Abstract
Background & objectives:
Logistic and financial constraints limit application of several available immunohistochemical (IHC) markers and molecular analysis in every case of synovial sarcoma, diagnosed in our settings. Recently, TLE1 has been recognized as a robust IHC marker for diagnosing a synovial sarcoma. Here, we present IHC features of synovial sarcomas, including TLE1 expression in these cases and in some other tumours.
Methods:
Conventional sections from 42 synovial sarcomas (30 retrospective & 12 prospectively diagnosed) were subjected to TLE1 IHC staining, including 21 tumours confirmed with molecular testing. TLE1 immunostaining was graded from 0, 1+, 2+, 3+, with 2+ or 3+ grades interpreted as positive staining.
Results:
Of the 42 tumours, 26 (61.9%) were of monophasic spindle cell type, 13 biphasic type (30.9%), two (4.7%) calcifying type and remaining one (2.3%) was a poorly differentiated synovial sarcoma. On immunohistochemistry (IHC), tumours were positive for epithelial membrane antigen (EMA) (26/34, 76.4%), cytokeratin (CK)7 (6/10, 60%), CK/MNF116 (6/21, 28.6%), B cell lymphoma 2 (BCL2) (36/37, 97.3%), cluster of differentiation molecule 99 (MIC2) (23/31, 74.1%) and transducin-like enhancer of split 1 (TLE1) (40/42, 95.2%), while negative for CD34 in all 21 tumours, wherever performed. TLE1 was also positive in tumour controls, including schwannomas (5/5, 100%), neurofibromas (2/2, 100%), malignant peripheral nerve sheath tumors (2/12, 17%) and Ewing sarcomas (4/10, 40%). TLE1 sensitivity for diagnosis of synovial sarcomas was 95.2 per cent. Its overall specificity was 63.7 per cent, whereas with regards to tumors forming its closest differential diagnoses, its specificity was 72 per cent.
Interpretation & conclusions:
Although molecular confirmation is the diagnostic gold standard for synovial sarcoma, TLE1, in view of its high sensitivity may be a useful marker within the optimal IHC panel comprising EMA, BCL2, MIC2, CD34 and CK7, especially on small biopsy samples, for substantiating a diagnosis of synovial sarcoma. Awareness of TLE1 expression in other tumours and its correct interpretation are necessary.
Keywords: Immunohistochemistry of synovial sarcoma, molecular analysis of synovial sarcomas, synovial sarcoma, TLE1, t(X; 18) (SYT-SSX)
Synovial sarcoma accounts for 10-15 per cent of adult soft tissue sarcomas. Although, according to the World Health Organization (WHO) classification of bone and soft tissue tumours, synovial sarcoma is a distinct morphological, clinical and genetically defined tumour entity, it can occur over a wide age-range and almost anywhere in the body1. It is predominantly identified in extremities of young adult males in 80-90 per cent cases, most commonly around the knee joint. On histopathology, its various subtypes include biphasic, monophasic spindle cell type, monophasic epithelial type, poorly differentiated (round) cell type and calcifying type1. Considerable clinicopathological heterogeneity within this tumour creates a diagnostic challenge in sorting it out from its differential diagnoses1–3. The value of an exact diagnosis of a synovial sarcoma lies in the fact that it is a relatively more chemosensitive sarcoma among other adult soft tissue sarcomas. Currently, several immunohistochemical (IHC) markers are available, namely epithelial membrane antigen (EMA), cytokeratin (CK) cocktails, specific CKs like CK7, CK19 and vimentin, along with additional markers like BCL-2, calponin, MIC-2, S100-P, CD56 (cluster of differentiation 56), E-cadherin, β-catenin and matrix metalloproteinase-2 that have been utilized in its objective diagnosis4–10. Further, synovial sarcoma is characterized by a highly specific, SYT-SSX fusion oncogene, as a result of t(X; 18) translocation, wherein SSX gene on chromosome X fuses to SYT gene on chromosome 18. The resultant SYT-SSX fusion oncoprotein brings together transcriptional activation (SYT) and repression (SSX) that seems to be involved in the oncogenesis of this tumour through transcriptional dysregulation, inducing epigenetic changes that silence key tumour suppressor genes11–15. Analysis of SYT-SSX transcripts is regarded as the diagnostic ‘gold standard’ for a synovial sarcoma12. This fusion transcript is detected by fluorescent in-situ hybridization (FISH) and reverse transcriptase-polymerase chain reaction (RT-PCR) technique in cases of diagnostic dilemmas, including in cases occurring at unexpected sites and when IHC profile is inconclusive for diagnosis of a synovial sarcoma16. Logistic considerations and financial constraints limit routine application of several IHC markers and molecular techniques in every case diagnosed in limited resource settings like in India.
Therefore, there is a need for identification of a sensitive and a specific IHC marker for this sarcoma. Gene expression profiling studies have unraveled TLE1 (Transducin-Like enhancer of split-1) as a useful diagnostic marker for a synovial sarcoma17. TLE1 is one of the four TLEs that encode human transcriptional repressors homologous to the Drosophilia coexpressor groucho18. Differential expressions of TLE2, 3 and 4 have also been noted in a synovial sarcoma18–20. Initially, Terry et al17, through their study on tissue microarray sections, observed high sensitivity and specificity of TLE1 in diagnosis of a synovial sarcoma. Subsequently, there have been very few studies regarding the utility and validation of this IHC marker on this sarcoma, all from the west21–23. While four studies17,21–23 have shown its utility as a fairly sensitive and a specific marker and further postulated its potential as a ‘robust’ biomarker for synovial sarcoma, Kosemehmetoglu et al24 reported its high sensitivity, but limited specificity in the diagnosis of a synovial sarcoma. Here, we present IHC expression of synovial sarcomas, including TLE1 expression in these cases and in other tumours, with intent to identify the potential of TLE1 as a useful marker within the optimal IHC panel for synovial sarcoma. Further, the study was also aimed to explore the utility of TLE1 in terms of its comparison with molecular analysis, in our settings.
Material & Methods
The study included 42 synovial sarcomas, including 30 retrospective and 12 prospectively diagnosed tumours at department of Pathology, Tata Memorial Hispital, Mumbai, over a 7-year period. The retrospective cases were retrieved from our pathology department database. The study samples were available in form of formalin-fixed, paraffin-embedded tissue blocks, with or without stained slides (21, 50%), biopsy specimens (8, 19%) and tumour resection specimens (13, 30.9%). Hematoxylin and eosin stained (H & E) microsections were accessible in all cases.
