Abstract
Background:
Stress has negative effect on health and type 2 diabetes patients may be at an increased risk. Abnormally high levels of free radicals and the simultaneous decline of antioxidant defense mechanisms can increase lipid peroxidation and insulin resistance. The objective of the present study was to demonstrate the efficacy of yogic practice in geriatric patients with type 2 diabetes mellitus and also to compare the efficacy with the state of glycaemic control.
Materials and Methods:
Seventy three (73) healthy elderly patients of type 2 diabetes mellitus in the age group of 60 to 70 years with a history of diabetes for 5 to 10 years and with poor glycaemic control (HbA1c >8 %) residing in Kozhikode district were recruited for the study. The subjects were divided into three groups according to their glycaemic control. Group I with HbA1c 8.6–9.7 %, group II with HbA1c 9.8–10.7 % and group III with HbA1c 10.8–12.7 %. Participants did yogic practice under the supervision of experienced trainer, daily 90 minutes and for three months. Biochemical estimation of HbA1c, glucose, lipid profile, cortisol, ferritin, malondialdehyde (MDA) and catalase activity were carried out on 0 day and 90th day. Seventy patients participated in a comparable control session.
Results:
The participants in the test group showed statistically significant (P < 0.001) decrease in glucose, HbA1c, lipids, cortisol, ferritin, MDA and significant increase in catalase activity after yogic practice.
Conclusions:
Yoga may improve risk profiles induced by stress in geriatric patients with type 2 diabetes and may have promise for the prevention or delay in diabetes complications. And at all stages of the disease a significant improvement can be achieved by yogic practice in geriatric diabetes.
Keywords: Geriatric, glycaemia, HbA1c, stress, yogic practice
INTRODUCTION
It is widely recognized that stress may have negative effects on health and that patients with type 2 diabetes may be at an increased risk. Stress is a term commonly used in psychological, biological and medical sciences. The concept of stress was developed in the 1930s by the endocrinologist Hans Selye. It is defined as “the non specific response of the body to any demand”. The experience of stress is associated with the release of counter regulatory hormones including cortisol and energy mobilization. Cortisol counteracts with insulin and causes enhanced glucose production by hepatic gluconeogenesis and by inhibiting the peripheral utilization of glucose[1] and contributes to the development of the entire spectrum of the metabolic syndrome, including visceral obesity, insulin resistance and dyslipidemia.[2]
Oxidative stress due to the production of reactive oxygen species including free radicals and peroxides by cells during metabolism of oxygen induces hyperglycaemia. Free radicals are very reactive chemical species and can cause damage to cellular proteins, membrane lipids and nucleic acids eventually leading to cell death.[3] Hydroperoxides have toxic effects on cells both directly and through degradation to highly toxic hydroxyl radicals. They may also react with transition metals like iron or copper to form stable aldehydes such as malondialdehydes that damage cell membranes. Peroxyl radicals can remove hydrogen from lipids, producing hydroperoxides that further propagate the free-radical pathway. Increased oxidative stress is a widely accepted phenomenon in the development and progression of diabetes and its complications.[4] Cells manifest potent antioxidant defenses against reactive oxygen species (ROS). Detoxifying enzymes that convert ROS to less reactive molecules are superoxide dismutase, catalase and glutathione peroxidase.
Iron takes part in the formation of highly toxic free radicals such as hydroxide and superoxide anions, which can induce lipid peroxidation.[5] Levels of plasma ferritin, a biomarker of iron stores, are also elevated in persons with prevalent diabetes as compared with non diabetic controls. Elevated iron stores may induce diabetes through a variety of mechanisms, including oxidative damage to pancreatic beta cells, impairment of hepatic insulin extraction by the liver, and interference with insulin's ability to suppress hepatic glucose production.[6]
Most developed countries in the world have accepted the chronological age of 65 years as a definition of “elderly”. In the year 2002, the number of elderly people in India was about 75 million and it is projected to rise to 9 % of the total population by 2016 and from global perspective the elderly will constitute one third of the total population of the world by 2050. Health economic models have suggested that benefits of treating glycaemia may be less in older than in younger patients.[7] Interest in and the use of complementary and alternative medicine has recently expanded in many countries around the world. Through the use of complementary therapies, diabetic patients can be alleviated from complications due to the chronic course of the disease, the debilitation of complications and threat of death, as well as the complexities of treatment plans.[8]
Yoga is an ancient Indian psychological and physical exercise regimen and a number of controlled studies exist on the effectiveness of Yoga on diabetes mellitus.[9] Yoga includes meditation, relaxation, control of breathing and various physical postures (asanas). Regular practice of Yoga establishes natural harmony and functional balance between various organ systems, leading to better health and a feeling of well-being. Yogic practice strengthens and increases the tone of weak muscles and helps with conscious control over autonomic functions of the body.[10] It has been used clinically as a therapeutic intervention and its practice develops correct breathing patterns, behavioral modification and dietary control through mental discipline.[11,12]
Although, Yoga may not be able to cure diabetes completely in elderly patients, it can complement with the lifestyle changes which are necessary to keep the symptoms in diabetics under control and protect them from long term complications. The advantages of Yoga as an option for physical activity in diabetes are linked to a wider lifestyle package with relaxation and stress management.[13] So, the objective of this study is to evaluate the impact of yogic practice on stress and oxidant/antioxidant system in elderly type 2 diabetic patients. For that, we estimated a few related biochemical parameters glucose, HbA1c, lipid profile, cortisol, MDA, ferritin and catalase activity levels in blood before and after 3 months of yogic practice. Changes were observed and were compared with the control group. They were matched with respect to age, socio-economical status and glycaemic level in base line parameters.
