Abstract
The partial intravenous anesthesia technique (PIVA) is used to lower the inspired concentration of an inhalational anesthetic by concurrent use of injectable drugs. This technique reduces the incidence of undesirable side-effects and provides superior quality of anesthesia and analgesia. Drugs commonly used for PIVA include opioids, alpha-2 adrenergic agonists, injectable anesthetic agents, and lidocaine. Most are administered by intravenous infusion.
Résumé
Anesthésie intraveineuse partielle chez les chats et les chiens. La technique d’anesthésie intraveineuse partielle (TAIP) est utilisée pour réduire la concentration inspirée d’un anesthésique inhalé par l’utilisation concomitante de médicaments injectables. Cette technique réduit l’incidence des effets secondaires indésirables et procure une qualité supérieure d’anesthésie et d’analgésie. Les médicaments communément utilisés pour la TAIP incluent des opioïdes, des agonistes adrénergiques alpha-2, des agents anesthésiques injectables et de la lidocaïne. La plupart sont administrés par infusion intraveineuse.
(Traduit par Isabelle Vallières)
Introduction
The concept of “balanced anesthesia” has existed for a long time. Balanced anesthesia techniques incorporate a combination of drugs, with each providing different components of anesthesia such as analgesia and muscle relaxation. This approach enables lower dosages of the main general anesthetic to be used and limits potentially harmful side-effects while providing good quality anesthesia (1). Early forms of balanced anesthesia often involved the use of opioids, nitrous oxide, and peripheral neuromuscular blockers. While this combination is still used to provide good quality anesthesia, balanced anesthesia techniques have evolved over the past 2 to 3 decades to involve a single drug or a combination of drugs administered by injectable infusion. This change has arisen from the introduction of new drugs and less expensive computerized infusion pumps (2).
In human anesthesia, computer pharmacokinetic modelling and target controlled infusions are designed to achieve and maintain a predefined plasma concentration by varying the infusion rate according to predetermined data. While the target controlled infusion technique is entering specialist veterinary anesthesia, simple intravenous infusion techniques are commonly used and involve administration of a continuous or constant rate infusion (CRI), or variable rate infusion (VRI), depending on circumstances (2,3). Infusion rates in veterinary medicine are often extrapolated from experience or pharma-cokinetic profiles obtained from bolus intravenous injection.
Terminology has also evolved over time. The administration of combinations of intravenous infusions of anesthetic drugs and analgesics for maintenance of anesthesia is called total intravenous anesthesia (TIVA). Infusion of drugs can also be used to reduce the inspired concentration of inhalational anesthetic agents and is called partial intravenous anesthesia (PIVA). Partial intravenous anesthesia or TIVA techniques have been used for many years for anesthetic management of patients with neurological conditions (4). The potent vasodilatory nature of modern volatile agents can cause detrimental increase in intracranial pressure for patients with poor cerebral autoregulation. The PIVA or TIVA technique allows for the use of very low concentrations of volatile agent or none at all, respectively. Application of similar anesthetic management to other areas such as cardiac anesthesia followed when it was realized that PIVA and TIVA techniques can provide superior hemodynamic stability (5,6). These techniques are now applied to many other medical and veterinary situations.
Total intravenous anesthesia (TIVA) is commonly used for anesthesia where there are no facilities for inhalational anesthesia such as in some rural spay/neuter programs or within the critical care unit for patients requiring ventilator support (7,8). The use of PIVA techniques alongside inhalational anesthesia may appear to be costly and more trouble than benefit, and this may be true for routine, short elective surgery. There is, however, utility in using PIVA to assist in maintenance of anesthesia and the technique has become popular in specialist veterinary anesthesia for certain procedures and in critically ill patients and/or for pain. The term “anesthetic-sparing” effect of drugs used for PIVA is preferred in the clinical setting to the term “reduction of minimum alveolar concentration (MAC),” which is defined under controlled laboratory conditions. The advantages of PIVA are listed as:
Good, stable quality of anesthesia — PIVA techniques provide a consistent level of receptor occupation by maintaining plasma concentration. The infusion can be terminated at the end of the surgical or anesthetic period or continued into the recovery phase until deemed unnecessary.
Better hemodynamic support. Some drugs used for PIVA have little negative impact on hemodynamic stability when administered at clinical infusion rates. These hemodynamically stable infusions can also have powerful volatile anesthetic-sparing ability. Isoflurane and sevoflurane have vasodilatory properties and negative impact on cardiac contractility, both of which can lead to hypotension. The anesthetic-sparing ability of the infusions can allow for a reduction in the inspired concentration of the volatile agent and therefore reduce their negative effects on hemodynamic stability (9). Ilkiw et al (9) found a considerable improvement in cardiovascular function when a fentanyl infusion was used and the enflurane concentration was reduced by about 65%. They cautioned that an anticholinergic agent may be required to reverse severe bradycardia caused by fentanyl (9). The ability to reduce respiratory depression, however, may not be as noticeable as the injectable drugs may have their own effects on respiratory function that can be compounded by the concurrent use of volatile agents. Therefore, ventilatory support may still be required.
