Abstract
A 3.7-kg, 3-year-old intact female domestic shorthaired cat was presented with the chief complaint of anorexia and lethargy of 3 days duration with a noticeable decrease in body condition and a large open wound on her ventral caudal abdomen. A diagnosis of acute mastitis with gland abscessation was made. The patient was successfully treated with oral antibiotics and open wound management using surgical debridement and lavage followed by wound dressings using honey.
Résumé
Mammite nécrotique féline. Une chatte domestique intacte pesant 3,7 kg et âgée de 3 ans a été présentée pour des plaintes portant principalement sur l’anorexie et l’abattement depuis 3 jours avec une dégradation marquée de la condition physique et une grande plaie ouverte sur l’abdomen caudal ventral. Un diagnostic de mammite aiguë avec un abcès glandulaire a été posé. La patiente a été traitée avec succès à l’aide d’antibiotiques oraux et la plaie ouverte a été gérée à l’aide d’un parage chirurgical et d’un lavage suivis d’un pansement de la plaie avec du miel.
(Traduit par Isabelle Vallières)
Mastitis is an uncommon reproductive disease of canines, and even more infrequently, felines (1). It occurs when there is bacterial infection of the mammary tissue resulting from an ascending infection, trauma, or unsanitary surroundings (2). The most commonly isolated causative organisms include Escherichia coli, staphylococci, and streptococci (3). Mastitis ranges in severity from acute and fulminant to chronic low-grade disease (4). Clinical signs most commonly include a firm, painful, swollen, discolored mammary gland which may yield abnormal secretion. Affected animals may be anorexic, pyrexic, depressed, and lethargic while nursing ill-thriving neonates (5). In severe cases, affected glands may become abscessed or develop gangrene. Diagnosis is based on history, clinical signs, and cytological evaluation of milk. Most cases of mastitis can be managed on an outpatient basis (6). Treatment involves the use of antibiotics to which the causative organism is susceptible and which are able to penetrate and concentrate in milk and mammary tissue, and are not harmful to nursing neonates (1). In cases in which glands become necrotic, neonates should be removed and hand-raised on milk replacer; the affected gland should be surgically drained and/or debrided followed by copious lavage and open wound management (4,6).
Case description
A 3-year-old, intact female domestic shorthaired cat was presented as a new patient to a mixed animal practice in central Ontario. The cat was 2 wk postpartum with her fifth litter and was nursing 5 kittens. Upon initial presentation, the owners reported that they had noticed a lump that formed acutely adjacent to a nipple. The cat had a 3-day history of anorexia and lethargy and her owners were concerned that she appeared to have recently lost a significant amount of weight.
On physical examination, the cat was depressed but alert and responsive. Her mucous membranes were pink but tacky. Her capillary refill time was < 2 s and she had a significantly prolonged skin tent. She was pyrexic (rectal temperature of 39.8°C) with a heart rate of 200 beats/min and a respiratory rate of 36 breaths/min. Her body condition was 3.5/9. On her left mammary chain, the third gland was noted to be very swollen, firm, and warm. No other abnormalities were evident on physical examination. A 21-day course of oral antibiotics, amoxicillin/clavulanic acid (Clavaseptin; Vétoquinol Canada, Lavaltrie, Quebec), was started, 150 mL of electrolytes (Plasmalyte A; Baxter Healthcare Corporation, Deerfield, Illinois, USA) were administered subcutaneously and the owner was instructed to soak the affected gland with a warm epsom salt solution every 8 to 12 h, at a concentration of 1 to 2 tbsp dissolved in 250 mL of warm water. The cat was also sent home with Medical Recovery food (Royal Canin Canada, Guelph, Ontario) to help increase her daily caloric intake.
Three days later, the cat was presented again on an emergency basis. The affected gland had ruptured. The resulting defect was approximately 2 to 3 cm in diameter superficially and was overlaid by a black necrotic flap of skin. All other mammary glands were swollen and hard. The most cranial glands were erythematous.
