Abstract
Background & objectives:
Purified protein derivative (PPD) is currently the only available skin test reagent used worldwide for the diagnosis of tuberculosis (TB). The aim of this study was to develop a Mycobacterium tuberculosis specific skin test reagent, without false positive results due to Bacillus Calmette-Guerin (BCG) vaccination using recombinant antigens.
Methods:
Proteins in PPD IC-65 were analyzed by tandem mass spectrometry and compared to proteins in M. tuberculosis culture filtrate; 54 proteins were found in common. Top candidates MPT64, ESAT 6, and CFP 10 were overexpressed in Escherichia coli expression strains and purified as recombinant proteins. To formulate optimal immunodiagnostic PPD cocktails, the antigens were evaluated by skin testing guinea pigs sensitized with M. tuberculosis H37Rv and BCG.
Results:
For single antigens and a cocktail mixture of these antigens, best results were obtained using 3 μg/0.1 ml, equivalent to 105 TU (tuberculin units). Each animal was simultaneously tested with PPD IC-65, 2 TU/0.1 ml, as reference. Reactivity of the multi-antigen cocktail was greater than that of any single antigen. The skin test results were between 34.3 and 76.6 per cent the level of reactivity compared to that of the reference when single antigens were tested and 124 per cent the level of reactivity compared to the reference for the multi-antigen cocktail.
Interpretation & conclusions:
Our results showed that this specific cocktail could represent a potential candidate for a new skin diagnostic test for TB.
Keywords: Purified protein derivative (PPD), recombinant antigens, skin test, tuberculosis
Tuberculin (purified protein derivative, PPD) is currently the only available skin test reagent used for the diagnosis of tuberculosis (TB) or for detection of latent TB infection (LTBI). Although tuberculin skin test (TST) has a remarkable sensitivity and has been in use for five decades to identify people infected with Mycobacterium tuberculosis, it has a lower specificity to TB, when compared to other mycobacterial species in the M. tuberculosis complex, such as BCG1,2. Cross-reactions due to vaccination with BCG, an attenuated strain of M. bovis or exposure to, or infection with environmental mycobacteria (EM) can also lead to false positive results. Romania has a high level of endemic TB, and in 2008 the notification rate for TB disease (prevalence of TB) was 105.9 cases per 100,000 people3.
The TST with the current PPD (PPD IC-65) was introduced in 1965 in Romania and since then it has been utilized as an in vivo assay for M. tuberculosis infection surveillance and TB diagnosis. Recently published studies4,5 showed that the efficacy of PPD IC-65 was equal to PPD RT23, the tuberculin most widely used globally for skin testing6. The development of an improved skin test reagent for in vivo diagnosis of TB with higher specificity and sensitivity, using recombinant antigens specific for M. tuberculosis and not found in M. bovis BCG or environmental mycobacteria, could represent a useful tool for an accurate diagnosis of TB and can aid in the prevention and control of the disease.
Protein antigens present in the culture filtrate of M. tuberculosis including 10 kDa culture filtrate protein (CFP10), 6 kDa early secretory antigen target (ESAT-6) and immunogenic protein MPT 64 (MPT64) were found to distinguish TB patients from BCG-vaccinated subjects and could induce strong immune responses to TB7,8. Thus, culture filtrate proteins constitute prime candidates as potential tools for a diagnostic skin test9. Most of these proteins, which have been characterized by gene cloning and nucleotide sequencing10–12 are potent antigens13. A new tuberculin should contain many antigens, since cocktails of multiple antigens are needed to elicit strong delalyed type hypersensitivity (DTH) responses13 and to cover the broad spectrum of antigen recognition by different individuals14. Colangeli et al15 showed that cocktails of M. tuberculosis complex-specific antigens elicit DTH responses that distinguish TB infection from sensitization with nontuberculous mycobacteria.
Three dominant antigens secreted by M. tuberculosis during its early and active growth phase, ESAT-6, CFP10, and MPT64, were present in PPD IC-65, and thus these may elicit strong, specific DTH responses. These proteins, known to induce strong immune responses to TB were found to distinguish TB patients from BCG-vaccinated subjects16.
The development of a new tuberculin that will allow discrimination by a skin test of LTBI from vaccination with BCG without false positive results due to BCG vaccination, based on specific antigens will have to maintain the PPD sensitivity to emphasize TB infection. In the present study, we evaluated the diagnostic potential of intradermal recombinant mycobacterial antigens in guinea pigs as a pre-clinical study for human use.
Material & Methods
The study was performed between 2008-2010 at Mycobacterial Antigen and Enzymology & Applied Microbiology Laboratories, Cantacuzino Institute, Bucharest, Romania.
