Abstract
INTRODUCTION:
The management of acute respiratory distress syndrome (ARDS) was investigated with the use of heliox in an experimental model.
OBJECTIVES:
To investigate whether heliox can be considered a new therapeutic approach in ARDS.
METHODS:
ARDS was designed in Wistar albino male rats, 250-300 g in weight, by intratracheal instillation of physiological saline solution. Anesthezied and tracheotomized rats with ARDS were pressure-controlled ventilated. At the end of 210 min, helium gas was tried. All rats were assigned to two groups: Group 1 (n = 10) was the control group, and was given no treatment; group 2 (n = 7) was given heliox (He: O2 = 50:50). The heliox group received heliox for 1 h continously. Rats were continued to be kept on a ventilator through the experiment. Two hours after the last inhalation, both lungs of the rats were excised for both histopathological examination and immunohistochemical evaluation.
STATISTICAL ANALYSIS USED:
Histopathological grading were expressed as median interquartile range. Mann–Whitney U-test was used to assess the relationships between the variables.
RESULTS:
The infiltation of neutrophils were decreased in rats treated with heliox. Edema in the interstitial and intraalveolar areas was less than that of the control rats. Also, the diminishing of perivascular and/or intraalveolar hemorrhage was apperant. Hyaline membrane (HM) formation decreased in the heliox group compared with the control group. Decreased inducible nitric oxide synthase expression was shown via immunohistochemical examination in the heliox group.
CONCLUSION:
The present study histopathologically indicated the effectiveness of heliox in the decreasing of neutrophil infiltation, interstitial/intraalveolar edema, perivascular and/or intraalveolar hemorrhage and HM formation in ARDS. Besides the known effect of heliox in obstructive lung disease, inhaled heliox therapy could be associated with the improvement of inflamation in ARDS.
Keywords: Acute respiratory distress syndrome, edema, heliox, hyaline membrane, inhalation, rat
Acute respiratory distress syndrome (ARDS) is characterized by diffuse inflammation of the lung in response to various pulmonary (gastric aspiration, pneumonia, inhalational injury, trauma) or extrapulmonary (sepsis, trauma, pancreatitis, multiple transfusions) insults.[1,2] In response to various inflammatory stimuli, myeloperoxidase (MPO) released from activated neutrophils and the inducible isoform of nitric oxide synthase (iNOS) activated by endothelial cells can cause alveolar damage.[3–7] Histopathological findings of ARDS are classified as acute, subacute and chronic phases. In the acute (exudative) phase (the first 1-6 days), interstitial and alveolar edema with accumulation of polymorphonuclear neutrophil leukocytes (PMNL), macrophages and red blood cells in the alveoli are seen. Prominent hyaline membrane (HM) occurs as well. In the subacute phase (the next 7-14 days), some of the edema is usually reabsorbed. The proliferation of alveolar epithelial type II cells can be seen for the repairment of alveolar epithelium. In the chronic phase (after 14 days), alveolar macrophages are settled in the alveoli and, often, more fibrosis with ongoing evidence of alveolar epithelial repair is seen.[1]
The current treatment modalities and new therapeutic approaches in ARDS primarily focused on the resolution of alveolar edema. These treatments include glucocorticoids, surfactants, inhaled nitric oxide, antioxidants and various anti-inflammatory treatments. Unfortunately, to date, none of these pharmacologic treatments has proven to be effective.[8] We hypothesized that helium gas might have a beneficial role in the treatment of ARDS. Helium is an odorless, non-explosive and biologically inert gas. It has been used for a long time as a mixture with oxygen, known as heliox, in the treatment of upper and lower airway obstruction, such as asthma, chronic obstructive pulmonary disease, bronchiolitis and bronchopulmonary dysplasia.[9,10] Heliox has the following benefits: Reduces resistance to flow within the airways and consequently decreases the work of breath during spontaneous and non-invasive positive-pressure ventilation; provides an optimal patient-ventilator interaction; and reduces destructive hemodynamic effects.[11,12] Additionally, heliox is used in children during bronchoscopy,[13] even as a treatment for pneumothorax[14] and hyperammonemia.[15] Besides the known effects of heliox, we hypothesized that heliox would have a possible role in the treatment of ARDS.