All tumours were critically reviewed by BR for inclusion in the study, as per diagnostic criteria for a synovial sarcoma1–3. Twenty-one tumours (50%), including those either occurring at uncommon sites, with variable histopathological features or with equivocal IHC results, were confirmed with molecular analysis. The remaining 21 tumours comprised biphasic types (6), calcifying variants (2) and monophasic synovial sarcomas (13), all that had classic clinical presentation, histopathological features and IHC profile, including at least positive expression of the IHC markers, namely EMA and/ or CK, BCL2 and MIC2 and negative expression of CD3424.
Immunohistochemistry (IHC) was performed by immunoperxoidase method using MAC H2 Universal HRP-Polymer detection kit, Biocare, CA, USA, including 3’-3’-diaminobenzidine tetrahydrochloride (DAB) as the chromogen. Appropriate positive and negative controls were included. The various IHC markers performed in various cases in the present study enlisted in Table I. TLE1 staining was performed in 42 synovial sarcomas. TLE1 (ab15587-200) rabbit polyclonal to TLE1, (Abcam, USA) was the antibody used in the present study. The antigen retrieval method used was Heat induction24, standardized, with Pascal using Tris-EDTA as the buffer that gave best result. The antibody results in positive cases, including cases confirmed with translocation results were validated with serial dilutions and 1:250 (as per manufacturers’ recommendations) was found to be the optimal dilution that revealed intense nuclear staining with nil background staining. TLE1 staining was also performed on 70 tumours, other than synovial sarcomas that were retrospectively diagnosed. These also included tumours that form differential diagnosis of synovial sarcoma.
Table I.
List of various antibody markers in the present study
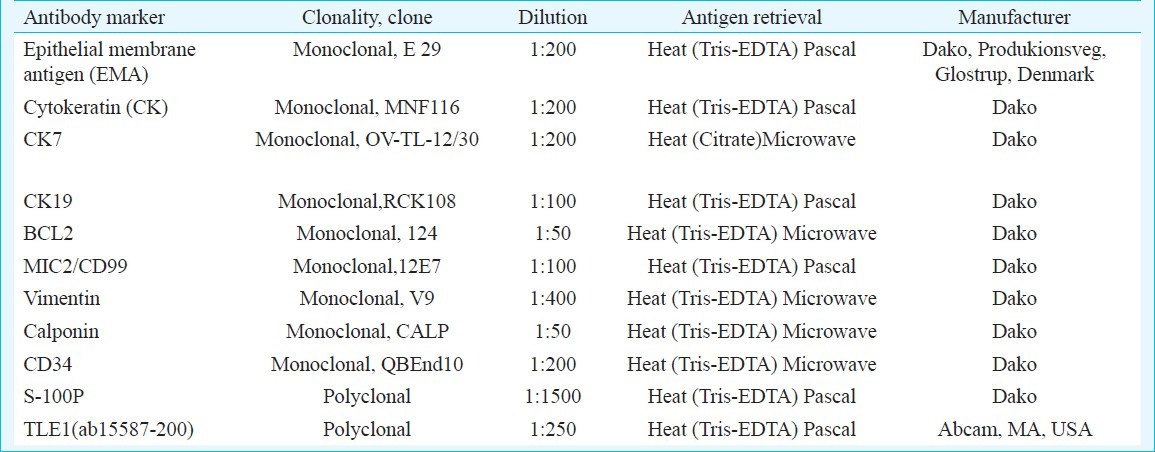
Interpretation of IHC results: For various IHC markers of epithelial differentiation, namely EMA, cytokeratins (CK), CK7 and CK19, staining was considered as positive when tumour cells showed discrete cytoplasmic membranous staining in case of epithelial membrane antigen and cytoplasmic staining in case of CKs within in 1-5 per cent tumour cells and onwards. For MIC2 and BCL-2, diffuse cytoplasmic and/or membranous staining for the former and cytoplasmic and or nuclear membrane staining within tumour cells for the latter were considered as positive staining. Only diffuse cytoplasmic staining with calponin was considered as positive.
TLE1 immunostaining was graded as 0 (Nil), 1+, 2+, 3+. Less than 5 per cent staining of tumour cell nuclei against background was considered as nil staining; 5-25 per cent staining of tumour cell nuclei was considered as grade 1+; 25-50 per cent as grade 2+ and more than 50 per cent intense staining of tumour nuclei was considered as grade 3+ staining. In cases of moderate staining of tumour nuclei more than 50 per cent, these were counted as grade 2+ staining. Only 2+ and 3+ staining was considered as positive staining22,24. Internal controls, including epithelial and endothelial cell staining and external controls in form of tumours confirmed with molecular testing that showed 2+ or 3+ TLE1 staining, were included.
Two cases were referred from elsewhere, in form of paraffin embedded tissue blocks for only SYT-SSX analysis. In all, 21 (50%) tumours were analyzed for general and further, specific transcripts i.e. SYT-SSX, SYT-SSX1, SYT-SSX2, by RT-PCR technique.
Molecular analysis: The paraffin blocks were subjected to RT-PCR analysis. Total RNA was isolated using Optimum™ FFPE RNA Isolation kit (Ambion Diagnostics, USA). This was reverse transcribed using cDNA synthesis kit (Gibco-BRL, USA). The primer sequences were as follows;
For SYT-SSX translocation detection, PCR was done using following primers; SYT (Forward): 5’ CCA GCA GAG GCC TTA TGG ATA 3’ SSX (Reverse): 5’ TTT GTG GGC CAG ATG CTT C 3’ Further, for SYT-SSX1 and 2 translocation detection, PCR was done using following primers; SYT (Forward): 5’ CAA CAG CAA GAT GCA TAC CA 3΄ SSX1 (Reverse): 5’ GGT GCA GTT GTT TCC CAT CG 3’ SSX2 (Reverse): 5’ GGC ACA GCT CTT TCC CAT CA 3’ The PCR products were analyzed on 10% polyacrylamide gel, which showed a positive band for t(X; 18) a translocation including chimeric transcripts SYT-SSX, SYT-SSX1 and SYT-SSX2. The base pair size of bands for individual transcript was 98 bp for the general transcript i.e. SYT-SSX and 331 bp for specific transcripts, namely SYT-SSX1, as well as for SYT-SSX2, but with different primers. The house keeping genes were FKHR of 220 bp size and ABL genes of 114 bp size; 2 μl of cDNA from test sample was subjected to molecular analysis.
Statistical analysis: Overall, sensitivity and specificity of TLE 1 staining for synovial sarcomas was calculated. Specificity of TLE1 for diagnosis of synovial sarcomas was calculated with regards to its common differential diagnoses including malignant peripheral nerve sheath tumours (MPNSTs), Ewing's sarcomas and low-grade fibromyxoid sarcomas that can show overlapping immunohistochemical results.
