Abstract
OBJECTIVES:
We aimed to evaluate the validity of the BodyMedia's SenseWear™ Armband (BSA) device in estimating total sleep time (TST) in patients with obstructive sleep apnea (OSA).
METHODS:
Simultaneous overnight recordings of in-laboratory polysomnography (PSG) and BSA were performed on (1) 107 OSA patients (mean age of 45.2 ± 14.3 years, mean apnea hypopnea index of 43 ± 35.7/hr and (2) 30 controls matched with OSA patients for age and body mass index. An agreement analysis between the PSG and BSA scoring results was performed using the Bland and Altman method.
RESULTS:
There was no significant difference in OSA patients between BSA and PSG with regard to TST, total wake time, and sleep efficiency. There was also no significant difference in the controls between BSA and PSG with regard to TST, total wake time, and sleep efficiency. Bland Altman plots showed strong agreement between TST, wake time, and sleep efficiency for both OSA and the controls. The intraclass correlation coefficients revealed perfect agreement between BSA and PSG in different levels of OSA severity and both genders.
CONCLUSION:
The current data suggest that BSA is a reliable method for determining sleep in patients with OSA when compared against the gold standard test (PSG). BSA can be a useful tool in determining sleep in patients with OSA and can be combined with portable sleep studies to determine TST.
Keywords: Actigraphy, armband, polysomnography, portable monitoring, sleep apnea, sleep duration, sleep-disordered breathing, type 4 sleep study
Obstructive sleep apnea (OSA) is a common sleep disorder[1] with serious medical complications, including hypertension,[2,3] atherosclerosis, stroke,[4,5] and insulin resistance.[6] For proper diagnosis and treatment, patients need to undergo an overnight sleep study (level I in-lab polysomnography [PSG]) in the hospital attended by a sleep technologist. Due to the limited resources and beds assigned for sleep studies, patients may have to wait for longer periods before undergoing sleep studies, which results in significant delay in their diagnosis and treatment. In addition, level I in-lab PSG (neuro-cardio-pulmonary monitoring) is time- and labor-consuming procedure and needs good expertise to perform and interpret the study. Therefore, unattended portable devices like level III (cardio-pulmonary monitoring) and type IV (single or dual channels) sleep studies at home that record the cardiopulmonary parameters have been proposed to diagnose OSA. However, a major limitation of these devices is the fact that they do not record sleep as electroencephalography (EEG) monitoring is not included. This limitation influences the accuracy of the study as it may underestimate the apnea hypopnea index (AHI) (as total sleep time [TST] cannot be assessed) and does not help the treating clinician to know if the patient was asleep at home or not. Estimation of TST during portable sleep studies will help giving better estimation of severity of sleep-disordered breathing and a better estimation of AHI. Therefore, devices that utilize accelerometer (actimetry) to estimate sleep have been used in some studies to give an estimation of sleep. The American Academy of Sleep Medicine (AASM) practice parameter (2007) endorsed the combination of wrist actimetry and a validated way of monitoring respiratory events as an alternative method to measure TST in OSA patients.[7] As OSA is a disease characterized by excessive body movements during sleep, the validity of actimetric devices is assessing TST in patients with sleep disordered breathing has been questioned in some previous studies.[8,9] Nevertheless, other studies reported reasonable estimation of TST in OSA patients.[10,11] The conflicting reported results could be related to the use of different actimetric devices. Actimetric devices use different data collection methods and different scoring algorithms. The SenseWear Pro Armband™ (Body Media, Pittsburgh, PA) (BSA) utilizes a dual axis accelerometer, which is different from accelerometers of most wrist actimeters (actigraphy). Therefore, this case-control study was designed to assess the accuracy of the BSA in estimating sleep duration in OSA patients and matched healthy controls.
