Abstract
Background & objectives:
Pyrazinamide is an essential component of first line anti-tuberculosis regimen as well as most of the second line regimens. This drug has a unique sterilizing activity against Mycobacterium tuberculosis. Its unique role in tuberculosis treatment has lead to the search and development of its structural analogues. One such analogue is 5-chloro-pyrazinamide (5-Cl-PZA) that has been tested under in vitro conditions against M. tuberculosis. The present study was designed with an aim to assess the activity of 5-Cl-PZA, alone and in combination with first-line drugs, against murine tuberculosis.
Methods:
The minimum inhibitory concentration (MIC) of 5-Cl-PZA in Middlebrook 7H9 broth (neutral pH) and the inhibitory titre of serum from mice that received a 300 mg/kg oral dose of 5-Cl-PZA 30 min before cardiac puncture were determined. To test the tolerability of orally administered 5-Cl-PZA, uninfected mice received doses up to 300 mg/kg for 2 wk. Four weeks after low-dose aerosol infection either with M. tuberculosis or M. bovis, mice were treated 5 days/wk with 5-Cl-PZA, at doses ranging from 37.5 to 150 mg/kg, either alone or in combination with isoniazid and rifampicin. Antimicrobial activity was assessed by colony-forming unit counts in lungs after 4 and 8 wk of treatment.
Results:
The MIC of 5-Cl-PZA against M. tuberculosis was between 12.5 and 25 μg/ml and the serum inhibitory titre was 1:4. Under the same experimental conditions, the MIC of pyrazinamide was >100 μg/ml and mouse serum had no inhibitory activity after a 300 mg/kg dose; 5-Cl-PZA was well tolerated in uninfected and infected mice up to 300 and 150 mg/kg, respectively. While PZA alone and in combination exhibited its usual antimicrobial activity in mice infected with M. tuberculosis and no activity in mice infected with M. bovis, 5-Cl-PZA exhibited antimicrobial activity neither in mice infected with M. tuberculosis nor in mice infected with M. bovis.
Interpretation & conclusion:
Our findings showed that 5-Cl-PZA at doses up to 150 mg/kg was not active in chronic murine TB model. Further studies need to be done to understand the mechanism and mode of inactivation in murine model of tuberculosis.
Keywords: Experimental chemotherapy, mouse, pyrazinamide, tuberculosis
Pyrazinamide (PZA), an analog of nicotinamide, is a first-line anti-tuberculosis drug with a unique sterilizing activity1,2. Its addition to the combined anti-tuberculosis regimen including isoniazid, rifampin, and ethambutol contributed to the reduction of treatment duration and thus led to the modern 6-month short-course therapy for tuberculosis1,3. PZA is a pro-drug that undergoes amide hydrolysis by the mycobacterial pyrazinamidase (a nicotinamidase) to pyrazinoic acid (POA)4. POA acts against Mycobacterium tuberculosis, but the exact biochemical basis of action has not been fully established. It has been hypothesized that POA either disrupts M. tuberculosis membrane transport and energetics, or inhibits fatty acid synthetase I (FAS I)5–7. Pyrazinamidase is encoded by the pncA gene and mutations in pncA that abolish the amidase activity confer PZA resistance in strains of M. tuberculosis8–10. PZA-susceptible and resistant isolates are generally susceptible to POA in vitro, but POA is not active in vivo11.
A series of esters of POA and 5-substituted POA derivatives have been reported to enhance in vitro activity against both PZA-susceptible and PZA-resistant M. tuberculosis, as well as against the naturally PZA-resistant M. bovis, M. kansasii, and M. avium isolates12,13. One such derivative, 5-chloropyrazinamide (5-Cl-PZA), has a minimum inhibitory concentration (MIC) of 25 μg/ml against M. smegmatis, which is far lower than that of PZA (4000 μg/ml). Both 5-Cl-PZA, along with its acid form, 5-chloropyrazinoic acid (5-Cl-POA), were evaluated for their in vitro activity against M. tuberculosis, M. bovis (inherently resistant to PZA owing to a lack of nicotinamidase)4, and several non-tuberculous mycobacteria by a broth dilution method, and 5-Cl-PZA was more active than PZA against all organisms tested14. At neutral pH, MICs of PZA and 5-Cl-PZA against M. tuberculosis range from 32 to 2048 μg/ml and from 8 to 32 μg/ml, respectively12–14. MICs of POA and 5-Cl-POA ranged from 16-64 mg/ml and from 64-256 μg/ml, respectively12–14. Thus MICs of 5-Cl-PZA and POA for M. tuberculosis are more favourable than those of PZA and 5-Cl-POA. In addition, PZA-resistant isolates retain susceptibility in vitro to 5-Cl-PZA, POA, and 5-Cl-POA, suggesting that 5-Cl-PZA does not require activation by mycobacterial pyrazinamidase. This is also supported by the observation that 5-Cl-PZA, unlike PZA, is active against M. bovis with an MIC of 8 μg/ml under in vitro conditions and, unlike PZA, is active even at a neutral pH12–14.