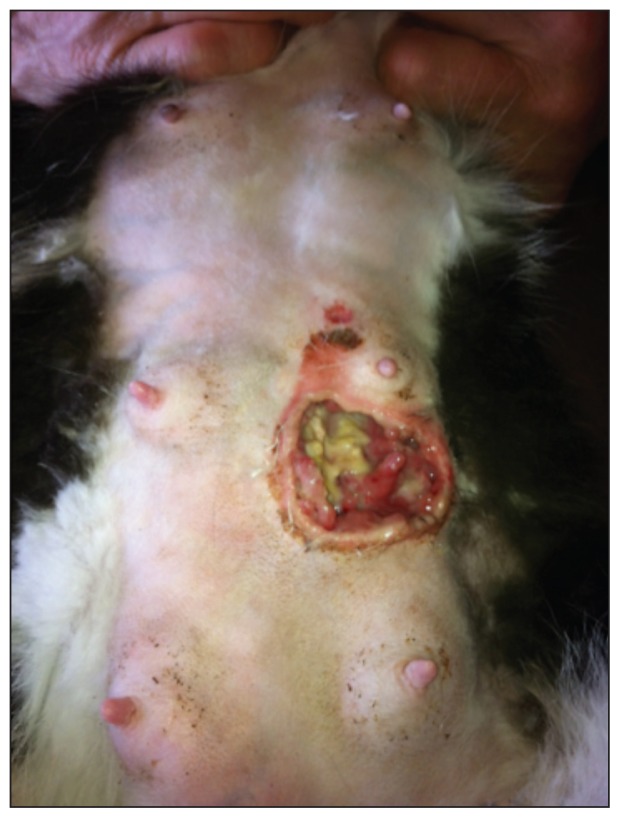
The final diagnosis was acute fulminant mastitis of the second-most caudal left mammary gland with abscessation and gangrenous involvement. The cat’s entire ventral abdomen was shaved and the wound was surgically debrided and explored. The necrotic flap and a significant amount of caseous subcutaneous tissue were removed. Once debrided, the wound was 6 cm diameter and 2.5 cm deep (Figure 1). A swab of the wound was sent for aerobic and anaerobic culture and sensitivity. The wound was then lavaged with a dilute betadine solution, non-pasteurized honey was applied to the wound and the cat’s abdomen was wrapped in a soft padded bandage. The cat was sent home with dilute betadine solution, non-pasteurized honey, bandaging material, epsom salts, and kitten milk replacer with the instructions to continue the previously prescribed oral antibiotics and to lavage the wound twice per day with the dilute betadine solution and follow this with a non-pasteurized honey wound dressing. All non-abscessed mammary glands were also to be soaked in dilute warm epsom salts twice a day. The owners were advised to stop the kittens from nursing the queen and feed them kitten milk replacer.
Figure 1.
Patient 3 days after initial presentation.
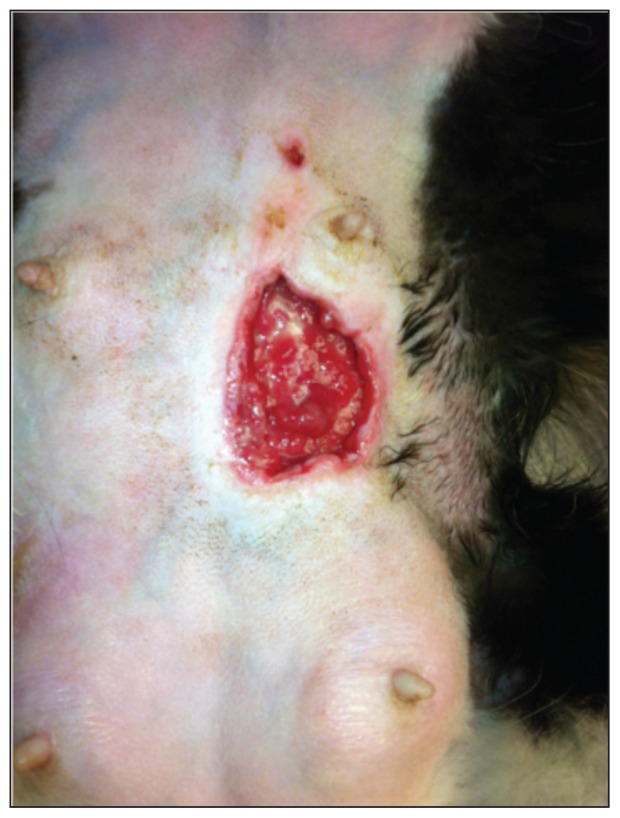
The cat was presented 3 d later for a re-evaluation. She was bright, alert, and responsive with an appropriate mentation state. All vital parameters were within normal limits. The wound was covered in a healthy layer of granulation tissue, was free of purulent/necrotic debris (Figure 2), and was smaller in diameter, measuring 4.5 cm wide. The swelling and erythema of the other mammary glands had resolved. The owner reported that the cat’s activity and appetite were back to normal and that the kittens had transitioned well onto the milk replacer and were steadily increasing in weight each day. The culture swab yielded hemolytic E. coli (4+) and Staphylococcus aureus (2+). Both organisms were sensitive to amoxicillin/clavulanate, ampicillin, cefovecin, cefoxitin, cephalothin, clindamycin, enrofloxacin, gentamicin, marbofloxacin, orbifloxacin, tetracycline, and trimethoprim/sulfonamide.
