Abstract
Context:
Stress is a state of mental or emotional strain or tension, which can lead to underperformance and adverse clinical conditions. Adaptogens are herbs that help in combating stress. Ayurvedic classical texts, animal studies and clinical studies describe Ashwagandha as a safe and effective adaptogen.
Aims:
The aim of the study was to evaluate the safety and efficacy of a high-concentration full-spectrum extract of Ashwagandha roots in reducing stress and anxiety and in improving the general well-being of adults who were under stress.
Settings and Design:
Single center, prospective, double-blind, randomized, placebo-controlled trial.
Materials and Methods:
A total of 64 subjects with a history of chronic stress were enrolled into the study after performing relevant clinical examinations and laboratory tests. These included a measurement of serum cortisol, and assessing their scores on standard stress-assessment questionnaires. They were randomized to either the placebo control group or the study drug treatment group, and were asked to take one capsule twice a day for a period of 60 days. In the study drug treatment group, each capsule contained 300 mg of high-concentration full-spectrum extract from the root of the Ashwagandha plant. During the treatment period (on Day 15, Day 30 and Day 45), a follow-up telephone call was made to all subjects to check for treatment compliance and to note any adverse reactions. Final safety and efficacy assessments were done on Day 60.
Statistical Analysis:
t-test, Mann-Whitney test.
Results:
The treatment group that was given the high-concentration full-spectrum Ashwagandha root extract exhibited a significant reduction (P<0.0001) in scores on all the stress-assessment scales on Day 60, relative to the placebo group. The serum cortisol levels were substantially reduced (P=0.0006) in the Ashwagandha group, relative to the placebo group. The adverse effects were mild in nature and were comparable in both the groups. No serious adverse events were reported.
Conclusion:
The findings of this study suggest that a high-concentration full-spectrum Ashwagandha root extract safely and effectively improves an individual's resistance towards stress and thereby improves self-assessed quality of life.
Keywords: Adaptogen, high-concentration full-spectrum Ashwagandha root extract, stress, Withania somnifera
INTRODUCTION
Stress is a condition arising from external physical or mental overload. It can make a person feel embattled, nervous, anxious or otherwise less capable of full and normal response to environmental demands. Prolonged exposure to stress can unbalance the mental and physiological state of a person, thereby leading to other illnesses like depression, high blood pressure, cardiac diseases and metabolic disorders. Such conditions, rooted in mental or emotional factors, are rapidly increasing in prevalence and emerging as major global diseases. It is not surprising, therefore, that an increasing fraction of the population is seeking medical help to overcome stress nowadays.
Adaptogens are herbs that improve an individual's ability to cope with stress. These herbs in times of increased stress, normalize the physiological process of the body and help the body adapt to changes.[1] A recent definition of an adaptogen is, “a … class of metabolic regulators which increase the ability of an organism to adapt to environmental factors and avoid damage from such factors.” Ideally, an adaptogen should: a) decrease stress-induced damage, b) be safe and produce a beneficial effect even if the number of administrations is more than required, c) be devoid of any negative effects such as withdrawal syndromes and d) not influence the normal body functions more than necessary.[2] Ashwagandha is one adaptogen that possesses all of the characteristics listed above.
Ashwagandha, botanically known as Withania somnifera Dunal, is a member of the Solanaceae family. It is commonly known as Indian Ginseng or Winter Cherry. The literal meaning of the word “Ashwagandha” is “smell of horse”. The herb is so named for two reasons. One reason is that the fresh roots of the herb emit the smell of horse. The second reason is that there is a commonly held belief that a person consuming extracts of the herb may develop the strength and vitality similar to that of a horse.[3] This herb has a central and prominent place in Ayurvedic medicine. Ashwagandha is referred to as a “royal herb” because of its multifarious rejuvenative effects on the human body. It is a multipurpose herb that acts on various systems of the human body: the neurological system, the immune system, the energy-production system, the endocrinal system and the reproductive system.