The present study was planned to evaluate the dose-dependent activity of 5-Cl-PZA alone and in combination with isoniazid and rifampicin in mice infected with M. tuberculosis or M. bovis and compare it with that of pyrazinamide.
Material & Methods
The study has been conducted at the Center for Tuberculosis Research, Johns Hopkins University, Baltimore, Maryland, USA.
Bacterial strains: M. tuberculosis H37Rv and M. bovis (Ravenel) were passaged in mice, frozen in 1 ml aliquots, and stored at -80°C before use. For infection, aliquots of each species were thawed and sub-cultured in Middlebrook 7H9 broth (Fisher, USA) supplemented with 10 per cent oleic acid-albumin-dextrose-catalase (OADC; Difco, USA) and 0.05 per cent Tween 80 (Sigma, USA).
Animals: Female BALB/c mice aged 4 to 6 wk were purchased from Charles River (Wilmington, MA, USA). The study protocol and procedures were approved by the Institutional Animal Care and Use Committee at Johns Hopkins.
Antimicrobials: PZA was purchased from Acros Organics (Morris Plains, NJ, USA). Isoniazid (INH) and rifampicin (RIF) were purchased from Sigma. 5-Cl-PZA was synthesized by one of the authors (JTW)14. For in vitro studies, 5-Cl-PZA was solubilised in DMSO and diluted with distilled water. For administration to mice, homogeneous suspensions of 5-Cl-PZA were prepared in a 0.25 per cent carboxy methylcellulose formulation (CMC). Stock solutions/suspensions of all drugs were prepared weekly in distilled water and stored at 4°C as described previously15.
Determination of MIC: The MIC was determined by the broth dilution method16. Middlebrook 7H9 broth supplemented with 10 per cent OADC and containing serial two-fold concentrations of 5-Cl-PZA ranging from 0.625 to 100 μg/ml were inoculated with 0.1 ml of the 10-3 dilution of a log phase broth culture of M. tuberculosis H37Rv with an optical density corresponding to approximately 108 cfu/ml. Isoniazid at two-fold concentrations ranging from 0.015 to 0.6 μg/ml served as positive control while drug free broth and PZA at two-fold concentrations ranging from 0.625 to 100 μg/ml served as negative controls. The MIC was defined as the lowest concentration at which no visible growth was observed after 14 days incubation at 37°C.
Determination of the serum inhibitory titre (SIT): SIT was determined by the broth dilution method. Briefly, mice were orally administered 5-Cl-PZA at 300 mg/kg and, after anaesthesia, bled via cardiac puncture 30 min later. Two-fold dilutions of serum to a maximum of 1:32 were performed in Middlebrook 7H9 broth + OADC without Tween 80. All vials were inoculated with 0.1 ml of the 10-3 dilution of the same broth culture of M. tuberculosis H37Rv that was used for MIC determination. Serum from mice administered INH at 10 mg/kg or PZA at 300 mg/kg and blood collected 30 min after dosing were used as positive and negative controls, respectively, while serum from untreated mice was taken as a standard control for growth. After incubation at 37°C for 14 days the SIT was defined as the highest dilution of serum that prevented visible growth.
Multi-doses tolerability of 5-Cl-PZA: To test whether multiple doses of 5-Cl-PZA would be toxic during chemotherapy, uninfected mice were orally given 5-Cl-PZA at two-fold doses ranging from 37.5 to 600 mg/kg/day for two weeks (five days/week), monitored for survival, behaviour and body weight changes in comparison with untreated controls. No haematology or serum chemistry parameters were studied.