Methods
Animals
Wistar albino male rats, weighing 250-300 g, were obtained from the Experimental Animal Research Center, Cukurova University Medical Faculty. The animals were kept in a temperature-(21 ± 2°C) and humidity (60 ± 5%)-controlled room in which a 12-12 h light-dark cycle was maintained. Animals were fed a standard rat chow diet and had access to water ad libitum, and were synchronized by the maintenance of controlled environmental conditions. The experiment was performed in accordance with the guidelines for Animal Research from the National Institute of Health and approved by the Committee on Animal Research at Cukurova University, Turkey.
Experimental design
Rats were anesthezied with 1.25 mg/kg body weight xylazine (Rompum, Bayer, Brazil, 2% solution) and 80 mg/kg body weight ketamine (Ketalar, Pfizer, USA, 50 mg/ml) intraperitoneally and instrumented in a manner previously described by German and Häfner et al.[16,17] A catheter was placed into one carotid artery and partial arterial oxygen pressure (PaO2) and partial arterial carbon dioxide pressure (PaCO2) were measured by a blood gas analyzer (The cobas b 121 system, Roche, Germany). A tracheotomy was performed and the trachea was canullated with polyethylene tubing, internal diameter (ID) 1.5 mm. The rats were pressure-controlled ventilated (Evita 4 Neoflow, Dräger, Germany) with 100% oxygen at a respiratory rate of 30 breaths/min, inspiration–expiration ratio of 1:2 and peak inspiratory pressure of 15 cm H2O at positive end-expiratory pressure (PEEP) of 2 cm H2O. At the start of the experiment, PaO2 and PaCO2 were evaluated under the described ventilatory settings. Before lavage, the peak inspiration pressure was raised to 28 cm H2O and the PEEP was raised to 8 cm H2O. Rat lung parenchyma lavage was applied with 5 ml × 6 ml of physiological saline solution per animal every half an hour through 210 min. The ventilation setting was not changed during the whole experimental period.
Two groups were assigned. Group 1 (n = 10) was the control group, which was given no treatment. Group 2 (n = 7) received helium gas mixture with oxygen (helium: oxygen = 50:50). At 2 h after the last lavage, the control group rats were sacrified. Helium was supplied in 4,000 L gas cylinders (Orya Medical, Turkey) containing 79% helium and 21% oxygen, and were pressurized at approximately 200 bars. Supplemental oxygen was administered via the oxygen tank. Heliox was delivered as a mixture of 50% helium and 50% oxygen by directly connecting the tanks to the ventilator (Avea Viasys Healthcare, USA). Rats breathed heliox for 1 h continously. Rats were continued to be kept on the ventilator adjusted with 50% oxygen at a respiratory rate of 30 breaths/min, inspiration-expiration ratio of 1:2, peak inspiratory pressure of 28 cm H2O at PEEP of 8 cm H2O. After receiving the heliox inhalation, 2 h later, the rats were sacrified. The right (upper, middle, accessory and lower lobes) and left lungs (one lobe) of both groups were examined.
Light microscopic examination
Tissues were fixed in 10% neutral buffered formalin and were embedded in paraffin. Sections of tissue were cut at 5 μm, mounted on slides and stained with H and E. Slides were coded and evaluated (Olympus conventional CX21 light microscope) without any knowledge of the sacrifice time. The severity of pathological features was graded according to the percentage of lung field (infiltration of PMNL, interstitial/intraalveolar edema, perivascular and/or intraalveolar hemorrhage and HM formation) as: Grade 0 (clear lung paranchyma), grade 1 (25% of lung paranchyma), grade 2 (50% of lung paranchyma), grade 3 (75% of lung paranchyma) and grade 4 (100% of lung paranchyma).[16]
Immunohistochemical examination
Samples were embedded in paraffin, cut into 5-μm-thick sections, mounted on glass slides for the localization iNOS and MPO. Prior to visualization, the sections were treated with xylene to remove the paraffin and rehydrated using ethanol. Specific labeling was detected by a biotin-conjugated goat anti-rabbit immunoglobulin G (IgG) and avidinbiotin peroxidase complex (DAKO North America Inc., Biotinylated Link Universal, streptavidin HRP Rabbit, Mouse, goat; K 0690). The iNOS antibody (Ab-1 Rabbit primary antibody, Neomarker Rabbit PAb (RB-1605-P1) at a dilution of 1:50 and the MPO (Ab-1 Rabbit polyclonal antibody thermo scientific RB-373 -A1) at a dilution of 1:100 were used. The sections were treated with citrat buffer (pH 6).
iNOS and MPO reactivity were examined in at least 10 randomly selected areas of both lungs at the ×400 magnification. Type II pneumocytes and neutrophils were evaluated with iNOS and MPO staining, respectively. The intensity of stained type II pneumocytes with iNOS was graded according to the percentage of lung field as 0 = clear lung paranchyma, 1 ≤ 25% of lung paranchyma, 2 = 25-50% of lung paranchyma, 3 = 50-75% of lung paranchyma and 4 ≥ 75% of lung paranchyma. Neutrophils were assessed using the following scale as per the lung samples as: 0 = no extravascular neutrophil; 1 ≤ 10 leukocytes; 2 = 10-45 neutrophils and 3 ≥ 45 neutrophils.[18] The final mean of the scores from the sections of the lungs was calculated in each category for each rat.