Figure 2.
Patient 3 days after starting open wound.
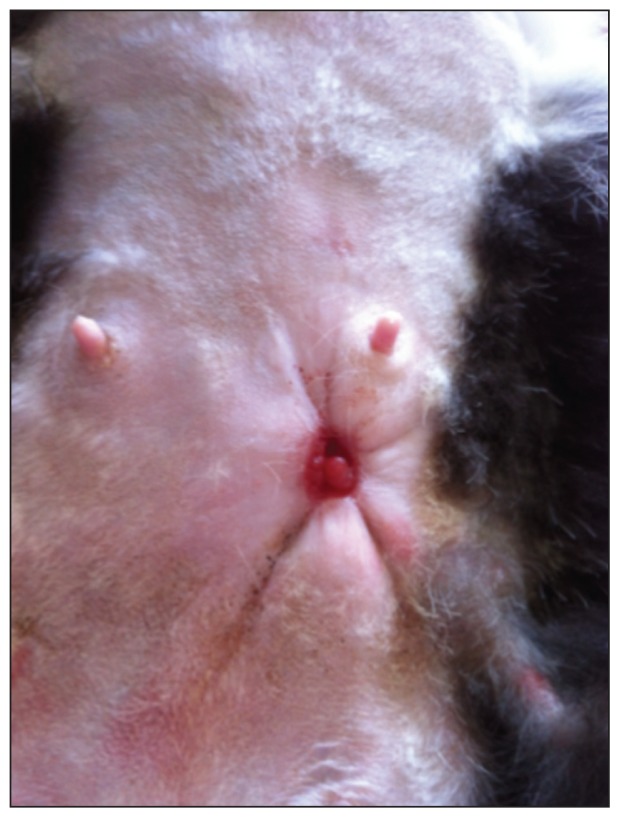
The owners were advised to continue with oral antibiotics and wound lavage with honey bandaging. The following week the cat was seen for another assessment. She had remained stable at home, tolerating all advised therapeutics. Her overall condition had remained unchanged from her previous visit. The only notable exception was her abdominal wound, which had decreased in size, with a diameter of 0.5 cm. The corresponding nipple was located in the center of the scar (Figure 3).
Figure 3.
Patient 10 days after starting open wound.
Discussion
Mastitis in dogs and cats is poorly understood. One study that involved clinical, bacteriological, cytological, hematological, and pathological analyses revealed that mastitis may lead to subclinical disease and that presence of bacteria and leukocyte increases are the main features of mastitis in bitches. In contrast to ruminant mastitis, increased leukocytes are present in adjacent mammary glands and not only in the individually affected ones (3). To the author’s knowledge, this is the first report of mastitis in a cat resulting in gangrene of the affected mammary gland.
The diagnosis of mastitis is based on history, clinical signs, and physical findings in a lactating female (2). Cytologic evaluation of the milk will identify an inflammatory response and intracellular bacteria can often be observed (4). When multiple mammary glands are involved, bitches may neglect their young or refuse to allow them to nurse, resulting in young that are weak, unthrifty, and crying (5,6). The tentative diagnosis in this case was made during initial presentation based on the history, clinical signs, and physical findings. Milk evaluation and cytology were not performed as any attempt to express the gland resulted in significant discomfort to the patient. A complete blood (cell) count (CBC) and biochemistry were offered but refused due to financial constraints. A CBC may reveal a left shift and biochemistry and is useful for assessing the severity of systemic illness (2,4). The diagnosis in this case was confirmed when the patient was presented 3 d later with abscessation and gangrene of the affected gland. Cases involving abscessation and necrosis of the mammary glands are severe, can develop rapidly, and are considered to be an emergency (4).
Culture and sensitivity tests should be performed on milk, fluid from a lanced abscess, and from necrotic tissue whenever possible (4). The bacteria that are implicated are frequently opportunistic pathogens and predisposing factors must usually be present for disease to develop (7). The source of infection in this queen was not identified; however, ascending infection via nursing or mammary gland trauma was highly suspected. Escherichia coli, a Gram-negative facultative anaerobe, is a normal part of the intestinal microflora in mammals (7) and the gastrointestinal microflora is the usual source of infection. Endotoxins liberated from the E. coli cell wall can gain access to the systemic circulation and activate the host systemic inflammatory response (7). The systemic clinical signs exhibited by the queen in this report are likely due to a combination of this systemic inflammatory response to E. coli endotoxin as well as the biological and metabolic stress that a lactating female goes through in the period directly following parturition. When uncontrolled, this response can trigger multiorgan failure; therefore, endotoxemia is a potentially fatal outcome to mastitis caused by E. coli(7). Escherichia coli has also been isolated from the organs of septicemic puppies of diseased bitches (1). Staphylococcus is often a member of the normal microflora inhabiting feline and canine skin and mucous membranes. Like E. coli, opportunistic infection of the mammary gland with Staphylococcus species occurs in the presence of predisposing factors and staphylococci have been shown to be a major cause of mastitis in lactating bitches (7).