Aerosol infection: Female BALB/c mice (Charles River) aged 4 to 6 wk were aerosol-infected using the Inhalation Exposure System (Glas-col Inc., Terre Haute, IN) with either M. tuberculosis or M. bovis. A log-phase broth culture at an optical density (at 600 nm) of approximately 1.0 was diluted 50-fold and used for infection. After infection, mice were randomly distributed into treatment groups (five mice per group per time point). A subgroup of 5 untreated mice were euthanized on the day after infection to determine the number of cfu implanted in the lungs and on the day of treatment initiation to determine baseline cfu counts. Quantitative lung cultures were performed on selective 7H11 plates (Becton Dickinson, USA), as described previously17.
Drug treatment: All drugs were administered once daily 5 days a week in 0.2 ml of phosphate buffered saline (PBS) by oesophageal cannula. RIF was given 1 h prior to other drugs to avoid an adverse pharmacokinetic interaction15–17. The drug doses were INH 25 mg/kg, RIF 10 mg/kg, and PZA 150 mg/kg. The rationale for the use of these drug doses has been described previously17.
Dose-ranging activities of 5-Cl-PZA alone and in combination: Control animals received one of the following drug regimens: RIF-INH-PZA, RIF-PZA, RIF-INH, RIF alone, and PZA alone (Table I). PZA was used at two doses, 150 and 75 mg/kg to compare with 5-Cl-PZA (Table I). Test mice received 5-Cl-PZA alone at two-fold doses ranging from 37.5 to 150 mg/kg to determine the minimum effective dose (MED) and the minimum bactericidal dose (MBD) of 5-Cl-PZA (Table I). In the combination regimens, 5-Cl-PZA was substituted for PZA in RIF-INH-PZA and RIF-PZA regimens, but only at the 150 mg/kg dose (Table I). Negative controls went untreated. The mice were euthanized after 4 and 8 wk of treatment for assessment of lung cfu counts.
Table I.
Activity of 5-Cl-pyrazinamide (5-Cl-PZA) against M. tuberculosis in mice
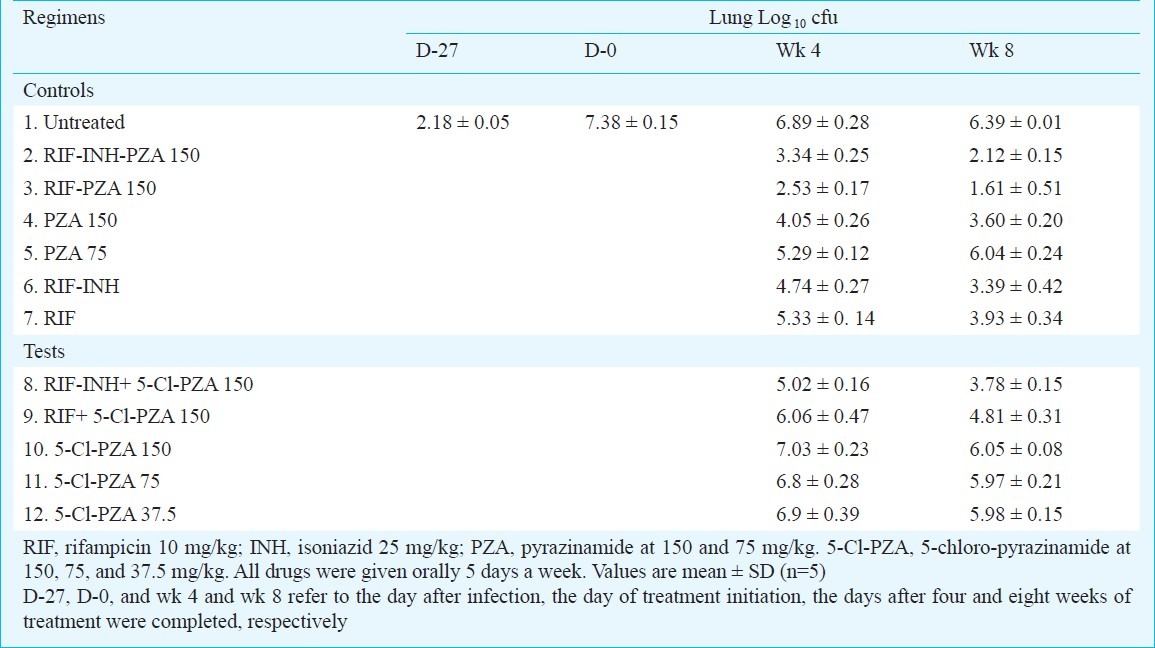
To confirm that 5-Cl-PZA does not require activation by mycobacterial pyrazinamidase like PZA, the activities of 5-Cl-PZA and PZA alone and in combination with either RIF or RIF-INH were compared in mice infected with M. tuberculosis and M. bovis. Because M. bovis lacks pyrazinamidase, a similar activity of 5-Cl-PZA against M. tuberculosis and M. bovis infected mice would mean that the 5-Cl-PZA activity is independent of pyrazinamidase activity. PZA served as a negative control for this experiment as it has no activity against M. bovis owing to lack of activation.