MATERIALS AND METHODS
This prospective study was conducted among type 2 diabetes mellitus patients during March 2009 to December 2010. The following two procedures for the recruitment of subjects were adopted: (i) From among patients who visited the diabetology department of the Malabar Institute of Medical Science Ltd, Kozhikode and who were residents of Kozhikode district, Kerala, India; (ii) news paper advertisements were published by the investigators in the local dailies giving information regarding the scientific study, and inviting subjects to volunteer for the study.
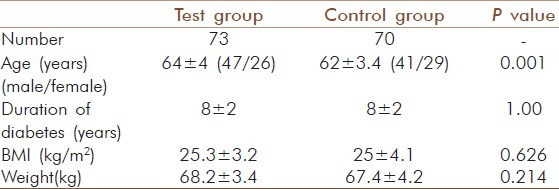
Patients who were clinically diagnosed to have complications like coronary artery disease, renal disease, malignancies, acute infections and other major complications were excluded from the study. A total of 143 healthy elderly patients of type 2 diabetes mellitus in the age group of 60 to 70 years with a history of diabetes for 5 to 10 years and with poor glycaemic control (HbA1c > 8 %) residing in Kozhikode district were recruited for the study. Informed consent was obtained from all the subjects involved in the study and approval of the institutional ethics committee was also obtained. All the subjects belonged to the “middle class” by socioeconomic considerations. They were literate and all were living with their families. The participants had non vegetarian food habits, and the diet patterns were also mostly similar and this status was maintained during the study. Prior to the study, all patients were on conventional medicine (metformin) and during the study period they were advised to continue the same medicine without alteration of the dosage. The demographic characteristics are reported in Table 1.
Table 1.
Demographic characteristics of participants (mean±SD)
An educational program was conducted for the participants to generate basic awareness in yogic practice in daily life. The importance of Yoga and its beneficial effect in diabetic patients was stressed. Those who were interested in yogic practice joined in the test group and others remained as controls. Seventy three patients who joined in the test group, practiced Yoga in Yogabharathy Yoga Centre, Vatakara, Kozhikode, for a period of three months under the supervision of experienced trainer for 90 min a day and six days a week. Every subject received personalized attention and supervision during Yoga session. Each Yoga session consisted of 15 min of pranayamas (breath-control exercises), 10 min of warm-up exercises, 50 min of asanas (yogic postures), and 15 min of supine relaxation in savasana.[14] All the participants were in good health and they did Yoga practice with obvious enthusiasm; they appeared to be in a relaxed manner throughout the exercise period and liked the rhythmic movements of Yoga. The 70 patients in the control group were asked to maintain their routine activities such as walking and other non specific exercises and not to begin any activity program during the course of this study. The total patients were again subdivided into three groups according to their HbA1c value: group 1 with HbA1c 8.6–9.7 %, group 2 with HbA1c 9.8–10.7 % and group 3 with HbA1c 10.8–12.7 %. Fasting blood glucose, cholesterol, triglyceride, HDL-cholesterol were estimated using Siemens Dimension clinical chemistry analyzer and HbA1c in Bio-rad D 10 analyzer, cortisol and ferritin in Cobas E 411 Immunoassay analyzer. Lipid peroxidation was determined by measuring malondialdehyde formed by thiobarbituric acid (TBA) reaction.[15] Catalase activity was estimated by measuring the rate of decomposition of H2O2.[16] All the parameters were assessed in zero day and 90th day in fasting blood sample in both test and control groups. All estimates were presented as means +/- SE. In this single-blinded study the clinician (who conducted the biochemical assessments) was blind to the group status of the patients.
Statistical analysis
The normality of the variables considered was tested using Kolmogorov - Smirnov test and we found that except triglyceride and HDL - cholesterol, all other parameters followed normal distribution. So we tested the effect of yogic practice on the levels of triglyceride and HDL - cholesterol using Wilcoxon Signed Rank test and paired t test for other variables. The difference between the triglyceride levels of control and test group at base line values of TGL and HDL-cholesterol were analyzed by Mann–Whitney Test and independent sample t test for other variables. SPSS 16.0 program and Microsoft Excel 2003 were used to perform statistical analysis. Level of significance was set at P < 0.05.