Treatment of mastitis is often initiated immediately using broad-spectrum antibiotics effective against E. coli, Streptococcus, and Staphylococcus until culture and sensitivity results are available (2,6). Escherichia coli from healthy dogs are frequently multi-drug resistant with resistance to ampicillin, tetracycline, and trimethoprim being common (8). Staphylococcal isolates are often, but not always, susceptible to beta-lactamase-resistant synthetic penicillins, first generation cephalosporins, aminoglycosides, and fluoroquinolones (7). Sensitivity testing is therefore important. Amoxicillin/clavulanate is considered a safe first choice as it penetrates and concentrates in milk and is safe for nursing puppies and kittens (1,2). The recommended oral dose in bitches and queens is 15 mg/kg BW, q12h for 2 to 3 wk (9). Fluid therapy is recommended if systemic involvement occurs. When glands are abscessed or necrotic, neonates should be removed and given supplemental artificial milk, if not at an appropriate age for weaning, and be frequently weighed to monitor growth (1,6). Affected glands should then be appropriately lanced, if not already open, drained and/or debrided surgically, lavaged, and treated as an open wound (1,6). For the queen herein, open wound management included the application of a honey dressing. Honey has antibacterial properties and enhances both granulation and epithelialization of wounds (10,11).
Inhibition of lactation can be of benefit in certain circumstances and can be achieved using Cabergoline (Dostinex; Pfizer Canada, Kirkland, Quebec) administered orally at a dose of 5 μg/kg BW, q24h for 5 to 7 d (1). This option was offered at the time of diagnosis, but was declined by the owner, partially due to cost. In addition, for non-abscessed glands, hot compresses or soaks are recommended to help encourage drainage and ease discomfort (6).
Acknowledgments
The author thanks the veterinarians and staff of North Simcoe Veterinary Services and Dr. Cathy Gartley of the Ontario Veterinary College for their advice, guidance and encouragement. CVJ
Footnotes
Ms. Wilson will receive 50 copies of her article free of charge courtesy of The Canadian Veterinary Journal.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Wiebe VJ, Howard JP. Pharmacologic advances in canine and feline reproduction. Top Companion Anim Med. 2009;24:71–99. doi: 10.1053/j.tcam.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jutkowitz LA. Reproductive emergencies. Vet Clin Small Anim. 2005;35:397–420. doi: 10.1016/j.cvsm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Ververidis HN, Mavrogianni VS, Fragkou IA, et al. Experimental staphylococcal mastitis in bitches: Clinical, bacteriological, cytological, haematological and pathological features. Vet Microbiol. 2007;124:95–106. doi: 10.1016/j.vetmic.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Traas AM, O’Connor C. Postpartum emergencies. International Veterinary Emergency and Critical Care Symposium. 2009 [Google Scholar]
- 5.Nelson RW, Couto CG. Small Animal Internal Medicine. 4th ed. St Louis, Missouri: Mosby Elsevier; 2009. p. 946. [Google Scholar]
- 6.Hopper K. Pyometra, mastitis and uterine prolapse. International Veterinary Emergency and Critical Care Symposium; New Orleans, Louisiana, USA. August 9–13, 2003. [Google Scholar]
- 7.Graham EM, Taylor DJ. Bacterial reproductive pathogens of cats and dogs. Vet Clin Small Anim. 2012;42:561–582. doi: 10.1016/j.cvsm.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Wedley AL, Maddox TW, Westgarth C, et al. Prevalence of antimicrobial-resistant Escherichia coli in dogs in a cross-sectional, community-based study. Vet Rec. 2011;168:354–358. doi: 10.1136/vr.d1540. [DOI] [PubMed] [Google Scholar]
- 9.Plumb DC. Plumb’s Veterinary Drug Handbook. 6th ed. Ames, Iowa: Blackwell Publishing Professional; 2008. pp. 51–53. [Google Scholar]
- 10.Mathews KA. Honey or sugar dressings for managing wounds: How sweet it is. NAVC Conference. 2010:1559–1561. [Google Scholar]
- 11.O’Connell K, Wardlaw JL. Unique therapies for difficult wounds. Today’s Veterinary Practice. 2011;1:10–16. [Google Scholar]