Results
Each vial of broth was inoculated with 6.2 × 105 cfu. After 7 days of incubation, no visible growth was observed in tubes containing 0.06 and 12.5 μg/ml for INH and 5-Cl-PZA, respectively. After 14 days of incubation, the MIC remained the same for INH but, in the case of 5-Cl-PZA, doubtful growth was observed at 12.5 μg/ml and no growth at 25 μg/ml. Therefore, the MIC of INH was 0.06 μg/ml and the MIC of 5-Cl-PZA was between 12.5 and 25 μg/ml. In the case of PZA, visible growth was observed at all concentrations tested, up to 100 μg/ml, both at days 7 and 14, confirming the lack of in vitro activity of PZA at neutral pH.
Each tube of serially-diluted serum for SIT received the same inoculum as did those for the MIC experiment, i.e., 6.2 × 105 cfu. After 14 days incubation at 37°C, there was doubtful growth in the tube containing the 1:32 dilution and no growth in the tube containing the 1:16 dilution of serum from mice administered 10 mg/kg of INH. There was no growth in the tube containing the 1:4 dilution of serum from mice administered 300 mg/kg of 5-Cl-PZA. Therefore, the SITs of INH (after a 10 mg/kg dose) and 5-Cl-PZA (after a 300 mg/kg dose) were 1:16 and 1:4, respectively, demonstrating that 5-Cl-PZA was not only bioavailable but also achieved active serum concentrations. Keeping in view that the MICs of INH and 5-Cl-PZA were 0.06 and 12.5-25 μg/ml, respectively, one may extrapolate from the SIT data that serum concentrations of about 1 μg/ml of INH and 50 to 100 μg/ml of 5-Cl-PZA were present at the time of sampling. Broth containing either no drug or serum from mice administered 300 mg/kg of PZA showed growth at all dilutions, hence the SIT was nil for PZA.
The multi-dose tolerability of 5-Cl-PZA in BALB/C mice was carried out only up to a 300 mg/kg daily dose and not, as planned, up to a dose of 600 mg/kg because the suspension of 5-Cl-PZA in 0.25 per cent CMC was homogenous only up to 300 mg/kg. All animals survived the 2-week treatment well, with no observable adverse effects or changes in body weight.
The day after aerosol infection, the mean lung cfu counts was 2.18 ± 0.05 log10. Treatment was initiated 28 days later, when the mean lung cfu count was 7.38 ± 0.15 log10. No spontaneous mortality was recorded during the course of the study. As shown in Table I, during the treatment period, untreated control mice experienced a spontaneous decrease in lung cfu counts to 6.89 ± 0.28 and 6.40 ± 0.01 log10 units at weeks 4 and 8, respectively. On average, the standard regimen of RIF-INH-PZA reduced the lung cfu counts to 3.30 and 2.18 log10 after 4 and 8 wk of treatment, respectively. As usual, the 2-drug combination RIF-PZA reduced the mean lung cfu counts by about one log10 more than the RIF-INH-PZA combination18. Conversely, the reduction in cfu counts induced by RIF-INH was one log10 less than the reduction induced by RIF-INH-PZA after 4 and 8 wk of treatment. A limited response was observed with RIF alone, as the mean lung cfu counts were reduced by approximately 2 log10 less than by RIF-INH-PZA combination. PZA alone at 150 mg/kg was one log10 more active than RIF alone at week 4 and as active as RIF alone at week 8. Finally, the activity of PZA alone at 75 mg/kg was, at week 4, similar to that of RIF alone but no further killing was observed between week 4 and 8 (Table I).
Activity of 5-Cl-PZA alone and in combination: Treatment with 5-Cl-PZA alone at any dose between 37.5 and 150 mg/kg did not result in any decrease of lung cfu counts, even after 8 wk of treatment (Table I).
