSO, peripheral nerves and salivary glands. Only antibodies to Manduca allatostatin, eclosion hormone and adipokinetic homones failed to react or showed inconsistent staining with any described cells or structures (Table 1). None of the antibodies used in this study showed positive reaction in the ROC, which was originally considered a major neurohaemal organ in ticks (Sonenshine 1991).
Bombyxin-like IR was distributed only on the dorsal side of the synganglion (Figs. 2, 3a). Three pairs of medium-sized neurons were observed in the anterior prothocerebrum (PcIN1–3), whereas an additional three pairs of small neurons were stained in the posterior prothocerebrum (PcDM1–3). A single pair of neurons was detected in the cheliceral lobe (ChD) and one pair of giant neurons was present in the opisthosomal ganglion (OsDM). Strong reaction was also detected in intrinsic cells of the LSO (Figs. 2, 3b).
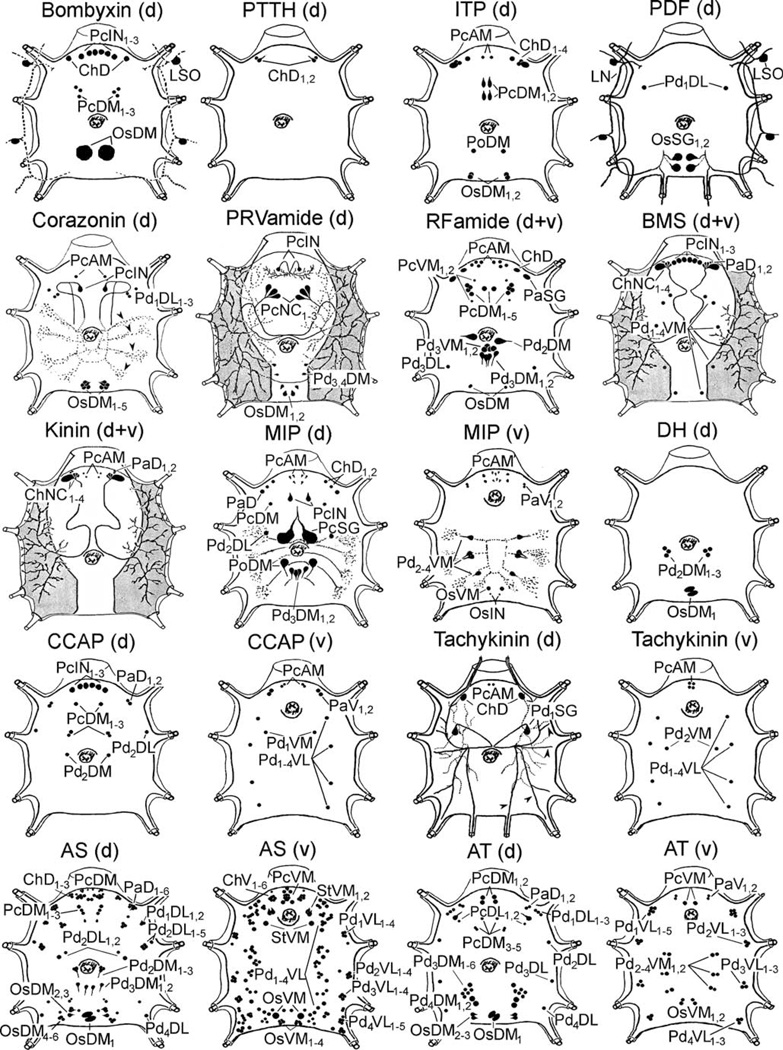
Fig. 2.
Representations of all peptidergic neurons described in this study (an explanation of abbreviations of labelled peptidergic neurons is given in Nomenclature of peptidergic neurons; d dorsal, v ventral)
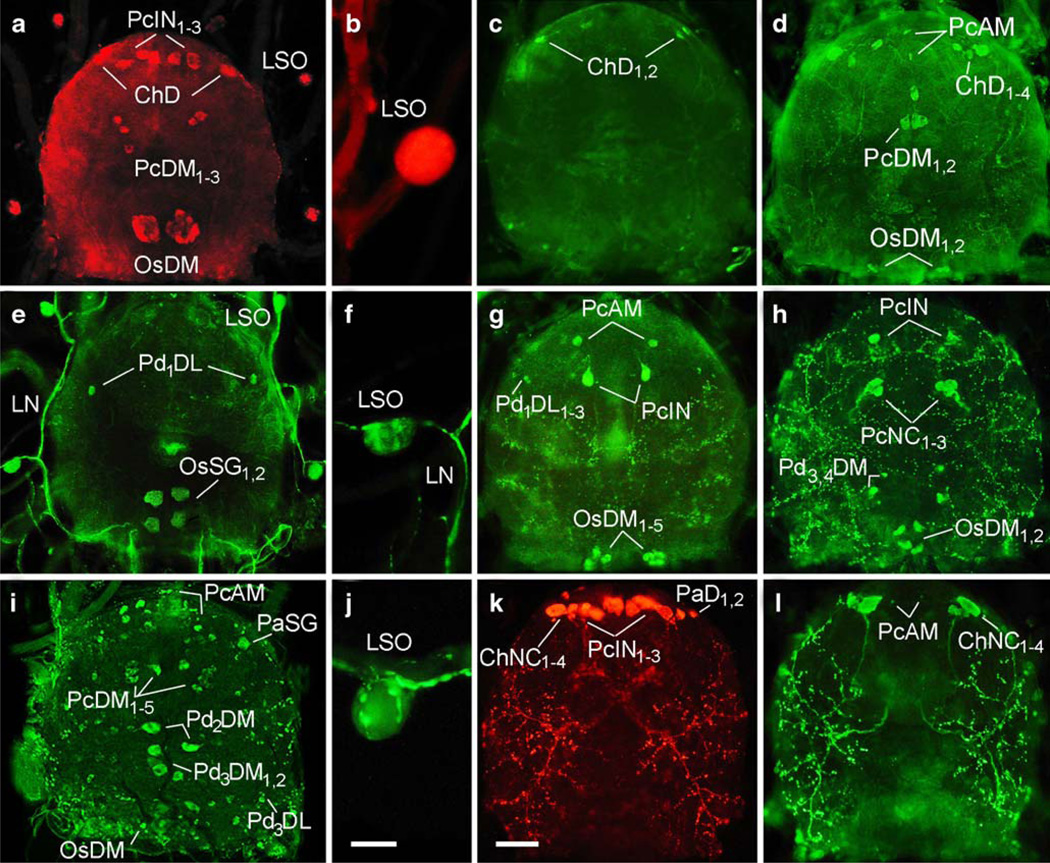
Fig. 3.
Neuropeptide immunoreactivity in synganglia (dorsal side) and lateral segmental organs (abbreviations as in Nomenclature of peptidergic neurons). a, b Bombyxin-like IR in the synganglion and intrinsic cells of the LSO. c PTTH-like IR in the anterior synganglion. d ITP-like IR in the synganglion. e, f PDF-like IR in the synganglion, lateral nerves (LN) and intrinsic cells of the LSO. g Corazonin-like IR in the synganglion. h PRVamide-like IR in the synganglion. i, j RFamide-like IR in the synganglion and putative neurohaemal axon terminals containing varicosities in the LSO. k Myosupressin-like IR in anterior neurons and putative neurohaemal areas in the dorsal periganglionic sheath of the synganglion. l Kinin-like IR in anterior synganglion neurons and the same neurohaemal areas as in k. Bars 50 µm (a, c–e, g–i, k, l), 20 µm (b, f, j)
Prothoracicotropic hormone (PTTH)-like IR (Figs. 2, 3c) was restricted to two pairs of small lateral neurons in the cheliceral lobes (ChD1,2). As in insects, no other neurons were stained with this monoclonal antibody.
Ion transport peptide (ITP)-like immunostaining was found on the dorsal synganglion (Figs. 2, 3d); three pairs of medial neurons in the prothocerebrum (PcAM and PcDM1,2), four neurons in each cheliceral lobe (ChD1–4), one neuronal pair in the postoesophageal region (PoDM) and two pairs of neurons in the opistosomal lobe (OsDM1–2).