RESULTS
Of the 143 geriatric patients in the study with type 2 diabetes, there were 88 males and 55 females. Subjects matched according to age and gender and the mean age were similar in control and test groups [Table 1]. There was no significant gender-specific difference in demographic and biochemical parameters measured in the study. All 143 patients completed the study by participating in the three months assessment. Significant decrease (P = 0.000) was observed for HbA1c after 3 months yogic practice in group I (9.40 ± 0.050 to 8.36 ± 0.05 %), group II (10.17 ± .046 to 8.9 ± 0.048 %) and group III (11.22 ± 0.069 to 10 ± 0.086 %). In contrast to this, a significant increase with P = 0.005 in control group I (9.27 ± 0.049 to 9.36 ± 0.057 %) and P=0.000 in control group II (10.17 ± 0.063 to 10.31 ± 0.083 %) and control group III (11.48 ± 0.187 to 11.80 ± 0.178 %) was observed. The percentage reduction in HbA1c of test group I, II and III was 11.0, 12.0 and 12.2 respectively [Table 2]. There was also a significant decrease (P =0.000) observed in the test group of fasting glucose, total cholesterol, LDL-cholesterol, triglyceride and T.chol/HDL ratio. For glucose in test group I the values decreased from 179.7 ± 6.23 to 140 ± 6.02 mg/dl (20 %), but in control group I a significant increase (P = 0.005) from 148 ± 2.08 to 150.97 ± 2.28 mg/dl was observed [Table 3]. In test group II the decrease was from 184.12 ± 7.07 to 146.81 ± 5.53 mg/dl (20 %) and in control group II the significant increase (P=0.036) was from 179.81 ± 6.63 to 183.71 ± 6.89 mg/dl [Table 4]. In test group III the decrease was from 176.93 ± 8.7 to 142.21 ± 7.93 mg/dl (19 %) and in control group III there were no significant changes observed [Table 5]. For cholesterol in test group I the changes were from 229.88 ± 6.13 to 202.82 ±4.67 mg/dl (11.7 %) and no significant change noticed in control group I(223.46 ± 8.01 to 226.05 ± 7.54 mg/dl) [Table 3]. In test group II the decrease was from 239.08 ± 6.86 mg/dl to 207.27± 5.61 (15.3 %) and no significant changes in control group II (215.29 ± 9.51 to218.38 ± 9.01mg/dl) [Table 4]. In test group III the decrease was from 224.93 ± 5.85 to 197 ± 5.38 mg/dl (13.6 %) and a significant increase (P = 0.035) was observed (215.17 ± 8.65 to 221.92 ± 10.24 mg/dl) in control group III [Table 5]. In LDL- cholesterol the decrease in the test group I was from 154.21 ± 5.79 to 126.39 ± 4.92 mg/dl (18 %) and the significant increase (P = 0.029) in control group I was from 147.84 ± 6.45 to 152.76 ± 6.01 mg/dl [Table 3]. In test group II the decrease was from 159.96 ± 6.62 to 127.42 ± 4.77 mg/dl (20 %) and increase in the control group II was not significant (133.38 ± 9.68 to 137.19 ± 8.84 mg/dl) [Table 4]. In test group III the decrease was from 146.71 ± 6.56 to 120.86 ± 5.60 mg/dl (13.6 %) and a significant increase (P = 0.001) from 137.25 ± 10.62 to 145 ± 11.3 mg/dl was observed in control group III [Table 5]. For triglyceride in test group I the decrease was from 159.39 ± 10.28 mg/dl to 122.88 ± 7.78 mg/dl (23 %) and the increase observed in control group I from 141.05 ± 15.1 to151.78 ± 15.02 mg/dl was also significant (P = 0.00) [Table 3]. In test group II the decrease was from 178.58 ± 13.92 to 138.65 ± 10.45 mg/dl (22 %) and in control group II a significant increase (P = 0.021) was observed from 158.62 ± 18 to 166.86± 16.67 mg/dl [Table 4]. In test group III the decrease was from 159.86 ± 15.2 to 131.14 ± 11.96 mg/dl (18 %) and the increase in control group III was not significant (117.58 ± 13.10 mg/dl to 127.08 ± 10.98 mg/dl) [Table 5]. For T.chol/HDL ratio the decrease in the test group I was from5.47 ± 0.21 to 4.17 ± 0.14 mg/dl (23 %) and in control group I the increase observed from 5.15 ± 0.30 to 5.5 ± 0.29 mg/ dl was significant (P = 0.00) [Table 3]. In test group II the decrease was from 5.77 ± 0.29 to 4.14 ± 0.16 mg/dl (29 %) and increase in the control group II was not significant (4.67 ± 0.37 to 4.81 ± 0.33 mg/ dl) [Table 4]. In test group III the decrease was from 5.16 ± 0.38 to 4.07 ± 0.27 mg/dl (21 %) and the increase observed in control group III from 4.25 ± 0.44 to 4.58 ± 0.44 mg/dl was significant (P = 0.000) [Table 5]. For HDL-cholesterol in test group I a significant increase (P = 0.00) was observed from 43.45 ± 1.58 to 47.51 ± 2.03 mg/dl and the decrease observed in control group I from 46.03 ± 1.83 to 42.78 ± 1.78 mg/dl was also significant (P = 0.000) [Table 3]. In test group II the change was from 43.35 ± 2.32 to 48.1 ± 2.83 mg/dl (P = 0.000) and in control group II the decrease was not significant (50.52 ± 3.48 to 47.76 ± 2.39 mg/dl) [Table 4]. In test group III the increase was from 46. ± 2.92 to50.79 ± 2.55 mg/dl (P = 0.001) and in control group III the decrease was not significant (54.52 ± 3.71 to 51.5 ± 2.65 mg/dl) [Table 5]. The percentage of increase in test groups I, II and III were 9.3, 13.2 and 10.0 % respectively.
Table 2.
HbA1c values before and after yogic practice of test groups and comparison with control groups
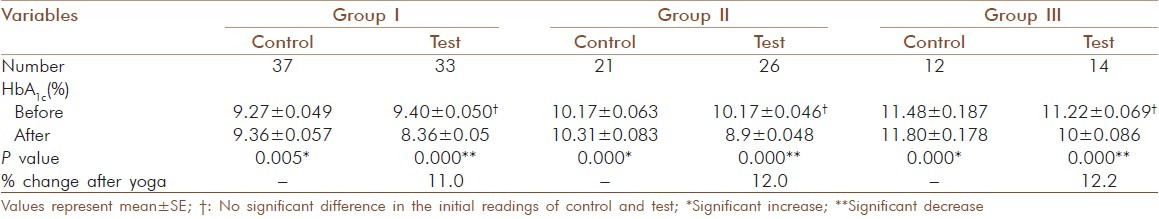
Table 3.