Recovery may be smoother and many drugs can be continued into the postoperative phase. Many of the drugs are sedatives and their combination with analgesics can allow a quieter and more controlled transition from general anesthesia into recovery and awareness. The infusion rates can be gradually decreased to allow the patient to completely recover in a controlled manner. There is still a requirement for close monitoring of the patient during the postoperative phase to avoid excessive sedation and potential loss of airway reflexes.
Most PIVA techniques provide analgesia as well as sedation. The analgesia can be used to reduce isoflurane concentration and can be maintained from before surgical intervention to well into the recovery phase, if required.
Many drugs have been used for PIVA and their benefits may change depending on the species in which the technique is used. Studies have shown that morphine, lidocaine, and ketamine infusions reduce isoflurane requirements by 48%, 29%, and 25%, respectively (10). This reduction allows the inspired isoflurane concentration to be decreased to about 1% or less. Monitoring of the patient and provision of life support are still necessary. Accidental relative overdose of the volatile agent may occur with clinician discomfort to reduce vaporizer settings from more familiar settings when PIVA techniques are not applied. Monitoring the usual signs of anesthetic depth is still applicable with PIVA and this helps to offset any fear of sudden awakening. In most situations, the infusion rates are decided upon and the volatile agent is adjusted accordingly. However, adjustments can also be made in the infusion rate: for example, if there is a response to surgical stimulation, more analgesic can be administered instead of increasing isoflurane concentration. If low arterial blood pressure is observed, the isoflurane concentration may be relatively too high, or the infusion rate of the hemodynamic-sparing drugs may be too low. Suggested infusion rates are listed in Table 2.
Table 2.
Suggested loading doses and infusion rates for isoflurane- or sevoflurane-anesthetized dogs and cats. Use lower end of infusion rates when combining 2 or more drugs
| Drug | Loading dose (mg/kg BW) intravenous route | Intravenous infusion rate (mg/kg BW per hour) |
|---|---|---|
| Morphine | 0.15–0.3 slow | 0.1–0.2 |
| Hydromorphone | 0.05–0.10 | 0.05–0.10 |
| Fentanyl | 0.003–0.005 | 0.006–0.040 |
| Remifentanil | Fentanyl: 0.003–0.005 (for longer action) | 0.006–0.040 |
| Sufentanil | 0.001–0.002 | 0.002–0.006 |
| Dexmedetomidine | 0.0005–0.002 | 0.0005–0.002 |
| Midazolam | 0.2–0.4 | 0.02–0.05 |
| Ketamine | 0.5–1.0 | 0.3–0.6 |
| Propofol | 4.0–6.0 | 12.0–30.0 |
| Alfaxalone | 1.0–2.0 | 4.0–9.0 |
| DOG ONLY | ||
| Lidocaine | 1.0–2.0 | 1.8–6.0 |
BW — body weight
Review of intravenous infusion pharmacokinetics
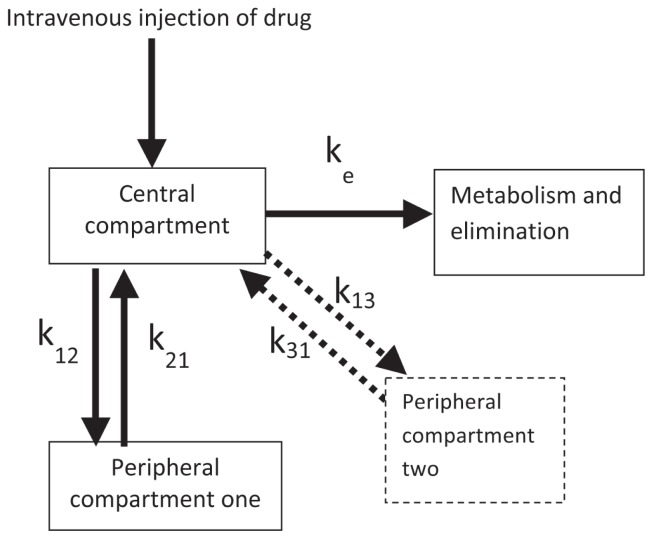
Some understanding of the pharmacokinetics of the drug used may be required. Infusions are used to maintain plasma concentrations of a drug which in turn, will maintain effective receptor occupation and the desired effect. An intravenous bolus injection of anesthetic and sedative drugs will directly increase plasma concentrations and allow rapid delivery to the sites where the drugs achieve their effects. The time it takes to achieve peak effect is called “effect-site equilibrium.” The circulation is regarded as the central compartment in Figure 1. The circulation delivers the drug to tissues receiving a high portion of cardiac output (brain, heart, kidneys) and the drug rapidly crosses biological membranes to a second compartment (Figure 1). As the drug plasma concentration in the central compartment declines through elimination processes (metabolism through liver and extra-hepatic sites), the drug in the peripheral compartment re-enters the central compartment to undergo elimination. In this way, the individual “recovers” from the effects of the drug. This pharmacokinetic model is termed a 2-compartment model. If another compartment is involved (tissues which receive less portion of cardiac output such as muscle, fat, and bone) a 3-compartment model is applied (Figure 1; dashed box and arrows). In order to maintain the desired effects, repeated boluses of drugs are required as the effects of the previous bolus wane. This can result in erratic maintenance of the desired effects. To maintain drug plasma concentrations and reliable effects, an appropriate infusion rate should account for distribution of the drug throughout the tissues and also compensate for elimination processes (Figure 2).
