Abstract
Background & objectives:
The role of oxidative stress in the development of diabetes mellitus and its vascular complications are extensively studied. Hyperglycaemia causes oxidative damage by generation of reactive oxygen species and results in the development of complications. The present study was undertaken with the objective of exploring the anti-hyperglycaemic potential of polyphenolic enriched extract of Ichnocarpus frutescens in streptozotocin induced (n-STZ) neonatal diabetic rats (pups) for six weeks and to study oxidative stress and antioxidant status.
Methods:
Two days old pups were rendered diabetic by single injection of streptozotocin (90 mg/kg body wt, ip). At the end of the treatment period, the level of blood glucose, serum biochemical markers, serum lipid levels and liver malondialdehyde, tissue antioxidant levels were measured.
Results:
A marked rise was observed in the levels of fasting blood glucose (230.33 mg/dl), lipid profiles, lipid peroxidative products and a significant decrease in tissue antioxidants (superoxide dismuatase, catalase and reduced glutathione) and serum high density lipoprotein cholesterol levels in STZ treated rats. Oral administration of polyphenolic extract (150 and 300 mg/kg body wt, po) decreased fasting blood glucose levels (187.66 and 170.50 mg/dl, respectively) of STZ-treated diabetic rats significantly (P<0.01), when compared with control rats. In addition, the polyphenolic extract showed favourable effect (P<0.01) on the reduced tissues antioxidants level, liver glycogen level, high density lipoprotein level, with significant (P<0.01) reduction of elevated lipid peroxidation products. Histopathological study of the pancreas showed the protective role of polyphenolic extract.
Interpretation & conclusions:
Our study showed the antioxidant of effect polyphenolic extract of I. frutescens in STZ induced experimental diabetes. The results also suggested that this polyphenolic rich extract could be potentially useful for hyperglycaemia treatment to correct the diabetic state.
Keywords: Antioxidants, diabetes mellitus, Ichnocarpus frutescens, neonatal rats, oxidative stress, polyphenolic extract, streptozotocin
Diabetes mellitus is the fastest growing metabolic disorder in the world and a major cause of morbidity in developing countries1. Diabetes mellitus is shown to be associated with increased oxidative stress, which could be a consequence of either increased production of free radicals or reduced antioxidant defenses2. Oxygen free radicals are formed disproportionately in diabetes mellitus by glucose oxidation, non-enzymatic glycation of proteins and the subsequent degradation of glycosylated proteins. Diabetic complications are also associated with overproduction of free radicals and accumulation of lipid peroxidation by-products. Enhanced oxidative stress has been well documented in both experimental and human diabetes mellitus3. Thus attempt has been made to reduce the oxidative stress in patient with diabetes by supplementation with naturally occurring antioxidants4. The major goals of antioxidant treatment have been to reduce oxidative stress by preventing or delaying the progression or reversing the complications of diabetes. Medicinal plants often contain substantial amounts of antioxidants such as polyphenols, flavonoids, anthocyanins and tannins.
Ichnocarpus frutescens L. Br. (common name: Sarsaparilla, local name: Paalvalli; Family: Apocynaceae) has been used as folk medicine and as an ingredient in Ayurvedic and Unani preparations for diseases of blood, skin, for headache, snake bite and inflammation. Leaves of I. frutescens are rich in polyphenols and flavonoids. Distribution of various phenolic acid compounds and flavonoids in the leaves of I. frutescens has been systematically studied and well documented5,6. The decoction of leaves of I. frutescens is used in the treatment of jaundice and diabetes, and this plants is also used by the tribals of Karnataka and Utter Pradesh for treating diabetes and jaundice7. Our earlier studies showed hepatoprotective, anti-hyperlipidemic, in vitro anti-oxidant properties and attenuation of diabetic complications with I. frutescens8,9. This study was carried out to evaluate the antioxidant potential of polyphenol enriched extract (PPE) of I. frutescens on streptozotocin induced diabetes in neonatal (n-STZ)-type II Wistar rats.