Glucose and lipid profile values before and after yogic practice of test group I and comparison with control group I
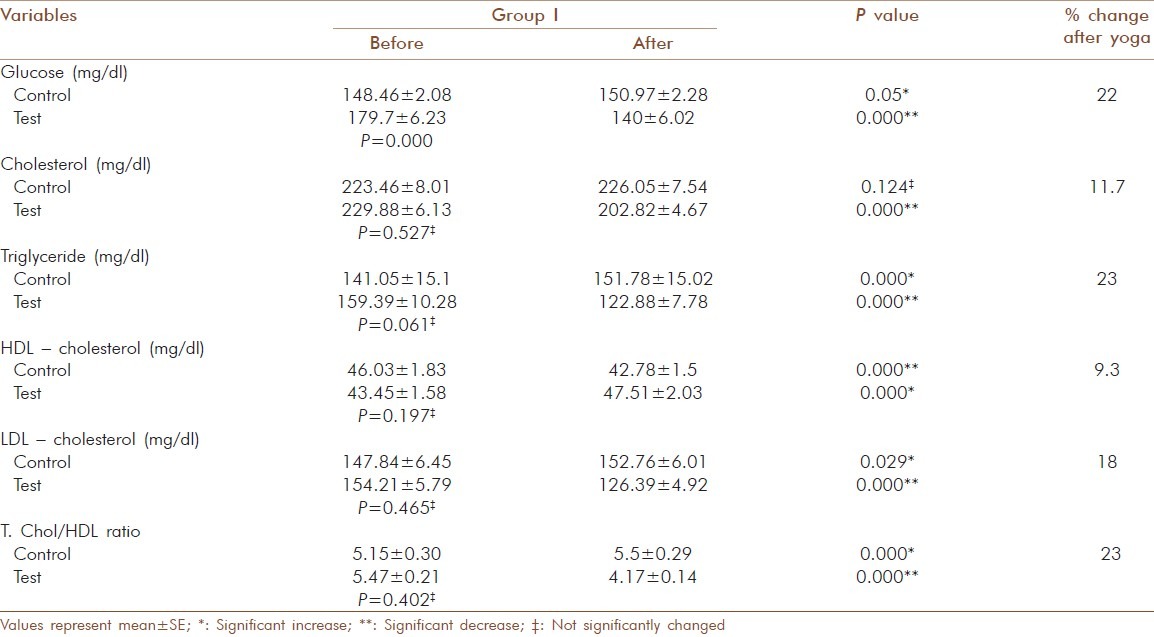
Table 4.
Glucose and lipid profile values before and after yogic practice of test group II and comparison with control group II
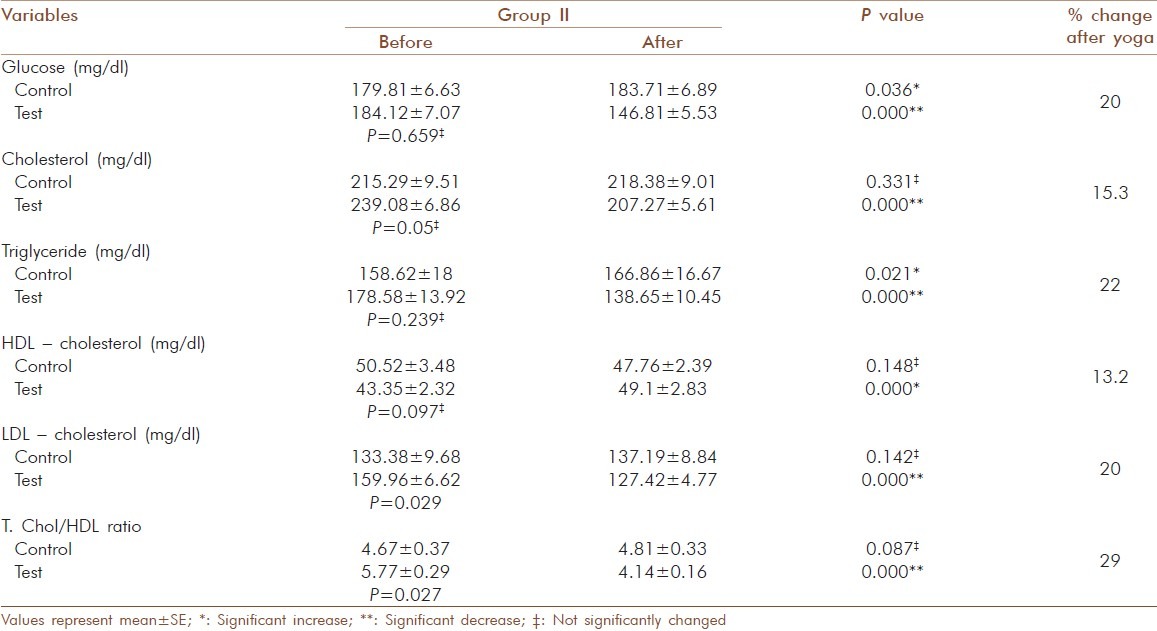
Table 5.