The addition of 5-Cl-PZA to RIF, or to RIF+INH, did not demonstrate any benefit i.e., the combination of RIF + 5-Cl-PZA was not more active than RIF alone, and the 3-drug combination RIF+INH+5-Cl-PZA was not more active than RIF+INH (Table I). Overall, 5-Cl-PZA did not demonstrate activity in M. tuberculosis-infected mice either alone or in combination with the first-line drugs, RIF and INH. There was even a trend towards a negative impact of the 5-Cl-PZA on the antimicrobial activity of the combined drugs.
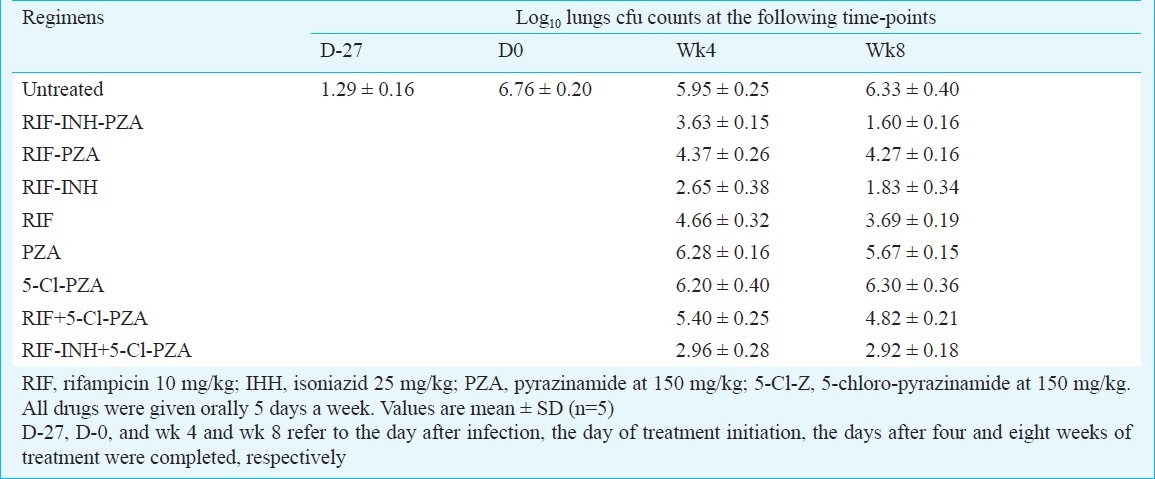
Comparative activity of PZA and 5-Cl-PZA in M. bovis infected mice: The aerosol infection of mice resulted in the lung implantation of 1.29 ± 0.16 log10 cfu of M. bovis. At treatment initiation, 28 days later, the mean lung cfu count was 6.76 ± 0.20 log10. All mice survived throughout the study. In untreated controls, the cfu counts spontaneously decreased to nearly 5.95 ± 0.25 log10 at week 4 and remained at the same level at week 8 (Table II). In mice treated with PZA alone or 5-Cl-PZA alone, the lung cfu counts at weeks 4 and 8, were not different than those in untreated controls, indicating lack of activity for PZA and 5-Cl-PZA in mice infected with M. bovis (Table II). Similar conclusions may be drawn for the activity of both drugs given in combination with RIF or RIF+INH. Addition of either PZA or 5-Cl-PZA to any combination did not significantly increase the bactericidal activity of RIF or RIF+INH.
Table II.
Activity of 5-Cl-pyrazinamide (5-Cl-PZA) alone or in combination with first line drugs in mice infected with M. bovis
Discussion
Despite the relatively promising in vitro and ex vivo activity at neutral pH of 5-Cl-PZA against M. tuberculosis, the present study showed lack of activity of 5-Cl-PZA in mice infected with M. tuberculosis or M. bovis. MIC was found to be between 12.5 and 25 μg/ml, in agreement with the reported MICs in the range of 8-32 μg/ml4,5. In addition, the ex vivo data provided by the SIT demonstrated that serum concentrations between 50 and 100 μg/ml were likely achieved in mice administered 300 mg/kg of 5-Cl-PZA. If the blood levels were proportional to the dose, one might expect blood levels to be in the range of 25-50 μg/ml in mice administered 150 mg/kg of 5-Cl-PZA. Such levels would be at or above the MIC and, therefore, may be associated with some in vivo activity. Actually, no antimicrobial activity was observed in mice infected either with M. tuberculosis or with M. bovis. It is curious, however, that at both 4 and 8 wk, in the 5-Cl-PZA+RIF regimens the addition of 5-Cl-PZA appeared antagonistic to the action of RIF against M. bovis. The same antagonistic effect was observed on treatment of M. tuberculosis with 5-Cl-PZA+RIF at week 8 but not, with PZA-RIF. This effect suggests, with regard to both mycobacterial species, either that 5-Cl-PZA is influencing the population of organisms normally susceptible to RIF or that 5-Cl-PZA has a negative pharmacological interaction with RIF.