Material & Methods
Chemicals and instruments: Streptozotocin (STZ) was purchased from Sigma Chemical Co (MO, USA). Glibenclamide and pentobarbitone sodium have been purchased from Ranbaxy Laboratories, India. Anthrone reagent, bovine serum albumin (BSA), thiobarbituric acid, reduced glutathione (GSH), nitroblue tetrazolium, trichloroacetic acid, nicotinamide adenine dinucleotide (NADH), Ellman's reagent [5,5’-dithiobis-(2-nitrobenzoic acid)] were purchased from SISCO Research Laboratories Private Limited, Mumbai, India. Serum biochemical assay kits for aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin, total cholesterol (TC), triglycerides (TGs) and total protein were purchased from Span Diagnostics Limited, Mumbai, India. Fasting blood glucose (FBG) levels were estimated by glucose oxidase peroxidase reactive strips (Accu-chek, Roche Diagnostics, USA). All other chemicals and solvents were of analytical grade. The equipment used were ultraviolet spectrophotometer (Spekol 1200, Japan), homogenizer (Remi, India), cold centrifuge (Remi, India), pH meter (Systronics, India), rotary vacuum evaporator (Superfit, India),lyophilizer (Instrumentation India, India), and light microscope (Kyowa, Japan).
Plant materials: The fresh leaves of I. frutescens were collected from delta region of Cauvery River, Thiruchirappalli, India, in February 2005 and authenticated at Botanical Survey of India, Central National Herbarium Howrah, India (Ref No: CNH/I -I/87/2005-TECH/1326). An authentic voucher specimen was deposited in the Herbarium of Division of Pharmacognosy, Phytotherapy Research Laboratory, Department of Pharmaceutical Technology, Jadavpur University, Kolkata, India.
Extraction of polyphenol enriched extract (PPE): The leaves were air dried for one wk at room temperature without exposure to sunlight and coarsely powdered. The leaf powder (300 g) was macerated for 5 days at room temperature three times with 800 ml of hydro-alcoholic mixture (double distilled water: 99% absolute alcohol; 30:70% vol/vol). The three macerates were combined and concentrated using a rotary evaporator under reduced pressure at <35°C. The residue was first dissolved in water and aqueous layer was washed with petroleum ether several times until a clear upper layer of petroleum ether was obtained. The concentrated solution of lower layer was extracted four times with 200 ml of ethyl acetate containing glacial acetic acid (10 ml/l) each time. The four ethyl acetate extracts were combined, evaporated to remove ethylacetate and polyphenolic extract (PPE) of I. frutescens was obtained as a lyophilized powder and stored at -70°C.
Animals: Two day old Wistar rat (M/s Ghosh Enterprises, Kolkata, India) were housed with their respective mother in macrolon cages under standard laboratory conditions. The mother rats were fed with commercial diet from Hindustan Lever Ltd (Bangalore, India) and free access to water during the experiments. The study protocol was approved by the Institutional Animal Ethical Committee (IAEC) of the Jadavpur University, Kolkata, India.
Induction of experimental type II diabetes: Wistar rat pups (n=40) were injected (ip) with 90 mg/kg STZ in 0.9 per cent sodium chloride solution. Control pups received equivalent vol of 0.9 per cent sodium chloride solution alone. Twelve weeks after the injection of STZ, animals exhibiting FBG>150 mg/dl were considered as neonatal- STZ (n-STZ)-diabetic10. These animals were divided into five groups. The treated control (glibenclamide 600 μg/mg) and treated diabetic rats received polyphenolic extract (150 and 300 mg/kg, po) for six wk.
After the last treatment of polyphenolic extract (after 6 wk), rats were fasted overnight and sacrificed by cervical decapitation. Blood was collected and serum was used for the estimation of biochemical parameters. Liver, kidney, pancreas and heart tissues were excised immediately and stored in ice-cold containers. The tissues were homogenized with appropriate buffer, centrifuged and the supernatant was collected. Tissue antioxidant estimations were carried out in the homogenates on the same day of sacrifice.
Assay of biochemical parameters: FBG levels were estimated by glucose oxidase peroxidase reactive strips11 (Accu-Chek, Roche Diagnostics, USA). The biochemical parameters evaluated were serum lipid profiles12,13 (TGs and TC), liver biomarkers such as, bilirubin14, AST15, ALT15 and ALP16, serum total protein17 using diagnostics kits. High density lipoprotein cholesterol18 (HDL-C) and low density lipoprotein cholesterol19 (LDL-C) were estimated by standard methods using spectrophotometric method.