Background literature: Being the most commonly used and extensively studied adaptogen, a large volume of literature is available for Withania somnifera.[1] This herb has been studied as adaptogenic, antioxidant, anticancer, anxiolytic, antidepressant, cardioprotective, thyroid modulating, immunomodulating, antibacterial, antifungal, anti-inflammatory, neuroprotective, cognitive enhancing and hematopoietic agent. Ashwagandha contains a range of constituents like withanolides, sitoindosides and other alkaloids that are pharmacologically and medicinally important. These chemicals protect cells from oxidative damage and disease.[4–7] Results from a battery of tests, conducted to identify the anti-stress activity of sitoindoside VII and sitoindoside VIII implied that both sitoindosides produce anti-stress activity.[8] Sitoindoside IX and X were tested in rats for immunomodulatory and central nervous system effects related to stress, memory and learning. A significant reduction was noticed in the incidence of stress-induced gastric ulcers.[9]
Various trials conducted in the past on Ashwagandha exhibited positive results for its adaptogenic and stress-reducing activity. However most of the trials were either in-vivo or in-vitro. Only a limited literature is available on the stress-reducing effects of Ashwagandha root extracts on humans. Our primary objective was to evaluate the efficacy of high-concentration full-spectrum Ashwagandha root extract in reducing stress and anxiety, and its role in improving general well-being in adults under stress.
The high-concentration full-spectrum Ashwagandha root extract used in this study is the KSM-66 Ashwagandha extract, provided by Ixoreal Biomed, Hyderabad, India. The extract is drawn only from the roots of the Ashwagandha plant, using no other parts like leaves. It is standardized to withanolide content of at least 5% as measured by HPLC. It is produced by a unique extraction process, based on the principles of ‘green chemistry’, without using alcohol or any synthetic solvents.
MATERIALS AND METHODS
This was a single-centered, prospective, double-blind, randomized, placebo-controlled trial, primarily to evaluate the efficacy of high-concentration full-spectrum Ashwagandha root extract in reducing stress and anxiety and in improving general well-being in adults under stress. A total of 64 subjects complaining of mental stress were enrolled into the study for a duration of 60 days.
The study was conducted in accordance with the Indian Council for Medical Research Guidelines for research in humans (ICMR-GCP) and the Declaration of Helsinki (2008), and was approved by the institutional review committee at Asha Hospital in Hyderabad, India.
Participants
Subjects were enrolled into the study if they met the following inclusion criteria: (1) of age between 18 and 54 years, (2) free of psychiatric conditions other than stress (3) have a score less than 15 on the World Health Organization-five (WHO-5)[10] well-being index and a score of at least 14 on the Perceived Stress Scale (PSS)[11] and (4) could read and write English. On the WHO-5 well-being index, lower scores correspond to higher levels of stress.
Subjects were excluded if they met any one of the following exclusion criteria: (1) they were suffering from any chronic physical, hormonal or psychiatric illness, (2) they were using certain hormonal birth-control measures, (3) they were currently on any medication on a regular basis, (4) they were currently taking any herbal preparations or formulations containing Ashwagandha, ginseng, ginkgo biloba, Brahmi or related herbs, (4) they were pregnant or lactating, (5) they had substance dependence (6) they had abnormal laboratory or ECG findings.
Interventions
The recruitment process started with a pre-screening meeting, which offered an introductory lecture on stress and was part of a set of stress-management talks. The meeting was attended by participants from a wide cross-section of the population, which included doctors, students, self-employed persons, executives and employees of Information Technology firms. These sub-populations work under stress and perceive themselves as being under stress. The purpose of the study was explained to attendees at the pre-screening meetings, who were then invited to be a part of the study. Those who volunteered to be a part of the study were given the WHO-5 health questionnaire so that their stress levels could be assessed. The participants who had a score of 15 or lower on the WHO-5 well-being index, reported high stress levels as measured by the PSS, and who were interested in further participation were asked to visit the research site for further evaluation.