Bacterial strains and products: M. tuberculosis H37Rv was obtained from Pasteur Institute, Paris, France. M. bovis BCG Romanian substrain was initially obtained by Cantacuzino Institute from Pasteur Institute, kept and continuously utilized for BCG vaccine production in Romania. Mycobacteria were cultured on the surface of Sauton medium, reaching full growth after 6-8 wk at 37°C.
Escherichia coli BL15 expression strain was grown with standard liquid and solid media. PPD IC-65, master batch # 20, was prepared in Cantacuzino Institute, Bucharest, Romania. The internationally used 2 TU PPD RT23 dosage is considered equivalent to the 5 TU PPD-S formulation17.
Digestion of the whole sample in solution: One mg of PPD IC-65 Master batch #20 was reduced in 6M guanidine HCl with dithiothretol (DTT), alkylated with iodacetamide, exchanged into 50 mM ammonium bicarbonate, dried, resuspended in 10 per cent ACN/ 0.2 M ammonium bicarbonate, and proteins digested with trypsin (1:50 E/S ratio). Digested PPD was analyzed by liquid chromatography-mass spectrometry (LC-MS) using a nano-spray liquid chromatography-linear trap quadrupole (LC-LTQ), data extracted using BioWorks (Thermo Finnigan, San Jose, CA), searched against the M. tuberculosis genome (GenBank accession number AL123456, 3991 entries) using Sequest (Thermo Finnigan, USA) and Mascot (Matrix Science, London, UK) and analysis validated using Scaffold (Proteome Software, Portland, OR) as described previously18.
In solution digestion of the crude PPD: Digested PPD was analyzed by LC-MS using a nano-spray LC-LTQ and triplicate injections. Tandem mass spectra were extracted and charge state deconvoluted by Bioworks version 3.3 (Thermo Finnigan). All MS/MS samples were analyzed using Sequest (Thermo Fisher Scientific, USA; version v.27, rev. 11) and X! Tandem (The GPM, thegpm.org; version 2007.01.01.1). Both search engines were set up to search the Mtb_V3 database (GenBank accession number AL123456, 3991 entries) assuming digestion with trypsin, fragment ion mass tolerance of 1.00 Da, parent ion tolerance of 1.5 Da, and a maximum of 4 missed cleavages. Oxidations of methionine and iodoacetamide derivative of cysteine were specified in Sequest and X! Tandem as variable modifications.
Scaffold (version Scaffold_3_00_01, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if these could be established at greater than 95.0 per cent probability as specified by the Peptide Prophet algorithm19. Peptide identifications were also required to exceed specific database search engine thresholds. Sequest identifications required at least deltaCn scores of greater than 0.10 and XCorr scores of greater than 1.5, 2.2, 3.0 for single-, double-, and triple-charged peptides, X! Tandem identifications required at least - Log (Expect Scores) scores of greater than 2.0. Protein identifications were accepted if these could be established at greater than 99.0 per cent probability and contained at least two identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm20. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
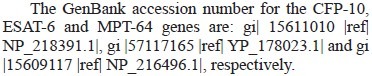
Bacterial strains, plasmids, growth conditions and DNA manipulations: General DNA manipulations were performed as previously described21. The M. tuberculosis H37Rv gene Rv3874, encoding CFP10, was amplified from chromosome DNA as template. The PCR product was inserted into the vector pET24a and the expression construct was transformed into E. coli BL15. The recombinant strains were grown in 2YT medium supplemented with kanamycin and chloramphenicol to an absorbance of 1.5 at 600 nm. Overexpression was induced with 1mM isopropyl-β-D-thiogalactoside for 3 h at 37°C. The cells were then harvested by centrifugation and served as source for protein purification.
Recombinant plasmids pMRLB.7 and pMRLB.12A containing Rv3875 and Rv1980c genes respectively, obtained through the TB Vaccine Testing and Research Materials Contract (Colorado State University, NIH/NIAID Contract #HHSN266200400091C), were introduced into E. coli BL 15 to overexpress ESAT-6 and MPT64 proteins. The recombinant strains were grown in 2YT medium supplemented with 100 μg/ml ampicilline and 30 μg/ml chloramphenicol (Sigma, USA) to an absorbance of 0.5 at 600 nm, then overexpression was induced by isopropyl-β-D-thiogalactoside induction (0.25 mM for ESAT-6 and 0.5 mM for MPT64, final concentrations) for 12 h at 25°C (ESAT-6) or 4 h at 37°C (MPT64). The cells were then harvested by centrifugation and served as the source for protein purification.