Results
Forty two synovial sarcomas were identified in 21 (50%) males and 21 (50%) females, with age ranging from 2-60 yr (mean 25.6, median 25 yr). Most tumours (31, 55.3%) were noted in lower extremities, including thigh (15), knee (6), foot/ankle (6) and leg (4); upper extremities (7, 12.5%), including hand (3), arm (3) and forearm (1); head and neck region (4, 7.14%), including neck (1), hypopharynx (1), laryngopharynx (1), larynx (1); inguinal region (3, 5.3%); retroperitoneum (2, 3.5%); kidney (2, 3.5%); and one each (1.7%) in lung; paravertebral region; perineum; pelvis and in iliac region. Two cases lacked details regarding site of occurrence and were referred from elsewhere as soft tissue tumours, in form of paraffin blocks, for molecular analysis.
On histopathology, 26 tumours (61.9%) were of monophasic spindle cell type that displayed “herring-bone” pattern, fascicular pattern, focal myxoid areas and palisading pattern with intervening ‘scar-like’ collagen, the latter feature conspicuously noted in two tumours. Thirteen tumours (30.9%) were of biphasic type, two (4.6%) of calcifying type and one (2.3%) of poorly differentiated synovial sarcoma.
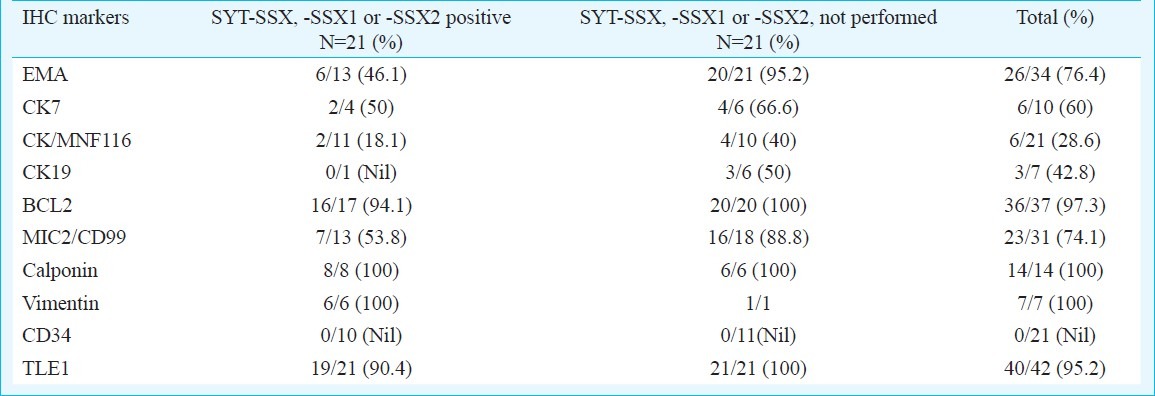
On IHC, tumours were positive for EMA (26/34, 76.4%), CK7(6/10, 60%), CK/MNF116 (6/21, 28.6%), CK19 (3/7, 42.8%), BCL2 (36/37, 97.3%), MIC2 (23/31, 74.1%), calponin (14/14, 100%), vimentin (7/7, 100%), while negative for CD34 in all 21 tumours, wherever performed (Table II).
Table II.
Immunohistochemical profile including TLE1 expression of synovial sarcomas (n=42), including tumours with positive molecular results (n=21) and those without molecular testing (n=21)
Twenty one synovial sarcomas (50%) were confirmed with positive translocation results in form of general and specific transcripts, namely SYT-SSX, SYT-SSX1 and SYT-SSX2. Nine tumours (42.8%), positive for SYT-SSX, occurred in leg (2), knee (2), thigh (1), hand (1), arm (1), kidney (1) and retroperitoneum (1). Eight tumours (38%), positive for SYT-SSX1, occurred in thigh (2); unknown soft tissue sites (2), laryngopharynx (1), lung (1), paravertebral region (1) and foot (1). Four tumours (19%) were positive for SYT-SSX2, including those occurring in hypopharynx (1), kidney (1), larynx (1) and pelvis (1).
TLE1 immunostaining was positive in 40 of 42 synovial sarcomas (95.2%), with 30 tumours displaying 3+ staining, 10 displaying 2+ staining and two displaying 1+ staining. Staining pattern was uniform within spindly and epithelioid cells in biphasic synovial sarcomas. Two cases with unavailable details, including IHC results, but with positive translocation results for SYT-SSX1, displayed positive (3+) TLE1 staining. Nineteen of the 21 tumours (90.4%) confirmed by molecular analysis cases displayed positive TLE1 staining. Among 15 tumours negative for CK, 13 displayed TLE1 positivity. All 8 tumours negative for EMA, displayed TLE1 positivity. A single tumour negative for BCL2 staining displayed TLE1 negativity Table II, Figs 1–4).
Fig. 1.
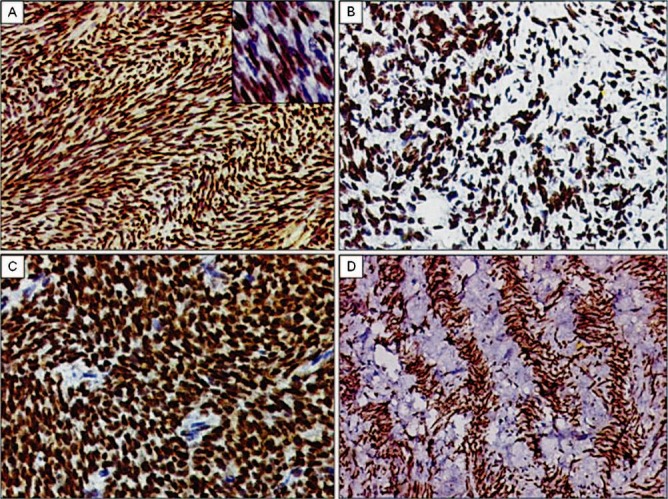
TLE1 expression in spectrum of monophasic spindle cell type synovial sarcomas. A. Diffuse TLE1 positivity (3+) highlighting herring-bone like pattern in a monophasic type of synovial sarcoma. DAB × 200. Inset. Higher magnification showing intense nuclear positivity. DAB × 400. B. TLE1 positivity in a tumour with focal myxoid differentiation. DAB × 200. C. TLE1 positivity (3+) in a synovial sarcoma exhibiting hemangiopericytomatous pattern. DAB × 400. D. TLE1 positivity (3+) in a case of monophasic synovial sarcoma exhibiting prominent palisading/scarring pattern. DAB × 200.