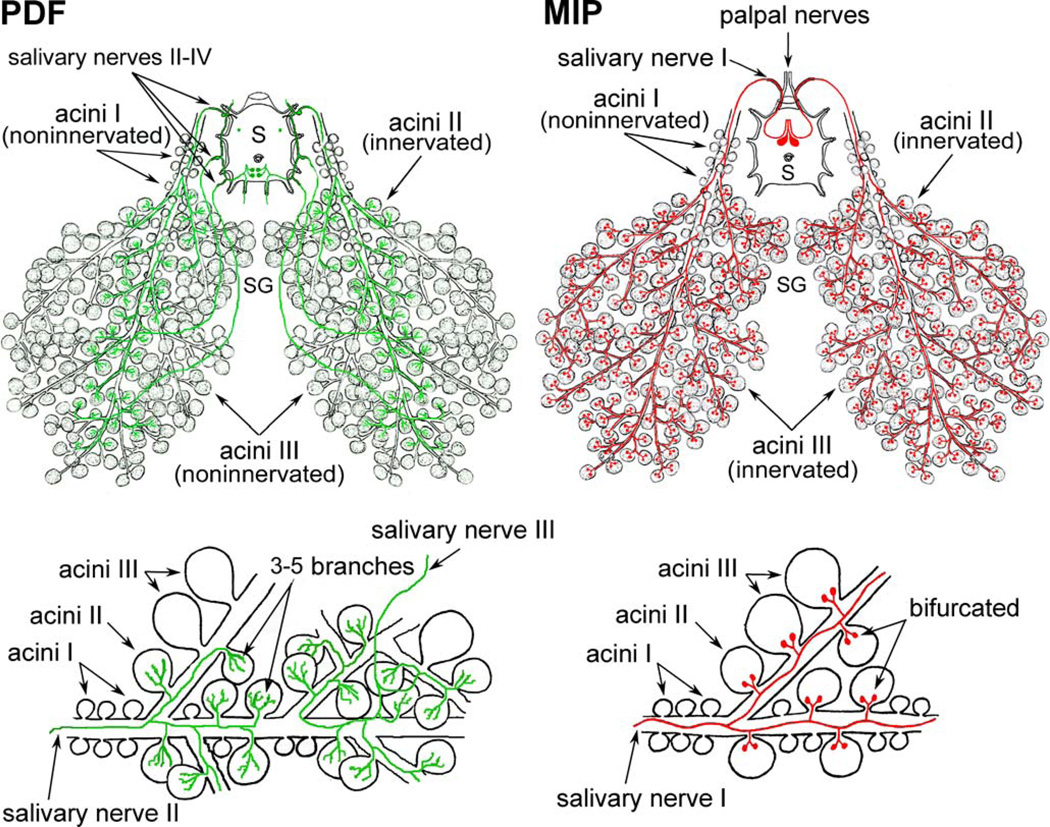
Pigment dispersing factor (PDF)-like IR was restricted to one pair of small neurons in the first pedal lobe (Pd1DL) and two pairs of large cells in the opisthosomal lobe (Figs. 2, 3e). These large opisthosomal lobe neurons projected axons into lateral and opisthosomal nerves (Figs. 2, 3e,f). Each axon in the lateral nerve sent branches into salivary nerves II–IV to innervate the salivary glands (see bellow). These neurons were named OsSG1,2 to indicate their association with salivary glands. Immunoreactive lateral nerves were connected with two pairs of the LSO whose intrinsic cells also showed strong PDF-like IR (Figs. 2, 3f).
Corazonin-like IR was observed in four prothocerebral neurons (PcAM and PcIN), three pairs of small cells in the first pedal lobe (Pd1DL1–3) and clusters of five paired neurons in the opistosomal lobe, viz. OsDM1–5 (Figs. 2, 3g). PcIN neurons showed the colocalization of corazonin-like and MIP-like IR (Fig. 4a–c). Since PcIN project posteriorly a loop of paired axons that arborize in each pedal lobe, they are identified as interneurons (Figs. 2, 3g).
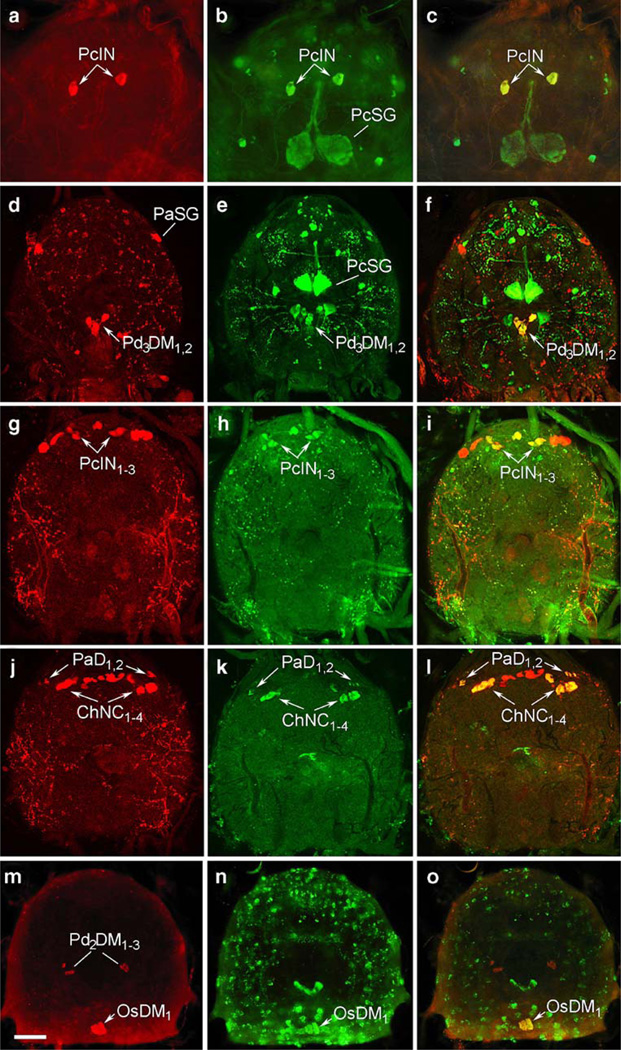
Fig. 4.
Identification of central neurons in tick synganglia by using double-staining with neuropeptide antibodies. a–c Double-staining with antibodies to corazonin (a, red) and MIP (b, green) revealed the colocalization of these peptides in the prothocerebral interneurons (PcIN) as shown in c (yellow, arrows). d–f Coexpression of RFamidelike (d, red) and MIP-like (e, green) peptides was detected in medial pedal neurons (PdDM31,2) as presented in f (yellow, arrows). g–i Colocalization of BMS-like (g, red) and CCAP-like IR (h, green) in prothocerebral interneurons (PcIN1–3) as seen in i (yellow, arrows). j–l BMS-like peptide (j, red) was also coexpressed with kinin-like peptides (k, green) in palpal neurons (PaD1,2) and cheliceral neurosecretory cells (ChNC1–4) as revealed in l (yellow, arrows). m–o Colocalization of DH-like (m, red) and allatostatin-like IR (n, green) was observed in OsDM1 neurons of the opisthosomal lobe (o, yellow, arrows). Bar 40 µm (a–c), 50 µm (d–o)
PRVamide-like IR was detected only in the dorsal region of the synganglion. We detected a pair of interneurons (PcIN) projecting short arborizing axons in the anterior prothocerebrum (Figs. 2, 3h). Posterior prothocerebrum contained three pairs of putative neurosecretory cells (PcNC1–3) projecting contralateral axons to the periganglionic sheath where they branched into elaborate arborizations. A pair of neurons was stained in each pedal lobe 3, 4 (Pd3,4DM) and two pairs of cells were observed in the opistosomal lobe (OsDM1,2).
RFamide-like IR was detected in a cluster of four paired small neurons in the anterior-medial prothocerebrum (PcAM) and seven other paired neurons were found more posteriorly in the medial prothocerebral lobe (PcDM1–5 and PcVM1,2; Figs. 2, 3i). A single neuron was stained in each cheliceral lobe (ChD). Another neuron in each palpal lobe projected axons into the palpal and salivary nerves I. Axonal projections of these palpal neurons terminated along main ducts of salivary glands and were therefore named PaSG (Figs. 2, 3i, 4d,f; see below). Several neurons were detected in pedal lobes: two pairs of ventro-medial neurons (Pd3DV1–2), a pair of dorso-medial neurons (Pd2DM), a pair of dorso-lateral neurons (Pd3DL) and two paired neurons showing co-expression of RFamide-like and MIP-like peptides (Pd3DM1,2; Fig. 4d–f). A pair of small neurons was found in the opistosomal lobe (OsDM). RFamide-like IR was also observed in axon terminals on the LSO surface (Fig. 3j). Therefore, the LSO may serve as neurohaemal organs for some neurosecretory cells of the synganglion, but their identity has not been determined.
Bombyx myosupressin (BMS)-like IR was prominent in three clusters of anterior dorsal neurons. Three pairs of neurons in the prothocerebrum showed colocalization of BMS-like and CCAP-like IR (Fig. 4g–i). Double-stained axon terminals of these neurons were detected in the lateral neuropile of the pedal lobes indicating that they were interneurons (PcIN1–3). Four pairs of neurons in the cheliceral lobes projected immunoreactive axons posteriorly to form elaborate arborizations in the periganglionic sheath and were therefore considered as neurosecretory cells (ChNC1–4; Figs. 2, 3k). Two additional paired neurons were found in the palpal lobes (PaD1,2). ChNC1–4 and PaD1,2 showed double-labelling with antibodies against BMS and kinin (Fig. 4j–l). Five pairs of ventral segmental neurons were detected in the pedal lobes (Pd1–4VM; Fig. 2).
Kinin-like IR was restricted to two pairs of small anterior prothocerebral neurons (PcAM), four pairs of anterior putative neurosecretory cells in the cheliceral lobes (ChNC1–4) and two pairs of small neurons in the palpal lobes (PaD1,2; Figs. 2, 3l, 4k,l). As described above, the two latter cell types produced kinin-like and BMS-like substances (Fig. 4j–l). Numerous immunoreactive axon terminals in the sheath apparently originating from ChNC1–4 indicated their neurohaemal function.
