Abstract
AIM: The aim of this study is to validate the accuracy of HER2 assessment on biopsies by comparing matched biopsy/surgical material from the same patients. METHODS: HER2 status was evaluated by immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) in 103 cases of gastric and gastroesophageal junction cancers in coupled biopsy and surgical material. RESULT: Complete concordance between IHC and FISH results on biopsy versus surgical samples was noted in 80% and 95% of cases, respectively. At comprehensive comparison, including IHC and FISH data on biopsy and surgical samples, 89% of biopsies were predictive of HER2 status in surgical samples, whereas 11% showed variable inconsistencies. The majority of these (10 of 12 cases) showed IHC score 0/1+ on biopsy but were all IHC positive and amplified at surgery; in particular, three (3 of 35; 8.5%) IHC score 0 and four (4 of 16; 25%) IHC score 1+ cases were FISH amplified on biopsy material also, whereas the remaining three cases were FISH non-amplified on biopsy. The percentage of cases, which were FISH amplified with IHC score 1+ or 2+ on biopsies, were similar (25% and 33%, respectively) and they also shared a similar grade of amplification. These data suggest that both IHC score 1+ and 2+ on biopsy material represent “equivocal cases” that may merit further investigation. CONCLUSIONS: The predictive value of HER2 IHC in biopsies is high. FISH analysis should be considered for IHC score 2+ and 1+ biopsy cases. Approximately 8% of cases will not be accurately predicted by biopsy evaluation.
Introduction
Despite a slow decrease in incidence, gastric cancer is still one of the leading causes of cancer-related deaths worldwide [1]. Early-stage carcinomas may be cured by surgery alone; however, advanced gastric carcinoma (GC) or gastroesophageal junction carcinoma (GEJC), whether resectable or unresectable, still present with a dismal prognosis [2–4]. New therapeutic regimens and drugs, both in the neoadjuvant and adjuvant settings, are therefore eagerly awaited.
Gastric cancerogenesis is a multistep process and the understanding of the molecular events involved is increasing rapidly [5]. HER2, part of the epidermal growth factor receptor family, is a proto-oncogene located on chromosome 17q21 [6], which encodes for a transmembrane tyrosine-kinase receptor. Its importance was first recognized in breast, as up to 20% to 25% of cancers show overexpression of HER2 with prognostic and predictive importance [7,8]. Amplification of HER2 in gastric cancer has been reported in the literature since the 1980s [9,10], and a recent systematic analysis has highlighted its prognostic significance [11]. However, it is only with the introduction of the anti-HER2 drug trastuzumab (Herceptin; Hoffmann-La Roche, Basel Switzerland) that these findings have become of major interest. The studies were conducted mainly on gastric cancer and reported HER2 overexpression rates between 8.2% and 53% [12], whereas the percent of positivity ranged between 20% and 25% in esophageal and junctional adenocarcinomas [13,14]. In 2010, the Trastuzumab for Gastric Cancer (ToGA) study [15] evaluated the use of the anti-HER2 drug trastuzumab in combination with chemotherapy (capecitabine and cisplatin or fluorurouracil and cisplatin) versus chemotherapy alone. A significant survival advantage was observed in the trastuzumab group with no significant increase in toxic side effects; these results led to Food and Drug Administration (FDA) and European Medicine Agency (EMEA) approval for the use of anti-HER2 therapy in advanced HER2-positive GC and GEJC [16].
Correct and reproducible evaluation of HER2 status in GC and GEJC is essential in the selection of patients who may be candidates for anti-HER2 therapy. In breast cancer, HER2 evaluation is determined by immunohistochemistry (IHC) as the first method of choice; in equivocal cases (IHC score 2+), gene amplification requires confirmation by fluorescence in situ hybridization (FISH) [17]. Whereas IHC method sensitivity and specificity vary greatly depending on the antibody and method used, FISH is more standardized and less variable and is therefore considered the gold standard for HER2 status assessment [18]. However, the HER2 evaluation scoring system for breast carcinoma has been shown to be poorly applicable in gastric cancer because staining is more heterogeneous and incomplete membrane immunoreactivity is more frequent in the latter [19]. For this reason, a different scoring system for HER2 expression in the stomach has been proposed by Hoffman et al. [20].
In the Western world, approximately half of gastric and junctional cancer patients are diagnosed when the neoplasm is at an unresectable stage and these patients are the potential target for trastuzumab therapy [21]. In such cases, the only available tissue for HER2 testing is either endoscopic or more rarely laparoscopic biopsies; in both situations, the tissue sample is generally scanty. It is therefore important to define the predictive accuracy of endoscopic biopsies in the evaluation of HER2 status when compared with surgical material. Some studies have investigated the reliability of biopsy material, but none focused on this specifically and systematically [19,22–24].