Assay of tissue antioxidant enzymes: Liver glycogen was estimated by the standard method and expressed as mg/g of liver tissue20. Thiobarbituric acid reactive substances (TBARS) and GSH21,22 were estimated in all the tissues (liver, heart, pancreas, and kidney). Further, the activities of antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT) were assayed by standard methods23,24.
Histopathological studies: For histopathological studies, rats from control and experimental groups were perfused with 10 per cent neutral formalin solution. Pancreas was removed immediately from the rat; paraffin sections of 5 μm thickness were made and stained by hematoxylin-eosin (H&E) stain. After staining, the sections were observed under light microscope.
Statistical analysis: Data were statistically evaluated by using one-way analysis of variance (ANOVA), followed by Dunnett ‘t-test’ using GraphadInstat Statistical software version 4.01(San Diego, CA, USA).
Results
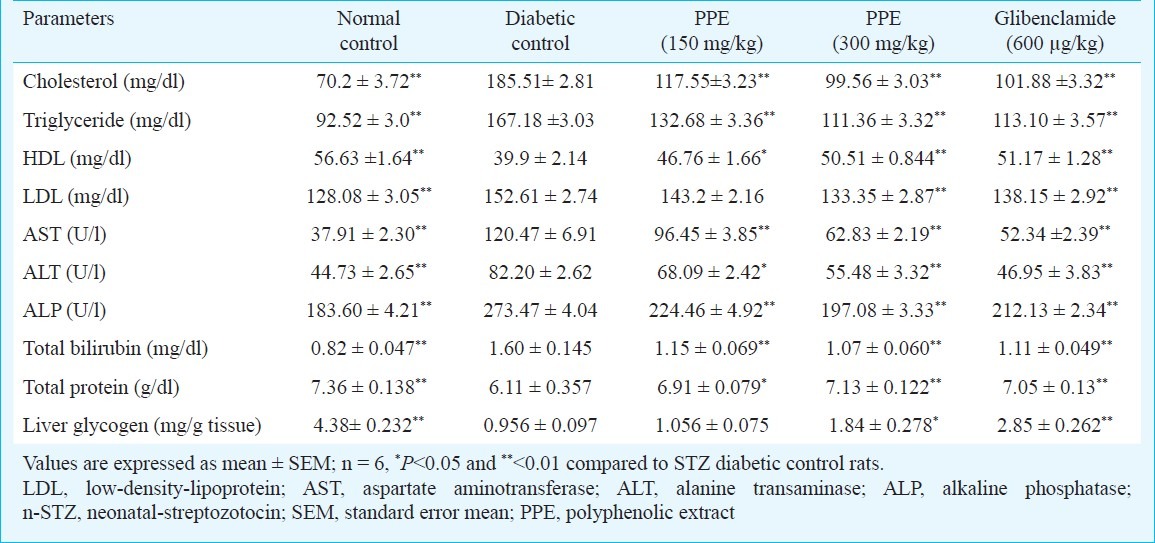
Tables I and II show the changes in the level of FBG, TGs, TC, AST, ALT, ALP and total protein in normal and experimental groups of rats. There was a significant elevation in FBG, AST, ALT, ALP, TGs, TC, LDL-C and while the levels of HDL-C, protein decreased during diabetes when compared to control group. Administration of polyphenolic extract brought back the levels to near normal values as that of standard drug glibenclamide. Streptozotocin induced diabetic rats showed a significant decrease in body weight compared to normal rats. Oral administration of polyphenol extract showed a significant increase (P<0.01) in body weight when compared to untreated diabetic rats.
Table I.