Methods
Subjects
Consecutive patients referred to the University Sleep Disorders Center with a clinical suspicion of OSA between March 2008 and September 2009 were assessed by a sleep disorders specialist. Patients with a high clinical suspicion of OSA based on the presence of loud interrupted snoring, daytime sleepiness, or witnessed apneas in the absence of symptoms of other sleep disorders were included. Exclusion criteria were chronic pulmonary diseases, elevated PaCO2, congestive heart failure, neuromuscular diseases, home oxygen or mechanical ventilation usage, and age <18 years old. The study was approved by our institutional review board (IRB) in the College of Medicine at King Saud University. An informed consent was obtained from all participants. The control group consisted of healthy subjects with no self-reported symptoms of sleep-disordered breathing matched with cases for age, body mass index (BMI), and gender. All controls underwent PSG and those with AHI < 5 were recruited (n = 30).
Protocol
All participants underwent a simultaneous sleep study with the BSA device and in-lab level I PSG. Standard in-lab PSG was performed monitoring brain activity (EEG; electrodes placed at C1-A4, C2-A3, O1-A4, O2-A3, F3A2, F4A1); muscle tone (electromyogram of the chin and both legs), eye movements (electrooculogram), heart rate (electrocardiogram), oxygen saturation (finger pulse oximeter), chest and abdominal-wall movements (thoracic and abdominal belts), airflow (thermistor and nasal prong pressure transducer), sleep position (body position sensor), and snoring (microphone). PSG recording was performed using Alice® 5 diagnostic equipment (Respironics Inc., Murrysville, Pennsylvania, USA). Manual scoring of the electronic raw data was completed in accordance with established criteria.[12] Time in bed was defined as the recording time from lights off to lights on. TST was defined as the total time spent asleep during time in bed. Sleep efficiency was defined as the percentage of time in bed when the subject was asleep 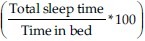 . Wake time was defined as time in bed minus TST. Apnea was defined as a drop in the peak thermal sensor excursion greater than or equal to 90% of the baseline for at least 10 seconds. At least 90% of the duration of the event had to meet the amplitude reduction criteria. The event was scored as obstructive apnea in the presence of continued respiratory effort and central apnea if it was associated with absent inspiratory effort throughout the entire period of absent airflow. Hypopnea was defined as a reduction in the airflow of ≥50% of the baseline that lasted for more than 10 seconds, resulting in a ≥3% decrease in oxygen saturation from the pre-event baseline or an arousal. At least 90% of the duration of the event had to meet the amplitude reduction criteria.[12] AHI was calculated by dividing all apnea-hypopnea episodes by TST. OSA was defined according to the International Classification of Sleep Disorders (ICSD 2005): (1) an AHI ≥5 events/hour with evidence of respiratory effort during all or a portion of the event associated with one of the following: excessive daytime sleepiness or unrefreshing sleep, gasping or choking during sleep, witnessed apnea, or loud snoring; or (2) an AHI ≥15 events/hr with evidence of respiratory effort during all or a portion of the event.[13] We defined mild OSA as an AHI score from 5 to 15/h; moderate OSA as an AHI score from 15 to 30/h; and severe OSA as an AHI score greater than 30/h.[14] Desaturation index was defined as the number of desaturation episodes (≥3% decrease in oxygen saturation) per hour of sleep. Arousal was scored according to the AASM guidelines.[12] Arousal index was defined as the number of arousals per hour of sleep. The PSG scorer was blinded to the clinical data and the results of BSA device.