Glucose and lipid profile values before and after yogic practice of test group III and comparison with control group III
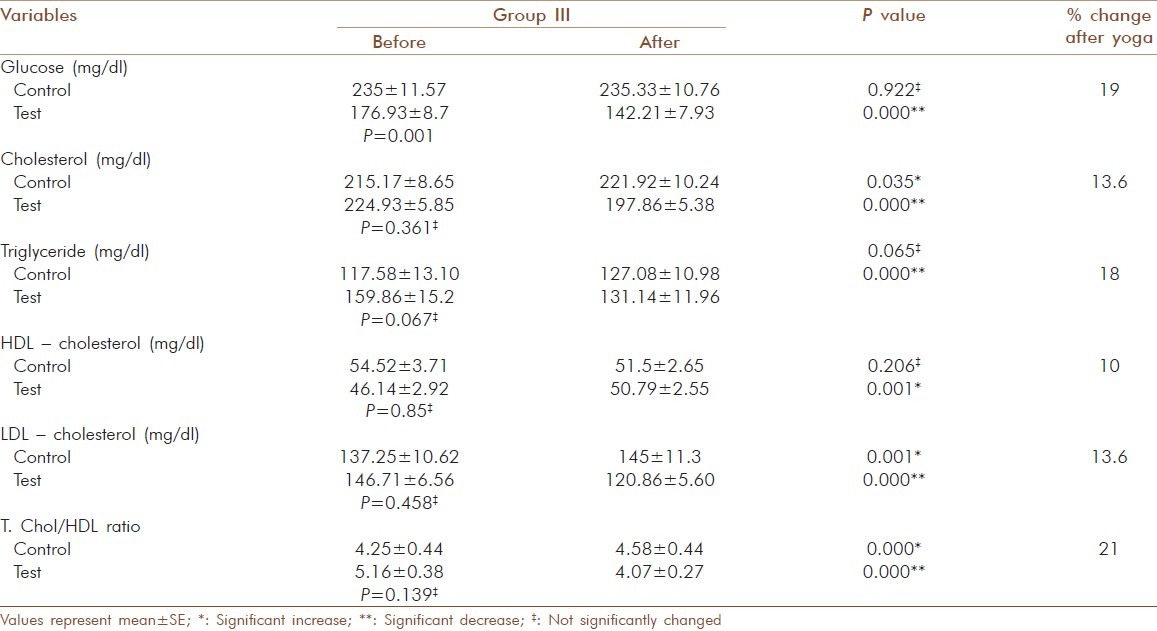
Significant decrease (P = 0.000) was observed for cortisol, ferritin and MDA in all three test groups. For cortisol in test group I the values decreased from 21.05 ± 0.67 to 17.05 ± 0.48 μg/dl (19 %) and the increase in control group I from 20.42 ± 0.65 to 20.49 ± 0.70 μg/dl was not significant [Table 6]. In test group II the decrease was from 24.58 ± 1.38 to19.43 ± 1.01 μg/dl (21 %) and in control group II there was no significant change observed (23.59 ± 0.72 to 23.87 ± 0.81μg/dl) [Table 7]. In test group III the decrease was from 27.82 ± 2.63 to 21.02 ± 1.72 μg/ dl (24.4 %) and in control group III a significant increase (P = 0.000) from 27.7 ± 1.00 to 28.69 ± 1.14 μg/ dl was observed [Table 8]. For ferritin in test group I the decrease was from 227.06 ± 6.61 to 194.03 ±6.5 ng/ dl (14.5 %), [Table 6] in test group II from 230 ± 6.8 to 196.85 ± 6.52 ng/ dl (14.5 %), [Table 7] in test group III from 252.57 ± 10.3 to220.29 ± 10.43 ng/ dl (14 %) and 10.43 ng/ dl (14 %) [Table 8] and no significant changes noticed in control groups (202.05 ± 10.24 to 202.05 ± 10.24 ng/dl).
Table 6.
Change in blood cortisol, ferritin, MDA and catalase activity in elderly diabetic patients before and after yogic practice in group I
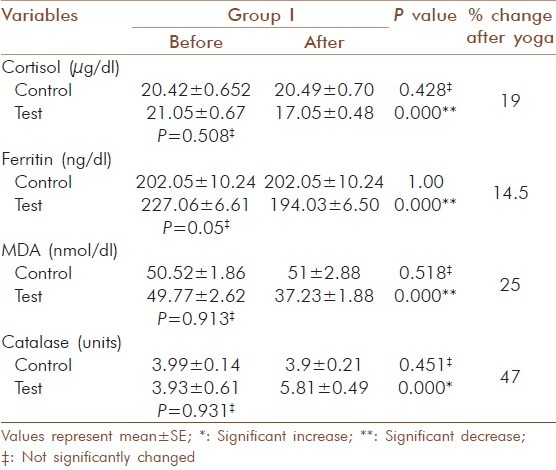
Table 7.
Change in blood cortisol, ferritin, MDA and catalase activity in elderly diabetic patients before and after yogic practice in group II
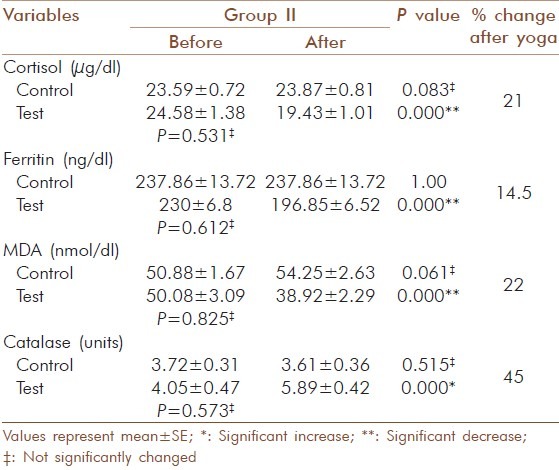
Table 8.