On Day 0 at the research site, after obtaining written consent from the subjects, their medical history was recorded. Clinical examination and laboratory assessments were carried out. Each subject was then assessed on the PSS. A qualified psychiatrist performed a clinical psychiatric examination on the subjects to check for the primary psychiatric disorders because the clinical trial protocol sought to exclude subjects with psychiatric disorders. Subjects with a PSS score of at least 14 were enrolled into the study.
On Day 0, serum cortisol levels were measured in the morning. The subjects were also administered three questionnaires: the PSS questionnaire again, the Depression Anxiety Stress Scale (DASS)[12] questionnaire and the 28-item version of the General Health Questionnaire[13] (referred to as GHQ-28 henceforth in this paper). After assessing the screening parameters, each subject was randomly assigned to either the study drug treatment group or the placebo control group, with a randomization ratio of 1:1 using a computer-generated randomization scheme. Subjects were provided a blinded kit containing capsules in a quantity adequate for the entire duration of the study. Subjects were instructed to consume one capsule two times a day after food with a glass of plain water, for 60 days. The study drug treatment group was given capsules, each containing 300 mg of high-concentration full-spectrum Ashwagandha root extract. Henceforth, in this research paper, this group will be referred to as the Ashwagandha group. The placebo-control group was given capsules containing a neutral substance. The capsules in the two groups were of identical size and form. To verify treatment compliance and to record any adverse events, a follow-up telephone call was made on Day 15, Day 30 and Day 45. After each telephone call, the findings were recorded in a case report form. On Day 60, final measurements of serum cortisol and the stress scale responses were performed. Data on safety and adverse effects of the study drug were collected on Day 60 also.
Data collection and analysis
On Day 0 and Day 60, data were collected by physiological measures in a laboratory and by survey responses to standard questionnaires. Additional data on compliance and adverse reactions were collected on Day 15, Day 30 and Day 45 through a telephone call.
Assay methodology
The efficacy of the high-concentration full-spectrum Ashwagandha root extract in reducing stress was assessed based on Day 0 and Day 60 levels of serum cortisol and the scores on the PSS, GHQ-28 and DASS questionnaires. Specifically, we compared the measures across the two groups on Day 0 and across the two groups on Day 60. We also compared the changes in the measures over the 60 days across the two groups. This allowed us to assess the effect of the drug on various dimensions of stress.
The safety of the Ashwagandha extract was assessed based on laboratory findings and incidence of adverse events. All adverse events reported by the subjects were recorded. Their potential relationship with the study drug was evaluated.
Statistical methods and analysis
A sample of 64 subjects was enrolled in the study. Three subjects were not compliant with the study protocol. For efficacy analysis, only the 61 subjects compliant with the protocol were included. For safety analysis, we considered all 64 subjects in the intent-to-treat population. This consisted of all those subjects who took at least one dose of either the treatment drug or the placebo.
Efficacy was evaluated based on both physiological and survey measures. For each measure, we computed the difference across the two groups on Day 0 and across the two groups on Day 60. We performed a t-test to assess the statistical significance of this difference. We also computed, within each group, the change in the measure over the 60 days, and measured the difference in these changes across the two groups. A t-test was used to assess the statistical significance of this difference. For robustness, we also used the Mann-Whitney test, which is a non-parametric test. All these assessments collectively allowed us to evaluate the effect of the drug on various dimensions of stress.
RESULTS
Background information on the subjects
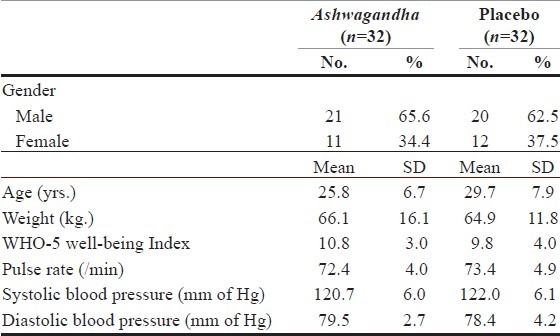
A total of 64 subjects (32 in each study group) were included in the study of which 41 (64%) were men, and 23 (36%) were women [Table 1]. After randomization, each group had 32 subjects. None of the enrolled subjects had any illness related to cardiovascular, respiratory, gastrointestinal, nervous, endocrine, urogenital systems or allergy/skin-related disorders. One subject reported a history of depression; however, at the time of enrollment he was free from psychiatric symptoms. The rest of the subjects were free from psychiatric illnesses. Two subjects from the Ashwagandha group and one subject from the placebo group were lost to follow-up. On Day 60, 61 subjects had completed the study.