Electron microscopic examination
Tissues of 1 mm3 thickness were immediately placed in 5% glutaraldehyde buffered at pH 7.4 with Millonig phosphate buffer for 4 h. Samples were subsequently fixed in 1% osmic acid for 2 h. After dehydration in acetone, they were embedded in araldite. Ultrathin sections were stained with uranyl acetate and lead citrate and evaluated (Jeol JEM 1400 Transmission Electron Microscope).
Statistical analysis
A computer program (SPSS 11.0) was used for statistical analysis. Histopathological grading was expressed as median (interquartile range [IQR]). While differences among the groups were detected, the Mann–Whitney U-test was used to assess the relationships between the variables. A P value < 0.05 was considered significant.
Results
Rats with a weight range of 230-270 g were studied. Before starting the experiment, the median blood PaO2 and PaCO2 values were 342.50 (IQR 94.07) and 53.90 (IQR 8.92) mmHg, respectively. After lavage (210 min later), the PaO2 decreased to 60.70 (IQR 14.22) mmHg and the PaCO2 increased to 74.70 (IQR 8.75) mmHg [Table 1].
Table 1.
Arterial blood gas parameters of control group
In the light microscopic examination, the control group had similar histopathological variables as PMNL infiltration, interstitial/intraalveolar edema, erythrocyte extravasation and HM formation readily observed during the acute phase of ARDS in humans [Figure 1a–d]. Electron microscope (EM) examination depicted thick basal lamina and increased intracytoplasmic vacualization of type II pneumocyte [Figure 2a]. Grading of the histopathological variables, medians and mean ± standard deviation (SD) values were presented in Table 2.
Figure 1.
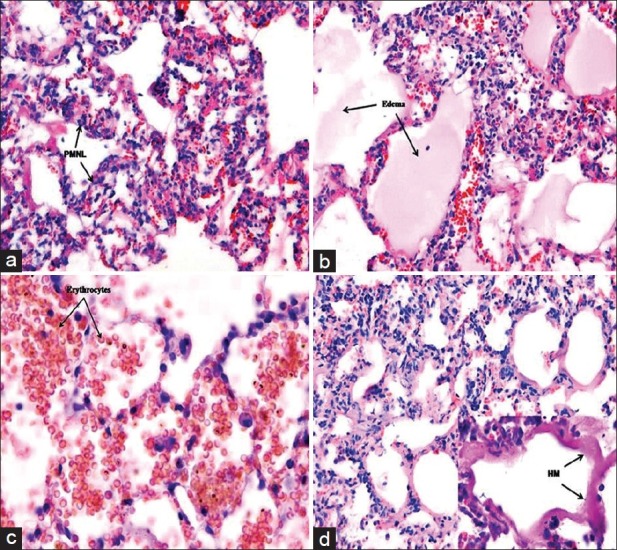
Light misroscopic examination (H and E, ×200) of control group. (a) infiltration of polymorphonuclear neutrophil leukocytes into the lung alveoli, (b) Interstitial and intraalveolar edema, (c) Perivascular and/or intraalveolar hemorrhage, (d) Hyaline membrane formation
Figure 2.
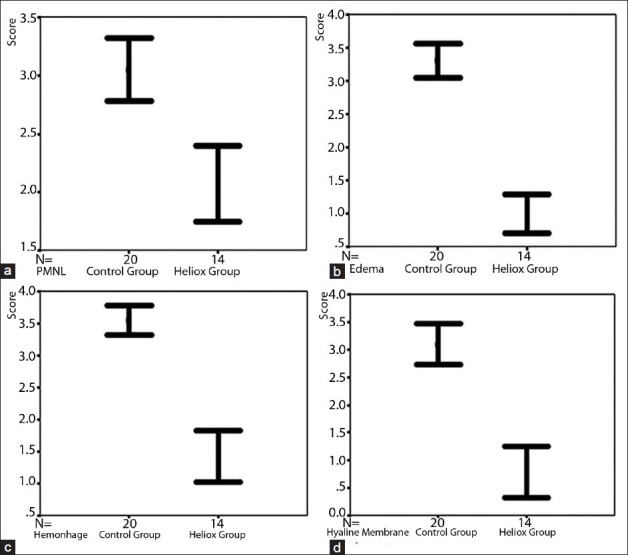
The comparison of both control and heliox group according to the polymorphonuclear neutrophil leukocytes infiltration, edema, hemorrhage and hyaline membrane formation
Table 2.