Figure 1.
Diagram showing the movement of a drug between the central compartment (systemic circulation) and peripheral compartment(s).
A 3-compartment model will include the peripheral compartment 2 with dashed lines. k12 and k21; k13 and k31 are rate constants associated with the to and fro transfer of drug between the compartments; ke is the rate constant for elimination from the central compartment.
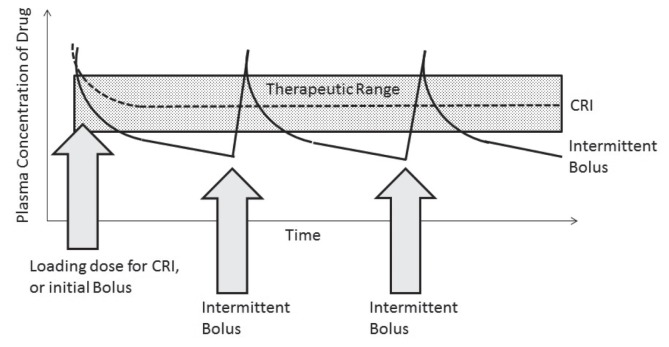
Figure 2.
Graph indicating the change in plasma concentration between intermittent bolus injections of a drug, and a continuous rate infusion with a loading dose.
Note that the plasma concentrations can decrease below the therapeutic range using the bolus technique.
If an infusion was commenced at the stated infusion rate, it would take 5 half-times (time for plasma concentrations to decline by 50%) to reach the appropriate plasma concentration and could result in considerable delay of observable effects (Table 1). Supplying enough of the drug to be distributed throughout the tissues requires a higher initial rate of infusion than required to offset elimination. Clinically, the “extra” amount of drug required for distribution throughout the tissues is provided by administering a bolus of the drug (the loading dose, LD) at the start of an infusion. Advanced techniques use preprogrammed target controlled infusions to take all pharmacokinetic requirements into effect for a particular drug and species (2,3).
Table 1.
Table indicating the relationship of half-times to the amount of drug eliminated from the plasma. Five half-times are required to eliminate 97% of the drug
| Number of half-times | Fraction of initial amount remaining | Percent of initial amount eliminated (%) |
|---|---|---|
| 0 | 1 | 0 |
| 1 | 1/2 | 50 |
| 2 | 1/4 | 75 |
| 3 | 1/8 | 88 |
| 4 | 1/16 | 94 |
| 5 | 1/32 | 97 |
| 6 | 1/64 | 98 |
Some drugs have a slightly longer effect-site equilibration time compared with other drugs, and the LD may take a few minutes for an observable effect. Fentanyl has a longer onset of action, measured in minutes, compared to induction drugs such as propofol, which have an onset of action measured in seconds. This slight delay may be clinically observable with opioids.
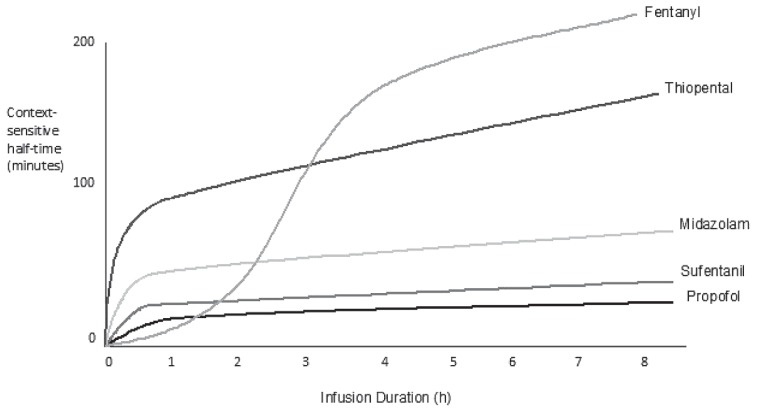
“Context sensitive half-time” is often quoted for infusions. The pharmacokinetics of drug elimination may depend on the length of time an infusion has been administered. It is the time for the concentration of drug in the plasma to decrease by 50% after discontinuing a continuous rate infusion (CRI) of a specific duration (context). This is dependent on lipid solubility and rate of elimination. The context sensitive half-time can increase with increasing duration of infusion with some drugs, and is not necessarily related to the elimination half time (Figure 3). For example, the context sensitive half time for fentanyl in humans can markedly increase after 3 to 4 h of infusion, and increase further with longer infusion duration. The changes in elimination are thought to be associated with different uptake, saturation, and release of the drug in various compartments of the body; for example, large amounts of fentanyl and sufentanil are taken up and stored in the lungs. A longer elimination half-time was not found after a 4-hour infusion period in dogs, but longer infusion periods have not been studied (11). Context sensitive half-time data for commonly used drugs are not readily available for veterinary patients. The clinician may have to rely on adjusting the infusion rates during lengthy infusion periods according to observable signs exhibited by the patient, or by measured parameters.
Figure 3.
Changes in context sensitive half-time with different duration (context) of infusion, indicating the potential for accumulation with long-term infusions. Data taken from studies performed in humans. (Reprinted with permission from Barash PG, Cullen BF, chapter 47; Figure 47-2 in “Clinical Anesthesia,” Wolters Kluwer Health).