Purification of mycobacterial proteins: The CFP10 protein was purified by ion exchange chromatography on DEAE-Sephacel, using an increasing salt gradient (0-0.5M NaCl), followed by size exclusion chromatography on AcA54. N-terminal His-tagged ESAT-6 and MPT64 proteins were purified by Nickel-nitriloacetic acid affinity chromatography using the QIA express system22. Protein concentration was measured according to Bradford23. SDS-PAGE was performed as described by Laemmli24. Purified proteins were conditioned in PBS and stored at -20°C before use.
DTH responses in guinea pigs: Twelve groups of six white guinea pigs (female : male ratio of 1:1) weighing approximately 300 g, obtained from Cantacuzino Institute Animal facility and maintained under specific pathogen free conditions, were tested. Four groups were sensitized with inactivated, dried M. tuberculosis H37Rv cells, mixed with incomplete Freund's adjuvant (0.4 mg/ml) by intramuscular (i.m.) injections in each of the four limbs, for a total of 0.5 ml /animal; 4 groups were similarly sensitized with live M. bovis BCG cells (0.4 mg/ml), and 4 groups were mock sensitized by intradermal (i.d.) injection of phosphate buffered saline.
The assay of potency by measuring DTH responses was performed according to European Pharmacopoeia (Eur. Ph.) 7.025 using inactivated M. tuberculosis cells. For sensitization, the guinea pigs were injected i.m. with a total of 0.5 ml suspension, divided between (each of) the four limbs. Five weeks after sensitization, animals were epilated on both flanks and three i.d. injections on left side were made with 3 μg/0.1 ml of each purified recombinant antigen (CFP10, ESAT-6, MPT64) and different cocktail mixtures (1 to 3 μg/ 0.1 ml per antigen) dissolved in phosphate buffered saline containing 0.05 per cent Tween and with the pH adjusted between 6.5 and 7.5. Each animal was also injected on the right flank with 2 TU/ 0.1 ml of PPD to control for sensitization and for the specificity of the assay. Skin lesions were measured and recorded 24 h after antigen injection.