Fig. 4.
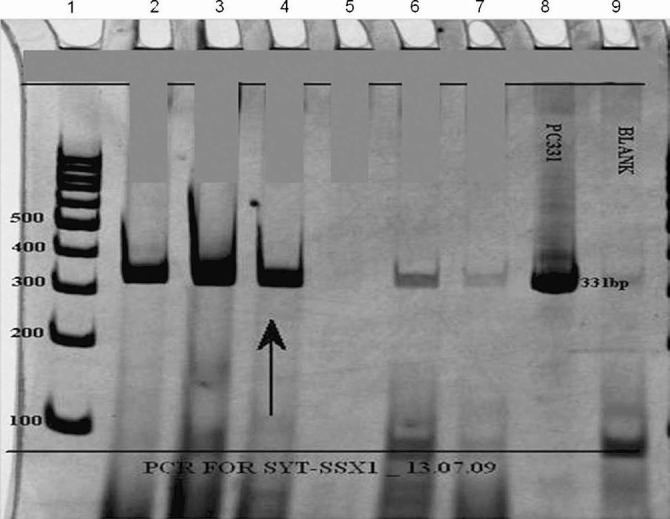
Polymerase chain reaction (PCR) analysis of SYT-SSX translocation using SYT and SSX1 primers. Reactions were subjected to electrophoresis on 10% polyacrylamide gel. Lane 1: the DNA size markers in base pairs (bp); Lanes 2 and 3: PCR run performed with cDNA from an already reported positive cases (331 bp) acting as positive control; Lane 4: PCR run performed with cDNA from test sample (arrow) showing positive band (331 bp); Lane 5: PCR run performed with cDNA from an already reported negative case acting as negative control; Lane 6: PCR run performed with cDNA from an earlier case, revealing weak band, interpreted as “inconclusive”; Lane 7: PCR run performed with cDNA from an unrelated tumour acting as negative control; Lane 8: Positive control DNA (pTZ57R/T-SYT-SSX1-331bp); Lane 9: PCR amplification without DNA template (Blank) to rule out contamination.
Fig. 2.
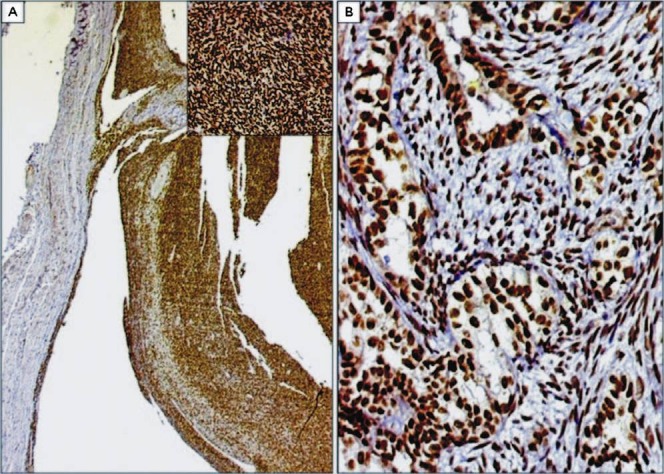
A. Cystic change in a case of monophasic synovial sarcoma exhibiting diffuse TLE1 expression. DAB × 40. Inset: Higher magnification showing intense TLE1 positivity within tumour cells arranged in hemangiopericytomatous pattern. DAB × 200. B. Intense TLE1 positivity in a case of biphasic synovial sarcoma. The glandular and spindly components display similar intensity of TLE1 expression. DAB × 200.
Fig. 3.
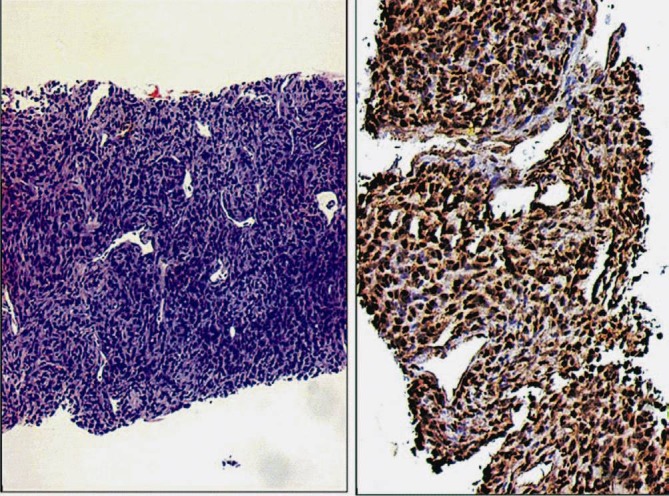
Case of a monophasic synovial sarcoma diagnosed on a small core biopsy. A. Tumour showing hemangiopericytomatous pattern. B. Diffuse, intense TLE1 positivity (3+). DAB × 200.
TLE1 immunostaining was also positive in other soft tissue tumours and sarcomas (26/70) (37.1%) including those that form differential diagnoses of a synovial sarcoma, as well as some other unrelated tumours. Other tumours displaying TLE1 positivity were schwannomas (5/5, 100%), neurofibromas (2/2), malignant peripheral nerve sheath tumours (2/12, 16.6%), desmoplastic small round cell tumours (DSRCTs) (single case confirmed with RT-PCR for EWS-WT1) (3/3, 100%), Ewing sarcomas/PNETs (five tumours confirmed with RT-PCR for EWS-FLI1) (4/10, 40%), low-grade fibromyxoid sarcomas (1/3, 33.3%) and a sclerosing epithelioid fibrosarcoma (1/1). Other tumours showing positive TLE1 immunostaining were rhabdomyosarcomas (2/2, 100%), pleomorphic sarcomas (2/4, 50%), adenocarcinomas (2/4, 50%) and leiomyosarcomas (1/5, 20%). All eight chordomas displayed negative TLE1 immunostaining (Fig. 5A–F). An isolated case of an undifferentiated sarcoma, composed of round to spindle cells, arising in the broad ligament that showed IHC features suggestive for a synovial sarcoma, but showed negative translocation results for synovial sarcoma, Ewing sarcoma and desmoplastic small round cell tumour, displayed positive TLE1 staining (Tables III, IV).
Fig. 5.