Drugs used for PIVA
Opioids
Opioids are ideal for intra-operative analgesia and to complement inhalational anesthesia. Opioids are often administered in premedication combinations and their activity can be lengthened by providing more of the drug intra-operatively. Opioids can also be administered by intravenous infusion during anesthesia. Opioids with high intrinsic activity at the mu-opioid receptor can be anesthetic sparing, especially in dogs, in which mu-opioid agonists can decrease the concentration of volatile agent required to maintain a surgical plane of anesthesia by as much as 50% to 60% (10,12,13). There is a ceiling effect with anesthetic sparing ability with opioids, and higher doses do not decrease volatile agent requirement further than this amount (9,14). In cats, the anesthetic sparing effect is less well appreciated with the same mu-opioid agonists and a 15% to 20% reduction in volatile anesthetic requirement is observed (15). The anesthetic sparing effect may be linked to the ability of mu-opioid agonists to stimulate the central nervous system in some individuals rather than cause depression. Central nervous system stimulation is more likely in cats using the higher dosages used in dogs. The opioids most commonly used for infusions in veterinary anesthesia include morphine, hydromorphone, fentanyl, sufentanil, and remifentanil (1,10,15–20). Anticholinergics may be required to correct bradycardia resulting from mu-opioid-induced increase in vagal tone, but otherwise mu-opioid agonist infusions provide good hemodynamic stability. Mu-opioid agonists also have respiratory depressant effects and support of ventilation may be required.
Remifentanil is unique because it is metabolized by plasma cholinesterases and its elimination is rapid once the infusion is terminated (21). A loading dose is not necessary for remifentanil as long as the infusion is commenced approximately 30 min before it is required in dogs, but over an hour is required to achieve stable plasma concentrations in cats. Therefore a loading dose may be advisable; fentanyl is a better choice to provide analgesia for the 20 to 30 min period than remifentanil. Alternative analgesic drugs or techniques must be used once remifentanil is stopped. Remifentanil can be used for post-operative analgesia, but it must be remembered that the context sensitive half-time is short and removal of infusion lines to take a dog out for a walk will cause plasma concentrations to plummet and the dog may soon be in pain. In a similar way, any technical problems in the delivery of remifentanil to the anesthetized patient will markedly change anesthetic depth and stability.
Alpha-2 adrenergic agonists
This group of drugs produces deep sedation in a number of species. They also provide good analgesia, which makes them useful for PIVA techniques (22). Visceral analgesia can be as powerful as opioid agonists in horses, and the combination of opioid and alpha-2 adrenergic drugs produces superior quality of analgesia in dogs (23,24). The combined sedative and analgesic qualities produce a powerful anesthetic sparing effect by up to 60% (22,25,26). The newer, potent alpha-2 adrenergic receptor specific drugs also appear to cause fewer cardiac arrhythmias and complications as long as it is recognized that these drugs also have effects on the cardiovascular system. All alpha-2 adrenergic agonists cause vasoconstriction, bradycardia, and decreased cardiac contractility (27). Infusions of alpha-2 adrenergic agents may still affect hemodynamic stability, even at the low infusion rates indicated in Table 2, and this should be anticipated. Heart rate is usually in the low normal range for the species and lowers cardiac output, although the systemic arterial blood pressure tends to be better maintained with use of these drugs (28). Hyperglycemia and increased urine production are also noticeable side-effects, and intravenous fluid therapy may be required to compensate for fluid losses. It is likely ventilatory support will be required, especially when these agents are used alongside opioids. It must be remembered that these drugs have a strong ability to reduce the concentration of volatile anesthetic agent and close attention must be paid to the depth of anesthesia.
Benzodiazepines
Benzodiazepines are useful for PIVA techniques as they have good sedative properties and hemodynamic stability (29). High infusion rates can reduce enflurane requirements by 55% in dogs (30). They are often used alongside mu-opioid agonists in critically ill patients (18). Diazepam can be used, but midazolam is more commonly employed for PIVA techniques because metabolism of diazepam into non-active products is rapid in the dog (31,32). These drugs are not effective analgesics, but they may provide greater centrally mediated muscle relaxation for some procedures. The author has found the antagonist, flumazenil [0.01 to 0.03 mg/kg body weight (BW)], to be useful when midazolam is used for long periods and a slow recovery with some hypoventilation is observed.
Injectable anesthetic agents
Ketamine
Ketamine is a dissociative anesthetic drug, but also has analgesic properties through its inhibitory action on N-methyl D-aspartate (NMDA) receptors. These receptors enhance pain transmission and are activated by continuous noxious stimulation (central sensitization), and are especially important in long-standing painful conditions. Ketamine has become popular for use intra-operatively and post-operatively for these analgesic properties (33). With intra-operative use, ketamine infusions can be anesthetic-sparing by around 25% at the same infusion rates used for postoperative analgesia (0.3 to 0.6 mg/kg BW per hour), but higher infusion rates (3.0 to 6.0 mg/kg BW per hour) can have a powerful anesthetic-sparing ability of 40% to 45% (12,34,35). Side-effects may include increased systemic blood pressure and body temperature, mydriasis, regurgitation, cardiac arrhythmias such as ventricular premature contractions, and dysphoria in conscious patients. The infusion rate can be decreased and the side-effects may subside. Ketamine infusions used as part of a PIVA technique provide good, stable anesthesia for patients with chronically painful conditions and patients with a great deal of central sensitization.