Table 1.
Demographic and baseline parameters of the subjects enrolled
Efficacy analysis
Perceived stress scale
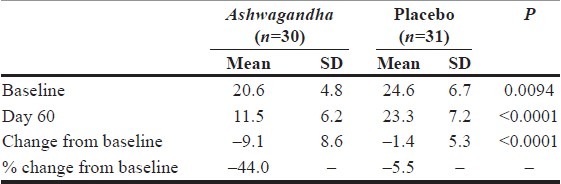
The PSS is perhaps the most commonly used psychological scale for assessing self-perception on the level of stress in one's life. The scale consists of ten items, with each item measuring the frequency or extent to which a certain stress-signaling event occurs. For each item, the score ranges from 0 to 4, with a score of zero connoting the absence or non-incidence of the corresponding stress-signaling event. The PSS score is obtained by summing the score across all ten items. The score ranges from 0 to 40, with a score of zero reflecting virtual absence of stress in one's life. Table 2 summarizes the data and the results from the key statistical significance tests. On completion of 60 days of treatment, there was only a 5.5% reduction in baseline PSS scores in the placebo group, compared to 44.0% in the Ashwagandha group. The difference in reduction in PSS scores between the two groups over the 60-day study period was highly statistically significant (P<0.0001).
Table 2.
Data analysis for perceived stress score data
Many psychometric scales do not lend themselves to ratio-scale interpretations and translation of increments or decrements to percentage changes. In contrast, all the psychometric scales used in this study are interpreted in the research literature as ratio-scaled. The crucial property of the scales used in this paper, which allows us to translate increments or decrements to percentage changes, is that the lowest score value is anchored to zero and corresponds to a state where there are virtually zero stress-signaling events. Therefore, each scale value can be interpreted as the absolute degree of self-assessed stress. This is consistent with the many research publications in the stress literature that use such scales.
General health questionnaire-28
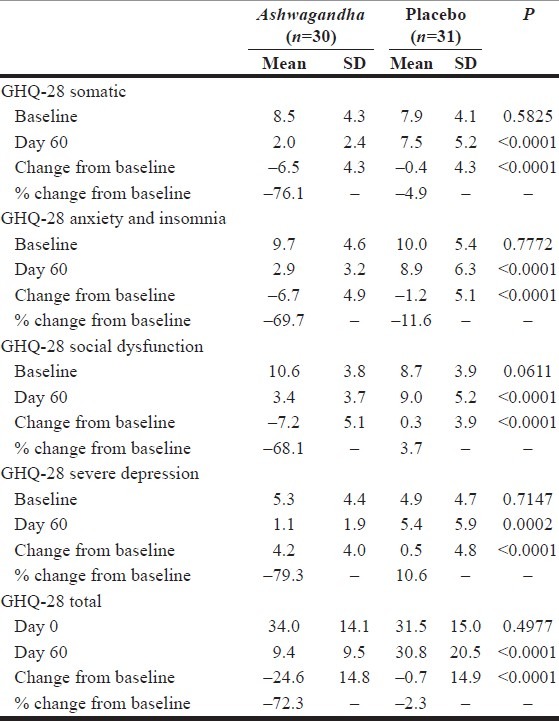
The GHQ-28 questionnaire (Goldberg and Hillier 1979) has four distinct item-subsets corresponding to stress categorized as ‘somatic’, ‘anxiety and insomnia’, ‘social dysfunction’ and ‘severe depression’. The score on each scale item measures the frequency or extent to which a certain stress-signaling event occurs. For each item-subset, the score ranges from 0 to 21. A score of zero corresponds to absence or non-incidence of a certain category of stress-signaling events. A score of 21 corresponds to a high frequency of these events. It is common to also report a total score, which represents a measure of overall stress, aggregated over all four item-subsets. Table 3 summarizes the data and the results from the key statistical significance tests. The Ashwagandha experimental group and the placebo control group were similar with respect to the baseline scores for all of these item-subsets and the total score (P>0.05). However, by Day 60, the scores in the two groups diverged greatly and the average scores were significantly different (P<0.0001 for each item-subset and the total). Furthermore, the decrease in the stress measure over the study period of 60 days was considerably higher in the Ashwagandha group than in the placebo group (P<0.0001 for each item-subset and the total). In the Ashwagandha group, by Day 60 there was a significant reduction in scores corresponding to all of the item-subsets: 76.1% for the “Somatic” item-subset, 69.7% for the “Anxiety and Insomnia” item-subset, 68.1% for the “Social Dysfunction” item-subset, 79.2% for the “Severe Depression” item-subset. In contrast, in the placebo control group, the corresponding reductions in scores were much smaller: 4.9%, 11.6%, –3.7% and –10.6%, respectively. As can be readily seen, the difference is at least 58 percentage points and as high as 89 percentage points.