Myoinhibitory peptide (MIP)-like IR was observed in numerous neurons of various sizes (Figs. 2, 5a). About eight pairs of small neurons were located in the anterior prothocerebrum (PcAM), whereas the central region of the prothocerebrum contained two pairs of neurons (PcDM and PcIN; Fig. 2). Double-staining revealed the colocalization of MIP-like and corazonin-like IR in the PcIN (Fig. 4a–c). The posterior prothocerebrum contained a pair of giant neurons that innervated specific acini of salivary glands (see below). To indicate that these prothocerebral neurons were associated with the salivary gland, we named them PcSG (Fig. 4b,c,e,f). A pair of small dorsal neurons was observed in each cheliceral lobe (ChD1,2), whereas three small neurons were located in each palpal lobe (PaD and PaV1,2). On the dorsal side of the pedal region, we detected a pair of small medial neurons (Pd2DL), a pair of large medial neurons (PoDM) and two pairs of smaller medial neurons (Pd3DM1–2) that showed the co-expression of MIP-like and RFamide-like peptides (Figs. 2, 4d–f, 5a). The ventral side of pedal lobes 2–4 and the opisthosomal lobe contained 1–2 pairs of small medial neurons (Pd2–4VM, OsVM, OsIN; Figs. 2, 5a).
Fig. 5.
Neuropeptide immunoreactivity in the tick synganglion (for an explanation of abbreviations, see Nomenclature of peptidergic neurons). a Composite view of MIP-like IR in most identified dorsal and ventral neurons. b Composite confocal sections of the synganglion showing CCAP-like IR in all described neurons. c Tachykininlike IR in dorsal cell bodies and elaborate axonal arborizations of Pd1SG neurons. d Allatostatin-like IR in selected dorsal and ventral neurons. e, f Allatotropin-like IR in dorsal (e) and ventral (f) sides of the synganglion. Bar 50 µm
Crustacean cardioactive peptide (CCAP)-like IR was distributed in a number of small neurons (Figs. 2, 5b). The most prominent cluster of interneurons in the anterior prothocerebrum (PcIN1–3) showed the co-expression of CCAP-like and BMS-like peptides (Figs. 4g–i, 5b). Additional small neurons were observed in the posterior prothocerebrum (PcDM1–3) and palpal lobes (PaD1,2 and PaV1,2). Pedal lobes contain small paired neurons on the dorsal and ventral sides; two pairs were detected on the dorsal side of the pedal lobe 2 (Pd2DM, Pd2DL), whereas segmentally distributed neurons were observed on the ventral side of each pedal lobe 1–4 (Pd1VM, Pd1–4VL; Figs. 2, 5b).
Tachykinin-like IR was found in a cluster of about six small anterior neurons in the prothocerebrum (PcAM), a pair of dorsal larger cells in the cheliceral lobes (ChD) and a prominent pair of large dorso-lateral neurons in the first pedal lobe (Pd1SG; Figs. 2, 5c). Each Pd1SG neuron projected a main axonal branch towards the oesophagus and then turned laterally. In the central part of the synganglion, each lateral axon branched in four directions; anteriorly into the palpal nerve innervating the salivary glands (see below), laterally into pedal nerve II, posteriolaterally into the pedal nerves III and IV, and posteriorly into the opisthosomal nerve. Axons from the pedal nerve IV terminate in the lateral nerves. These two large neurons therefore innervated most main central nerves, whereas their smaller axonal branches arborized on the lateral periganglionic sheath. Small paired neurons Pd1–4VL and Pd2VM were found in pedal lobes on the ventral side.
Diuretic hormone (DH)-like IR was observed in three pairs of dorso-medial neurons in the pedal region (Pd2DM1–3) and in a pair of larger neurons in the opistosomal lobe (OsDM1; Figs. 2, 4m). Double staining revealed the colocalization of DH-like and allatostatin-like IR in OsDM1 neurons (Fig. 4m–o), which projected branching axons into the lateral, opistosomal and pedal nerves IV.
Diploptera allatostatin-like IR was detected in numerous small neurons distributed in clusters on the dorsal (~55 pairs) and ventral (~90 pairs) sides of the synganglion (Figs. 2, 5d). We observed ~25 pairs of dorso- and ventromedial prothocerebral neurons (PcDM, PcVM), two clusters of dorsal and ventral neurons (~3 and 6 pairs, respectively) in the cheliceral lobe (ChD1–3, ChV1–6) and ~6 pairs of cells in the palpal lobe (PaD1–6). Each pedal lobe contained ~15–20 dorso-lateral (PdDL) or ventrolateral (PdVL) neurons, whereas 2–3 dorso-medial neurons were observed in the pedal lobes 2, 3 (Pd2DM1–3, Pd3DM1,2). About 25 pairs of cells were found in the opistosomal lobe (Fig. 2, 5d). As described above, the largest pair of dorso-medial neurons in the opistosomal lobe (OsDM1) showed allatostatin-like and DH-like IR (Fig. 4m–o).
Allatotropin-like IR was found in a large number of neurons throughout the synganglion (Figs. 2, 5e,f). The prothocerebrum contained five pairs of dorso-medial (PcDM1–5), two pairs of dorso-lateral (PcDL1,2) and a pair of ventro-medial (PcVM) neurons (Fig. 5e,f). Paired neurons were found on the dorsal and ventral sides (PaD1,2, PaV1,2) of each palpal lobe (Fig. 5e). Most neurons were distributed in the pedal region; the first pedal lobe contained clusters of three dorso-lateral (Pd1DL1–3) and five ventro-lateral (Pd1VL1–5) neurons, whereas only one dorsal pair and three pairs of ventral neurons were found in more posterior pedal lobes 2–4 (Pd2–4DL, Pd2–4VL1–3). Coupled neurons were also distributed in more medial regions of pedal lobes 2–4 (Pd2–4VM1,2). A group of six paired dorso-medial neurons was detected in the pedal lobe 3 (Pd3DM1–6) and two larger paired dorso-medial cells were located in the pedal lobe 4 (Pd4DM1,2). The opistosomal lobe contained three pairs of dorsal neurons (OsDM1–3) and two pairs of ventral neurons (OsVM1,2).
Salivary gland innervation
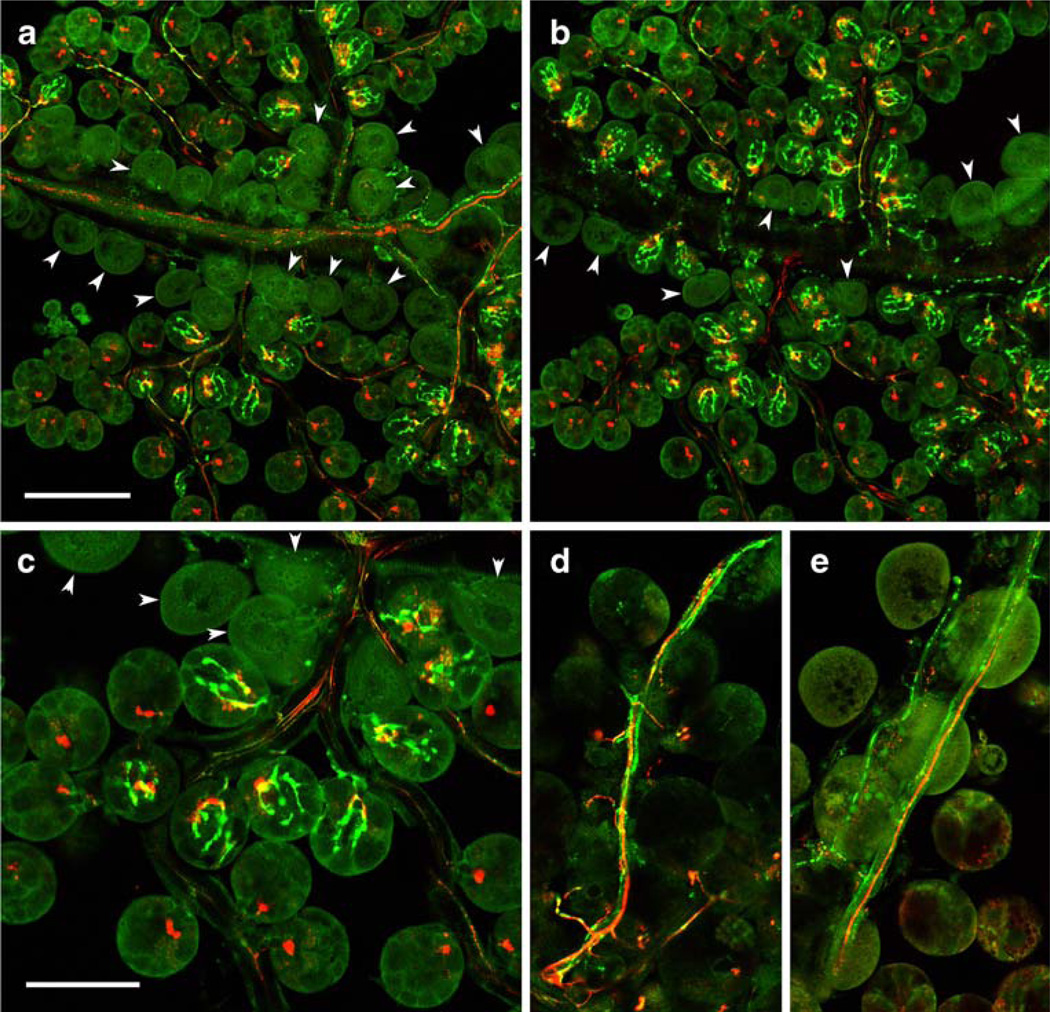
We identified four types of neurons in the synganglion, each innervating different parts of salivary glands. The PDF antiserum revealed the entire anatomy of paired OsSG1,2 neurons located in the dorso-medial part of the opisthosomal lobe. All four cells (Figs. 2, 3e) projected axons through the peripheral nerves to innervate specific acini of the salivary glands (Figs. 6, 7a-c). A single axon from each OsSG neuron entered the lateral nerve and projected three branches into the salivary nerves II–IV. These three posterior immunoreactive axons exhibited anastomosis along the major salivary ducts and terminated exclusively on acini type II (Figs. 6, 7a–c). Axon terminals of OsSG were characterized by three to five small branches containing varicosities (Fig. 7a–c).