Effect of polyphenolic extracts of I. frutescens on fasting blood glucose and body wt on streptozotocin induced neonatal diabetic rats
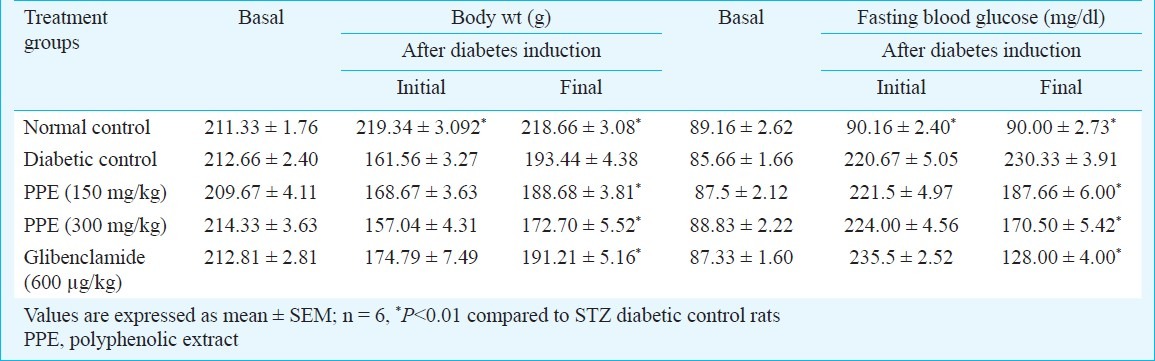
Table II.
Effect of polyphenolic extracts of I. frutescens on serum biochemical parameters and liver glycogen levels on streptozotocin induced neonatal diabetic rats
The antioxidant enzymes SOD, CAT and non-enzymatic GSH levels were determined in the liver, pancreas, heart and kidney of diabetic and diabetic rats treated with polyphenolic extract and glibenclamide (Table III). In the negative control rats, the highest antioxidant levels were found in the liver, followed by kidney, heart and pancreas. In diabetic rat liver, pancreas, heart and kidney the levels of SOD, CAT and GSH were decreased significantly compared to age matched control rats. Treatment with polyphenolic extract normalized the altered antioxidant levels of all tissues occurring due to diseases.
Table III.
Effect of polyphenolic extracts of I. frutescens on tissues antioxidant enzymes and lipid peroxidation levels in streptozotocin induced neonatal diabetic rats
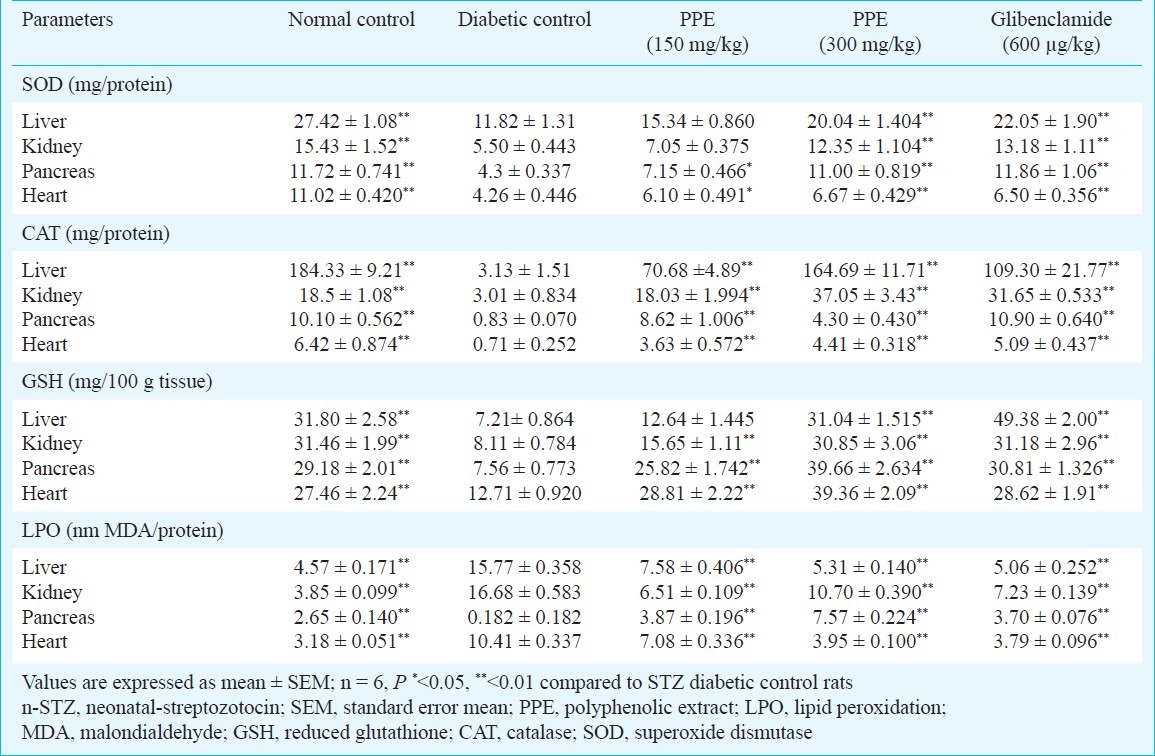
The lipid peroxide levels were increased significantly in liver, heart, kidney and pancreas in STZ-treated rats. The polyphenolic extract was able to reverse the altered peroxidative damage to near normal values (Table III).