The length and the width of the lesions were measured and the area calculated. To ensure that the suffering of the animals is kept to a minimum, all manipulations were performed according to the study protocol approved by local Animal Ethics Committee (IRB #0002508). All animal manipulations were carried out by trained technicians with more than 5 years experience in animal experimentation.
Results
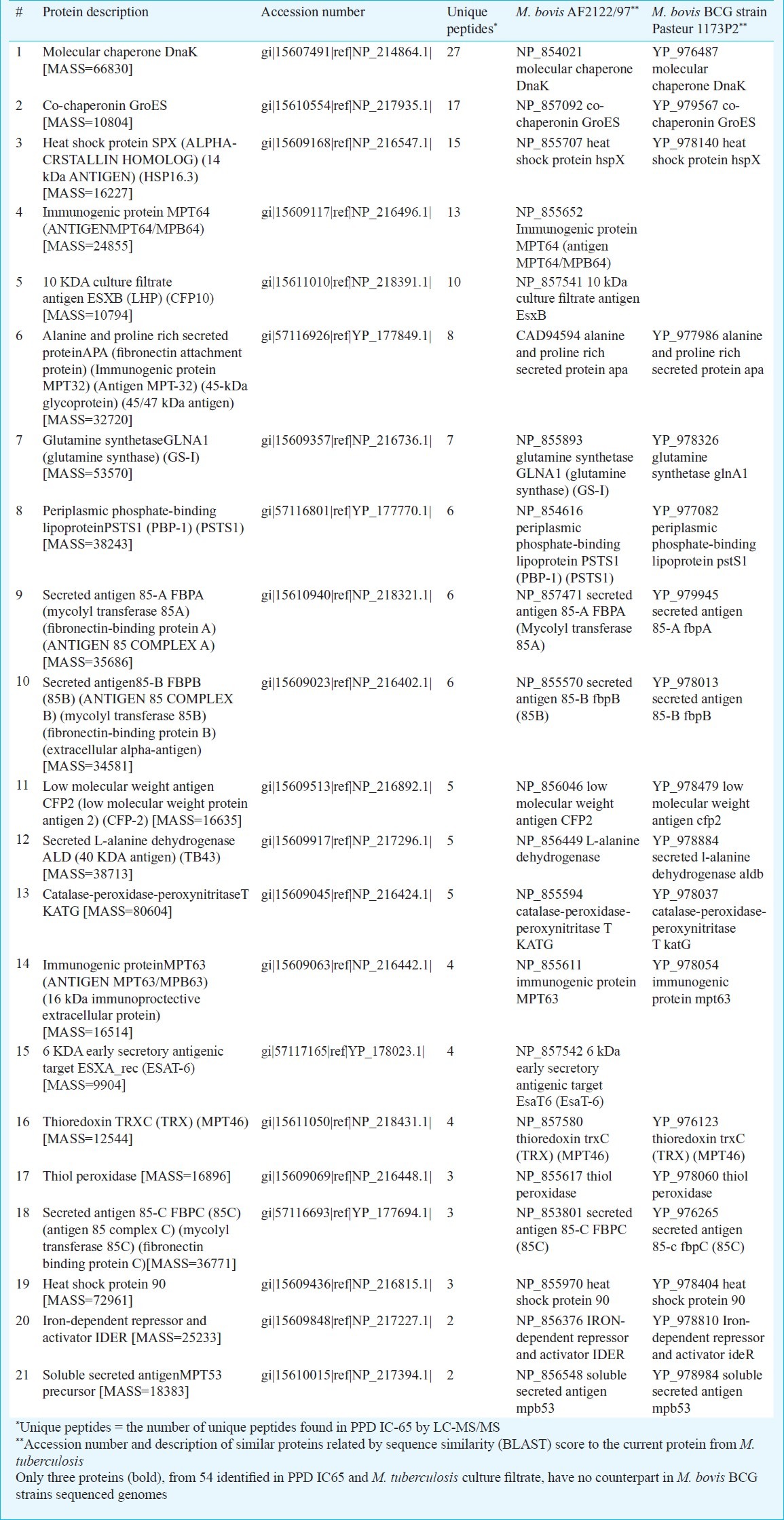
PPD IC-65 Master batch 20 was digested, analyzed by LC-MS/MS and interrogated against the M. tuberculosis genome. This led to the identification of 132 proteins in PPD IC-65 (data not shown). Of these, 54 proteins were found to be common with those identified in the M. tuberculosis culture filtrate26, 21 annotated as known function (Table I) and 33 as hypothetical, putative or possible proteins.
Table I.
Common proteins identified in PPD IC-65 and M. tuberculosis culture filtrate
A search was initiated for DTH-active antigens expressed by M. tuberculosis but not by M. bovis BCG. CFP10, ESAT-6 and MPT64 were over-expressed and purified (Fig. 1). Corresponding spots were identified in different culture filtrates of M. tuberculosis H37Rv (Fig. 2). The secreted antigens were clearly visible in the two-week old culture filtrate.
Fig. 1.
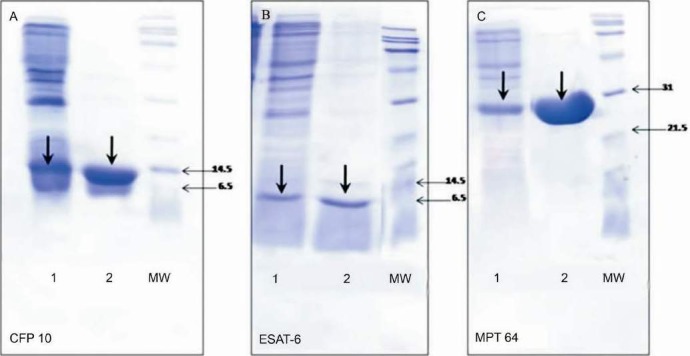
Purification of CFP-10 (A), ESAT-6 (B) and MPT-64 (C) recombinant proteins. BE, brute extract; El, purified protein. Molecular markers are indicated in kilodaltons to the right of each panel. The arrows show each specific antigen position.
Fig. 2.
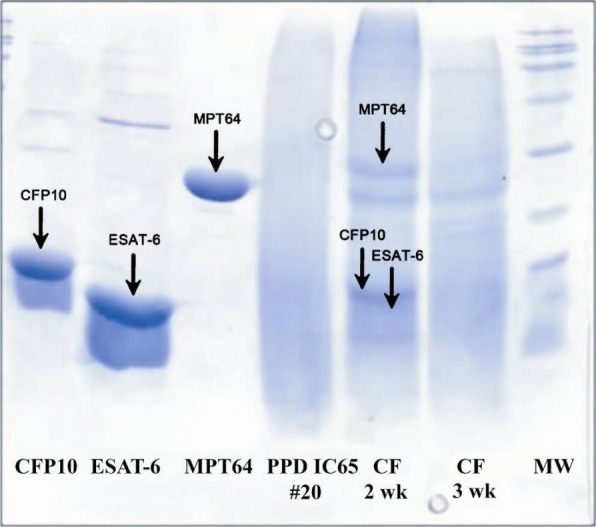
Presence of CFP-10, ESAT-6, and MPT-64 in two and eight week old, unheated culture filtrates of respectively, M. tuberculosis H37Rv, PPD IC-65 Master batch #20 and MW (SDS PAGE 15%).