Fig. 6.
Representations of identified central neurons innervating salivary glands (SG). PDF-like immunoreactive neurons (OsSG1,2, green) exit the synganglion (S) through lateral and salivary nerves IIIV to innervate specific acini type II. Branching axon terminals contain varicosities. MIP-like immunoreactive giant neurons (PcSG, red) exit through the salivary nerve I and innervate acini type II and III. Axons of these neurons lack varicosities and show typical bifurcating axon terminals
Fig. 7.
Neuropeptide immunoreactivity in the salivary gland innervation. a–c Merged consecutive confocal sections of salivary glands showing double-staining with antibodies to PDF (green) and MIP (red). a Dorsal side. b Ventral side. c Higher magnification. Note the PDF-like IR in axons containing varicosities along salivary ducts and multiple axon terminals exclusively on acini type II, whereas axons containing MIP-like IR terminate on acini II and III. Acini I located along the main salivary duct are not innervated (arrowheads). d Double-staining with antibodies to RFamide (green) and MIP (red). Note that RFamide-like IR is restricted to axons running along salivary ducts, whereas adjacent MIP-like immunopositive axons terminate on salivary acini II and III. e Tachykinin-like IR (green) and MIP-like IR (red) in branching axonal processes innervating ducts of salivary glands. Bars 100 µm (a, b), 50 µm (c–e)
The antibody to MIP reacted with a prominent pair of giant neurons in the prothocerebrum PcSG (Figs. 2, 4b–f, 5a; see above). Each neuron projected a single thick axonal process lacking varicosities in a loop, exited the synganglion through the palpal nerve and salivary nerve I and branched along the salivary ducts to terminate on salivary acini type II and III (Figs. 6, 7a–c). Each axonal branch showed characteristic bifurcating axon terminals at the base of these acini, which were clearly different from those stained with the PDF antibody (Figs. 6, 7a–c).
A single RFamide-like immunoreactive neuron in each palpal lobe (PaSG, Figs. 2, 3i, 4d,f) projected an axon through the palpal nerve and salivary nerve I and branches along the main ducts of salivary glands (Fig. 7d). The numerous varicosities along this axon indicated possible release sites of RFamide-like peptide(s) on the surface of salivary ducts (Fig. 7d). These axons never terminated on the secretory acini of salivary glands.
The anterior axonal branch of each tachykinin-like immunoreactive neuron (Pd1SG; Figs. 2, 5c) innervated the ducts of the salivary glands. Fine axonal projections arborizing along salivary ducts contained numerous varicosities but did not innervate salivary acini (Fig. 7e).
Peripheral nervous system
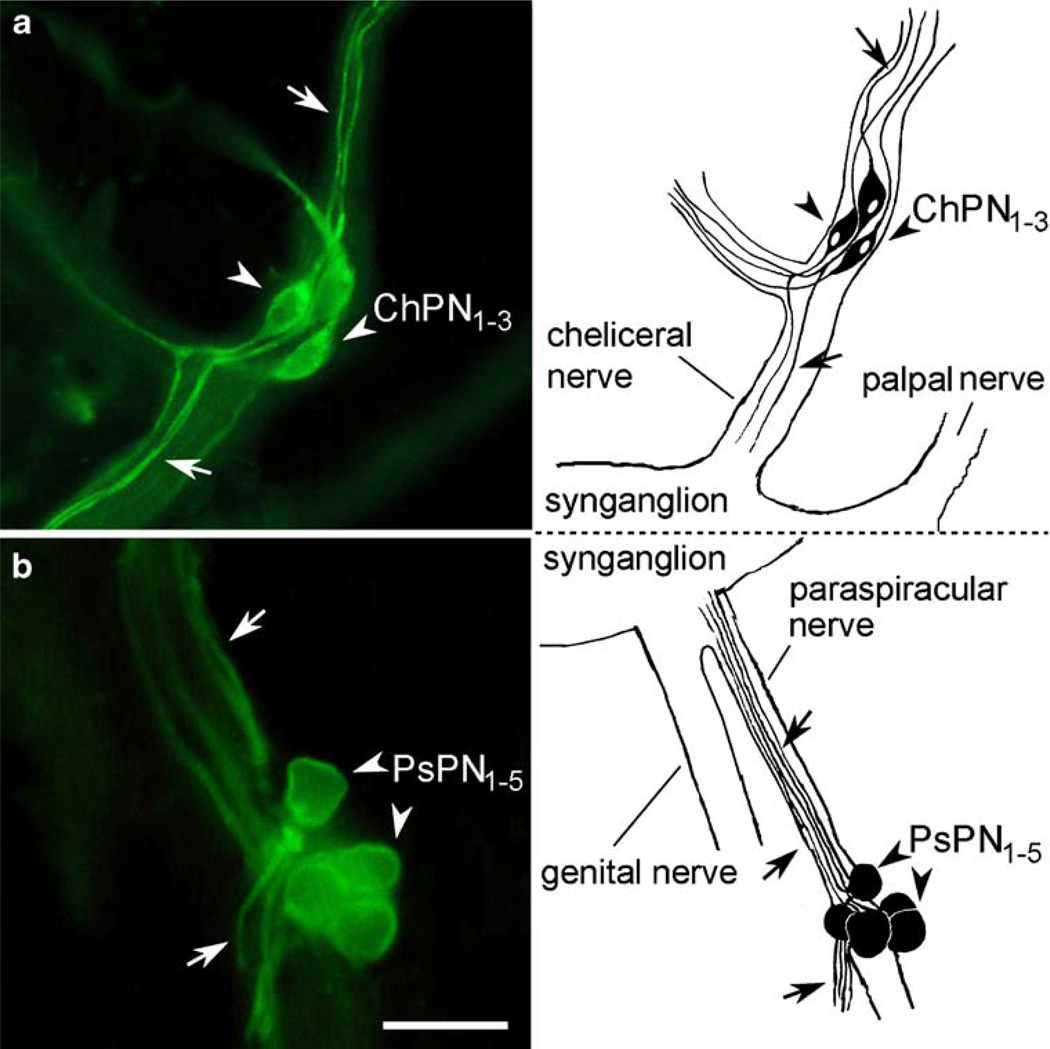
Strong allatotropin-like IR was observed in three to six peripheral neurons contained in the cheliceral nerves. Since these cells had not been described before, we named them the cheliceral peripheral neurons (ChPN1–3). The ChPN1–3 were elongated bipolar neurons, each projecting a single process into the periphery and another opposite bifurcating processes into the synganglion and a side branch of the cheliceral nerve (Fig. 8a).
Fig. 8.
Allatotropin-like IR in the peripheral nervous system. a Cluster of three cheliceral peripheral neurons (ChPN1–3, arrowheads) in each cheliceral nerve. These bipolar neurons project axons into the periphery and into the synganglion (arrows). b Group of five paraspiracular peripheral neurons (PsPN1–5, arrowheads) projecting axons into the synganglion and opposite processes into the periphery through a side branch of the paraspiracular nerve (arrows). Bar 5 µm
We also immunostained five neurons attached to the paraspiracular nerves and named them the paraspiracular peripheral neurons (PsPN1–5; Figs. 8a,b). Each PsPN had a round cell body projecting a single axon into the synganglion and another opposite process into a peripheral side branch of the paraspiracular nerve (Fig. 8b).
Discussion
Synganglion
Several tick species are medically important exoparasites and vectors of serious human pathogens. Thus, it is surprising that so little is known about the regulation of their growth, feeding and reproduction. Numerous studies of other invertebrate and vertebrate models indicate that neuropeptides control these physiological and behavioural processes essential for normal development and survival. Evidence for the presence of neuropeptides in ticks is limited to scarce immunohistochemical staining with antibodies to insect or vertebrate neuropeptides (Davis et al. 1994; Zhu et al. 1995; Zhu and Oliver 1991, 2001). So far, only one neuropeptide related to insect periviscerokinins has recently been identified by MALDI TOF/TOF mass spectrometry in specific central neurons of the ticks Ixodes ricinus and Boophilus microplus (Neupert et al. 2005). The primary structures, functions and cellular sources of other neuropeptides possibly produced by ticks are enigmatic. The genome project of the tick Ixodes scapularis (Hill and Wikel 2005) has recently accelerated progress in the identification of genes encoding putative bioactive peptides (Šimo and Park 2008). To provide more information about the presence of tick bioactive compounds and to associate them with appropriate physiological roles, we have determined the expression patterns of multiple neuropeptides in the CNS, putative neurohaemal/endocrine organs and salivary glands of the tick R. appendiculatus. For this purpose, we have used antibodies against identified and functionally characterized insect and crustacean neuropeptides. Our immunohistochemical study complements previously published observations but has also led to several interesting and novel findings.