Median, interquartile range values and scores of control and heliox group according to the infiltration of polymorphonuclear neutrophil leukocytes into the lung alveoli, interstitial/intraalveolar edema, perivascular and/or intraalveolar hemorrhage and hyaline membrane formation
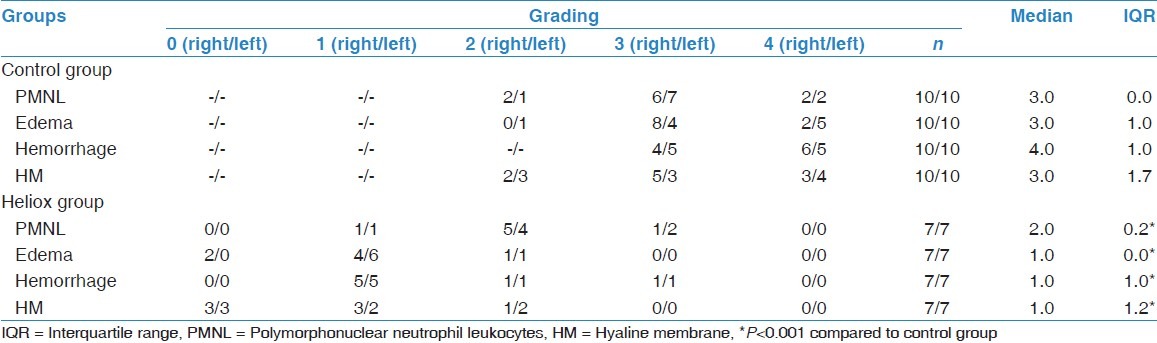
The infiltration of PMNL, interstitial/intraalveolar edema, perivascular and/or intraalveolar hemorrhage and HM formation were affected by heliox. The median severity of infiltration of PMNL was 2.0 (IQR 0.2) in rats receiving heliox inhalation. There was a statistically prominent difference for the peribronchial and perivascular neutrophil infiltration between rats that had heliox and those that did not have any treatment (P < 0.001). The median severity of edema (1.0, IQR 0.0) showed that interstitial and intraalveolar edema decreased compared with rats not receiving heliox inhalation. The significance was statistically different (P < 0.001). In addition, the extravasation of erythrocytes to the perivascular and/or intraalveolar areas diminished prominently in the heliox group, with a median of 1.0 (IQR 1.0). The difference was considered to be statistically significant (P < 0.001). Heliox-treathed rats with a median of 1.0 (IQR 1.2) had a lesser severity of HM formation. While comparing rats that were treated with heliox and that were not, decreased HM formation was observed in the heliox group. The statistical difference was significant (P < 0.001) [Table 2, Figure 3]. Decreased HM formation and marked type II pneumocytes in the heliox group were displayed in the light microscopic [Figure 4] and EM examinations [Figure 2b], respectively.
Figure 3.
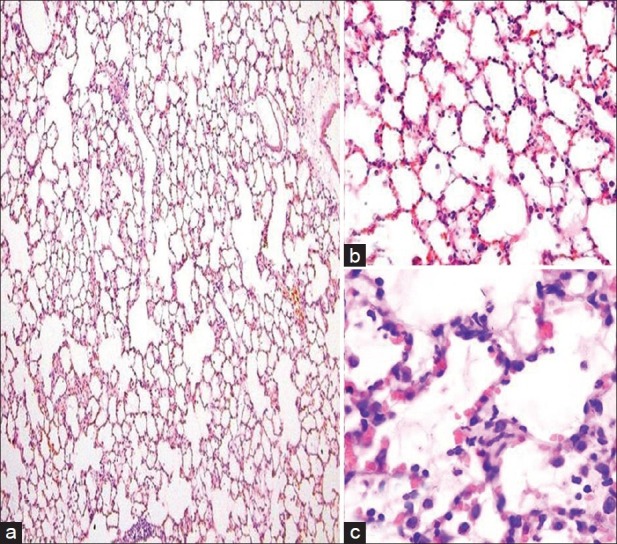
Light microscopic examination. (a) H and E, ×100, (b) H and E, ×200, (c) H and E, ×400). The histopathological signs in acute phase of acute respiratory distress syndrome such as polymorphonuclear neutrophil leukocytes infiltration, alveolar edema, hemorrhage and hyaline membrane formation were decreased after heliox inhalation
Figure 4.