Propofol
Propofol is a rapidly metabolized injectable anesthetic with rapid recovery characteristics in dogs even following long periods of propofol infusion. Propofol has been extensively studied as part of TIVA, but has also been recommended for PIVA techniques (36,37). A sedation level of CNS depression rather than anesthetic level has been used in critical care settings with low infusion rates. The sub-anesthetic infusion rates can also be used alongside volatile anesthetic. The PIVA technique with a propofol/opioid infusion combination has been used for anesthetic management of patients requiring intracranial surgery to reduce the vasodilatory effect from volatile anesthetics, but can equally be applied to other situations where volatile agent administration requires limitation. The eye reflexes and position changes with different anesthetic depth are similar between propofol and the volatile agent so judgment of anesthetic depth and subsequent vaporizer adjustment is easily performed. Propofol can accumulate in cats because glucuronidation of propofol occurs at a slower pace; therefore, PIVA techniques require attention to gradual decrease in propofol infusion rates, or prolonged recoveries might follow (38).
Alfaxalone
Alfaxalone is a steroid-based injectable anesthetic for use in cats and dogs and has the same mechanism of action as propofol and thiopental. Alfaxalone is similar to propofol in terms of pharmacokinetics and it would be expected that it can also be easily incorporated into TIVA and PIVA techniques. Reports of use of alfaxalone have been published for TIVA but not for PIVA techniques (39,40). Alfaxalone infusions for TIVA have been reported in cats, with some delayed recoveries which may have been due to concurrently administered drugs (41). The usefulness of alfaxalone for PIVA/TIVA in cats remains to be fully evaluated.
Miscellaneous drugs
Lidocaine
Lidocaine can have sedative actions in conscious dogs at relatively high infusion rates (> 50 μg/kg BW per minute) (42) and can be used to produce anesthetic-sparing effects of 20% to 40% in dogs. There are many recommendations for using lidocaine infusions for PIVA (12,43,44). It is also thought that lidocaine may have some analgesic effects, although this useful effect may be more relevant for inflammatory conditions. Lidocaine infusions have not been reported in anesthetized cats, but a bolus dose of lidocaine was found to be hemodynamically depressant; therefore, its use for reduction of volatile agent was not recommended (45). The author has successfully used lidocaine infusions for PIVA in cats with painful inflammatory conditions at less than half the dosages used in dogs, alongside other analgesic drugs such as remifentanil and ketamine. Further studies may help to clarify whether lidocaine infusion techniques are a useful tool in the anesthetic management of cats. Bupivacaine is not a good substitute for intravenous infusions because of its cardiotoxic properties.
Combinations of drugs used for PIVA
Selected combinations of the drugs discussed can be used for more powerful anesthetic-sparing effects in extremely debilitated patients or those with severely painful conditions. Close attention is required to anesthetic depth and the vaporizer setting may even be reduced to 0% with some combinations in some individuals, especially if they are debilitated (5,10,18,43). Not all the potential choices need to be used, but depending on the situation, usually 1 to 3 drugs may be selected along with a volatile agent. Mu-opioid agonists are almost always selected for their excellent analgesia with hemodynamic stability; alpha2 adrenergic agonists for sedation and analgesia, lidocaine for its anti-inflammatory and sedative effects, ketamine for its good analgesic effects with control of central sensitization processes, benzodiazepines for hemodynamic stability, and injectable anesthetic drugs for hemodynamic stability and powerful anesthetic-sparing effects. A common combination is morphine with lidocaine and ketamine (MLK) (10). Some may replace the morphine with fentanyl (FLK) to obtain further analgesia and anesthetic-sparing effects. The drug combination can also be used postoperatively if close monitoring is maintained. All 3 drugs can be combined in the same fluid bag (10). The author has found that ketamine is the most likely to produce undesirable side-effects and prefers to administer ketamine infusions separately so the infusion rate can be adjusted if necessary without changing the rates of the other drugs. The use of MLK in cats should be avoided, and infusions without lidocaine should be used instead (45).
Infusion apparatus
Drugs can be combined in intravenous fluid therapy or within a separate fluid bag if the clinician is prepared to do the appropriate calculations. Although providing infusions in fluid bags is much less expensive, it can result in inaccurate delivery of drug and difficulty in making changes in infusion rate for 1 drug and not another. Intravenous fluid pumps can be used to give better infusion rates for fluid bags, and some can be adapted to draw drug from a syringe. Syringe drivers or syringe pumps can be programmed to deliver the desired infusion rate by depressing the plunger of a syringe, and changes are easily made. The less expensive versions use 1 size of syringe and have only 1 mode (mL/h) so some calculation is required. The more expensive pumps can take a variety of syringe sizes and can be programmed with the patient body weight, concentration of drug in the syringe and infusion mode (mL/kg BW per hour, μg/kg BW per minute, etc). Long microbore tubing extension sets are available with low priming volumes, and are useful for attaching the drug syringe to the patient fluid therapy line and avoid excessive drug waste.