Reaction of the bombyxin antibody in the synganglion of R. appendiculatus (this study) is strikingly similar to that revealed with the insulin antibody in the ixodid tick D. variabilis (Davis et al. 1994). Both antibodies immunostain similar prothocerebral, cheliceral and opisthosomal neurons. Insulin-like substance has also been detected in the posterior opisthosomal region of the argasid tick O. parkeri (Zhu and Oliver 1991). These data indicate that ticks produce neuropeptide(s) related to the insulin/bombyxin family in a conserved set of neurons. The possible role of these neuropeptide(s) in metabolic and/or reproductive processes is indicated by reduced insulin-like IR detected after blood feeding and mating in males and females of D. variabilis (Davis et al. 1994). Metabolic and growth regulating functions have been determined for bombyxin in the moth Bombyx mori (Iwami 2000) and insulin-related peptides in the fly Drosophila melanogaster (Colombani et al. 2005).
Cockroach allatostatin-like IR has been described in the synganglion of the American dog tick D. variabilis (Zhu and Oliver 2001); one to nine pairs of stained cells are distributed in each lobe, except for the pedal lobes, which lack immunoreactive neurons. Weaker allatostatin-like IR in freshly ecdysed females accumulate in unfed ticks one month later (Zhu and Oliver 2001). Using the same antibody, we have observed strong allatostatin-like IR in a considerably larger number of neurons in all regions of the synganglion of R. appendiculatus. At least 150 pairs of neurons have been detected in the synganglion of R. appendiculatus compared with ~30 pairs in D. variabilis. Accordingly, the position of allatostatin-like immunoreactive cells often differs in each species. These differences may indicate the functional diversification of allatostatin-like peptides in the different ticks. Indeed, various bioassays in insects indicate that allatostatins ending in FGLamide are multifunctional neuropeptides. They suppress JH biosynthesis only in some cockroaches and few other hemimetaboulous insects, whereas the same or closely related peptides suppress gut and oviduct contractions or inhibit vitellogenin production in various cockroaches, locusts, moths or flies (Bendena et al. 1999; Gäde and Hoffmann 2005).
RFamides belong to a large family of neuropeptides widely distributed in the nervous and endocrine organs of all animals studied. Various neuropeptides sharing the same RFamide C-terminal are encoded by five to six different genes in insects (Roller et al. 2008). Immunohistochemical staining with the FMRFamide antibody indicates that these neuropeptides are also present in ticks. FMRFamide-like imunoreactive neurons are located in various regions of the synganglion of D. variabilis and O. parkeri. FMRFamide-like immunoreactive processes in coxal muscles suggest that these peptides control locomotory activity (Zhu et al. 1995). In this study, we have confirmed the expression of RFamide-related peptides in the synganglion and describe, for the first time, the presence of these peptides in axonal processes on the LSO surface and along salivary ducts. These data indicate that RFamides control a variety of central and peripheral functions, including the activity of secretory cells in the LSO and salivary ducts.
Kinin-like and periviscerokinin-like IR have been described in the synganglion of I. ricinus and B. microplus. The latter neuropeptide has been identified by using MALDI-TOF/TOF and named Ixori-PVK (PALIPFPRV-NH2) since it shows high sequence homology with members of insect periviscerokinins (Neupert et al. 2005). Its C-terminal sequence (−PRVamide) is shared with preecdysis triggering hormone (PETH) and, thus, it is not surprising that our PETH antibody has revealed a similar staining pattern to that of the antibody to PVK. We conclude that the PETH antibody probably reacts with PVK in the synganglion of R. appendiculatus. Moreover, the distribution pattern of kinin-like IR is almost identical in R. appendiculatus (this study) and I. ricinus (Neupert et al. 2005) indicating their conserved function(s) in both species.
Neuropeptide expression patterns in the tick synganglion are, in some cases, comparable with those described in the insect CNS. For example, the restricted PTTH-like IR in only two pairs of lateral neurons of the tick resembles that in the paired PTTH-expressing neurosecretory cells (type III) in moths and Drosophila (Mizoguchi et al. 1990; Žitňan et al. 1993, 1995). Segmental neurons Pd1–4VL showing CCAP-like IR in the tick pedal lobes are similar to interneurons 704 producing CCAP in the abdominal ganglia of many insects (Žitňan and Adams 2005), whereas posterior OsDM1 neurons containing DH/allatostatin-like IR resemble the L3 neurosecretory cells expressing the same neuropeptides in a moth (Davis et al. 1997). Likewise, large OsSG1,2 neurons showing PDF-like IR in the tick are similar to abdominal PDF neurons in a locust and a fly (Nässel et al. 1993; Park et al. 2000; Persson et al. 2001). These staining patterns indicate that some neurons and their active molecules have conserved functions.
The staining patterns with other neuropeptide antibodies appear to be unique for the tick synganglion and are difficult to associate with cellular neuropeptide expression in the CNS of other studied arthropods. This suggests the functional shift of the tick peptidergic network and its modification for the ectoparasitic life characterized by relatively short periods of specific feeding behaviour and prolonged quiescent periods in each developmental stage. Further studies are necessary to reveal the functions of the individual peptidergic neurons and their active messengers in ticks.
Salivary gland innervation
The neuroanatomy of the peripheral nervous system including the innervation of the salivary glands has been described by using classical histological, histochemical and ultrastructural techniques. The innervation of the salivary glands by a pair of nerves from the second pedal ganglion (lobe) was reported for the first time in Haemaphysalis flava (Saito 1960) and the innervation of nongranular and granular alveoli by individual axons was detailed in D. variabilis by Coons and Roshdy (1973). Later, Obenchain and Oliver (1976) reported the innervation of the salivary glands of D. variabilis and Amblyomma tuberculatum by palpal, lateral and paraspiracular nerves. The salivary glands of B. microplus are innervated by fine nerves branching from the palpal nerves and pedal nerves I–III (Binnington 1978). This innervation is involved in the direct control of salivary gland secretion, presumably through the release of catecholamines (e.g. dopamine) from the synganglion, although their cellular sources in the CNS are unknown (Megaw 1977). Quantitative radioenzymatic assay for catecholamines and immunohistochemical staining for tyrosine hydroxylase indicate that specific cells in salivary acini are a major source of dopamine, whereas only a minor component of dopamine is contained in the nerves of Amblyomma hebraeum (Kaufman et al. 1999).
Tick salivary glands are composed of three distinct types of acini (alveoli); nongranular (type I) and granular acini (types II and III) interconnected with salivary ducts. Nongranular acini I are attached to the main salivary duct in the anterior region of the salivary gland and each acinus is composed of a single central cell and several pyramidal, peritubular and constrictor cells. Granular acini II and III differ in their number and structure of granule-secreting cells and acini III are the most peripheral (Coons and Roshdy 1973; Binnington 1978; Megaw and Beadle 1979; Krolak et al. 1982). Salivary gland acini are innervated by axons containing neurosecretory vesicles but their origin has not been determined (Coons and Alberti 1999). Using antibodies to PDF, MIP, RFamide and tachykinin, we have identified several peptidergic neurons innervating salivary glands. Each type of peptidergic neuron produces distinct neuropeptide(s) and has a highly characteristic anatomy and, especially, axon terminals. For example, the OsSG neurons producing PDF-like peptide innervate only acini type II and exhibit anastomosis into three to five axon terminals containing varicosities, whereas the PcSG neurons expressing MIP-like substances show characteristic bifurcating axon terminals for the innervation of acini II and III. MIP-like IR in two to three axon terminals has also been detected in acini II and III of Ixodes scapularis (L. Šimo and Y. Park, unpublished). The PaSG neurons showing RFamide-like IR and the Pd1SG neurons producing tachykinin-like peptide(s) innervate only salivary ducts and never terminate on secretory salivary cells. A similar innervation of salivary acini and ducts has been observed with antibodies to serotonin, dopamine, octopamine and FMRFamide in several insect species. In vitro bioassays indicate that serotonin and dopamine control insect salivation through cyclic AMP, although the precise mechanisms of their action need further studies (Ali 1997). Certain FMRFamide-like peptides from the blowfly Calliphora vomitoria stimulate fluid secretion from isolated salivary glands, whereas other related peptides are inactive (Duve et al. 1992).