. Wake time was defined as time in bed minus TST. Apnea was defined as a drop in the peak thermal sensor excursion greater than or equal to 90% of the baseline for at least 10 seconds. At least 90% of the duration of the event had to meet the amplitude reduction criteria. The event was scored as obstructive apnea in the presence of continued respiratory effort and central apnea if it was associated with absent inspiratory effort throughout the entire period of absent airflow. Hypopnea was defined as a reduction in the airflow of ≥50% of the baseline that lasted for more than 10 seconds, resulting in a ≥3% decrease in oxygen saturation from the pre-event baseline or an arousal. At least 90% of the duration of the event had to meet the amplitude reduction criteria.[12] AHI was calculated by dividing all apnea-hypopnea episodes by TST. OSA was defined according to the International Classification of Sleep Disorders (ICSD 2005): (1) an AHI ≥5 events/hour with evidence of respiratory effort during all or a portion of the event associated with one of the following: excessive daytime sleepiness or unrefreshing sleep, gasping or choking during sleep, witnessed apnea, or loud snoring; or (2) an AHI ≥15 events/hr with evidence of respiratory effort during all or a portion of the event.[13] We defined mild OSA as an AHI score from 5 to 15/h; moderate OSA as an AHI score from 15 to 30/h; and severe OSA as an AHI score greater than 30/h.[14] Desaturation index was defined as the number of desaturation episodes (≥3% decrease in oxygen saturation) per hour of sleep. Arousal was scored according to the AASM guidelines.[12] Arousal index was defined as the number of arousals per hour of sleep. The PSG scorer was blinded to the clinical data and the results of BSA device.
BodyMedia's senseWear™ armband
According to the manufacturer's instructions, the BSA was placed over the triceps muscle of the right arm, at the midpoint between the acromion and olecranon processes of all participants during PSG monitoring. The BSA is a portable sensing device, 8.8 × 5.6 × 2.1 cm in size and 82 g in weight that can provide information regarding the total energy expenditure, TST, and circadian rhythm.[15] The sensors in the BSA measure skin temperature, galvanic skin response, heat flux from the body, and movement. These physiological data are then processed by advanced algorithms to calculate and report total energy expenditure, metabolic physical activity, and sleep duration in free-living environment.[15,16] However, in this study, we analyzed data related to accelerometry (movement) only to validate the detection of sleep and wake in patients with OSA using BSA. The BSA accelerometer is similar to wrist actimeter (actigraphy), except for the fact that BSA is worn over the arm and it utilizes a dual axis accelerometer. The accelerometer uses a micro-electro-mechanical sensor (MEMS) device that detects and measures motion. The built-in accelerometer has a scale of ±2 g and a sensitivity of 167 mV/g. Data about sleep for both BSA and PSG were classified in a binary form into wake = 0 and sleep (any stage) = 1. The BSA is limited to estimating wake and sleep in 1 minute epochs. The computers recording the data of the PSG and BSA were synchronized to a standard time and the data analysis window for the BSA was marked to match the lights out and lights on from PSG. The sensor was monitored 32 times per second, and data tracked over a period of 1 minute.[17] Minute-by-minute data from the BSA were analyzed by algorithms using Body Media® InnerView® Research Software (version 5.1) provided by BodyMedia, Inc.[16]
Statistical analysis
The BSA software creates one excel sheet for each individual patient, which gave 137 excel sheets. We developed a MS Excel macro that extracts sleep data from each patient's worksheet and match it with the patient's demographic data and finally stored in a single excel sheet. This macro made it easy for the analysis and more practical for clinical research use. Data were summarized by the mean and standard deviation (SD) and number and percent for categorical variables. Data were also stratified by gender and OSA severity. Comparisons were done using paired sample t-test for normally distributed variables. If normality test failed, Wilcoxon matched pair signed rank test was used. Comparisons among categorical variables were done using Chi-squared (χ2) test. Paired sample correlation was used to assess the strength of the relationship between sleep duration, wake duration, and sleep efficiency using PSG and BSA data. The difference in TST measured by PSG and BSA was expressed as a mean difference or as an absolute difference. An agreement analysis between the PSG and BSA scoring results was performed using the Bland and Altman method.[18] The Bland-Altman plot represents the difference between sleep duration, wake duration, and sleep efficiency using PSG and BSA data against the mean value of sleep duration, wake duration, and sleep efficiency on both PSG and BSA devices. The limits of agreement were defined as mean ± 1.96 SD. Further assessment of agreement between PSG and BSA was done using the Intraclass Correlation Coefficients (ICC). The IBM SPSS Statistics 19.0 and MS Excel 2007 were used in the analysis. P value ≤0.05 was considered to be significant.