Section from the non-diabetic vehicle treated rats showed normal architecture of pancreas (Fig. a) and no histopathological alterations were observed in these animals. Pancreatic section from control diabetic rats (Fig. b) showed minute and reduced number of islets. Section from polyphenolic extract treated diabetic rats showed regenerating tiny islets (Fig. c) which could be comparable to that of non-diabetic control rats. These histological observations showed the protective role of polyphenolic extract on pancreas in STZ induced diabetic rats.
Fig.
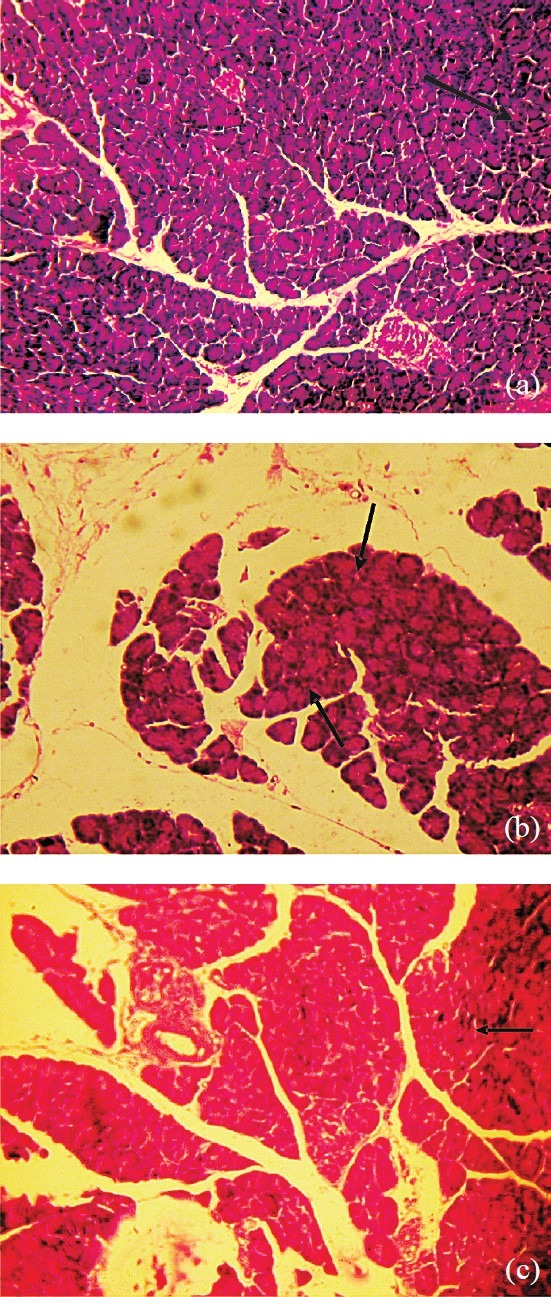
(a) Section from pancreas of non-diabetic rat showing normal islets (→). (b). Section from pancreas of diabetic control rat showing minute islets (→) with disturbed arrangement. (c). Section from pancreas of PPE treated diabetic rat showing recovery ofislets and its arrangement (→) that are comparable to that of non-diabetic rat (a-c: H & E stain, 100 X).