The complex innervation of salivary glands by neurons producing PDF-, MIP-, tachykinin- and RFamide-like peptides probably represents an adaptation for the specialized functions associated with the ectoparasitic life of ticks. Our preliminary data indicate a marked decrease of MIP-like and PDF-like IR in salivary gland innervation during the feeding of the adult R. appendiculatus. We speculate that each neuronal type producing distinct neuropeptides controls the activity of specific secretory cells or duct contractions of feeding ticks. Type II and III acini contain characteristic cuticular valvae, whereas type I acini lack these valvae (Binnington 1978). The central lumen of acini II and III opens via these valvae into salivary ducts. The type III acini of female ixodid ticks swell up as they accumulate fluid. Contractions of myoepithelial cells then force the fluid out into expanding salivary ducts (Coons et al. 1994; Lamoreaux et al. 1994, 2000). These observations indicate that MIPlike and PDF-like peptides modulate the opening of valvae and/or myoepithelial cell contractions during tick feeding, in order to release the content of specific secretory cells into the host’s blood.
Additional tachykinin-like and RFamide-like IR has been observed in specific secretory cells of tick salivary glands (not shown in this paper). The active production of small bioactive compounds by salivary glands is not surprising, since tachykinin-related peptides (eleidosin and sialokinins) are produced by the salivary secretory cells of the curled octopus Eledone cirrhosa (Anastasi and Erspamer 1963) and the yellow fewer mosquito Aedes aegypti (Champagne and Ribeiro 1994), respectively.
LSO as secretory glands
Staining with paraldehyde-fuchsin and vital dyes indicates that the LSO are secretory glands (Chow and Wang 1974; Obenchain and Oliver 1975; Panfilova 1978). They increase in size during feeding of females Ixodes persulcatus, as shown by Panfilova (1978). This author has proposed that the LSO are similar to neurohaemal perisympathetic organs of insects and that their size increase correlates with vitellogenesis in female oocytes. Ultrastructural studies suggest that LSO cells exhibit profiles characteristic for steroid-hormone-secreting tissues (Binnington 1981). These organs are likely candidates for endocrine functions but the nature of their secretion(s) has not been determined (Sonenshine 1991). Our immunohistochemical staining with antibodies against bombyxin and PDF indicate that the LSOs are composed of peptidergic endocrine cells and therefore probably do not produce ecdysteroids. RFamide-like IR in axons and their terminals on the LSO surface also indicates a neurohaemal function of these organs reminiscent of the corpora cardiaca in insects.
Peripheral nervous system
Our antibody to allatotropin has revealed clusters of novel peripheral neurons in the cheliceral and paraspiracular nerves (ChPN and PsPN). These bipolar neurons project axons into the synganglion and periphery. In insects, similar bipolar link neurons (L1) located in the periphery close to spiracles innervate the heart and neurohaemal transverse nerves or perisympathetic organs. Immunohistochemical and in situ hybridization techniques have shown that L1 neurons are peptidergic and express CCAP, FLRFamide-related neuropeptide (F10 or myosupressin) and non-amidated ITP-like peptide (ITPL; Davis et al. 1993; Dai et al. 2007; Lu et al. 2002). Crustacean hyperglycemic hormone (CHH)-like peptides related to ITPL are expressed in neurosecretory cells of the peripheral pericardial organs of crabs (Dircksen et al. 2001). These crab cells are probably homologous to insect L1 neurons (Dircksen and Heyn 1998). It will be interesting to determine whether peptidergic cells in the peripheral nervous systems of ticks, crabs and insects are of the same origin.
Concluding remarks
The results of our study indicate that ticks produce a large variety of neuropeptides by various types of central and peripheral neurons and by endocrine cells. The peptidergic nature and anatomy of various neurons, neurohaemal/neuromodulatory structures and endocrine cells has been described here for the first time in ticks. The physiological functions of these neuropeptides and cells are unknown and require further studies. Some of these peptidergic structures are probably adapted for the ectoparasitic life of ticks and themodulation of the host haemostatic and immune responses.
Acknowledgements
We are grateful to Drs. M.E. Adams, H. Dircksen, C.J.P. Grimmelikhuijzen, D. Kodrík, A. Mizoguchi, D.R. Nässel, L. Schoofs and Y. Tanaka for providing antibodies to AKH, corazonin, bombyxin, PTTH, ITP, MIP, BMS, leucokinin, tachykinin and PDF.
This work was supported by Slovak grant agencies: Agentúra na podporu výskumu a vývoja (APVV-51-039105) and Vedecká grantová agentúra (VEGA 2-6090-26 and 2/6155/26).
Contributor Information
Ladislav Šimo, Institute of Zoology, Slovak Academy of Sciences, Dúbravská Cesta 9, 84506 Bratislava, Slovakia; Department of Entomology, Kansas State University, Manhattan, KS 66506-4004, USA.
Mirko Slovák, Institute of Zoology, Slovak Academy of Sciences, Dúbravská Cesta 9, 84506 Bratislava, Slovakia.
Yoonseong Park, Department of Entomology, Kansas State University, Manhattan, KS 66506-4004, USA.
Dušan Žitňan, Email: dusan.zitnan@savba.sk, Institute of Zoology, Slovak Academy of Sciences, Dúbravská Cesta 9, 84506 Bratislava, Slovakia.
References
- Ali DW. The aminergic and peptidergic innervation of insect salivary glands. J Exp Biol. 1997;200:1941–1949. doi: 10.1242/jeb.200.14.1941. [DOI] [PubMed] [Google Scholar]
- Anastasi A, Erspamer V. The isolation and amino acid sequence of eleidosin, the active endecapeptide of the posterior salivary glands of Eledone. Arch Biochem Biophys. 1963;101:56–65. doi: 10.1016/0003-9861(63)90533-2. [DOI] [PubMed] [Google Scholar]
- Balashov YS. Iksodovyje kleshchi - parazity i perenoschiki infektsij (in English: Ixodid ticks – parasites and vectors of diseases) Sankt-Peterburg: Nauka; 1998. [Google Scholar]
- Bendena WG, Donly BC, Tobe SS. Allatostatins: a growing family of neuropeptides with structural and functional diversity. Ann NY Acad Sci. 1999;897:311–329. doi: 10.1111/j.1749-6632.1999.tb07902.x. [DOI] [PubMed] [Google Scholar]
- Binnington KC. Sequential changes in salivary gland structure during attachment and feeding of the catle tick, Boophilus microplus. Int J Parasitol. 1978;8:97–115. doi: 10.1016/0020-7519(78)90004-8. [DOI] [PubMed] [Google Scholar]
- Binnington KC. Ultrastructural evidence for the endocrine nature of the lateral organs of the cattle tick, Boophilus microplus. Tissue Cell. 1981;13:475–490. doi: 10.1016/0040-8166(81)90020-3. [DOI] [PubMed] [Google Scholar]
- Binnington KC. Histology and ultrastructure of the acarine synganglion. In: Gupta AP, editor. Arthropod brain. Its evolution, development, structure, and functions. New York: Wiley; 1987. pp. 95–109. [Google Scholar]
- Binnington KC, Tatchell RJ. The nervous system and neurosecretory cells of Boophilus microplus (Acarina, Ixodidae) Z Wiss Zool. 1973;185:193–206. [Google Scholar]
- Champagne DE, Ribeiro J. Sialokinin I and II. Vasodilatory tachykinins from the yellow fever mosquito Aedes aegypti. Proc Natl Acad Sci USA. 1994;91:138–142. doi: 10.1073/pnas.91.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang ES, Kaufman WR. Endocrinology of Crustacea and Chelicerata. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive molecular insect science, vol 3. Amsterdam-Tokyo: Elsevier Pergamon; 2005. pp. 805–842. [Google Scholar]
- Chow YS, Wang CH. Neurosecretory cells and their ultrastructures of Rhipicephalus sanguineus (Latreille) (Acarina, Ixodidae) Acta Arachnol. 1974;25:53–67. [Google Scholar]
- Chow YS, Lin SH, Wang CH. An ultrastructural and electrophysiological study of the brain of the brown dog tick Rhipicephalus sanguineus (Latreille) Chin Biosci. 1972;I:83–92. [Google Scholar]
- Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin- Villemant C, Antoniewski C, Carre C, Noselli S, Leopold P. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310:667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- Coons LB, Alberti G. The Acari-ticks. In: Harrison FW, Foelix R, editors. Microscopic anatomy of invertebrates, vol 8B. Chelicerate Arthropoda. New York: Wiley-Liss; 1999. pp. 267–514. [Google Scholar]