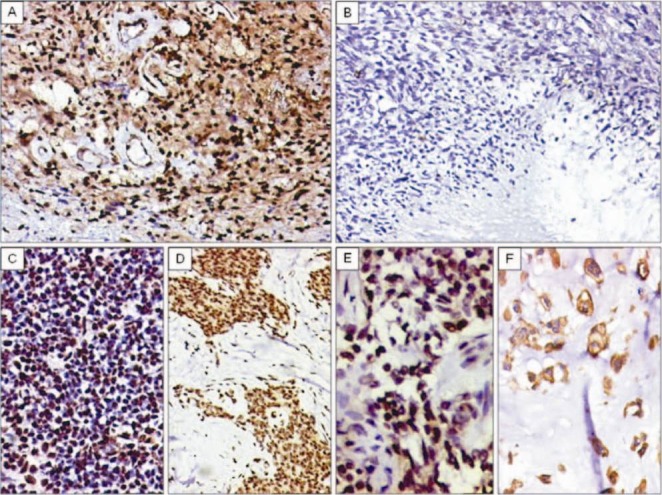
A. Diffuse TLE1 positivity in a neurilemoma/schwannoma. TLE1 positivity also noted within endothelial cells of vessels. DAB × 200. B. TLE1 negativity in MPNST (high-grade) DABx 200C. C. TLE1 positivity (2+) in a case of Ewing sarcoma/PNET. DAB × 200. D. TLE1 positivity (3+) in a desmoplastic small round cell tumour (DSRCT). DAB × 200. E. TLE1 positivity (2+) noted in a case of adamantinoma. DAB × 400. F. Negative nuclear staining, but positive cytoplasmic staining for TLE1 in chordoma. DAB × 400.
Table III.
TLE1 expression in tumours (n=70) other than synovial sarcoma
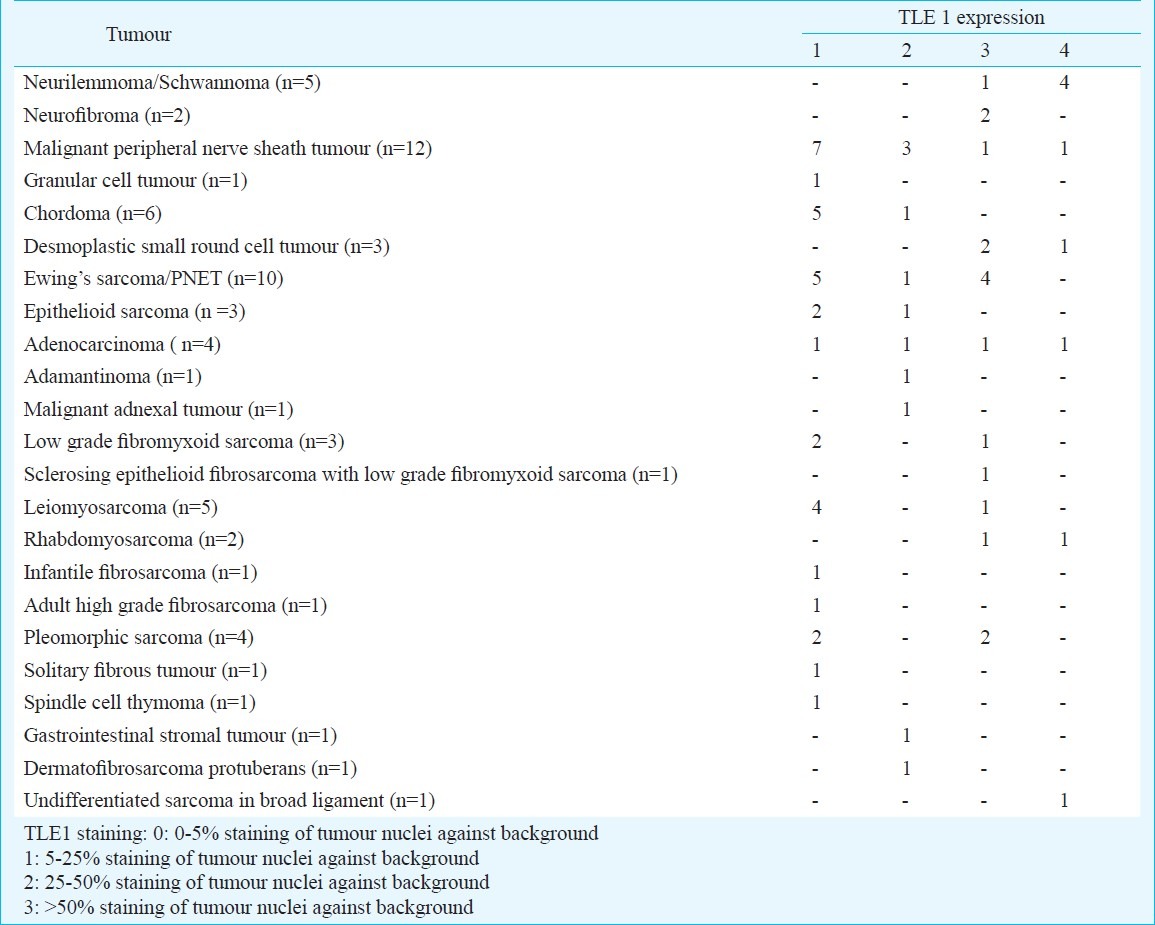
Table IV.
Tumours showing positive TLE1 expression
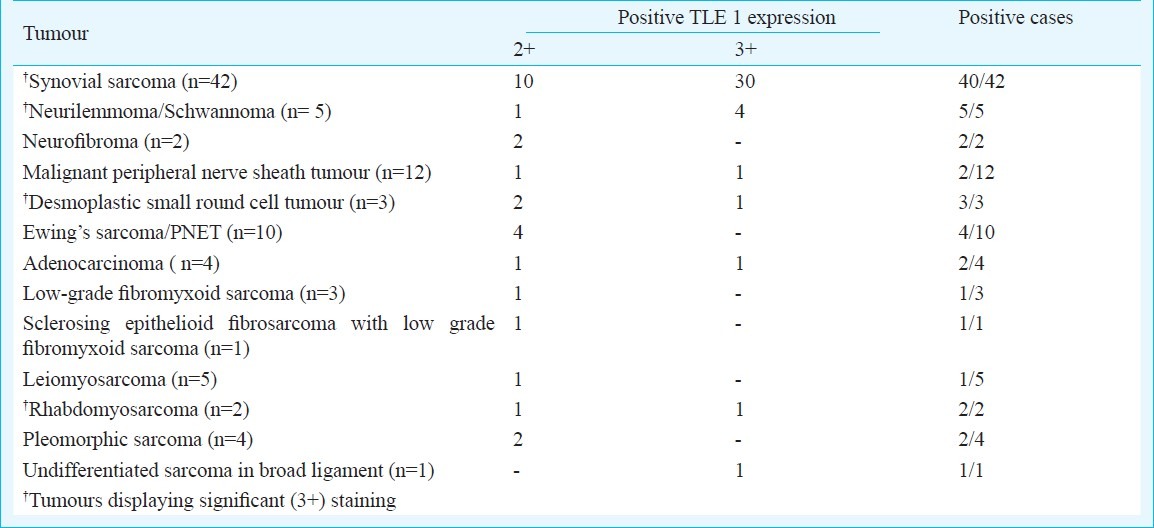
Overall sensitivity of TLE1 staining in synovial sarcomas was 95.2 per cent and in cases that were confirmed with molecular results, the same was 90.4 per cent. Its overall specificity was 63.7 per cent, and specificity for synovial sarcomas with regards select differential diagnoses, despite IHC, was 72 per cent. Besides various tumours, we also observed TLE1 positivity was also observed in endothelial cells, basal keratinocytes and adipocytes.