Results
A total of 113 OSA patients underwent simultaneous PSG and BSA monitoring. Six patients were excluded due to data loss or short evaluation duration. Complete data were available for 107 OSA patients and 30 control subjects. Characteristics of OSA patients and controls are summarized in Table 1. There were no differences between OSA patients and controls with regard to age, BMI, or gender. On the other hand, AHI, desaturation index, and arousal index were significantly higher in the OSA group.
Table 1.
Comparison between obstructive sleep apnea patients and controls

Comparison between polysomnography and bodyMedia's senseWear™ armband
Among OSA patients, there was no significant difference between BSA and PSG in the following parameters: TST (186.9 ± 98.5 min vs 184.9 ± 99.5 min; P = 0.71), total wake time (70.9 ± 62.4 min vs 72.9 ± 62.5 min; P = 0.71), and sleep efficiency (72.6 ± 19%, 71.3 ± 22.7%; P = 0.52) [Table 2]. Moreover, among the controls, there was no significant difference between BSA and PSG with regard to TST (290.4 ± 105.5 min vs 301.1 ± 96.5 min; P = 0.32), total wake time (74 ± 71.1 min vs 63.2 ± 61.5 min; P = 0.32), and sleep efficiency (79.8 ± 17.63 min, 83.3 ± 14.6 min; P = 0.23). Table 3 demonstrates the correlation between PSG and BSA in both OSA patients and controls. A strong correlation was demonstrated between both devices in TST (r = 0.84; P < 0.001) and total wake time (0.61; P < 0.001) in OSA patients.
Table 2.
Comparison between polysomnography and bodyMedia's senseWear™ armband
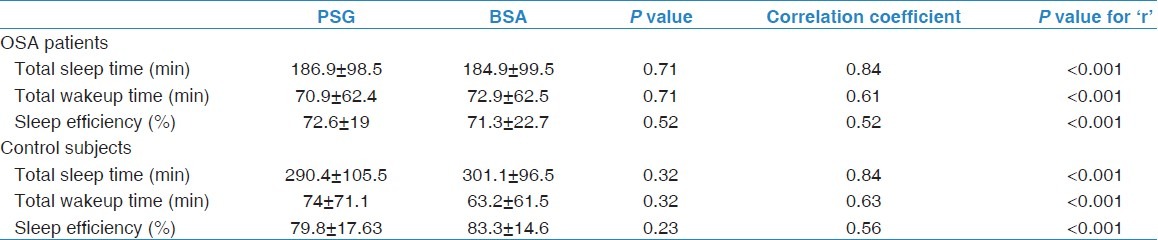
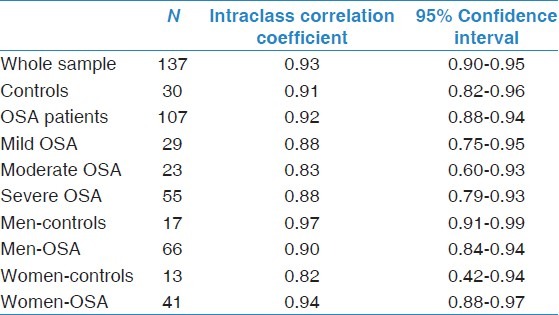
Table 3.
Intraclass correlation coefficients for total sleep time between polysomnography and BodyMedia's senseWear™ armband
Bland-Altman plots were used to demonstrate the agreement of both devices [Figure 1]. For TST, the plots revealed good agreement between PSG and BSA in both OSA patients and controls. The identity line and line of mean difference almost coincide on the plot [Figure 1a]. The plot of wake time shows a trend for increased difference between the two methods when wake time increases [Figure 1b]. On the other hand, sleep efficiency plot reveals a trend for the difference to get larger when sleep efficiency reduces [Figure 1c].