- Coons LB, Roshdy MA. Fine structure of the salivary glands of unfed male Dermacentor variabilis (Say) (Ixodoidea: Ixodidae) J Parasitol. 1973;59:900–912. [PubMed] [Google Scholar]
- Coons LB, Roshdy MA, Axtell RC. Fine structure of the central nervous system of Dermacentor variabilis (Say), Amblyomma americanum (L.), and Argas arboreus Kaiser, Hoogstraal, and Kohls (Ixodoidea) J Parasitol. 1974;60:687–698. [PubMed] [Google Scholar]
- Coons LB, Lessman CA, Ward MA, Berg RH, Lamoreaux WJ. Evidence of a myoepithelial cell in tick salivary glands. Int J Parasitol. 1994;24:551–562. doi: 10.1016/0020-7519(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Dai L, Žitňan D, Adams ME. Strategic expression of ion transport peptide gene products in central and peripheral neurons of insects. J Comp Neurol. 2007;500:353–367. doi: 10.1002/cne.21192. [DOI] [PubMed] [Google Scholar]
- Davis HH, Dotson EM, Oliver JH., Jr Localization of insulinlike immunoreactivity in the synganglion of nymphal and adult Dermacentor variabilis (Acari: Ixodidae) Exp Appl Acarol. 1994;18:111–122. doi: 10.1007/BF00055035. [DOI] [PubMed] [Google Scholar]
- Davis NT, Homberg U, Dircksen H, Levine RB, Hildebrand JG. Crustacean cardioactive peptide-immunoreactive neurons in the hawkmoth Manduca sexta and changes in their immunoreactivity during postembryonic development. J Comp Neurol. 1993;338:612–627. doi: 10.1002/cne.903380410. [DOI] [PubMed] [Google Scholar]
- Davis NT, Veenstra JA, Feyereisen R, Hildebrand JG. Allatostatin-like-immunoreactive neurons of the tobacco hornworm, Manduca sexta and isolation and identification of a new neuropeptide related to cockroach allatostatins. J Comp Neurol. 1997;385:265–284. doi: 10.1002/(sici)1096-9861(19970825)385:2<265::aid-cne6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Dircksen H, Heyn U. Crustacean hyperglycemic hormone-like peptides in crab and locust peripheral intrinsic neurosecretory cells. Ann NY Acad Sci. 1998;839:392–394. [Google Scholar]
- Dircksen H, Böcking D, Heyn U, Mandel C, Chung JS, Baggerman G, Verhaert P, Daufeldt S, Plösch T, Jaros PP, Waelkens E, Keller R, Webster SG. Crustacean hyperglycemic hormone (CHH)-like peptides and CHHprecursor related peptides from pericardial organ neurosecretory cells in the shore crab, Carcinus maenas are putatively spliced and modified products of multiple genes. Biochem J. 2001;356:159–170. doi: 10.1042/0264-6021:3560159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duve H, Johnsen AH, Sewell JC, Scott AG, Orchard I, Rehfeld JF, Thorpe A. Isolation, structure, and activity of -Phe-Met- Arg-Phe-NH2 neuropeptides (designated calliFMRFamides) from the blowfly Calliphora vomitoria. Proc Natl Acad Sci USA. 1992;89:2326–2330. doi: 10.1073/pnas.89.6.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Shoura SM. Ultrastructure of the lateral organs in larval Argas (Persicargas) arboreus (Ixodoidea: Argasidae) Exp Appl Acarol. 1989;7:231–237. [Google Scholar]
- Gäde G, Hoffmann KH. Neuropeptides regulating development and reproduction in insects. Physiol Entomol. 2005;30:103–121. [Google Scholar]
- Grimmelikhuijzen CJP, Spencer AN. FMRFamide immunoreactivity in the nervous system of the medusa Polyorchis penicillatus. J Comp Neurol. 1984;230:361–371. doi: 10.1002/cne.902300305. [DOI] [PubMed] [Google Scholar]
- Hill CA, Wikel SK. The Ixodes scapularis genome project: an opportunity for advancing tick research. Trends Parasitol. 2005;21:151–153. doi: 10.1016/j.pt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Holmes SP, He H, Chen AC, Ivie GV, Pietrantonio PV. Cloning and transcriptional expression of a leucokinin-like peptide receptor from the southern cattle tick, Boophilus microplus (Acari: Ixodidae) Insect Mol Biol. 2000;9:457–465. doi: 10.1046/j.1365-2583.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- Holmes SP, Barhoumit R, Nachman RJ, Pietrantonio PV. Functional analysis of a G protein-coupled receptor from the southern cattle tick Boophilus microplus (Acari: Ixodidae) identifies it as the first arthropod myokinin receptor. Insect Mol Biol. 2003;12:27–38. doi: 10.1046/j.1365-2583.2003.00384.x. [DOI] [PubMed] [Google Scholar]
- Iwami M. Bombyxin: an insect brain peptide that belongs to the insulin family. Zool Sci. 2000;17:1035–1044. doi: 10.2108/zsj.17.1035. [DOI] [PubMed] [Google Scholar]
- Kaufman WR. Harris KF. Current topics in vector research. New York: Praeger Scientific; 1983. The function of tick salivary glands; pp. 215–247. [Google Scholar]
- Kaufman WR, Sloley BD, Tatchell RJ, Zbitnew GL, Diefenbach TJ, Goldberg JI. Quantification and cellular localization of dopamine in the salivary gland of the ixodid tick Amblyomma hebraeum. Exp Appl Acarol. 1999;23:251–265. [Google Scholar]
- Kim Y-J, Žitňan D, Cho K-H, Mizoguchi A, Schooley D, Adams ME. Central peptidergic ensembles associated with organization of an innate behavior. Proc Natl Acad Sci USA. 2006;103:14211–14216. doi: 10.1073/pnas.0603459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolak JM, Ownby CL, Sauer JR. Alveolar structure of salivary glands of the lone star tick, Amblyomma americanum (L.): unfed females. J Parasitol. 1982;68:61–82. [PubMed] [Google Scholar]
- Lamoreaux WJ, Needham GR, Coons LB. Fluid secretion by isolated tick salivary glands depends on an intact cytoskeleton. Int J Parasitol. 1994;24:563–567. doi: 10.1016/0020-7519(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Lamoreaux WJ, Needham GR, Coons LB. Evidence that dilation of isolated salivary ducts from the tick Dermacentor variabilis (Say) is mediated by nitric oxide. J Insect Physiol. 2000;46:959–964. doi: 10.1016/s0022-1910(99)00205-x. [DOI] [PubMed] [Google Scholar]
- Lees K, Bowman AS. Tick neurobiology: recent advances and the post-genomic era. Invert Neurosci. 2007;7:183–198. doi: 10.1007/s10158-007-0060-4. [DOI] [PubMed] [Google Scholar]
- Liang JG, Zhang J, Lai R, Rees HH. An opioid peptide from synganglia of the tick, Amblyomma testindinarium. Peptides. 2005;26:603–606. doi: 10.1016/j.peptides.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lomas LO, Turner PC, Rees HH. A novel neuropeptideendocrine interaction controlling ecdysteroid production in ixodid ticks. Proc R Soc Lond [Biol] 1997;264:589–596. doi: 10.1098/rspb.1997.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Lee KY, Horodyski FM, Witten JL. Molecular characterization and cell-specific expression of a Manduca sexta FLRFamide gene. J Comp Neurol. 2002;446:377–396. doi: 10.1002/cne.10205. [DOI] [PubMed] [Google Scholar]
- Marzouk AS, Mohamed FSA, Khalil GM. Neurohemalendocrine organs in the camel tick, Hyalomma dromedarii (Acari: Ixodoidea: Ixodidae) J Med Entomol. 1985;22:385–391. [Google Scholar]
- Megaw MWJ. The innervation of the salivary gland of the tick, Boophilus microplus. Cell Tissue Res. 1977;184:551–558. doi: 10.1007/BF00220978. [DOI] [PubMed] [Google Scholar]
- Megaw MWJ, Beadle DJ. Structure and function of the salivary glands of the tick Boophilus microplus Canestrini (Acarina: hodidae) Int J Insect Morphol Embryol. 1979;8:67–83. [Google Scholar]
- Mizoguchi A, Ishizaki H, Nagasawa H, Kataoka H, Isogai A, Tamura S, Suzuki A, Fujino M, Kitada C. A monoclonal antibody against a synthetic fragment of bombyxin (4K-prothoracicotropic hormone) from the silkmoth, Bombyx mori ; characterization and immunohistochemistry. Mol Cell Endocrinol. 1987;51:227–235. doi: 10.1016/0303-7207(87)90032-3. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Oka T, Kataoka H, Nagasawa H, Suzuki A, Ishizaki H. Immunohistochemical localization of prothoracicotropic hormone-producing neurosecretory cells in the brain of Bombyx mori. Dev Growth Differ. 1990;32:591–598. doi: 10.1111/j.1440-169X.1990.00591.x. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Cantera R, Karlsson A. Neurons in the cockroach nervous system reacting with antisera to the neuropeptide leucokinin I. J. Comp Neurol. 1992;322:45–67. doi: 10.1002/cne.903220105. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Shiga S, Mohrherr CJ, Rao KR. Pigment-dispersing hormone-like peptide in the nervous system of the flies Phormia and Drosophila : immunocytochemistry and partial characterization. J Comp Neurol. 1993;331:183–198. doi: 10.1002/cne.903310204. [DOI] [PubMed] [Google Scholar]