Discussion
Synovial sarcoma displays a diverse clinicopathological spectrum as noted in the present study. Even though it is an aggressive sarcoma, it is amenable to treatment modalities, including chemotherapy. Hence, its correct identification is vital. Several IHC markers are employed for its objective diagnosis and in differentiating it from it's diagnostic mimics. The diagnostic challenge is further amplified with limited biopsy material, wherein focal expression, especially of epithelial markers, might be lacking, thereby creating a challenge in exact recognition, especially of monophasic spindle cell and poorly differentiated subtypes of synovial sarcoma.
Although an extensive panel of IHC markers is available for diagnosing a synovial sarcoma, there has been no single, fairly specific and sensitive marker for the same. In the present study, markers displaying high sensitivity and reasonable specificity comprised EMA, BCL2, and MIC2. Noteworthy, expression of MIC2 in synovial sarcomas is cytoplasmic, rather than diffuse cytoplasmic membranous positivity, as noted in Ewing sarcoma/primitive neuroectodermal tumor (PNET). Vimentin and calponin display high sensitivity, but low specificity. CK expression in the present study was low as we have MNF116, rather than AE1/AE3 that is a broad spectrum cytokeratin. Of the specific CKs, namely CK7 and CK19, CK7 had reasonable sensitivity and specificity. Negative expression of CD34 was useful in ruling out other differentials like a solitary fibrous tumour (SFT) and/or a hemangioperictyoma.
Although molecular analysis remains the diagnostic ‘gold standard’, there are limitations in subjecting every case to molecular confirmation in limited resource settings. Even though t(X; 18) (p11.2; q11.2) SYT–SSX is positive in more than 90% synovial sarcomas, resulting in SYT-SSX1 and SYT-SSX2 transcripts; SYT–SSX4 is another variant transcript resulting from the t(X; 18) translocation. t(X; 20) (p11.2; q13.3) translocation resulting in SSL18L1/SSX has also been documented in a synovial sarcoma25. Primers for the latter two transcripts are presently unavailable in our laboratory. Besides, cryptic X; 18 rearrangements, although rare, have been documented26,27. There can be rare situations of PCR failures, wherein dual confirmation with FISH, which is relatively expensive, becomes imperative. Therefore, there is a need for a marker with higher sensitivity and reasonable specificity that could obviate routine application of expensive molecular techniques, especially in cases with limited biopsy specimens. SYT has also been recommended as another IHC marker for synovial sarcoma. However, it is presumed to lack specificity, in view of its expression in native form28.
Gene expression studies have identified TLE1, a gene, related to WnT pathway and this has been found to be consistently overexpressed in synovial sarcoma, in various studies19,20.
There are only limited studies, with none from our subcontinent on TLE1 expression on synovial sarcomas17,21–24. All these studies have observed high sensitivity ranging from 82-100 per cent. In the present study, positive staining was observed in 95.2 per cent synovial sarcomas. Among 15 tumours negative for CK, 13 displayed TLE1 positivity. All 8 tumours negative for EMA, displayed TLE1 positivity. This reinforces high sensitivity of TLE1, when compared with epithelial markers. A single tumour negative for BCL2 staining displayed TLE1 negativity, reinforcing a similar high sensitivity of both these markers. The reasons for weak staining in two of our study cases, confirmed with molecular analysis are not known. Whereas four studies17,21,22,23 revealed high sensitivity and specificity of TLE1 for synovial sarcoma, Kosemehmetoglu et al24 in their study, concluded this to be a non-specific marker, in view of its positive expression in other tumours. TLE1 positivity was found in 26 out of 70 tumours, leading to an overall specificity of 63.7 per cent. Other tumours displaying TLE1 positivity were all schwannomas as also observed by others24, who observed 82 per cent positivity in schwannomas, but was in contrast to the result of Terry et al17, who noted TLE1 positivity in 31% regular schwannomas and in 17 per cent cellular schwannomas.
Among the differential diagnosis of synovial sarcoma, TLE1 positivity was noted in 16.6 per cent MPNSTs, 40 per cent Ewing sarcomas, while its negativity was observed in epithelioid sarcomas and in a single case of a solitary fibrous tumour and adult fibrosarcoma, respectively. Terry et al17 documented TLE1 positivity in 5 per cent MPNSTs; in 8 per cent of Ewing sarcomas/PNETs; in none of the epithelioid sarcomas and in 30 per cent of solitary fibrous tumors, Kosemehmetoglu et al24 observed TLE1 positivity in 30 per cent MPNSTs; in 33 per cent epithelioid sarcomas; in 20 per cent solitary fibrous tumours, but in none of the Ewing sarcomas. The reason for much lesser staining in the former study was usage of tissue array, instead of standard tissue sections. In the present study, nine tumours other than synovial sarcoma revealed 3+ staining for TLE1. Besides four schwannomas, these included a single case, each, of a DSRCT, a MPNSTs, a rhabdomyosarcoma and an undifferentiated round cell sarcoma, respectively. Another example of TLE1 positivity as in malignant mesotheliomas29. This tumour is rarely a differential diagnosis of a synovial sarcoma and can be objectively identified with markers like calretinin and HBME1. Besides an excellent sensitivity (95.2%) and high negative predictive value, we, like others22,23 observed intense TLE1 positivity as fairly useful in differentiating a synovial sarcoma from its mimics. Its specificity, with regards its closest differential diagnoses, even with IHC was 72 per cent. Further, its specificity with regards to only MPNSTs was 83.3 per cent.