Among all possible speculations about the overall lack of in vivo efficacy, the simplest and the most likely is that the ratio of Cmax/MIC obtained with 150 mg/kg of 5-Cl-PZA is not high enough to result in antimicrobial activity in vivo in adequate drug exposure. Another possibility is that 5-Cl-PZA unlike PZA lacks bactericidal activity and is just a bacteriostatic drug. It is emphasized here that this model does not permit discrimination of bacteriostatic activity because one cannot demonstrate growth inhibition against non-actively multiplying bacteria. However, activity in this model appears to indicate the potential for “sterilizing” activity, evidenced by activities of RIF and PZA. At the 150 mg/kg dose, the activity of 5-Cl-PZA might be insufficient to inhibit fatty acid synthase 1 (FAS1), especially when the Cmax is close to the MIC. Another possible explanation might be the in vivo instability of 5-Cl-PZA5.
Our experiments were performed in the mouse model of chronic TB disease. Mice were infected with a limited inoculum of 2.18 ± 0.05 log10 cfu and were kept without treatment for 4 wk in order to establish in the lungs a stable population that is contained by the specific immunity. Evidence of the efficacious immune containment is provided by the fact that at 28 days after infection, the size of the bacillary population was 7.38 ± 0.15 log10 M. tuberculosis cfu and was followed with small but spontaneous decline in the cfu count in untreated controls. Similar experimental conditions were realized in mice infected with M. bovis. Thus the activity of PZA and 5-Cl-PZA alone and in combination was tested against a non-actively-multiplying bacillary population, particularly favourable to reveal the sterilizing activity of a drug regimen. Under these experimental conditions, RIF and PZA alone were quite active against M. tuberculosis and, as usual18–20 the combination RIF+PZA was the most potent of all combinations against M. tuberculosis. Thus, the lack of any effect of 5-Cl-PZA alone and the lack of any additive effect of the combination of 5-Cl-PZA with RIF did not result from an experimental artifact. It rather demonstrated a lack of 5-Cl-PZA activity in a mouse at the tested dose of 150 mg/kg. It is possible that the compound would be active at a higher dose than 150 mg/kg, but interest in a PZA derivative that would be given at a higher dose than PZA is unlikely because of the risks of potential toxicity.
It is well-known that anti-tuberculosis chemo-therapy is strongly dependent upon interactions between the host, the pathogen, and the chemotherapeutic agent. The unique role of PZA in chemotherapy is a consequence of a precise balance in these interactions. The contradiction between the ex vivo antimycobacterial activity of 5-Cl-PZA and the absence of activity in the mouse model of infection suggests the importance of identifying the mode of inactivation in the murine model, whether it be metabolic instability or poor pharmacokinetics. The development of more potent analogues logically depends on structural modifications that lead to better tolerance by the diseased host yet yield compounds that still retain the desired antibacterial properties.
Acknowledgment
Authors acknowledge the generous donation of 5-hydroxypyrazinoic acid by Lonza Ltd, Muenchensteinerstrasse 38, CH-4002 Basel, Switzerland. This work was supported by the National Institutes of Health financial grant.