Figure 1.
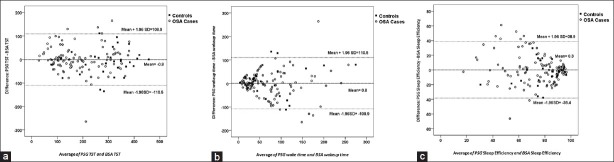
A Bland-Altman agreement plot for (a) total sleep time, (b) wake time, and (c) sleep efficiency. The dotted lines are the mean difference ±1.96 SD. The continuous line represents the identity line
ICC were calculated to measure the association between TST measured by PSG compared to that measured by BSA. The overall ICCs were >0.8, indicating a perfect agreement between the two devices [Table 3].
Comparison between polysomnography and bodyMedia's senseWear™ armband by subgroups
Analysis of the agreement in TST estimation between PSG and BSA was further analyzed by subgroups of gender and OSA severity. Bland-Altman plot revealed good agreement between BSA and PSG in both men and women [Figure 2]. The ICC revealed perfect agreement between BSA and PSG in different levels of OSA severity and both genders [Table 3].
Figure 2.
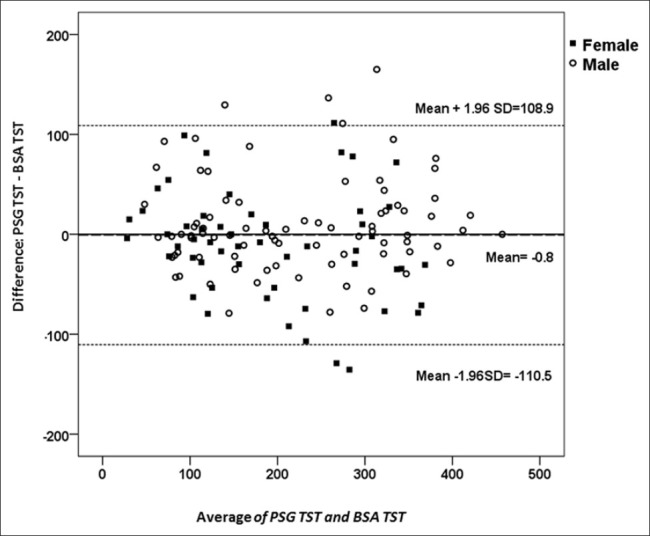
A Bland-Altman agreement plot for total sleep time in men and women with obstructive sleep apnea
Figure 3 shows the percentage of measurements of categories of Absolute Difference in TST in OSA patients and controls. In the figure, we can observe no important differences between the two groups, indicating that BSA has good detection for sleep in OSA patients.
Figure 3.
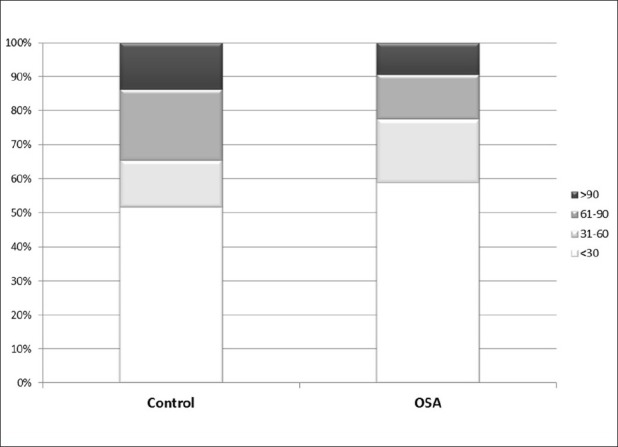
Categories of absolute difference in total sleep time from polysomnography and BSA by apnea hypopnea index category
Discussion
Our results showed that the accelerometer built in the BSA provided a good estimation of TST in OSA patients when compared with PSG. This is the first case-control study to assess the validity of BSA in estimating TST in a large sample of OSA patients and to include a matched control group without sleep-disordered breathing. A recent study using 50 adults with a clinical suspicion of sleep-disordered breathing referred for an overnight sleep study reported a good estimation of TST and a poor estimation of wake time by BSA.[19]
As BSA determines sleep/wake status in one minute epochs rather than 30 seconds epochs as in PSG; during analysis, we used TST as a summary measure rather than pursuing epoch-by-epoch analysis to estimate sensitivity and specificity. In this study, we compared estimates of TST measured by PSG and BSA concurrently. This kind of design allowed us to assess the agreement of BSA estimation of TST with the currently gold-standard method to assess TST (PSG), and then to assess this agreement in the subgroups of men and women and OSA severity. The results showed that BSA provided strong estimates of TST in both OSA patients and controls, both genders and different levels of OSA severity. In addition, we found a good agreement between PSG and BSA in detecting wake time. Moreover, ICC showed perfect agreement between BSA and PSG. However, the agreement was not very good when sleep efficiency was low. As sleep efficiency decreases, the agreement between the two devices decreases. This finding concurs with previous studies using wrist actimetric devices that demonstrated lower agreement between actimetry and PSG when sleep efficiency was low.[10,11,20]