Discussion
The aim of the present study was to demonstrate the efficacy of polyphenolic extract in the reduction of FBG level as well as to determine the recovery in altered biochemical variables indicative oxidative stress and various organ damages in rats with STZ induced diabetes. The high blood FBG levels were observed in STZ treated rats indicating the establishment of oxidative stress mediated diabetic state25. Administra-tion of graded dose of polyphenolic extract significantly decreased FBG concentration when compared to the diabetic control. Oral administration of polyphenolic extract resulted in a significant decrease in serum TC and TGs. The TGs, TC and VLDL-C contents in plasma registered a significant hike in diabetic control group, which was retrieved to near normalcy in polyphenolic extract treated groups. This observation indicates the lipid lowering potential of I. frutescens.
Increase in the plasma ALT, AST and ALP are observed in the condition in which pancreas, liver, kidney and heart are destroyed by STZ. Moreover, the activities of these enzymes have been used as indicators of tissue toxicity in experimental diabetes. Increased levels of AST, ALT and ALP were seen in STZ induced diabetic rats, over a six weeks period. Treatment with polyphenolic extract showed potential hepatoprotective activity as reported in our earlier study10.
Glycogen synthesis in the rat liver and skeletal muscles is impaired during diabetes26. The regulation of glycogen metabolism occurs by the multifunctional enzyme glycogen synthase and glycogen phosphorylase that play a major role in the glycogen metabolism27. The reduced glycogen store in rats with experimentally induced diabetes has been attributed to reduced activity of glycogen synthase and increased activity of glycogen phosphorylase. In the present study the experimental diabetic rats treated with polyphenolic extract and glibenclamide treated groups restored the level of hepatic glycogen by means of increasing the activity of glycogen synthase enzyme.
Measurement of tissue TBARS help to assess the extent of tissues damage and elevated TBARS observed in the various tissues of diabetic rat can be related to overproduction of lipid peroxidation by-products and diffusion from damaged tissues28. Enhanced TBARS and declined antioxidants observed in the liver, pancreas, heart and kidney of diabetic rats can be attributed to increased biomembrane lipid peroxidation process and thereby contributing to alteration in antioxidant status29. The decrease in thiobarbituric acid reactive substance (TBARS) of various tissues clearly showed the antioxidant property of polyphenolic extract. These findings suggest that the polyphenolic extract may exert antioxidant activity and protect the tissue from lipid peroxidation.
In the current study, the SOD, CAT and GSH activities of diabetic rat liver, pancreas, heart and kidney were significantly reduced. This may be due to the production of reactive oxygen free radicals that can themselves reduce the activity of these enzymes. The lowered glutathione level in diabetes has been considered an important indicator of increased oxidative stress30.
Flavonoids have been shown to be potential antioxidants in the treatment of STZ induced oxidative stress in diabetic rats31. It is possible that the delay in STZ induced oxidative stress in various tissues of polyphenolic extract treated rats is predominantly due to its antioxidant activity. Polyphenolic extract of I. frutescens may also act by either directly scavenging reactive oxygen metabolites due to the presence of various antioxidant compounds or by increasing the level of endogenous antioxidant molecules or enzymes. Further, pharmacological and chemical studies are required to explore the mechanism of action of active ingredient(s) responsible for the antioxidant activity observed.
Acknowledgment
The authors acknowledge to All India Council of Technical Education (AICTE), New Delhi, Government of India, for providing National Doctoral Fellowship (NDF-AICTE) to the first author (CTK).