Jagdis et al22 observed 100 per cent sensitivity and 96 per cent specificity, 92 per cent high positive predictive value of 92 and 100 per cent negative predictive value in TLE1 in synovial sarcoma. In view of its high sensitivity and limited specificity when compared with molecular analysis, which has 96 per cent specificity and sensitivity ranging from 75-100 per cent, in different studies13,30, TLE1 seems to be a useful marker for dual confirmation especially, when application of FISH techniques incurs an additionally substantial expenditure. In a recent prospective study, it was observed that utilization of TLE1 for diagnosing synovial sarcomas leads to a considerable reduction in cost and turnaround time, as compared to SYT results by FISH22. Utility of TLE1 in limited biopsy specimens was also observed in the present study. Besides, these authors22 were also able to substantiate diagnosis of synovial sarcoma with TLE1 staining in a case that was negative for SYT-SSX and subsequently disclosed cryptic SYT-SSX2 transcript. Apart from its expression in certain tumours, we noted aberrant TLE1 expression in a single case of adenocarcinoma and adamantinoma. The limitations in the present study include lack of testing this marker in more cases of hemangiopericytomas/SFTs and fibrosarcoma arising in a dermatofibrosarcoma protuberans (DFSP). Nonetheless, these tumours can be differentiated from synovial sarcoma, with application of other markers like CD34 that is extremely rarely noted in a synovial sarcoma, but is substantially positive in these two tumours. The second limitation was availability of tumours tested with molecular analysis in 50 per cent cases. This is as a result of financial constraints among our patients, as a result of which tumors at uncommon sites and equivocal pathological features are generally subjected to molecular analysis. This emphasizes upon the value of TLE 1 as a useful marker for more objective diagnosis of a synovial sarcoma. TLE1 expression relates to its involvement in control of hematopoiesis, neural differentiation and terminal epithelial differentiation. These could form the reasons for its positivity in tumours with epithelial differentiation, including “polyphenotypic” DSRCTs, in our study, a feature in contrast to other studies17,23. Similar to others24, we also noted TLE positivity in non-neoplastic tissues like endothelial cells, basal keratinocytes and adipocytes. Besides its diagnostic value, intense TLE1 positivity in most synovial sarcomas and in schwannomas can be presumed to be reflective of neural histogenesis, apart from the epithelial origin of a synovial sarcoma.
To sum up, although, molecular testing remains the diagnostic gold standard for a synovial sarcoma, TLE1 could be a useful IHC marker, including on small biopsies; in cases with classical histopathological features and for dual confirmation, in conjunction with molecular analysis, whenever necessary. Its inclusion in an optimal IHC panel formed by EMA, BCL2, MIC2 and CD34 along with CK7, for substantiating a histopathological diagnosis of a synovial sarcoma in cases occurring at unusual sites and with variable histopathological features, could reduce further requests for molecular testing, especially for TLE1 negative tumours. Awareness of TLE1 expression in other tumours, leading to its limited specificity, as well as its correct interpretation are necessary.
Acknowledgment
Authors acknowledge the technical support of Shrimati Rekha Thorat, Jyoti Kadam, Shriyut Amir Khan, Pritam Shinde and Mahendra Palker from Immunohistochemistry Laboratory at Tata Memorial Hospital, Parel, Mumbai and Shrimati Jyoti Bodake from the Molecular Pathology Laboratory at Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Khargar, Navi Mumbai, India.
References
- 1.Weiss SV, Goldblum R, editors. Enzinger and Weiss's soft tissue tumors. 4th ed. St Louis: Mosby; 2001. [Google Scholar]
- 2.Fisher C, Bruijn DRH, Geurts van Kessel A. Synovial Sarcoma. In: Fletcher CDM, Unni K, Mertens F, editors. Tumors of soft tissue and bone. Pathology and genetics. World Health Organization classification of tumors. Lyon: IARC Press; 2002. pp. 200–4. [Google Scholar]
- 3.Fisher C. Synovial sarcoma. Ann Diagn Pathol. 1998;6:401–21. doi: 10.1016/s1092-9134(98)80042-7. [DOI] [PubMed] [Google Scholar]
- 4.Machen SK, Fisher C, Gautam RS, Tubbs RR, Goldblum JR. Utility of cytokeratin subsets for distinguishing poorly differentiated synovial sarcoma from peripheral primitive neuroectodermal tumor. Histopathology. 1998;33:501–7. doi: 10.1046/j.1365-2559.1998.00562.x. [DOI] [PubMed] [Google Scholar]
- 5.Suster S, Fisher C, Moran CA. Expression of BCL-2 oncoprotein in benign and malignant spindle cell tumors of soft tissue, skin, serosal surfaces, and gastrointestinal tract. Am J Surg Pathol. 1998;22:863–72. doi: 10.1097/00000478-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Fisher C, Montgomery E, Healy V. Calponin and h-caldesmon expression in synovial sarcoma; the use of calponin in diagnosis. Histopathology. 2003;42:588–93. doi: 10.1046/j.1365-2559.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 7.Dei Tos AP, Wadden C, Calonje E, Sciot R, Pauwels P, Knight JC, et al. Immunohistochemical demonstration of glycoperotein 30/mic2 (CD99) in synovial sarcoma: a potential cause of dagnostic confusion. Appl Immunohistochem. 1995;3:168–73. [Google Scholar]
- 8.Pelmus M, Guillou L, Hostein I, Sierankowski G, Lussan C, Coindre JM. Monophasic fibrous and poorly differentiated synovial sarcoma: Immunohistochemical reassessment of 60 t(X; 18) (SYT-SSX)-positive cases. Am J Surg Pathol. 2002;26:1434–40. doi: 10.1097/00000478-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa T, Yokoyama R, Matsuno Y, Shimoda T, Hirohashi S. Prognostic significance of histologic grade and nuclear expression of beta-catenin in synovial sarcoma. Hum Pathol. 2001;32:257–63. doi: 10.1053/hupa.2001.22764. [DOI] [PubMed] [Google Scholar]