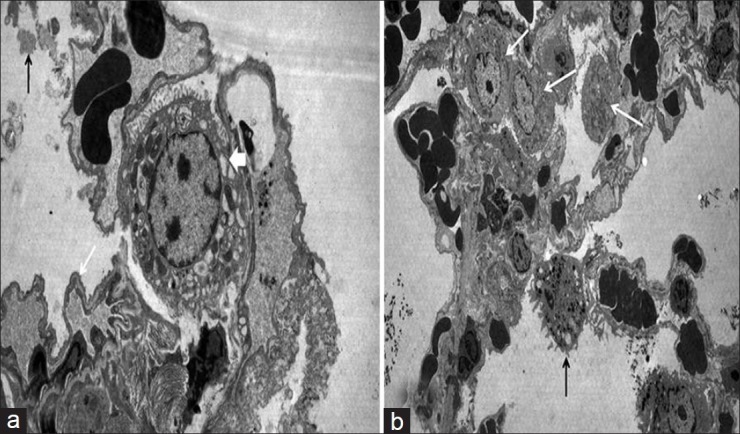
Electron microscope examination. (a) In control group: Granular dens stractures in alvoalar space (black arrow). Notice thick basal lamina on blood-air barrier area (white arrow) and increased intracytoplasmic vacualization of type II pneumocyte (thick white arrow), (b) In heliox group: Alveolar macrophage (black arrow) and increased type II pneumocytes (white arrow)
The iNOS and MPO immunoreactivity increased after lung parenchyma lavage with physiological saline solution [Figure 5a and b]. The heliox group, with a median score of 2 (IQR 1.2), had diminished iNOS immunoreactivity for perialveolar and perivascular neutrophil infiltration. The difference was statistically significant in the heliox group (P < 0.001). The immunohistochemical localization of MPO in perialveolar and perivascular leukocytes was slightly decreased in rats that inhaled heliox [Figure 5c and d]. No statistical difference was found [Table 3].
Figure 5.
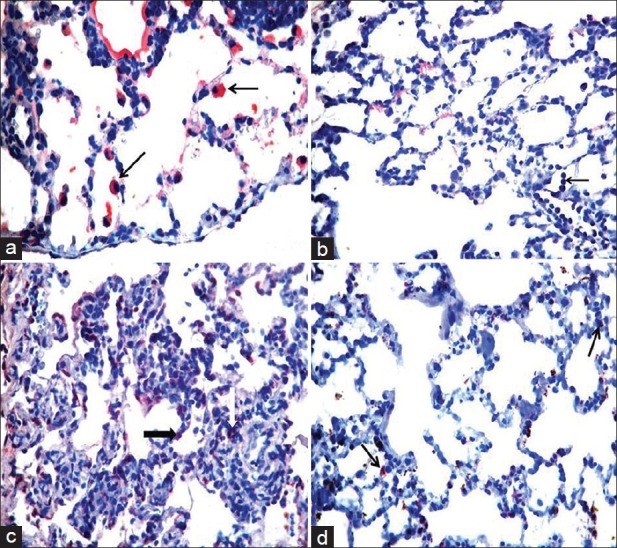
Immunohistochemical examination (H and E, ×400). (a) Mainly positive staining for isoform of nitric oxide synthase (iNOS) in type II pneumocytes for control group (black arrows), (b) Normally marked myeloperoxidase (MPO) staining in neutrophils (black arrow) for control group, (c) Negative staining for iNOS in heliox group, (d) Slightly decreased MPO staining in heliox group
Table 3.
Median and interquartile range values of isoform of nitric oxide synthase and myeloperoxidase immunoreactivity in control and heliox group

Discussion
In previous studies, it was shown that the helium–oxygen mixture was used in the treatment of severe asthma, cystic fibrosis, bronchoalveolitis, post-extubation pathologies and pharyngo–laryngeal edema.[9,19] The same studies indicated that scientific validation of heliox in airway diseases was inadequate. Also, it is usually less known that heliox can be used as a therapeutic agent in the treatment of acute respiratory distress[20] or ventilatiory-induced lung inflammation.[21] The major goals of this study were, first, to determine whether heliox inhalation could have an effect on the histopathological features of ARDS and, second, to determine whether iNOS and MPO activity were altered by heliox in lung tissue.