Drug calculations
Make an MLK solution in a 250-mL fluid bag and administer it at a fluid rate of 1.0 mL/kg BW/h. This solution can be used intra- and post-operatively and will provide 10 h of analgesia for a 25-kg dog, for example. Use another bag of fluids for fluid therapy and attach the drug combination to the fluid therapy line as close to the catheter as possible:
Morphine: Infusion rate 0.1 mg/kg BW per hour
Lidocaine: Infusion rate 3.0 mg/kg BW per hour
Ketamine: Infusion rate 0.3 mg/kg BW per hour
Remove 39.8 mL of fluid from a 250 mL bag of fluids to allow for adding the total volume of drug required and provide an appropriate drug concentration:
Morphine (25 mg/mL solution): Add 25 mg (1.0 mL) to obtain 0.1 mg/mL solution.
Lidocaine (20 mg/mL solution): Add 760 mg (38.0 mL) to obtain 3.0 mg/mL solution
Ketamine (100 mg/mL solution): Add 75 mg (0.75 mL) to obtain 0.3 mg/mL solution CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Ilkiw JE. Balanced anesthetic techniques in dogs and cats. Clin Tech Small Anim Pract. 1999;14:27–37. doi: 10.1016/S1096-2867(99)80024-3. [DOI] [PubMed] [Google Scholar]
- 2.Musk GC, Pang DS, Beths T, Flaherty DA. Target-controlled infusion of propofol in dogs for induction of anaesthesia. Vet Rec. 2005;157:766–770. doi: 10.1136/vr.157.24.766. [DOI] [PubMed] [Google Scholar]
- 3.Joubert KE, Keller N, DuPlessis CJ. A retrospective case series of computer-controlled total intravenous anaesthesia in dogs presented for neurosurgery. J S Afr Vet Assoc. 2004;75:85–89. doi: 10.4102/jsava.v75i2.458. [DOI] [PubMed] [Google Scholar]
- 4.Cole CD, Gottfried ON, Gupta DK, Couldwell WT. Total intravenous anesthesia: Advantages for intracranial surgery. Neurosurgery. 2007;61:5369–5378. doi: 10.1227/01.neu.0000303996.74526.30. [DOI] [PubMed] [Google Scholar]
- 5.Liehmann L, Mosing M, Auer U. A comparison of cardiorespiratory variables during isoflurane-fentanyl and propofol-fentanyl anaesthesia for surgery in injured cats. Vet Anaesth Analg. 2006;33:158–168. doi: 10.1111/j.1467-2995.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 6.Wong GLS, Morton S. Total intravenous anesthesia (TIVA) in pediatric cardiac anesthesia. Pediatric Anesthesia. 2011;5:560–566. doi: 10.1111/j.1460-9592.2011.03565.x. [DOI] [PubMed] [Google Scholar]
- 7.Ethier MR, Mathews KA, Valverde A, et al. Evaluation of the efficacy and safety for use of two sedation and analgesia protocols to facilitate assisted ventilation of healthy dogs. Am J Vet Res. 2008;69:1351–1359. doi: 10.2460/ajvr.69.10.1351. [DOI] [PubMed] [Google Scholar]
- 8.Ko J, Berman AG. Anesthesia in shelter medicine. Top Companion Anim Med. 2010;25:92–97. doi: 10.1053/j.tcam.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Ilkiw JE, Pascoe PJ, Haskins SC, Patz JD, Jaffe R. The cardiovascular sparing effect of fentanyl and atropine, administered to enflurane anesthetized dogs. Can J Vet Res. 1994;57:248–253. [PMC free article] [PubMed] [Google Scholar]
- 10.Muir WW, Wiese AJ, March PA. Effects of morphine, lidocaine, ketamine, and morphine-lidocaine-ketamine drug combination on minimum alveolar concentration in dogs anesthetized with isoflurane. Am J Vet Res. 2003;64:1155–1160. doi: 10.2460/ajvr.2003.64.1155. [DOI] [PubMed] [Google Scholar]
- 11.Sano T, Nishimura R, Kanazawa H, et al. Pharmacokinetics of fentanyl after single intravenous injection and constant rate infusion in dogs. Vet Anaesth Analg. 2006;33:266–273. doi: 10.1111/j.1467-2995.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 12.Steffey EP, Baggot JD, Eisele JH, et al. Morphine-isoflurane interaction in dogs, swine and Rhesus monkeys. J Vet Pharmacol Therap. 1994;17:202–210. doi: 10.1111/j.1365-2885.1994.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 13.Hellyer PW, Mama KR, Shafford HL, Wagner AE, Kollias-Baker C. Effects of diazepam and flumazenil on minimum alveolar concentrations for dogs anesthetized with isoflurane or a combination of isoflurane and fentanyl. Am J Vet Res. 2001;62:555–560. doi: 10.2460/ajvr.2001.62.555. [DOI] [PubMed] [Google Scholar]