To investigate the species specificity of the recombinant antigens, DTH reactions were measured in guinea pigs sensitized with live M. bovis BCG, with inactivated M. tuberculosis H37Rv and mock sensitized with PBS. Skin reactivity to CFP10, ESAT-6 and MPT64 was zero in BCG sensitized guinea pigs (Fig. 3, blue bars) and positive in animals sensitized with M. tuberculosis H37Rv (Fig. 3, green bars). The DTH responses induced by PPD 2 TU/0.1 ml was similar in BCG and M. tuberculosis H37Rv sensitized animals. The mock sensitized animals gave no DTH resposes to any of the antigens or to PPD (yellow bars).
Fig. 3.
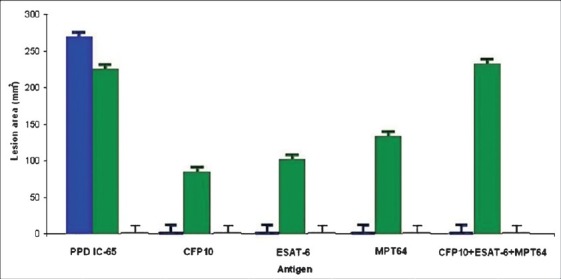
Immunological activity and specificity of recombinant antigens CFP-10, ESAT-6 and MPT-64. DTH responses to selected recombinant antigens were compared to PPD IC- 65 in guinea pigs immunized with living M. bovis BCG (blue bars), inactivated M. tuberculosis H37Rv (green bars) and PBS (yellow bars). Twelve groups of six guinea pigs were sensitized and skin tested 5 wk after sensitized by intradermal injection of 2 TU/0.1 ml of PPD and 3 μg / 0.1 ml of purified recombinant antigens and a cocktail mixture (1 μg / 0.1 ml of each). Results are expressed as the mean lesion area (in millimeters2), measured 24 h after antigen injection in each group of animals.
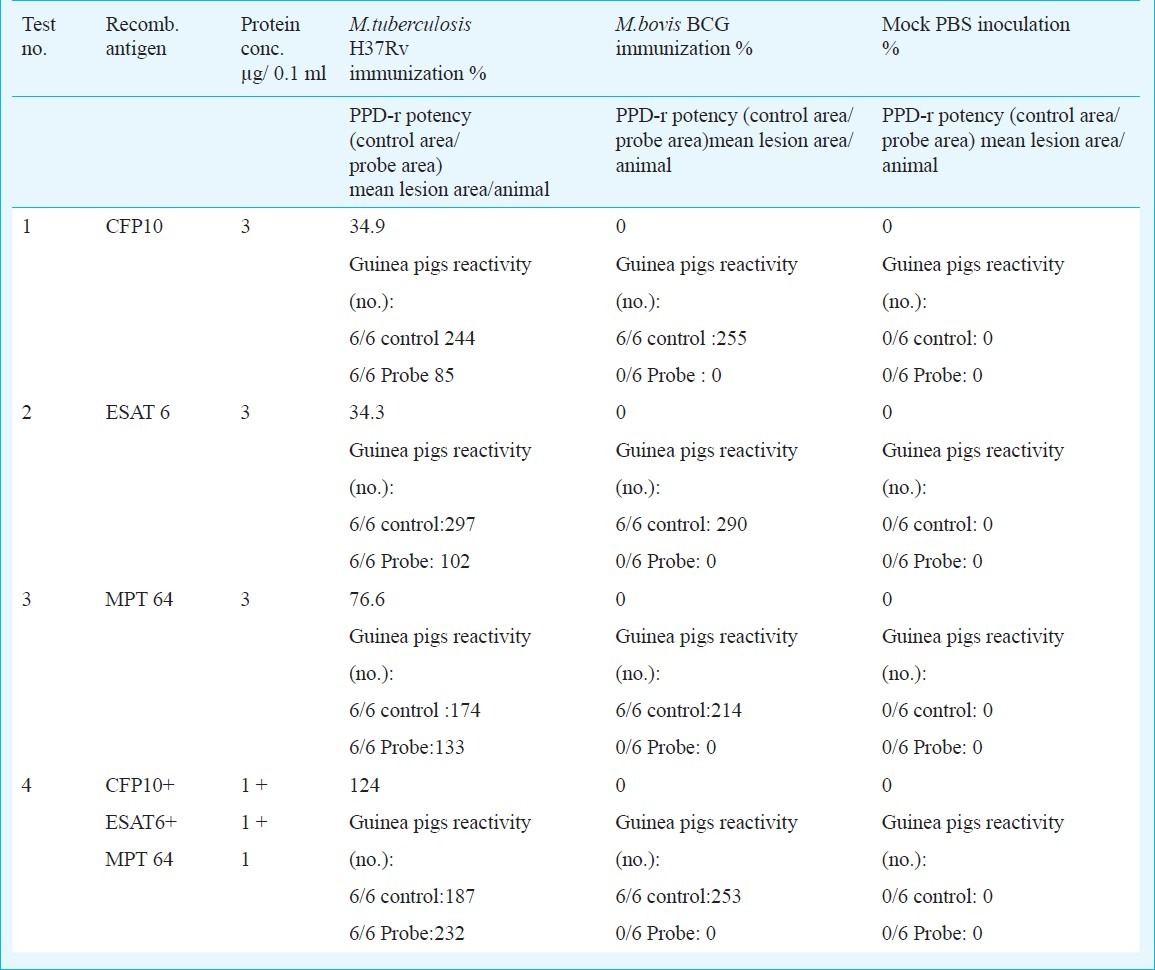
In order to formulate optimal specific immunodiagnostic cocktails, the recombinant antigens (individually or as a mixture) were evaluated by skin testing several guinea pigs groups sensitized with M. tuberculosis H37Rv and BCG, as controls. For single antigens, good results were obtained using 3 μg/0.1 ml of each recombinant protein, equivalent to 105 TU/0.1ml; while for the mixture, 1 μg of each recombinant antigen /0.1 ml, gave the highest reaction. Each animal was simultaneously tested with PPD IC-65, 2 TU/0.1 ml, as reference. The BCG controls did not react with any of these antigens, but elicited skin test responses with the reference. Reactivity of multi-antigen cocktails was greater than that of any single antigen, when compared to the reference: 34.3-76.6 per cent for single antigen tests and 124 per cent for cocktail (Table II).
Table II.
Potency assays results
Discussion