Change in blood cortisol, ferritin, MDA and catalase activity in elderly diabetic patients before and after yogic practice in group III
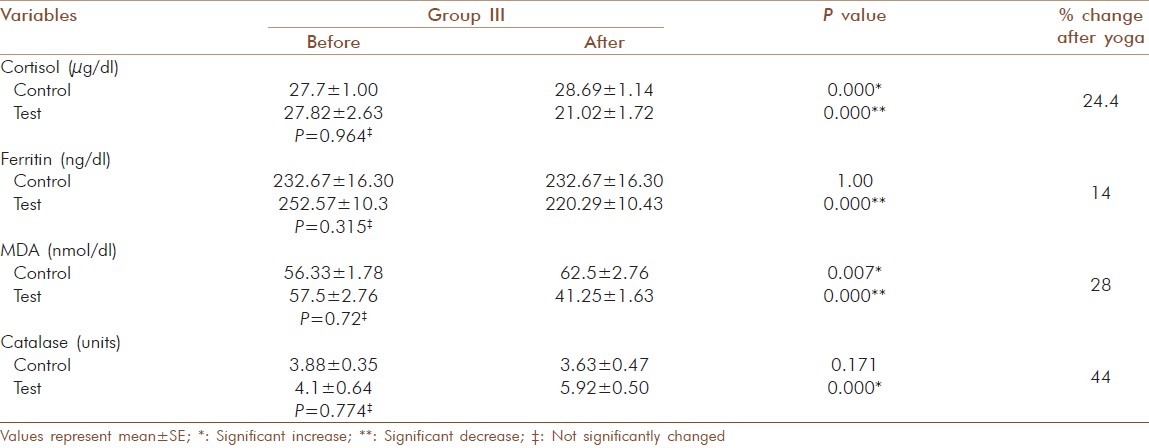
For MDA the decrease in the test group I was from 49.77 ± 2.62 to 37.23 ± 1.88 nmol/dl (25 %) and the increase in the control group I was not significant (50.52 ± 1.86 to 51± 2.88 nmol/dl) [Table 6]. In test group II the decrease was from 50.08 ± 3.09 to 38.92 ± 2.29 nmol/dl (22 %) and increase in the control group II was not significant (50.88 ± 1.67 to 54.25 ± 2.63 nmol/dl) [Table 7]. In test group III the decrease was from 57.5 ± 2.76 to 41.25 ± 1.63 nmol/ dl (28 %) and the increase observed in control group III from 56.33 ± 1.78 to 62.5 ± 2.76 nmol/dl was significant (P = 0.007) [Table 8]. The increase observed in catalyses activity was significant (P = 0.000) in all three test groups. In test group I the increase was from 3.93 ± 0.61 to 5.81 ± 0.49 units and the decrease observed in control group I was not significant (3.99 ±0.14 to 3.9 ±0.21 units) [Table 6]. In test group II the decrease was from 4.05 ± 0.47 to 5.89 ± 0.42 units and in control group II the decrease was not significant (3.72 ± 0.31 to 3.61 ± 0.36 units) [Table 7]. In test group III the increase was from 4.1 ± 0.64 to 5.92 ± 0.5 units (P=0.001) and in control group III the decrease was not significant (3.88 ± 0.35 to 3.63 ± 0.47 units) [Table 8]. The percentage of increase observed in test groups I, II and III were 47, 45 and 44 % respectively. There was no significant changes (P > 0.05) for the values of control and test group in initial observations except for glucose in group I (P = 0.000), LDL-cholesterol (P = 0.029) and T chol/HDL ratio (P = 0.029) in group II.
DISCUSSION
The number of people affected with type 2 diabetes has reached epidemic proportions worldwide. Diabetes is associated with a variety of metabolic abnormalities, principal among which is hyperglycaemia. Stress may aggravate the condition. The present study reveals the positive effects of yogic practice on stress, oxidative stress and antioxidant status in elderly type 2 diabetics over a 3 months period. Significant decrease was observed in fasting glucose, total cholesterol, triglyceride, LDL-cholesterol, T.chol/HDL ratio and a significant increase in the HDL - cholesterol after yogic practice in all the three test groups. Malhotra et al., have reported that an improvement in the lipid profile after practicing yogasana is associated with glycaemic control for the age group 30 to 60 years.[17] Our study was consistent with this and the group analysis indicated that after yogic practice, percentage of reduction in HbA1c was achieved more in group III and the improvement in lipid profile was more in group I. The favorable changes observed may be due to increased hepatic lipase and lipoprotein lipase at cellular level, which affects the metabolism of lipoprotein and thus increase uptake of triglycerides by adipose tissues.[18]
Serum cortisol levels decreased significantly in all three groups and a significant increase was observed in control group III. Michalsen et al., found out that Iyengar Yoga reduces stress-related diseases and it shows that yogic practice has an effect on hypothalamic - pituitary-adrenal (HPA) axis. It may alleviate the effects of stress and foster multiple positive downstream effects on the neuroendocrine system.[19] Our findings also correlated to that and in group analysis percentage of reduction in cortisol levels were more in group III and it seems to offer a promise that Yoga has a control on this disease at all stages.