- 14.Murphy MR, Hug CC., Jr The anesthetic potency of fentanyl in terms of its reduction of enflurane. Anesthesiology. 1982;57:485–488. doi: 10.1097/00000542-198212000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Ilkiw JE, Pascoe PJ, Tripp LD. Effects of morphine, butorphanol, buprenorphine, U50488H on the minimum alveolar concentration of isoflurane in cats. Am J Vet Res. 2002;63:1198–1202. doi: 10.2460/ajvr.2002.63.1198. [DOI] [PubMed] [Google Scholar]
- 16.KuKanich B, Hogan BK, Krugner-Higby LA. Pharmacokinetics of hydromorphone in healthy dogs. Vet Anaesth Analg. 2008;35:256–264. doi: 10.1111/j.1467-2995.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- 17.Hall RI, Murphy MR, Hug CC. The enflurane sparing effect of sufentanil in dogs. Anesthesiology. 1987;67:518–525. doi: 10.1097/00000542-198710000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Hellebrekers LJ, Sap R. Sufentanil-midazolam anaesthesia in the dog. J Vet Anaesth. 1992;19:69–71. [Google Scholar]
- 19.Ferreira TH, Aguiar AJA, Valverde A, Teixeira Neto FJ, Steagall PVM, Soares JHN. Effect of remifentanil hydrochloride administered via constant rate infusion on the minimum alveolar concentration of isoflurane in cats. Am J Vet Res. 2009;70:581–588. doi: 10.2460/ajvr.70.5.581. [DOI] [PubMed] [Google Scholar]
- 20.Michelsen LG, Salmenperä M, Hug CC, Jr, Szlam F, VanderMeer D. Anesthetic potency of remifentanil in dogs. Anesthesiology. 1996;84:865–872. doi: 10.1097/00000542-199604000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Hoke FJ, Cunningham F, James MK, Muir KT, Hoffman WE. Comparative pharmacokinetics and pharmacodynamics of remifentanil, its principal metabolite (GR90291) and alfentanil in dogs. J Pharm Exp Therap. 1997;281:226–232. [PubMed] [Google Scholar]
- 22.Ansah OB, Raekillo M, Vainio O. Correlation between serum concentrations following continuous intravenous infusion of dexmedetomidine or medetomidine in cats and their sedative and analgesic effects. J Vet Pharmacol Therap. 2000;23:1–8. doi: 10.1046/j.1365-2885.2000.00240.x. [DOI] [PubMed] [Google Scholar]
- 23.Muir W, Robertson JT. Visceral analgesia: Effects of xylazine, butorphanol, meperidine and pentozacine in horses. Am J Vet Res. 1985;46:2081–2084. [PubMed] [Google Scholar]
- 24.Grimm KA, Tranquilli WJ, Thurman JC, Benseon GJ. Duration of nonresponse to noxious stimulation after intramuscular administration of butorphanol, medetomidine, or a butorphanol-medetomidine combination during isoflurane administration in dogs. Am J Vet Res. 2000;61:42–47. doi: 10.2460/ajvr.2000.61.42. [DOI] [PubMed] [Google Scholar]
- 25.Pascoe PJ, Raekello M, Kuusela E, McKusik B, Granholm M. Changes in the minimum alveolar concentration of isoflurane and some cardio-pulmonary measurements during three continuous infusion rates of dexmedetomidine in dogs. Vet Anaesth Analg. 2006;33:97–103. doi: 10.1111/j.1467-2995.2005.00236.x. [DOI] [PubMed] [Google Scholar]
- 26.Souza SS, Intelisano TR, De Biagg CP, et al. Cardiopulmonary and isoflurane-sparing effects of epidural or intravenous infusion of dexmedetomidine in cats undergoing surgery with epidural lidocaine. Vet Anaesth Analg. 2010;37:106–115. doi: 10.1111/j.1467-2995.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- 27.Pypendop BH, Verstegen JP. Hemodynamic effects of medetomidine in the dog: A dose titration study. Vet Surg. 1998;27:612–622. doi: 10.1111/j.1532-950x.1998.tb00539.x. [DOI] [PubMed] [Google Scholar]
- 28.Carter JE, Campbell NB, Posner LP, Swanson C. The haemodynamic effect of medetomidine continuous rate infusions in the dog. Vet Anaesth Analg. 2010;37:197–206. doi: 10.1111/j.1467-2995.2009.00522.x. [DOI] [PubMed] [Google Scholar]
- 29.Jones DJ, Stehling LC, Zauder HL. Cardiovascular responses to diazepam and midazolam maleate in the dog. Anesthesiology. 1979;51:430–434. doi: 10.1097/00000542-197911000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Hall RI, Szlam F, Hug CC. Pharmacokinetics and pharmacodynamics of midazolam in the enflurane-anesthetized dog. J Pharmacokinet Biopharm. 1988;16:251–262. doi: 10.1007/BF01062136. [DOI] [PubMed] [Google Scholar]
- 31.Löscher W, Frey HH. Pharmacokinetics of diazepam in the dog. Arch Int Pharmacodyn Ther. 1981;254:180–195. [PubMed] [Google Scholar]