References
- 1.Gu P, Constantino L, Zhang Y. Enhancement of the antituberculosis activity of weak acids by inhibitors of energy metabolism but not by anaerobiosis suggests that weak acids act differently from the front-line tuberculosis drug pyrazinamide. J Med Microbiol. 2008;57:1129–34. doi: 10.1099/jmm.0.2008/000786-0. [DOI] [PubMed] [Google Scholar]
- 2.Wade MM, Zhang Y. Anaerobic incubation conditions enhance pyrazinamide activity against Mycobacterium tuberculosis. J Med Microbiol. 2004;53:769–73. doi: 10.1099/jmm.0.45639-0. [DOI] [PubMed] [Google Scholar]
- 3.Steele MA, Des Prez RM. The role of pyrazinamide in tuberculosis chemotherapy. Chest. 1988;94:845–50. doi: 10.1378/chest.94.4.845. [DOI] [PubMed] [Google Scholar]
- 4.Konno K, Nagayama H, Oka S. Nicotinamidase in mycobacteria: a method for distinguishing bovine type tubercle bacilli from other mycobacteria. Nature. 1959;184:1743–4. doi: 10.1038/1841743b0. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis. 2003;7:6–21. [PubMed] [Google Scholar]
- 6.Zhang Y, Wade MM, Scorpio A†, Zhang H, Sun Z. Mode of action of pyrazinamide: disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J Antimicrob Chemother. 2003;52:790–5. doi: 10.1093/jac/dkg446. [DOI] [PubMed] [Google Scholar]
- 7.Zimhony O, Cox JS, Welch JT, Vilchèze C, Jacobs WR., Jr Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis. Nat Med. 2000;6:1043–7. doi: 10.1038/79558. [DOI] [PubMed] [Google Scholar]
- 8.Konno K, Feldmann FM, McDermott W. Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am Rev Respir Dis. 1967;95:461–9. doi: 10.1164/arrd.1967.95.3.461. [DOI] [PubMed] [Google Scholar]
- 9.Scorpio A, Lindholm-Levy P, Heifets L, Gilman R, Siddiqi S, Cynamon M, et al. Characterization of the pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1997;41:540–3. doi: 10.1128/aac.41.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scorpio A, Zhang Y. Mutations in pncA a gene encoding pyrazinamidase / nicotin-amidase cause resistance to the antituberculosis drug pyrazinamide in M. tuberculosis. Nature Med. 1996;2:662–7. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- 11.Gangadharam PRJ, Iseman MD. Antimycobacterial drugs. In: Peterson PK, Verhoef J, editors. The antimicrobial agents annual. New York: Elsevier; 1986. pp. 17–40. [Google Scholar]
- 12.Cynamon MH, Gimi R, Gyenes F, Sharpe CA, Bergmann KR, Han HJ, et al. Pyrazinoic acid esters with broad spectrum in vitro antimycobacterial activity. J Med Chem. 1995;38:3902–7. doi: 10.1021/jm00020a003. [DOI] [PubMed] [Google Scholar]
- 13.Cynamon MH, Klemens SP, Chou TS. Antimycobacterial activity of a series of pyrazinoic acid esters. J Med Chem. 1992;35:1212–5. doi: 10.1021/jm00085a007. [DOI] [PubMed] [Google Scholar]
- 14.Cynamon MH, Speirs RJ, Welch JT. In Vitro antimycobacterial activity of 5-chloropyrazinamide. Antimicrob Agents Chemother. 1998;42:462–3. doi: 10.1128/aac.42.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyagi S, Nuermberger E, Yoshimatsu T, Williams K, Rosenthal I, Lounis N, et al. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob Agents Chemother. 2005;49:2289–3. doi: 10.1128/AAC.49.6.2289-2293.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams KN, Stover CK, Zhu T, Zhu T, Ogden A, Tasneen R, et al. Promising antituberculosis activity of the oxazolidinone PNU-100480 relative to that of linezolid in a murine model. Antimicrob Agents Chemother. 2009;53:1314–9. doi: 10.1128/AAC.01182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad Z, Nuermberger EL, Tasneen R, Pinn ML, Williams KN, Peloquin CA, et al. Comparison of the ‘Denver regimen’ against acute tuberculosis in the mouse and guinea pig. J Antimicrob Chemother. 2010;65:729–34. doi: 10.1093/jac/dkq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida D, Nuermberger EL, Tasneen R, Rosenthal I, Tyagi S, Williams K, et al. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob Agents Chemother. 2009;53:4178–84. doi: 10.1128/AAC.00830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosset J. The sterilizing value of rifampicin and pyrazinamide in experimental short-course chemotherapy. Bull Int Union Tuberc. 1978;53:5–12. [PubMed] [Google Scholar]
- 20.Grosset J, Truffot-Pernot C, Lacroix C, Ji B. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother. 1992;36:548–51. doi: 10.1128/aac.36.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]