Table 3.
Data analysis for GHQ-28 questionnaire data
Depression anxiety stress scale
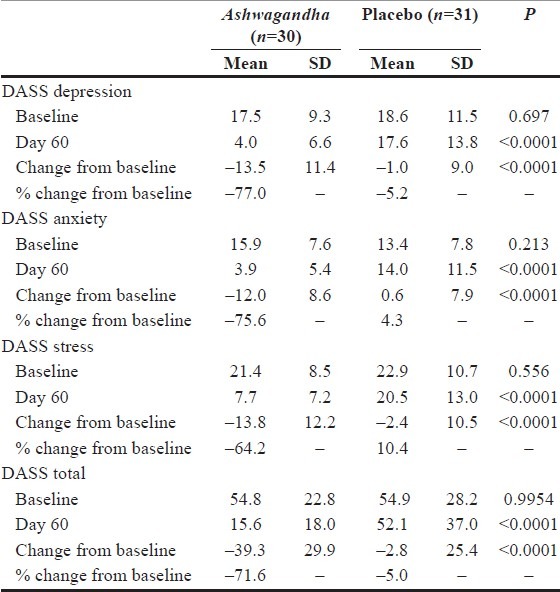
The DASS questionnaire (Lovibond and Lovibond 1995) has three distinct item-subsets corresponding to stress categorized as ‘depression’, ‘anxiety’ and ‘stress’. Like with the GHQ-28 scale, the score on each scale item measures the frequency or extent to which a certain stress-signaling event occurs. For each item-subset, the score ranges from 0 to 42, with a score of zero connoting the absence or non-incidence of the corresponding category of stress-signaling events. We also report the total score, which is a measure of overall stress, aggregated over all three item-subsets. Table 4 summarizes the data and the results from the key statistical significance tests. The two treatment groups were similar with respect to the baseline scores for all of these three DASS subsets (P>0.05). By Day 60, the scores in the two groups differed substantially and the average scores were significantly different (P<0.0001 for each item-subset and the total). Furthermore, relative to the placebo group, greater reductions were seen in the scores for all three subsets in the Ashwagandha group after 60 days of treatment (P<0.0001 for each item-subset and the total). In the Ashwagandha group, by Day 60 there was a significant reduction in scores corresponding to all of the item-subsets: 77% for the “Depression” item-subset, 75.6% for the “Anxiety” item-subset, 64.2% for the “Stress” item-subset. In contrast, in the placebo-control group, the corresponding reductions in scores were much smaller: 5.2%, – 4.3% and 10.4%, respectively. In the placebo group, there was a slight increase in the score in the ‘anxiety’ sub-subset and a small decrease in the scores in the ‘depression’ and ‘stress’ item-subsets.
Table 4.