- 32.Court MH, Greenblatt DJ. Pharmacokinetics and preliminary observations of behavioral changes following administration of midazolam to dogs. J Vet Pharmacol Ther. 1992;15:343–350. doi: 10.1111/j.1365-2885.1992.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 33.Wagner AE, Walton JA, Hellyer PW, Gaynor JS, Mama KR. Use of low doses of ketamine administered by constant rate infusion used as an adjunct for postoperative analgesia in the dog. J Am Vet Med Assoc. 2002;221:72–75. doi: 10.2460/javma.2002.221.72. [DOI] [PubMed] [Google Scholar]
- 34.Solano AM, Pypendop BH, Boscan PL, Ilkiw JE. Effect of intravenous administration of ketamine on the minimum alveolar concentration of isoflurane in anesthetized dogs. Am J Vet Res. 2006;67:21–25. doi: 10.2460/ajvr.67.1.21. [DOI] [PubMed] [Google Scholar]
- 35.Wilson J, Doherty TJ, Egger CM, Fidler A, Cox S, Rohrbach B. Effects of intravenous lidocaine, ketamine and the combination on the minimum alveolar concentration of sevoflurane in dogs. Vet Anaesth Analg. 2008;35:289–296. doi: 10.1111/j.1467-2995.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 36.Correa MDA, Aguiar AJDA, Teixeira Neto J, Mendes GDM, Steagall PVM, Lima AFdM. Effects of remifentanil infusion regimens on cardiovascular function and responses to noxious stimulation in propofol-anesthetized cats. Am J Vet Res. 2007;68:932–940. doi: 10.2460/ajvr.68.9.932. [DOI] [PubMed] [Google Scholar]
- 37.Kuusela E, Vainio O, Short CE, et al. A comparison of propofol infusion and propofol/isoflurane anaesthesia in dexmedetomidine premedicated dogs. J Vet Pharmacol Therap. 2003;26:199–204. doi: 10.1046/j.1365-2885.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- 38.Pascoe PJ, Ilkiw JE, Frischmeyer KJ. The effect of the duration of propofol administration on recovery from anesthesia in cats. Vet Anaesth Analg. 2006;33:2–7. doi: 10.1111/j.1467-2995.2005.00216.x. [DOI] [PubMed] [Google Scholar]
- 39.Ambros B, Duke-Novakovski T, Pasloske KS. Comparison of the anesthetic efficacy and cardiopulmonary effects of continuous rate infusions of alfaxalone-2-hydroxypropyl-beta-cyclodextrin and propofol in dogs. Am J Vet Res. 2008;69:1391–1398. doi: 10.2460/ajvr.69.11.1391. [DOI] [PubMed] [Google Scholar]
- 40.Suarez MA, Dzikiti BT, Stegmann FG, Hartman M. Comparison of alfaxalone and propofol administered as total intravenous anaesthesia for ovariohysterectomy in dogs. Vet Anaesth Analg. 2012;39:236–244. doi: 10.1111/j.1467-2995.2011.00700.x. [DOI] [PubMed] [Google Scholar]
- 41.Beths T, Touzot-Jourde G, Musk G, Pasloske K. Total intravenous anesthesia in cats: Evaluation of alfaxalone in hydroxypropyl-beta-cyclodextrin to induce and maintain anesthesia in feral and domestic cats undergoing neutering procedures. Vet Anaesth Analg; Abstracts presented at the 10th World Congress of Veterinary Anaesthesia; Sept 2009; Glasgow, UK. 2010. p. 58. [Google Scholar]
- 42.MacDougall LM, Hethey JA, Livingston A, Clark C, Shmon CL, Duke-Novakovski T. Antinociceptive, cardiopulmonary and sedative effects of five different infusion rates of lidocaine in conscious dogs. Vet Anaesth Analg. 2009;36:512–522. doi: 10.1111/j.1467-2995.2009.00480.x. [DOI] [PubMed] [Google Scholar]
- 43.Ortega M, Cruz I. Evaluation of a constant rate infusion of lidocaine for balanced anesthesia in dogs undergoing surgery. Can Vet J. 2011;52:856–860. [PMC free article] [PubMed] [Google Scholar]
- 44.Steagall PVM, Tiexeira Neto FJ, Minto BW, Campagnol D, Correa MA. Evaluation of the isoflurane-sparing effects of lidocaine and fentanyl during surgery in dogs. J Am Vet Med Assoc. 2006;229:522–527. doi: 10.2460/javma.229.4.522. [DOI] [PubMed] [Google Scholar]
- 45.Pypendop B, Ilkiw J. Assessment of the hemodynamic effects of lidocaine administered IV in isoflurane-anesthetized cats. Am J Vet Res. 2005;66:661–668. doi: 10.2460/ajvr.2005.66.661. [DOI] [PubMed] [Google Scholar]
- 46.Scholz J, Steinfath M, Schulz M. Clinical pharmacokinetics of alfentanil, fentanyl and sufentanil. An update. Clin Pharmacokinet. 1996;31:275–292. doi: 10.2165/00003088-199631040-00004. [DOI] [PubMed] [Google Scholar]
- 47.McFarlan CS, Anderson BJ, Short TG. The use of propofol infusions in paediatric anaesthesia: A practical guide. Paeditr Anaesth. 1999;9:209–216. [PubMed] [Google Scholar]
- 48.Turcant A, Delhumeau A, Premel-Cabic A, et al. Thiopental pharmacokinetics under conditions of long-term infusion. Anesthesiology. 1985;63:50–54. doi: 10.1097/00000542-198507000-00007. [DOI] [PubMed] [Google Scholar]