Under inflammatory conditions, large numbers of activated neutrophils release oxidant intracellular molecules that induce alveolar–capillary membrane injury.[4,22] We hypothesized that helium gas could have a beneficial effect on the pathological findings of ARDS. The infiltration of neutrophils in the peribronchial and perivascular areas was less in rats treated with heliox than that of rats not given heliox in our study. The knowledge of decreased PMNL infiltration related directly to the heliox inhalation is not avaliable in the literature. In a review presented by Antonelli et al.,[23] a relationship between heliox and leukocyte activation was established indirectly, with the expressing of bubble elimination in patients with decompression illnes. Bubbles in the venous vessels normally blocked the flow and induced hypoxia, which provoked oxidative perivascular stress, leading to leukocyte activation and adhesion to the endothelium. Also, Hyldegaard et al.[24] reported that heliox breathing could result in faster bubble resolution than oxygen breathing. It could be logical that heliox would indirectly decrease leukocyte activation and, consequently, the oxidative process. In our study, peribronchial and perivascular neutrophil infiltration were decreased in rats breathing heliox. From this point, a goal-directed investigation could be more important to determine whether heliox directly or indirectly had a role related to the decreasing of neutrophil infiltration and, consequently, oxidative stress in the acute phase of ARDS.
Alveolar/capillary damage is a significant factor contributing to edema and hemorrhage in the perivascular and/or intraalveolar areas in ARDS.[1,4] Despite the lack of a specific pharmacologic treatment, current therapeutic approaches (such as a fluid-conservative strategy, beta agonists and anti-inflammatory agents) have been primarily concerned about the resolution of edema fluid to the lung interstitium.[3,4] Although the data related to the effect of heliox on alveolar edema could not be found in previous studies, the severity of alveolar edema was demonstrated to decrease by heliox inhalation in our investigation. In addition, the extravasation of erythrocytes to perivascular and/or intraalveolar areas prominently diminished in rats receiving heliox inhalation. Therefore, it could very reasonably be argued that heliox might have a beneficial role in the alleviation of edema, and erythrocyte extravasation occured in the acute phase of ARDS. For this reason, the therapeutic effects of heliox on alveolar–capillary injury should be confirmed with further studies.
Heliox reduces the airways resistance and more readily reaches the alveoli.[25,26] Therefore, heliox allows to improve respiratory distress in patients with upper airway obstruction, including croup and post-extubation edema.[10,27] Frazier et al.[28] showed that heliox provided significant clinical improvement in acute inflammation of the upper airway. Therefore, it could be inferred from the former study that heliox might minimize oxidative stress. In our study, HM formation was noticeably attenuated in the heliox group. During inflammation, type II pneumocytes can be injuried, causing insuffucient surfactant to the HM.[4] At this point, if the antioxidative effect of heliox on type II pneumocytes could be proven with more pre-clinical studies, the reason of decreased HM formation would be elucidated. Previous studies mostly stated the clinical improvement, such as decreased respiratory rate, improved retractions or stridor, in patients receiving heliox.[25–27] Cambonie et al.[29] reported that heliox was shown to decrease the length of stay in patients with severe respiratory distress in the intensive care unit. While considering each of the histopathological signs attenuated by heliox in our observation, we thought that heliox could encourage physicians in the treatment of ARDS as well as obstructive pulmonary diseases. Therefore, it could be reasonable that heliox might have a beneficial role with other treatment modalities used during the course of ARDS.
Under inflammatory conditions, iNOS and MPO are activated.[6,7] In our study, the iNOS and MPO immunoreactivity increased after lung parenchyma lavage with physiological saline solution. The present study indicated that ARDS related to oxidative stress might be assessed by immunohistochemical examination on the basis of both iNOS and MPO reactivity in the lung paranchyma. Sittipunt et al.[30] demonstrated that iNOS was expressed in human alveolar macrophages before as well as after the onset of ARDS, and that iNOS expression persisted in the lungs of patients with sustained ARDS. The results from these studies suggested that oxidant stress mediated by NO appeared to be an important factor in the pathogenesis of ARDS. In our investigation, rats treated with heliox had lower iNOS intensity than rats receiving any inhalation treatment. Nawab et al.[21] presented in their study that heliox attenuated lung inflammation and lung mechanics in a neonatal animal model with acute lung injury (ALI) They noted decreased proinflammatory cytokines and MPO activity in the heliox-breathing animals. Although we found decreased MPO immunoreactivity in rats breathing heliox, the difference was not meaningful between rats that were treated with heliox and those that were not. Taken together, our study supported that heliox would have a possible role in the diminishing of lung inflammation in rats with ARDS.