Data analysis for depression–anxiety stress scale data
Serum cortisol levels
Serum cortisol is a frequently used biological marker of stress. For this reason, it may be useful to assess the effect of the treatment on cortisol level. Table 5 summarizes the data and the results from the key statistical significance tests. The serum cortisol levels are measured in μg/dL. Both the groups had similar serum cortisol levels at the baseline (P>0.05). By the end of the study period, the two groups’ average serum cortisol levels were considerably different (P=0.006). After 60 days of treatment, a reduction of 27.9% from baseline was observed in the Ashwagandha group. In contrast, a reduction of 7.9% was observed in the placebo-control group. The difference in the reduction of serum cortisol levels in the two groups on Day 60 was statistically significant (P=0.002) [Table 5 and Figure 1].
Table 5.
Data analysis for serum cortisol* data
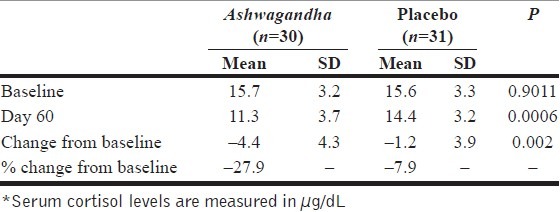
Figure 1.
Percentage change from baseline in PSS, GHQ-28, DASS, Serum cortisol
Safety analysis
Laboratory assessments
After 60 days of therapy, apart from the changes in the efficacy measures reported above, there were no significant changes in the laboratory parameters from baseline visit to final visit in both the groups except for the values of serum globulin and serum triglycerides. However, these differences were clinically insignificant.
Adverse events
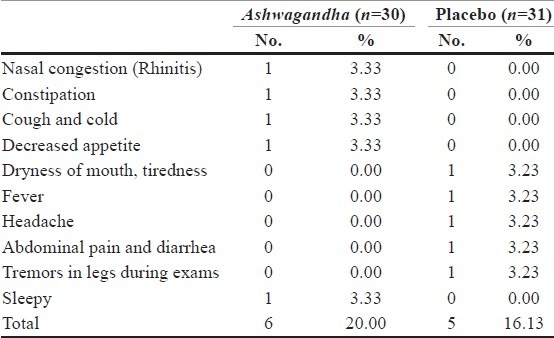
Data of adverse events were available for only 61 subjects as three subjects were lost to follow-up. There were six adverse effects reported in the Ashwagandha group and five in the placebo group [Table 6]. Nasal congestion (rhinitis), constipation, cough and cold, drowsiness and decreased appetite were seen in the Ashwagandha group. In the placebo-control group, dryness of mouth, tiredness, fever, headache, abdominal pain and diarrhea, tremors in legs during examination were seen. The incidences of adverse events were comparable in both the groups and the difference was not statistically significant. Moreover, all the adverse events were mild in nature and no known mechanisms relate these adverse events to the study drug.
Table 6.
Adverse events
DISCUSSION
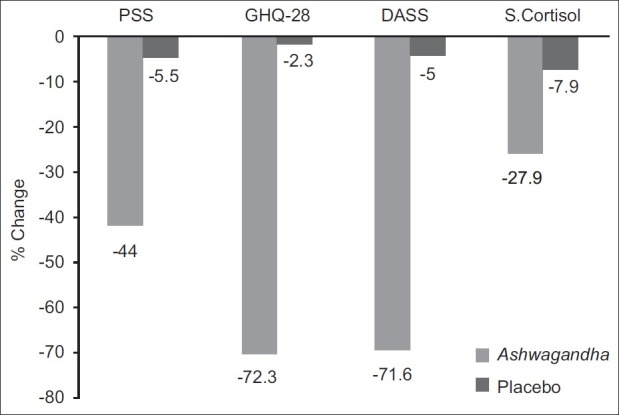
This study is unusual in that we use numerous measures of stress. In contrast, most other studies use only a small set of measures. The present study assessed the level of stress, depression, anxiety and general well-being of an individual using three different, extensively used sets of stress scales and serum cortisol level, a biochemical marker of stress. A graph summarizing the results across all these measures is depicted in Figure 1. Collectively, these measures speak not only to the focal aspects of stress but also to some antecedents and consequences of stress. As we discuss below, the results show that high-concentration full-spectrum Ashwagandha root extract has a significant effect on both sets of aspects related to stress.