Studies utilizing wrist actimetry to estimate TST in OSA patients have reported conflicting results. Although some reported that TST was underestimated by actimetry, others reported a good agreement with PSG and lack of significant effects of OSA severity and related respiratory arousal on the accuracy in detecting sleep/wake.[8,9,11] Hedner et al. reported that the agreement of sleep estimation measured by wrist actimetry and PSG declined with the increase in OSA severity.[8] In contrast, Wang et al. in a sample of 11 patients with OSA demonstrated fair agreement in sleep estimation between wrist actimetry and PSG.[11] In this study, BSA maintained good agreement at all levels of OSA severity. The conflicting data reported in different studies could be related to the different inherent characteristics of the devices used. It has been suggested that different actimetric devices may perform differently when estimating TST due to different collection strategies and scoring algorithms.[20] In addition, BSA is placed on the arm while other actimetric devices are placed around the wrist. This placement made the BSA less likely to be affected by the extraneous small movements of the wrist that can lead to overestimation of physical activity and hence underestimation of sleep. Moreover, the BSA uses a dual axis accelerometer, which may enhance the prediction of activity states that are important with respect to sleep. Therefore, it is difficult to generalize the results of other studies that used wrist actimetry (actigraphy) to BSA and the validation of new devices like BSA becomes essential.
The strength of this study is the selection of a large sample of middle-aged patients of both genders with different levels of OSA severity and the inclusion of a matched control group. Based on the limited available data, actimetric devices are not reliable in determining the presence or absence of breathing abnormalities in OSA patients.[21] The best utility of actimetric devices in OSA is in combination with stand-alone portable recording equipment that are used to screen for OSA and that do not have measures of wake or sleep. The addition of an actimetry such as BSA would allow the therapist to determine if all respiratory events actually occurred during sleep. Elbaz et al. compared an AHI estimated from a simple polygraphy using time in bed as an estimate of sleep duration and then using wrist actimetry-based TST as an estimate of sleep duration to PSG-derived AHI.[22] AHI improved the validity of estimated AHI when added to a simple polygraphy. In an updated Standards of Practice of the AASM, the authors concluded that actimetry is indicated for assessing TST among patients with OSA when PSG is not available.[7]
In summary, our data show that BSA is a good method for determining sleep in patients with OSA when compared against the gold standard test (PSG). The detection of sleep in OSA patients using BSA was comparable to that in the control group. BSA can be a useful tool in determining sleep in patients with OSA and can be combined with portable sleep studies to determine TST.
Acknowledgments
This project was partially funded by The National Plan for Sciences and Technology (King Saud University and King Abdulaziz City for Science and Technology).
Footnotes
Source of Support: The National Plan for Sciences and Technology (King Saud University and King Abdulaziz City for Science and Technology).