- Neupert S, Predel R, Russell WK, Davies R, Pietrantonio PV, Nachman RJ. Identification of tick periviscerokinin, the first neurohormone of Ixodidae: single cell analysis by means of MALDI-TOF/TOF mass spectrometry. Biochem Biophys Res Commun. 2005;338:1860–1864. doi: 10.1016/j.bbrc.2005.10.165. [DOI] [PubMed] [Google Scholar]
- Obenchain FD. Neurosecretory system of the American dog tick, Dermacentor variabilis (Acari: Ixodidae)IDiversity of cell types. J Morphol. 1974;142:433–446. doi: 10.1002/jmor.1051420406. [DOI] [PubMed] [Google Scholar]
- Obenchain FD, Oliver JH., Jr Neurosecretory system of the American dog tick, Dermacentor variabilis (Acari: Ixodidae). II. Distribution of secretory cell types, axonal pathways and putative neurohemal-neuroendocrine associations; comparative histological and anatomical implications. J Morphol. 1975;145:269–294. doi: 10.1002/jmor.1051450303. [DOI] [PubMed] [Google Scholar]
- Obenchain FD, Oliver JH., Jr Peripheral nervous system of the ticks, Amblyomma tuberculatum Marx and Argas radiatus Railliet (Acari: Ixodoidea) J Parasitol. 1976;62:811–817. [PubMed] [Google Scholar]
- Panfilova IM. Lateral”nye organy Ixodes persulcatus (Parasitiformes, Ixodidae) (in English: lateral organs in Ixodes persulcatus (Parasitiformes, Ixodidae) Zool Zh. 1978;57:190–196. [Google Scholar]
- Park JH, Helfrich-Förster C, Lee G, Liu L, Rosbash M, Hall JC. Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc Natl Acad Sci USA. 2000;97:3608–3613. doi: 10.1073/pnas.070036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson MG, Eklund MB, Dircksen H, Muren JE, Nässel DR. Pigment-dispersing factor in the locust abdominal ganglia may have roles as circulating neurohormone and central neuromodulator. J Neurobiol. 2001;48:19–41. doi: 10.1002/neu.1040. [DOI] [PubMed] [Google Scholar]
- Pound MJ, Oliver JH., Jr Synganglial and neurosecretory morphology of female Ornithodoros parkeri (Cooley) (Acari: Argasidae) J Morphol. 1982;173:159–177. doi: 10.1002/jmor.1051730204. [DOI] [PubMed] [Google Scholar]
- Prullage JB, Pound JM, Meola SM. Synganglial morphology and neurosecretory centers of adult Amblyomma americanum (L.) (Acari: Ixodidae) J Med Entomol. 1992;29:1023–1034. doi: 10.1093/jmedent/29.6.1023. [DOI] [PubMed] [Google Scholar]
- Rees HH. Hormonal control of tick development and reproduction. Parasitology. 2004;129:S127–S143. doi: 10.1017/s003118200400530x. [DOI] [PubMed] [Google Scholar]
- Roller L, Tanaka Y, Tanaka S. Corazonin and corazonin-like substances in the central nervous system of the pterygote and apterygote insects. Cell Tissue Res. 2003;312:393–406. doi: 10.1007/s00441-003-0722-4. [DOI] [PubMed] [Google Scholar]
- Roller L, Yamanaka N, Watanabe K, Daubnerová I, Žitňan D, Kataoka H, Tanaka Y. The unique evolution of neuropeptide genes in the silkworm Bombyx mori. Insect Biochem Mol Biol. 2008 doi: 10.1016/j.ibmb.2008.04.009. (in press) [DOI] [PubMed] [Google Scholar]
- Roshdy MA, Shoukrey NM, Coons LB. The subgenus Persicargas (Ixodoidea: Argasidae: Argas). 17. A neurohemal organ in A. (P.) arboreus Kaiser, Hoogstraal and Kohls. J Parasitol. 1973;59:530–544. [Google Scholar]
- Saito Y. Studies on ixodid ticks. Part IV. The internal anatomy in each stage of Haemaphysalis flava Neuman, 1897. Acta Med Biol. 1960;8:189–239. [Google Scholar]
- Schoofs L, Vanden Broeck J, De Loof A. The myotropic peptides of Locusta migratoria : structures, distribution, functions and receptors. Insect Biochem Mol Biol. 1993;23:859–881. doi: 10.1016/0965-1748(93)90104-z. [DOI] [PubMed] [Google Scholar]
- Šimo L, Park Y. Genomics and proteomics of neuropeptides in the black-legged tick Ixodes scapularis; Second Annual Arthropod Genomics Symposium, April 10–13, 2008, Kansas City.2008. [Google Scholar]
- Slovák M, Labuda M, Marley SE. Mass laboratory rearing of Dermacentor reticulatus ticks (Acarina, Ixodidae) Biologia (Bratislava) 2002;57:261–266. [Google Scholar]
- Sonenshine DE. Biology of ticks, vol 1. New York Oxford: Oxford University Press; 1991. [Google Scholar]
- Stangier J, Hilbich C, Dircksen H, Keller R. Distribution of a novel cardioactive neuropeptide (CCAP) in the nervous system of the shore crab Carcinus maenas. Peptides. 1988;4:795–800. doi: 10.1016/0196-9781(88)90124-6. [DOI] [PubMed] [Google Scholar]
- Stay B, Chan KK, Woodhead AP. Allatostatin-immunoreactive neurons projecting to the corpora allata of adult Diploptera punctata. Cell Tissue Res. 1992;270:15–23. doi: 10.1007/BF00381875. [DOI] [PubMed] [Google Scholar]
- Szlendak E, Oliver JH., Jr Anatomy of synganglia, including their neurosecretory regions, in unfed, virgin female Ixodes scapularis Say (Acari: Ixodidae) J Morphol. 1992;213:349–364. doi: 10.1002/jmor.1052130308. [DOI] [PubMed] [Google Scholar]
- Taneja-Bageshwar S, Strey A, Zubrzak P, Pietrantonio PV, Nachman RJ. Comparative structure-activity analysis of insect kinin core analogs on recombinant kinin receptors from southern cattle tick Boophilus microplus (Acari: Ixodidae) and mosquito Aedes aegypti (Diptera: Culicidae) Arch Insect Biochem Physiol. 2006;62:128–140. doi: 10.1002/arch.20129. [DOI] [PubMed] [Google Scholar]
- White K, Hurteau T, Punsal P. Neuropeptide FMRFamide-like immunoreactivity in Drosophila. Wilhem Roux’s Arch. 1986;142:131–182. doi: 10.1002/cne.902470403. [DOI] [PubMed] [Google Scholar]
- Yamanaka N, Žitňan D, Kim Y-J, Adams ME, Hua Y-J, Suzuki Y, Suzuki M, Suzuki A, Satake H, Mizoguchi A, Asaoka K, Tanaka Y, Kataoka H. Regulation of insect steroid hormone biosynthesis by innervating peptidergic neurons. Proc Natl Acad Sci USA. 2006;103:8622–8627. doi: 10.1073/pnas.0511196103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XX, Oliver JH., Jr Immunocytochemical localization of an insulin like substance in the synganglion of the tick Ornithodoros parkeri (Acari: Argasidae) Exp Appl Acarol. 1991;13:153–159. doi: 10.1007/BF01193666. [DOI] [PubMed] [Google Scholar]
- Zhu XX, Oliver JH., Jr Cockroach allatostatin-like immunoreactivity in the synganglion of the American dog tick Dermacentor variabilis (Acari: Ixodidae) Exp Appl Acarol. 2001;25:1005–1013. doi: 10.1023/a:1020664211999. [DOI] [PubMed] [Google Scholar]
- Zhu XX, Zhang WY, Oliver JH., Jr Immunocytochemical mapping of FMRFamide-like peptides in the argasid tick Ornithodoros parkeri and the ixodid tick Dermacentor variabilis. Exp Appl Acarol. 1995;19:1–9. doi: 10.1007/BF00051932. [DOI] [PubMed] [Google Scholar]
- Žitňan D, Adams ME. Neuroendocrine regulation of insect ecdysis. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive molecular insect science, vol 3. Amsterdam- Tokyo: Elsevier Pergamon; 2005. pp. 1–60. [Google Scholar]
- Žitňan D, Sehnal F, Bryant P. Neurons producing specific neuropeptides in the central nervous system of normal and pupariation-delayed Drosophila. Dev Biol. 1993;155:682–693. doi: 10.1006/dbio.1993.1063. [DOI] [PubMed] [Google Scholar]
- Žitňan D, Kingan TG, Kramer SJ, Beckage NE. Accumulation of neuropeptides in the cerebral neurosecretory system of Manduca sexta larvae parasitized by the braconid wasp Cotesia congregata. J Comp Neurol. 1995;356:83–100. doi: 10.1002/cne.903560106. [DOI] [PubMed] [Google Scholar]
- Žitňan D, Ross LS, Žitňanová I, Hermesman JL, Gill SS, Adams MA. Steroid induction of a peptide hormone gene leads to orchestration of a defined behavioral sequence. Neuron. 1999;23:523–535. doi: 10.1016/s0896-6273(00)80805-3. [DOI] [PubMed] [Google Scholar]
- Žitňan D, Hollar L, Spalovská I, Takáč P, Žitňanová I, Gill SS, Adams ME. Molecular cloning and function of ecdysis-triggering hormones in the silkworm Bombyx mori. J Exp Biol. 2002;205:3459–3473. doi: 10.1242/jeb.205.22.3459. [DOI] [PubMed] [Google Scholar]
- Žitňanová I, Adams ME, Žitňan D. Dual ecdysteroid action on the epitracheal glands and central nervous system preceding ecdysis of Manduca sexta. J Exp Biol. 2001;204:3483–3495. doi: 10.1242/jeb.204.20.3483. [DOI] [PubMed] [Google Scholar]