Significant increase in control group III shows that cortisol level increases along with the severity of diabetes. Oltmanns et al., reported that increasing cortisol is strongly associated with increasing diabetic pathophysiology, particularly in the variables that depend on hyperglycaemia.[20]
Increasing evidence suggests that oxidative stress plays a major role in the pathogenesis of type 2 diabetes mellitus. Oxidative stress generally causes damage to the membrane polyunsaturated fatty acids leading to the generation of MDA, a lipid peroxidation product and a marker of oxidative stress and its concentration are elevated in poorly controlled type 2 diabetic patients.[21] In this study the MDA levels were decreased significantly after yogic practice along with a decrease in glucose and lipids. But in the control group, MDA, glucose and lipid levels remained high and this indicated that there was a reduction in lipid peroxidation by yogic practice. And it provides added support for the protective effect of yogic practice against oxidative stress in diabetics. Other researchers have found significant reduction in plasma MDA by Sudharshana kriya Yoga in type 2 diabetes after a four month's practice.[22] Percentage of reduction of MDA in the group analysis was 25, 22 and 28 % respectively and the yogic impact was not less even though the glycaemic control was least in group III.
Abnormally high levels of free radicals, lipid peroxidation and simultaneous decline in antioxidant defense mechanisms can lead to damage of cellular organelles and enzymes. Antioxidant, enzyme-dependent defenses play an important role in scavenging free radicals produced under oxidative stress.[23] The present study revealed that catalase, an antioxidant enzyme that protects against damage caused by free radicals, increased significantly after yogic practice. Rate of increase in the catalase activity was 47, 45 and 44 % respectively in the three groups and the increase was relatively high in good glycaemic control group. The improved antioxidant status due to yogic practice may point to adaptive response to oxidative stress on increased enzyme biosynthesis.[24]
Ferritin levels may be increased in response to oxidative stress, nitric oxide signaling and to several inflammatory cytokines. These mechanisms are involved in the pathogenesis of the metabolic syndrome and could be a reflection of liver involvement in insulin resistance.[4] The reaction of ferritin with H2O2 can induce lipid peroxidation that plays roles in aging and oxidative stress-associated diseases.[25] Moderately increased ferritin levels are just a marker for the metabolic alterations that ultimately result in diabetes.[26] Ferritin level was decreased significantly after yogic practice in all the three groups (14.5, 14 and 13 %) and percentage of reduction was slightly higher in group I. Studies regarding the effect of Yoga on ferritin level in type 2 diabetics are virtually non existent. Our results are pragmatic with previous research which shows that the elevated ferritin level is characteristic of type 2 diabetes.[6] A significant reduction of ferritin level in Yoga practicing group corroborate the role of Yoga in oxidative stress. The observed changes may have occurred by reducing the activation and reactivity of the sympathoadrenal system and the hypothalamic pituitary adrenal axis and by reducing cortisol secretion. The Texas Heart Institute used Yoga as an adjunct to their cardiac rehabilitation program as a stress reliever and to improve musculoskeletal flexibility.[10]
The potential benefits of physical activity such as Yoga in elderly people are the improved insulin resistance and glucose tolerance, maximum oxygen consumption, increased muscle strength, improved lipid profile and improved sense of well being. However, aerobic exercise is hindered in many type 2 diabetic because of advancing age and other comorbid conditions and unlike aerobic exercise, Yoga postures are slow rhythmic movements which emphasize the stimulation of the organs and glands by easy bending and extensions which do not over-stimulate muscles but concentrate on glandular stimulation[10] and also induce a mild oxidative stress that stimulates the expression of certain antioxidant enzymes. This is mediated by the activation of redox-sensitive signaling pathways;[27] thus we can say that yogic practice is the best choice in older age.
This study findings corroborates the earlier findings and suggest that yogic exercise has therapeutic, preventive and protective effects in elderly type 2 diabetic patients by reducing stress, oxidative stress and by improving the antioxidant defense system and from the group analysis we can conclude that at all stages of the disease a significant improvement can be achieved by yogic practice in geriatric diabetes also. The major limitation of the present study was that the subjects could not be kept under uniform, identical study environment as all the subjects were staying at home with their families. The subjects were advised about the importance of strict adherence to metformin medication and proper control of their diet. The significant changes that occurred in the control group for some parameters may be due to the noncompliance with our instructions by few patients.