Effect of Ashwagandha on focal aspects of stress
In this study, some focal aspects of stress are measured by (a) the “Anxiety and Insomnia” item-subset of GHQ-28, (b) the “Stress” item-subset of the DASS, (c) the “Anxiety” item-subset of the DASS, and (d) the full item set of the PSS. As the results presented above show, the Ashwagandha group of subjects showed substantial reduction in all four of these measures of stress. The Ashwagandha group's subjects had reductions of 69.7%, 64.2%, 75.6% and 44.0%, respectively, on these four measures. In contrast, the placebo group's subjects saw reductions of only 11.6%, 10.4%, –4.3% and 5.5%, respectively. The differences are highly statistically significant, suggesting a substantial effect of Ashwagandha in improving the well-being of subjects with respect to these focal aspects of stress. This finding provides evidence of strong anti-stress adaptogenic activity of Ashwagandha. These results are consistent with the findings of previous in-vivo studies.[4,9] Similar results were reported by Andrade et al. in anxiety patients.[14]
Effect of Ashwagandha on antecedents and consequences of stress
This study also measured aspects of stress that can be seen as precursors or results of the focal phenomenon of stress. These correspond to either events and conditions that lead to stress or events and conditions that result from stress. In this study, these are represented by (a) the “Somatic” item-subset of GHQ-28, (b) the “Social Dysfunction” item-subset of GHQ-28, (c) the “Severe Depression” item-subset of GHQ-28, (d) the “Depression” item-subset of the DASS, (e) the level of serum cortisol. The Ashwagandha group's subjects had reductions of 76.1%, 68.1%, 79.2%, 77.0%, 27.9%, respectively, on these five measures. In contrast, the placebo group's subjects saw reductions of only 4.9%, –3.7%, –10.6% –5.2%, 7.9%. Once again, these differences are highly statistically significant, suggesting a substantial effect of Ashwagandha in improving the condition of the subjects.
Serum cortisol is a frequently cited correlate of stress and is therefore worth elaborating on in this discussion. Acute stress increases heart rate and arterial blood pressure, stimulates gluconeogenesis, glycogenolysis, lipolysis and hepatic glucose secretion. These in turn elevates the catecholamines and cortisol levels in the body.[15] Stress, either physical or mental leads to enhancement of ACTH secretion, which in turn increases cortisol levels; at times, the level may increase even 20-fold.[16] The results of our study shows that high-concentration full-spectrum Ashwagandha root extract reduces levels of serum cortisol, which elevates in stressful conditions. Similar findings were observed in previous study by Auddy et al. in patients with stress.[17]
These two sets of effects collectively suggest that high-concentration full-spectrum Ashwagandha root extract mitigates not only the focal aspects of stress but also some of the precursors, consequences and associated symptoms of stress. One can think of this as Ashwagandha helping both directly and indirectly. This suggests, therefore, that high-concentration full-spectrum Ashwagandha root extract possesses the ability to improve the overall well-being of a person.
Safety
An analysis of the adverse events recorded in this study indicates that high-concentration full-spectrum Ashwagandha root extract is safe and well tolerated as there were no serious adverse events. The side effects that were observed were mostly mild in nature and no known causal mechanisms relate them to the study drug. The side effects in both the groups were comparable. Insignificant changes in laboratory values were observed in both the groups. The results on safety in this study are consistent with previous studies on Ashwagandha, where generally there were no adverse events leading to drop outs or withdrawal symptoms.[17] Long-term administration of the roots of Ashwagandha was found to be safe also in animal studies.[18] A word of caution, however, those allergic to herbs belonging to the Solanaceae family are contraindicated for treatment with this herb.
Limitations
The study's sample size, while large enough to establish statistical significance, was not very large. There should be a larger study conducted, using a wider cross-section of the population. Furthermore, though the enrolled subjects were under stress, none had any psychological illness or any other systemic illness. Studies using a wider set of clinical backgrounds would be illuminating. There also need to be studies of longer duration so that we get a better understanding of the long-term effects of Ashwagandha on stress resistance.