The accurate diagnosis of TB infection by skin testing requires a new tuberculin, consisting of defined protein antigens that are unique to M. tuberculosis. A new tuberculin should contain many antigens, since cocktails of multiple antigens are needed to elicit strong DTH responses13,26 and to cover the broad spectrum of antigen recognition by different individuals, typical of TB. Lyashchenko et al13 formulated cocktails of two to eight specific antigens of M. tuberculosis complex purified from recombinant E. coli, which were evaluated by skin testing in guinea pigs sensitized with M. bovis BCG. The mapping of the DTH-inducing epitope of secreted protein MPT64 from M. tuberculosis has been performed since 199510. More recently, a double blind randomized phase 1 study was reported assessing the safety of intradermal-recombinant ESAT 6 compared to tuberculin and determining the human dose16 and the use of recombinant ESAT 6 in a skin test in human volunteers27.
To develop a better skin test reagent, without false positive results due to BCG vaccination, all the proteins present in a PPD IC-65 concentrated harvest were analysed by tandem mass spectrometry. The three top proteins present in both PPD IC-65 and M. tuberculosis culture filtrate: CFP10/ Rv3874, ESAT-6/ Rv3875 and MPT64/ Rv1980c were considered. These unique antigens, secreted by M. tuberculosis during its early and active growth phase, and absent from M. bovis BCG, Pasteur 1173P2 and Tokyo 172 strains, and also from M. bovis AF2122/97 strain could elicit strong, specific DTH responses in guinea pigs.
In our study all three recombinant antigens, known to be immunodominant secreted antigens, elicited specific DTH responses only in M. tuberculosis H37Rv sensitized guinea pigs and not in BCG sensitized animals. In all of the animal studies, intradermal CFP10, ESAT-6, MPT64 and their cocktail mixture were safe. All doses were tolerated well and induced specific skin test responses. In the future studies, the biopsy of the skin test site needs to be done, as confirmatory to detect the presence of mononuclear infiltrates.
An immune response to ESAT-6 was reported in individuals infected with M. kansasii and M. marinum, which are, however, relatively rare clinical entities28. Therefore, we intend to test the recombinant antigens and their mixture on guinea pigs sensitized with these environmental mycobacteria in order to confirm their specificity.
Our results supported earlier data13 that the cocktail mixture of the recombinant antigens induced a stronger DTH response in sensitized animals than each of the single antigens. A recent study, using a mixture of seven recombinant proteins (ESAT-6, CFP10, TB10.3, TB10.4, MTSP11, MPT70, and MPT83), evaluated the DTH reactions in Cavia porcellus in comparison with a standard PPD. When applied together at a concentration of each recombinant protein of 0.04 mg/ml, the intradermoreaction of the mixture in C. porcellus was significantly higher than that obtained by standard PPD29.