The treatment pressures of oxygen and helium were pointed in different studies performed in the obstructive pulmonary diseases.[11,15] In one study, both 50:50 and 80:20 of the helium: Oxygen breathings were investigated in the treatment of decompression illness.[31] Although there was no knowledge associated with the mixture of helium and oxygen in ARDS, we tried the mixture of helium–oxygen at a ratio of 50:50. It was found to be favorable on the histopathological signs of ARDS model in our study. In an experimental study done by Frazier et al.,[28] the reduction of MPO immunoreactivity was found to be lower at the higher pressure than 50:50 of the helium–oxygen mixture. Our study is the first study, to the best of our knowledge, to demonstrate the effect of heliox therapy given with a mixture of 50% helium and 50% oxygen on histopathological signs of ARDS. Frankly, we thought that if the exact mechanism of heliox could be proven with further studies, heliox might open a new age in the management of critically ill patients with ARDS. Also, we believed that this kind of practice could prevent the probable progression of ARDS to chronic lung disease. Therefore, heliox could be effective in ARDS when the quality of life of the critical care patients is taken into consideration.
Study limitation
The major limitation of this study was that the lung mechanics could not be studied. Despite this, the aim of our study was primarily to assess heliox function on the histopathological features of ARDS.
Conclusion
The present experimental study could hopefully improve our understanding in the use of heliox as a novel treatment agent in ARDS. Our study suggested that heliox inhalation might be a therapeutic bridge in case of allowing time for other therapies to become effective. Therefore, heliox could permit the clinical improvement of the acute phase of ARDS and could, probably, ameliorate the outcome. Moreover, the quality of life in terms of decreasing the duration of mechanical ventilation and hospitalization could be potentially increased in critically ill patients with ARDS. To confirm our hypothesis, further investigations are required to determine the potential effects of heliox as an adjunctive therapy in ARDS.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. Report of the American-European consensus conference on ARDS: Definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med. 1994;20:225–32. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 3.Tomashefski JF., Jr Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–66. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 4.Matthay MA, Zemans RL. The acute respiratory distress syndrome: Pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–63. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tasaka S, Amaya F, Hashimoto S, Ishizaka A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid Redox Signal. 2008;10:739–53. doi: 10.1089/ars.2007.1940. [DOI] [PubMed] [Google Scholar]
- 6.Lee CC, Lin NT, Hsu YH, Chen HI. Inducible nitric oxide synthase inhibition potentiates multiple organ dysfunction induced by endotoxin in conscious rats. J Cardiovasc Pharmacol. 2005;45:396–403. doi: 10.1097/01.fjc.0000157438.72483.ae. [DOI] [PubMed] [Google Scholar]
- 7.Camussi G, Ronco C, Montrucchio G, Piccoli G. Role of soluble mediators in sepsis and renal failure. Kidney Int Suppl. 1998;66:S38–42. [PubMed] [Google Scholar]
- 8.Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intensive Care Med. 2006;21:119–43. doi: 10.1177/0885066606287045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barach AL, Eckman M. The effects of ınhalatıon of helıum mıxed wıth oxygen on the mechanıcs of respıratıon. J Clin Invest. 1936;15:47–61. doi: 10.1172/JCI100758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers TR. Use of heliox in children. Respir Care. 2006;51:619–31. [PubMed] [Google Scholar]