CONCLUSION
The findings suggest that high-concentration full-spectrum Ashwagandha root extract improves an individual's resistance towards stress and thereby improves self-assessed quality of life. High-concentration full-spectrum Ashwagandha root extract can be used safely as an adaptogen in adults who are under stress.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Provino R. The role of adaptogens in stress management. Aust J Med Herbal. 2010;22:41–9. [Google Scholar]
- 2.Panossian A, Wikman G. Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Curr Clin Pharmacol. 2009;4:198–219. doi: 10.2174/157488409789375311. [DOI] [PubMed] [Google Scholar]
- 3.Shastry JLN., Dr . Ayurvedokta oushadha niruktamala, Chaukhambha Orientalia. Ist ed. Varanasi, India: 2001. p. 10. [Google Scholar]
- 4.Bhattacharya SK, Muruganandam AV. Adaptogenic activity of Withania somnifera: An experimental study using a rat model of chronic stress. Pharmacol Biochem Behav. 2003;75:547–55. doi: 10.1016/s0091-3057(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 5.Singh G, Sharma PK, Dudhe R, Singh S. Biological activities of Withania somnifera. Ann Biol Res. 2010;1:56–63. [Google Scholar]
- 6.Sharma V, Sharma S, Pracheta, Paliwal R. Withania somnifera: A rejuvenating ayurvedic medicinal herb for the treatment of various human ailments. Int J PharmTech Res. 2011;3:187–92. [Google Scholar]
- 7.Kulkarni SK, Dhir A. Withania somnifera: An Indian ginseng. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:1093–05. doi: 10.1016/j.pnpbp.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya SK, Goel RK, Kaur R, Ghosal S. Anti-stress activity of sitoindosides VII and VIII, new acylsterylglucosides from Withania somnifera. Phytother Res. 1987;1:32–7. [Google Scholar]
- 9.Ghosal S, Lal J, Srivastava R, Bhattacharya SK, Upadhyay SN, Jaiswal AK, et al. Immunomodulatory and CNS effects of sitoindosides IX and X, two new glycowithanolides from Withania somnifera. Phytother Res. 1989;3:201–6. [Google Scholar]
- 10.Bech P. Measuring the dimentions of psychological General Well-being by the WHO-5. QoL Newsletter. 2004;32:15–6. [Google Scholar]
- 11.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:386–96. [PubMed] [Google Scholar]
- 12.Lovibond SH, Lovibond PF. Manual for the depression anxiety stress scales. 2nd ed. Sydney: Psychology Foundation; 1995. ISBN 7334-1423-0. [Google Scholar]
- 13.Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychol Med. 1979;9:139–45. doi: 10.1017/s0033291700021644. [DOI] [PubMed] [Google Scholar]
- 14.Andrade C, Aswath A, Chaturvedi SK, Srinivas M, Raguram R. A double blind, placebo- controlled evaluation of the anxiolytic efficacy of an ethanolic extract of Withania somnifera. Indian J Psychiatry. 2000;42:295–301. [PMC free article] [PubMed] [Google Scholar]
- 15.Chrousos GP. Stress and disorders of the stress system. Nature reviews. Endocrinology. 2009;5:374–81. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 16.Guyton AC, Hall JE. Adrenocortical Hormones Textbook of Medical Physiology. 11th ed. Philadelphia: Saunders; 2006. pp. 944–60. [Google Scholar]
- 17.Auddy B, Hazra J, Mitra A, Abedon B, Ghosal S. A standardized Withania somnifera extract significantly reduces stress-related parameters in chronically stressed humans: A double-blind, randomized, placebo-controlled study. J Am Nutraceutical Assoc. 2008;11:50–6. [Google Scholar]
- 18.Sharma S, Dahanukar SA, Karandikar SM. Effects of long-term administration of the roots of ashwagandha and shatavari in rats. Indian Drugs. 1985;29:133–9. [Google Scholar]