Because of immunological specificity of CFP10, ESAT-6 and MPT64 for the M. tuberculosis complex further evaluation of these antigens or their cocktail should be done, as a reagent for TB-specific immunodiagnostic assays in ex vivo and in vivo human studies. We plan to test the recombinant proteins mixture, in comparison to a commercial PPD (PPD IC-65 as reference) on experimental groups of children with active TB and children who were BCG vaccinated at birth (with a post-vaccinal scar >3 mm) who have not been in contact with TB infected people. The test results will also be compared to those obtained by performing commercial blood assays, which detect interferon-γ (IFN-γ) release30.
The goal is to elicit a cellular response of the host to TB that will help us differentiate between TB infection and a successful vaccination. With a specificity compared to that of cytokine-derived assays such as the IFN-γ release assay, but not requiring venous puncture, the new PPD recombinant reagent will probably have a lower price and could represent a more affordable diagnostic test for high TB incidence in low income countries. Our data have demonstrated that in the case of a population with high TB incidence, as in Romania, the sensitivity of TST proved to be higher than IFN-γ release assay, even with BCG vaccinated people4.
Recently, a first–in–man open clinical trial of recombinant ESAT 6 and CFP10 antigens in a TB specific skin test reagent was reported31, which opens the road for the use of recombinant antigens as an improved TST.
Acknowledgment
This work was supported by The Romanian Ministry of Education, Research and Youth, under the 61-026/2007 grant. Authors thank to Karen Dobos and Agatha Wieczorek (Mycobacteria Research Laboratories, Department of Microbiology, Immunology & Pathology, Colorado State University, Fort Collins, USA) for their assistance in PPD IC-65 identification by LC-MS/MS.
References
- 1.Huebner RE, Schein MF, Bass JB., Jr The tuberculin skin test. Clin Infect Dis. 1993;17:968–75. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 2.Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192–204. [PubMed] [Google Scholar]
- 3.Ibraim E, Stoicescu P, Homorodean D, Popa C, Burecu M, Stoicescu I, et al. Tuberculosis in Romania.Problems and solutions. Pneumologia. 2010;59:6–12. [PubMed] [Google Scholar]
- 4.Stavri HR, Murgoci G, Ulea I, Popa LG, Popa M. Prospective comparison of two brands of tuberculin skin tests and Quantiferon-TB Gold in-tube assay performances for tuberculosis infection in hospitalized children. Maedica (Buchar) 2010;5:271–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Ulea I, Murgoci G, Popa ML, Popa L, Stavri H. Comparative study of RT-23 and IC-65 tuberculins tested on children with tuberculosis. Roum Arch Microbiol Immunol. 2010;69:75–8. [PubMed] [Google Scholar]
- 6.Haslov K, Ponce-de-Leon Rosales S, Rangel-Frausto S, Olesen Larsen S. Tuberculin PPD RT23: still going strong. Int J Tuberc Lung Dis. 1998;2:793–5. [PubMed] [Google Scholar]
- 7.Skjǿt RL, Oettinger T, Rosenkrands I, Ravn P, Brock I, Jacobsen S, et al. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-Cell antigens. Infect Immun. 2000;68:214–20. doi: 10.1128/iai.68.1.214-220.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar G, Dagur PK, Singh PK, Shankar H, Yadav VS, Katoch VM, et al. Serodiagnostic efficacy of Mycobacterium tuberculosis 30/32-kDa mycolyl transferase complex, ESAT-6, and CFP-10 in patients with active tuberculosis. Arch Immunol Ther Exp. 2010;58:57–65. doi: 10.1007/s00005-009-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen AB, Brennan P. Proteins and antigens of Mycobacterium tuberculosis. In: Bloom BR, editor. Tuberculosis: pathogenesis, protection, and control. Washington D.C: American Society for Microbiology; 1994. pp. 307–27. [Google Scholar]
- 10.Oettinger T, Holm A, Mtoni IM, Andersen ÅB, Hasløov K. Mapping of the delayed-type hypersensitivity-inducing epitope of secreted protein MPT64 from Mycobacterium tuberculosis. Infect Immun. 1995;63:4613–8. doi: 10.1128/iai.63.12.4613-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coler RN, Skeiky YAW, Ovendale PJ, Vedvick TS, Gervassi L, Guderian J, et al. Cloning of a Mycobacterium tuberculosis gene encoding a purified protein derivative protein that elicits strong tuberculosis-specific delayed-type hypersensitivity. J Infect Dis. 2000;182:224–33. doi: 10.1086/315677. [DOI] [PubMed] [Google Scholar]