- 11.Chevrolet JC. Helium oxygen mixtures in the intensive care unit. Crit Care. 2001;5:179–81. doi: 10.1186/cc1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gainnier M, Forel JM. Clinical review: Use of helium-oxygen in critically ill patients. Crit Care. 2006;10:241. doi: 10.1186/cc5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pingleton SK, Bone RC, Ruth WC. Helium-oxygen mixtures during bronchoscopy. Crit Care Med. 1980;8:50–3. doi: 10.1097/00003246-198001000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Barr J, Lushkov G, Starinsky R, Klin B, Berkovitch M, Eshel G. Heliox therapy for pneumothorax: New indication for an old remedy. Ann Emerg Med. 1997;30:159–62. doi: 10.1016/s0196-0644(97)70135-5. [DOI] [PubMed] [Google Scholar]
- 15.Barr J, Eshel G, Chen-Levy Z, Lahat E. Heliox use in the treatment of acute hyperammonemia. J Child Neurol. 2001;16:456–8. doi: 10.1177/088307380101600616. [DOI] [PubMed] [Google Scholar]
- 16.Germann PG, Häfner D. A rat model of acute respiratory distress syndrome (ARDS): Part 1. Time dependency of histological and pathological changes. J Pharmacol Toxicol Methods. 1998;40:101–7. doi: 10.1016/s1056-8719(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 17.Häfner D, Germann PG. A rat model of acute respiratory distress syndrome (ARDS) Part 2, influence of lavage volume, lavage repetition, and therapeutic treatment with rSP-C surfactant. J Pharmacol Toxicol Methods. 1999;41:97–106. doi: 10.1016/s1056-8719(99)00025-8. [DOI] [PubMed] [Google Scholar]
- 18.Aytacoglu BN, Calikoglu M, Tamer L, Coşkun B, Sucu N, Köse N, et al. Alcohol-induced lung damage and increased oxidative stress. Respiration. 2006;73:100–4. doi: 10.1159/000088680. [DOI] [PubMed] [Google Scholar]
- 19.Barthélémy L, Michaud A, Sebert P. Medical value of a respiratory mixture of oxygen and helium (Heliox) Rev Pneumol Clin. 1993;49:92–8. [PubMed] [Google Scholar]
- 20.Faisy C, Diehl JL, Guerot E, Rezgui N, Labrousse J. Use of the helium-oxygen mixture in pneumology practice. Rev Mal Respir. 1999;16:1063–73. [PubMed] [Google Scholar]
- 21.Nawab US, Touch SM, Irwin-Sherman T, Blackson TJ, Greenspan JS, Zhu G, et al. Heliox attenuates lung inflammation and structural alterations in acute lung injury. Pediatr Pulmonol. 2005;40:524–32. doi: 10.1002/ppul.20304. [DOI] [PubMed] [Google Scholar]
- 22.Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: Mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol. 2009;40:519–35. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonelli C, Franchi F, Della Marta ME, Carinci A, Sbrana G, Tanasi P, et al. Guiding principles in choosing a therapeutic table for DCI hyperbaric therapy. Minerva Anestesiol. 2009;75:151–61. [PubMed] [Google Scholar]
- 24.Hyldegaard O, Madsen J. Influence of heliox, oxygen, and N2O-O2 breathing on N2 bubbles in adipose tissue. Undersea Biomed Res. 1989;16:185–93. [PubMed] [Google Scholar]
- 25.Katz A, Gentile MA, Craig DM, Quick G, Meliones JN, Cheifetz IM. Heliox improves gas exchange during high-frequency ventilation in a pediatric model of acute lung injury. Am J Respir Crit Care Med. 2001;164:260–4. doi: 10.1164/ajrccm.164.2.2006105. [DOI] [PubMed] [Google Scholar]
- 26.Hurford WE, Cheifetz IM. Respiratory controversies in the critical care setting. Should heliox be used for mechanically ventilated patients? Respir Care. 2007;52:582–91. [PubMed] [Google Scholar]
- 27.Duncan PG. Efficacy of helium – Oxygen mixtures in the management of severe viral and post-intubation croup. Can Anaesth Soc J. 1979;26:206–12. doi: 10.1007/BF03006983. [DOI] [PubMed] [Google Scholar]
- 28.Frazier MD, Cheifetz IM. The role of heliox in paediatric respiratory disease. Paediatr Respir Rev. 2010;11:46–53. doi: 10.1016/j.prrv.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Cambonie G, Milési C, Fournier-Favre S, Counil F, Jaber S, Picaud JC, et al. Clinical effects of heliox administration for acute bronchiolitis in young infants. Chest. 2006;129:676–82. doi: 10.1378/chest.129.3.676. [DOI] [PubMed] [Google Scholar]
- 30.Sittipunt C, Steinberg KP, Ruzinski JT, Myles C, Zhu S, Goodman RB, et al. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:503–10. doi: 10.1164/ajrccm.163.2.2004187. [DOI] [PubMed] [Google Scholar]
- 31.Hyldegaard O, Madsen J. Effect of hypobaric air, oxygen, heliox (50:50), or heliox (80:20) breathing on air bubbles in adipose tissue. J Appl Physiol. 2007;103:757–62. doi: 10.1152/japplphysiol.00155.2007. [DOI] [PubMed] [Google Scholar]