Conflict of Interest: None declared.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Alharbi MS, Sharif MM, Alotaibi DA, Shaikh S, BaHammam AS. Prevalence and predictors of hypertension in Saudi patients with obstructive sleep apnea. Saudi Med J. 2010;31:585–6. [PubMed] [Google Scholar]
- 3.Fang J, Wheaton AG, Keenan NL, Greenlund KJ, Perry GS, Croft JB. Association of sleep duration and hypertension among US adults varies by age and sex. Am J Hypertens. 2012;25:335–41. doi: 10.1038/ajh.2011.201. [DOI] [PubMed] [Google Scholar]
- 4.Selim B, Won C, Yaggi HK. Cardiovascular consequences of sleep apnea. Clin Chest Med. 2010;31:203–20. doi: 10.1016/j.ccm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Lurie A. Cardiovascular disorders associated with obstructive sleep apnea. Adv Cardiol. 2011;46:197–266. doi: 10.1159/000325110. [DOI] [PubMed] [Google Scholar]
- 6.Tkacova R, Dorkova Z, Molcanyiova A, Radikova Z, Klimes I, Tkac I. Cardiovascular risk and insulin resistance in patients with obstructive sleep apnea. Med Sci Monit. 2008;14:CR438–44. [PubMed] [Google Scholar]
- 7.Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: An update for 2007. Sleep. 2007;30:519–29. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 8.Hedner J, Pillar G, Pittman SD, Zou D, Grote L, White DP. A novel adaptive wrist actigraphy algorithm for sleep-wake assessment in sleep apnea patients. Sleep 8. 2004;27:1560–6. doi: 10.1093/sleep/27.8.1560. [DOI] [PubMed] [Google Scholar]
- 9.Johnson NL, Kirchner HL, Rosen CL, Storfer-Isser A, Cartar LN, Ancoli-Israel S, et al. Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: A comparison of three data modes. Sleep. 2007;30:899–905. doi: 10.1093/sleep/30.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Wong KK, Dungan GC, 2nd, Buchanan PR, Yee BJ, Grunstein RR. The validity of wrist actimetry assessment of sleep with and without sleep apnea. J Clin Sleep Med. 2008;4:450–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. 1st ed. Westchester, Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 13.American Academy of Sleep Medicine. International classification of sleep disorders (ICSD): Diagnostic and coding manual. 2nd ed. Westchester (IL): American Academy of Sleep Medicine; 2005. [Google Scholar]
- 14.Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 15.BaHammam A, Alrajeh M, Albabtain M, Bahammam S, Sharif M. Circadian pattern of sleep, energy expenditure, and body temperature of young healthy men during the intermittent fasting of Ramadan. Appetite. 2010;54:426–9. doi: 10.1016/j.appet.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Malvolti M, Pietrobelli A, Dugoni M, Poli M, de Cristogaro P, Battistini N. A new device for measuring daily total energy expenditure (TEE) in free living individuals. Int J Body Compos Res. 2005;3:63. [Google Scholar]
- 17.Teller A. A platform for wearable physiological computing. Interact Comput. 2004;16:917–37. [Google Scholar]
- 18.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 19.O’Driscoll DM, Turton AR, Copland JM, Strauss BJ, Hamilton GS. Energy expenditure in obstructive sleep apnea: Validation of a multiple physiological sensor for determination of sleep and wake. Sleep Breath. 2012 doi: 10.1007/s11325-012-0662-x. [DOI] [PubMed] [Google Scholar]
- 20.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 21.Stone KL, Ancoli-Israel S. Actigraphy. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. Philadelphia, PA, USA: Elsevier Saunders; 2011. pp. 1668–75. [Google Scholar]
- 22.Elbaz M, Roue GM, Lofaso F, Quera Salva MA. Utility of actigraphy in the diagnosis of obstructive sleep apnea. Sleep. 2002;25:527–31. [PubMed] [Google Scholar]