- 12.Manca C, Lyashchenko K, Wiker HG, Usai D, Colangeli R, Gennaro ML. Molecular cloning, purification, and serological characterization of MPT63, a novel secreted antigen of Mycobacterium tuberculosis. Infect Immun. 1997;65:16–23. doi: 10.1128/iai.65.1.16-23.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyashchenko K, Manca C, Colangeli R, Heijbel A, Williams A, Gennaro ML. Use of Mycobacterium tuberculosis complex-specific antigen cocktails for a skin test specific for tuberculosis. Infect Immun. 1998;66:3606–10. doi: 10.1128/iai.66.8.3606-3610.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elhay MJ, Oettinger T, Andersen P. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect Immun. 1998;66:3454–6. doi: 10.1128/iai.66.7.3454-3456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colangeli R, Spencer JS, Bifani P, Williams A, Lyashchenko K, Keen MA, et al. MTSA-10, the product of the Rv3874 gene of Mycobacterium tuberculosis, elicits tuberculosis-specific, delayed-type hypersensitivity in guinea pigs. Infect Immun. 2000;68:990–3. doi: 10.1128/iai.68.2.990-993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arend SM, Franken WP, Aggerbeck H, Prins C, van Dissel JT, Thierry-Carstensen B, et al. Double-blind randomized Phase I study comparing rdESAT-6 to tuberculin as skin test reagent in the diagnosis of tuberculosis infection. Tuberculosis (Edinb) 2008;88:249–61. doi: 10.1016/j.tube.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Molina-Gamboa JD, Ponce-de-León-Rosales S, Rivera-Morales I, Romero C, Báez R, Huertas M, et al. Evaluation of the sensitivity of RT-23 purified protein derivative for determining tuberculin reactivity in a group of health care workers. Clin Infect Dis. 1994;19:784–6. doi: 10.1093/clinids/19.4.784. [DOI] [PubMed] [Google Scholar]
- 18.Mehaffy C, Hess A, Prenni JE, Mathema B, Kreiswirth B, Dobos KM. Descriptive proteomic analysis shows protein variability between closely related clinical isolates of Mycobacterium tuberculosis. Proteomics. 2010;10:1966–84. doi: 10.1002/pmic.200900836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–92. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 20.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 21.Evrin C, Straut M, Slavova-Azmanova N, Bucurenci N, Onu A, Assairi L, et al. Regulatory mechanisms differ in UMP kinases from Gram-negative and Gram-positive bacteria. J Biol Chem. 2007;282:7242–53. doi: 10.1074/jbc.M606963200. [DOI] [PubMed] [Google Scholar]
- 22.Crowe J, Döbeli H, Gentz R, Hochulu E, Stüber D, Henco K. ‘6xHis-Ni-NTA chromatography as a superior technique in recombinant expression protein expression/purification’. In: Harwood AJ, editor. Methods in molecular biology. Totawa: Humana Press, Inc; 1994. [DOI] [PubMed] [Google Scholar]
- 23.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Tuberculin purified protein derivative for human use. 01/2008:0151. Strasbourg: Council of Europe; 2010. European Pharmacopoeia 7.0; pp. 3162–4. [Google Scholar]
- 26. [accessed on May 1, 2010]. Available from: http://web.mpiib-berlin.mpg.de/cgi-bin/pdbs/2d-page/extern/menu_frame.cgi?gel=17 .
- 27.Wu X, Zhang L, Zhang J, Zhang C, Zhu L, Shi Y. Recombinant early secreted antigen target 6 protein as a skin test antigen for the specific detection of Mycobacterium tuberculosis infection. Clin Exp Immunol. 2008;152:81–8. doi: 10.1111/j.1365-2249.2008.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arend SM, van Meijgaarden KE, de Boer K, de Palou EC, van Soolingen D, Ottenhoff TH, et al. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J Infect Dis. 2002;186:1797–807. doi: 10.1086/345760. [DOI] [PubMed] [Google Scholar]
- 29.Malaghini M, Thomaz-Soccol V, Probst CM, Krieger MA, Petri H, Kritski A, et al. Recombinant antigen production for assays of intradermoreaction for diagnosis and surveillance of tuberculosis. J Biotechnol. 2011;156:56–8. doi: 10.1016/j.jbiotec.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Pai M, Kalantri S, Dheda K. New tools and emerging technologies for the diagnosis of tuberculosis: Part 1.Latent tuberculosis. Expert Rev Mol Diagn. 2006;6:413–22. doi: 10.1586/14737159.6.3.413. [DOI] [PubMed] [Google Scholar]
- 31.Bergstedt W, Tingskov PN, Thierry-Carstensen B, Hoff ST, Aggerbeck H, Thomsen VO, et al. First-in-man open clinical trial of a combined rdESAT-6 and rCFP-10 tuberculosis specific skin test reagent. PLoS ONE. 2010;5:e11277. doi: 10.1371/journal.pone.0011277. [DOI] [PMC free article] [PubMed] [Google Scholar]