ACKNOWLEDGMENTS
The authors are thankful to the participants with out whose cooperation the work would not have been completed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Surwit RS, van Tilburg MA, Zucker N, McCaskill CC, Parekh P, Feinglos MN, et al. Stress management improves long - term glycaemic control in type 2 diabetes. Diabetes care. 2002;25:30–4. doi: 10.2337/diacare.25.1.30. [DOI] [PubMed] [Google Scholar]
- 2.Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrino. 2005;30:1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Ramakrishna V, Jailkhani R. Evaluation of oxidative stress in Insulin Dependent Diabetes Mellitus (IDDM) patients. Diagn Pathol. 2007;2:22. doi: 10.1186/1746-1596-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, Oxidative Stress and Antioxidants: A Review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda Y, Suehiro T, Yamanaka S, Kumon Y, Takata H, Inada S, et al. Association between serum ferritin and circulating oxidized low density lipoprotein levels in patients with type 2 diabetes. Endocr J. 2006;53:665–70. doi: 10.1507/endocrj.k06-010. [DOI] [PubMed] [Google Scholar]
- 6.Jehn ML, Guallar E, Clark JM, Couper D, Duncan BB, Ballantyne CM, et al. A prospective study of plasma ferritin level and incident diabetes the atherosclerosis risk in communities (ARIC) study. Am J Epidemiol. 2007;165:1047–54. doi: 10.1093/aje/kwk093. [DOI] [PubMed] [Google Scholar]
- 7.Baruah MP, Kalra S, Unnikrishnan AG, Raza SA, Somasundaram N, John M, et al. Management of hyperglycemia in geriatric patients with diabetes mellitus: South Asian consensus guidelines. Indian J Endocrinol Metab. 2011;15:75–90. doi: 10.4103/2230-8210.81935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aljasir B, Bryson M, Al-Shehri B. Yoga practice for the management of type ii diabetes mellitus in adults: A systematic review. Evid Based Complement Alternat Med. 2010;7:399–408. doi: 10.1093/ecam/nen027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malhotra V, Singh S, Singh KP, Gupta P, Sharma SB, Madhu SV, et al. Study of Yoga asanas in assessment of pulmonary function in NIDDM patients. Indian J Physiol Pharmacol. 2002;46:313–20. [PubMed] [Google Scholar]
- 10.Nayak NN, Shankar K. Yoga: A therapeutic approach. Phys Med Rehabil Clin N Am. 2004;15:783–98. doi: 10.1016/j.pmr.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Gordon LA, Morrison EY, McGrowder DA, Young R, Fraser YT, Zamora EM, et al. Effect of exercise therapy on lipid profile and oxidative stress indicators in patients with type 2 diabetes. BMC Complement Altern Med. 2008;8:21. doi: 10.1186/1472-6882-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madanmohan D, Thombre P, Balakumar B, Nambinarayanan TK, Thakur S, Krishnanmurthy N, et al. Effects of yogic training on reaction time, respiratory endurance and muscle strength. Indian J Physiol Pharmacol. 1993;37:350–2. [PubMed] [Google Scholar]
- 13.Skoro-Kondza L, Tai SS, Gadelrab R, Drincevic D, Greenhalgh T. Community based Yoga classes for type 2 diabetes: An exploratory randomized controlled trial. BMC Health Serv Res. 2009;9:33. doi: 10.1186/1472-6963-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishiyama S, Carlsen J, Lawrence J, Small E, Zajdel D, Oken B. Yoga as an experimental intervention for cognition in multiple sclerosis. International J Yoga Thera. 2002;12:57–62. [Google Scholar]
- 15.Aoki N, Naito K, Yoshida N. Inhibition of platelet aggregation by protease inhibitors.Possible involvement of proteases in platelet aggregation. Blood. 1978;52:1–12. [PubMed] [Google Scholar]
- 16.Dobkin GB, Glantz MD. Colorimetric method for the quantitative determination of plasma catalase activity. Clin Chem. 1958;4:316–22. [PubMed] [Google Scholar]
- 17.Malhotra V, Singh S, Sharma SB, Gupta P, Prasad A, Tandon OP, et al. The Status of NIDDM Patients after Yoga Asanas: Assessment of Important Parameters. J Clin Diag Res. 2010;4:2652–67. [Google Scholar]
- 18.Singh S, Kyizom T, Singh KP, Tandon OP, Madhu SV. Influence of pranayamas and Yoga-asanas on serum insulin, blood Glucose and lipid profile in type 2 diabetes. Indian J Clin Biochem. 2008;23:365–8. doi: 10.1007/s12291-008-0080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michalsen A, Grossman P, Acil A, Langhorst J, Lüdtke R, Esch T, et al. Rapid stress reduction and anxiolysis among distressed women as a consequence of a three-month intensive Yoga program. Med Sci Monit. 2005;11:CR555–61. [PubMed] [Google Scholar]
- 20.Oltmanns KM, Dodt B, Schultes B, Raspe HH, Schweiger U, Born J, et al. Cortisol correlates with metabolic disturbances in a population study of type 2 diabetic patients. European J Endocrinol. 2006;154:325–31. doi: 10.1530/eje.1.02074. [DOI] [PubMed] [Google Scholar]
- 21.Peuchant E. Short-term insulin therapy and normoglycemia Effects on erythrocyte lipid peroxidation in NIDDM patients. Diabetes Care. 1997;20:202–7. doi: 10.2337/diacare.20.2.202. [DOI] [PubMed] [Google Scholar]
- 22.Agte VV, Tarwadi K. Sudarshan Kriya Yoga for treating type 2 diabetes. Altern Complemen Therapies. 2004;10:220–2. [Google Scholar]
- 23.Mahboob SM, Rahman MF, Grover P. Serum lipid peroxidation and antioxidant enzyme levels in male and female diabetic patients. Singapore Med J. 2005;46:322. [PubMed] [Google Scholar]
- 24.Lui D, Maulik N, Moraru II, Kreutzer DL, Das DK. Molecular adaptation of vascular endothelial cells to oxidative stress. Am J Physiol. 1993;264:715–22. doi: 10.1152/ajpcell.1993.264.3.C715. [DOI] [PubMed] [Google Scholar]
- 25.Yoon JH, Lee MS, Kang JH. Reaction of ferritin with hydrogen peroxide induces lipid peroxidation. BMB Rep. 2010;43:219–24. doi: 10.5483/bmbrep.2010.43.3.219. [DOI] [PubMed] [Google Scholar]
- 26.Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care. 2004;27:2422–8. doi: 10.2337/diacare.27.10.2422. [DOI] [PubMed] [Google Scholar]
- 27.Reid MB. Redox modulation of skeletal muscle contraction: what we know and what we dont. J Appl Physiol. 2001;90:724–31. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]