References
- 1.King H, Aubert R, Herman WH. Global burden of diabetes, 1995-2025: Prevalence, numerical estimates and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 3.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 4.Bursell SE, King GL. Can protein kinase C inhibition and vitamin E prevent the development of diabetic vascular complications? Diabetes Res Clin Pract. 1999;45:169–82. doi: 10.1016/s0168-8227(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 5.Singh RP, Singh RP. Flavonoids of the flowers of Ichnocarpus frutescens. J Indian Chem Soc. 1987;64:715–56. [Google Scholar]
- 6.Babita A, Mohamed A, Vijender S, Rajeev KS. Isolation and characterization of phytoconstituents from the stems of Ichnocarpus frutescens. Chin J Natural Med. 2010;8:401–4. [Google Scholar]
- 7.Parinitha M, Harish GU, Vivek NC, Mahesh T, Shivanna MB. Ethno-botanical wealth of Bhadra wild life sanctuary in Karnataka. Indian J Trad Knowledge. 2004;31:37–50. [Google Scholar]
- 8.Kumarappan CT, Vijayakumar M, Thilagam E, Balamurugan M, Thiagarajan M, Senthil S, et al. Protective and curative effects of polyphenolic extracts from Ichnocarpus frutescense leaves on experimental hepatotoxicity by carbon tretrachloride and tamoxifen. Ann Hepatol. 2011;10:63–72. [PubMed] [Google Scholar]
- 9.Kumarappan CT, Mandal SC. Alpha-glucosidase inhibitory activity and in vitro antioxidant activities of alcohol-water extract of Ichnocarpus frutescens leaves. Med Chem Res. 2008;17:219–33. [Google Scholar]
- 10.Portha B, Blondel O, Serradas P. The rat models of non-insulin dependent diabetes induced by neonatal streptozotocin. Diabet Metab. 1989;15:61–75. [PubMed] [Google Scholar]
- 11.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24–7. [Google Scholar]
- 12.Van Handel E, Zilversmit DB. Micromethod for direct determination of serum triglycerides. J Lab Clin Med. 1957;50:152–7. [PubMed] [Google Scholar]
- 13.Allain CC, Poon LS, Chan CSG. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 14.Malloy HT, Evelyn KA. The determination of bilirubin with the photometric colorimeter. J Biol Chem. 1937;119:481–90. [Google Scholar]
- 15.King J. The transaminases: alanine and aspartate transaminases.In: , editor. In: Van D, editor. Practical clinical enzymology. London: Van D. Nostrand Co; 1965. pp. 363–95. [Google Scholar]
- 16.King J. The hydrolases-acid and alkaline phosphatases. In: Van D, editor. Practical clinical enzymology. London: Van D. Nostrand Co; 1965. pp. 199–208. [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin's phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 18.Warnick GR, Nguyan T, Albers AA. Comparison of improved precipitation methods for quantification of high density lipoprotein cholesterol. Clin Chem. 1985;31:217–22. [PubMed] [Google Scholar]
- 19.Friedwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultra-centrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.Plummer DT. An introduction to practical biochemistry. New York: McGraw-Hill; 1978. Isolation and assay of glycogen from the liver and skeletal muscles of rats; pp. 182–4. [Google Scholar]
- 21.Ohkawa H, Onishi N, Yagi K. Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 22.Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 23.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:131–2. [PubMed] [Google Scholar]
- 24.Aebi H. Catalase. In: Bergmeyer HV, editor. Methods in enzymatic analysis. New York: Academic Press; 1974. pp. 674–84. [Google Scholar]
- 25.Wright E, Jr, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract. 2006;60:308–14. doi: 10.1111/j.1368-5031.2006.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akatsuka A, Singh TJ, Huang KP. Comparison of liver glycogen synthase from normal and streptozotocin induced diabetic rats. Arch Biochem Biophys. 1983;220:426–34. doi: 10.1016/0003-9861(83)90432-0. [DOI] [PubMed] [Google Scholar]
- 27.Janero DR. Malondialdehyde and thiobarbituric acid reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Rad Biol Med. 1990;9:515–40. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee B, Mukherjee JR, Chatterjee M. Lipid peroxidation, glutathione levels and change in glutathione-related enzyme activities in streptozotocin-induced diabetic rats. Immunol Cell Biol. 1994;72:109–14. doi: 10.1038/icb.1994.17. [DOI] [PubMed] [Google Scholar]
- 29.McLennan SV, Heffernan S, Wright L, Rae C, Fisher E, Yue DK, et al. Changes in hepatic glutathione metabolism in diabetes. Diabetes. 1991;40:344–8. doi: 10.2337/diab.40.3.344. [DOI] [PubMed] [Google Scholar]
- 30.Du Thie G, Crozier A. Plant derived polyphenolic antioxidants. Curr Opin Nutr Metab. 2000;3:447–51. doi: 10.1097/00075197-200011000-00006. [DOI] [PubMed] [Google Scholar]