- 10.Saito T, Oda Y, Sakamoto A, Tamiya S, Iwamoto Y, Tsuneyoshi M. Matrix metalloproteinase-2 expression correlates with morphological and immunohistochemical epithelial characteristics in synovial sarcoma. Histopathology. 2002;40:279–85. doi: 10.1046/j.1365-2559.2002.01345.x. [DOI] [PubMed] [Google Scholar]
- 11.Clark J, Rocques PJ, Crew AJ, Gill S, Shipley J, Chan AM, et al. Identification of novel genes, SYT and SSX, involved in the t(X; 18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet. 1994;7:502–8. doi: 10.1038/ng0894-502. [DOI] [PubMed] [Google Scholar]
- 12.Lasota J, Jasinski M, Debiec-Rychter M, Szadowska A, Limon J, Miettinen M. Detection of the SYT-SSX fusion transcripts in formaldehyde-fixed, paraffin-embedded tissue: a reverse transcription polymerase chain reaction amplification assay useful in the diagnosis of synovial sarcoma. Mod Pathol. 1998;11:626–33. [PubMed] [Google Scholar]
- 13.Guillou L, Coindre J, Gallagher G, Gebhard S, de Saint Aubain Somerhausen N, et al. Detection of the synovial sarcoma translocation t(X; 18) (SYT; SSX) in paraffin-embedded tissues using reverse transcriptase-polymerase chain reaction: a reliable and powerful diagnostic tool for pathologists.A molecular analysis of 221 mesenchymal tumors fixed in different fixatives. Hum Pathol. 2001;32:105–12. doi: 10.1053/hupa.2001.21130. [DOI] [PubMed] [Google Scholar]
- 14.Ladanyi M. Fusions of the SYT and SSX genes in synovial sarcoma. Oncogene. 2001;20:5755–62. doi: 10.1038/sj.onc.1204601. [DOI] [PubMed] [Google Scholar]
- 15.Ladayni M, Antonescu CR, Leung DH, Woodruff JM, Kawai A, Healey JH, et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multinstitutional retrospective study of 243 patients. Cancer Res. 2002;62:135–40. [PubMed] [Google Scholar]
- 16.Cole P, Ladanyi M, Gerald WL, Cheung NK, Kramer K, LaQuaglia MP, et al. Synovial sarcoma mimicking desmoplastic small round-cell tumor: critical role for molecular diagnosis. Med Pediatr Oncol. 1999;32:97–101. doi: 10.1002/(sici)1096-911x(199902)32:2<97::aid-mpo5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Terry J, Saito T, Subramanian S, Ruttan C, Antonescu CR, Goldblum JR, et al. TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol. 2007;31:240–6. doi: 10.1097/01.pas.0000213330.71745.39. [DOI] [PubMed] [Google Scholar]
- 18.Stifani S, Blaumueller CM, Redhead NJ, Hill RE, Artavanis-Tsakonas S. Human homologs of a Drosophila enhancer of split gene product define a novel family of nuclear proteins. Nat Genet. 1992;2:119–27. doi: 10.1038/ng1092-119. [DOI] [PubMed] [Google Scholar]
- 19.Allander SV, Ille PB, Chen Y, Antonescu CR, Bittner M, Ladanyi M, et al. Expression profiling of synovial sarcoma by cDNA microaarays association of ERBB2, IGFBP2, and ELF3 with epithelial differentiation. Am J Pathol. 2002;161:1587–95. doi: 10.1016/S0002-9440(10)64437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen TO, West RB, Linn SC, Alter O, Knowling MA, O’Connell JX, et al. Molecular characterization of soft tissue tumors: a gene expression study. Lancet. 2002;359:1301–7. doi: 10.1016/S0140-6736(02)08270-3. [DOI] [PubMed] [Google Scholar]
- 21.Knösel T, Heretsch S, Altendorf-Hofmann A, Richter P, Katenkamp K, Katenkamp D, et al. TLE1 is a robust diagnostic biomarker for synovial sarcomas and correlates with t(X; 18): analysis of 319 cases. Eur J Cancer. 2010;46:1170–6. doi: 10.1016/j.ejca.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 22.Jagdis A, Rubin BP, Tubbs RR, Pacheco M, Nielsen TO. Prospective evaluation of TLE1 as a diagnostic immunohistochemical marker in synovial sarcoma. Am J Surg Pathol. 2009;33:1743–51. doi: 10.1097/PAS.0b013e3181b7ed36. [DOI] [PubMed] [Google Scholar]
- 23.Foo WC, Cruise MW, Wick MR, Hornick JL. Immunohistochemical staining for TLE1 distinguishes synovial sarcoma from histologic mimics. Am J Clin Pathol. 2011;135:839–44. doi: 10.1309/AJCP45SSNAOPXYXU. [DOI] [PubMed] [Google Scholar]
- 24.Kosemehmetoglu K, Vrana JA, Folpe AL. TLE1 expression is not specific for synovial sarcoma: a whole section study of 163 soft tissue and bone neoplasms. Mod Pathol. 2009;22:872–8. doi: 10.1038/modpathol.2009.47. [DOI] [PubMed] [Google Scholar]
- 25.Storlazzi CT, Mertens F, Mandhal N, Gisselsson D, Isaksson M, Gustafson P, et al. A novel fusion gene, SS18L1/SSX1, in synovial sarcoma. Genes Chromosomes Cancer. 2003;37:195–200. doi: 10.1002/gcc.10210. [DOI] [PubMed] [Google Scholar]
- 26.Lestou VS, O’Connell JX, Robichaud M, Salski C, Mathers J, Maguire J, et al. Cryptic t(X; 18), ins (6; 18), and SYT-SSX2 gene fusion in a case of intraneural monophasic synovial sarcoma. Cancer Genet Cytogenet. 2002;138:153–6. doi: 10.1016/s0165-4608(02)00583-6. [DOI] [PubMed] [Google Scholar]
- 27.Torres L, Lisboa S, Cerveira N, Lopes JM, Lopes C, Teixeira MR. Cryptic chromosome rearrangement resulting in SYT-SSX2 fusion gene in a monophasic synovial sarcoma. Cancer Genet Cytogenet. 2008;187:45–9. doi: 10.1016/j.cancergencyto.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 28.He R, Patel RM, Alkan S, Hammadeh R, Weiss SW, Goldblum JR, et al. Immunostaining for SYT protein discriminates synovial sarcoma from other soft tissue tumors: analysis of 146 cases. Mod Pathol. 2007;20:522–8. doi: 10.1038/modpathol.3800766. [DOI] [PubMed] [Google Scholar]
- 29.Matsuyama A, Hisaoka M, Iwasaki M, Iwashita M, Hisanaga S, Hashimoto H. TLE1 expression in malignant mesothelioma. Virchows Arch. 2010;457:577–83. doi: 10.1007/s00428-010-0975-8. [DOI] [PubMed] [Google Scholar]
- 30.Inagaki H, Murase T, Otsuka T, Eimoto T. Detection of SYT-SSX fusion transcript in synovial sarcoma using archival cytologic specimens. Am J Clin Pathol. 1999;111:528–33. doi: 10.1093/ajcp/111.4.528. [DOI] [PubMed] [Google Scholar]