This study is aimed at the evaluation of: 1) the concordance between matched biopsy and surgical samples; 2) the comparison of two different commercially available HER2 antibodies; and 3) the comparison of IHC results with HER2 gene amplification as determined by FISH. The final goal of the study is therefore to validate the accuracy of HER2 assessment on endoscopic biopsies by comparing matched biopsy/surgical material from the same patients.
Materials and Methods
Study Group
All consecutive cases of GC accessioned at the Pathology Unit, Department of Surgical and Diagnostic Sciences (DISC), University of Genoa between 2004 and 2009 and all consecutive cases of GEJC accessioned at the Surgical Pathology and Cytopathology Unit, Department of Medicine (DIMED), University of Padua between 2006 and 2010 were reviewed.
Fifty cases of GC and 53 cases of GEJC were selected. Clinical information including patient's age, sex, neoadjuvant therapy, and type of surgical procedure were obtained from the patient's medical records. Twenty-eight patients were female and 75 were male; mean age was 69.5 years (range, 37–90).
Selection criteria included cases with available formalin-fixed and paraffin-embedded material from paired biopsy and surgical resections (total or partial gastrectomy or Ivor Lewis resections) in the absence of previous neoadjuvant therapy. From formalin-fixed and paraffin-embedded blocks, 4-µm-thick sections were cut and made available for subsequent analyses.
Pathologic Assessment
All cases were reviewed and reclassified in terms of histotype according to World Health Organization (WHO) 2010 [25], Laurén [26], and Ming [27] classifications, grade, and stage [28].
Two representative samples from the surgical resection specimens were selected as well as all diagnostic biopsy samples for each patient. Biopsy number for each case ranged between 2 and 13 samples (median = 5, total sum of 504 samples). Of these 504 samples, only 302 (60%) contained invasive carcinoma and were therefore available for HER2 status evaluation. For each selected paraffin block, ten 4-µm serial sections were cut. One section was stained with hematoxylin and eosin (H&E) and the others were mounted on SuperFrost Plus slides and made available for IHC and FISH analysis.
Immunohistochemistry
Two different IHC staining methods using automated staining devices were used: PATHWAY HER2/neu (4B5) rabbit monoclonal antibody (Ventana Medical Systems, Tucson, AZ) and Oracle HER2 Bond mouse monoclonal antibody (CB11) (Menarini Diagnostics, Florence, Italy).
The Ventana PATHWAY HER2/neu was performed at the University of Genoa using the Ventana BenchMark XT platform (Ventana Medical Systems), whereas the Menarini antibody test was performed at the University of Padua using the automated Microsystems Bondmax (Leica, Wetzlar, Germany), according to the manufacturer's instructions.
Briefly, tissue sections were deparaffinized and rehydrated. After epitope retrieval, endogenous peroxidase was blocked with 5% H2O2 for 10 minutes. Sections were then incubated with primary antibodies against HER2 (as per different protocol) and immunoperoxidase reaction was performed according to procedure. The slides were then counterstained with hematoxylin and coverslipped.
Fluorescence In Situ Hybridization
HER2 copy number was investigated by FISH, using the PathVysion HER2 DNA Probe Kit (Vysis, Downers Grove, IL). A fluorescence microscope (Olympus BX61, Hicksville, NY) was used for the evaluation of HER2 gene copy number, and image capture was performed with CytoVision 3.93 software.
The manufacturer's instructions were modified to optimize the technique. In brief, sections were incubated at 56°C overnight, deparaffinized in three series of Histoclear, and incubated with two series of absolute ethanol. Slides were pretreated with Vysis 1N NaSCN “pre-treatment solution” in a water bath at 81°C for 28 minutes. Enzymatic digestion was carried out with 260 mg of Vysis Protease I for 23 minutes for endoscopic biopsies and 28 minutes for surgical specimens, both in a water bath at 37°C.
Sections were then dried at room temperature before incubation with 6 µl of HER2/CEP17 probe mix. The slides and probe were then denatured at 73°C for 5 minutes and hybridized at 37°C overnight in a Thermobrite. On the second day, the sections were washed with Vysis post-hybridization wash buffer 1 (0.4x SSC/0.3% NP-40) at 73°C for 2 minutes in a water bath and then washed with post-hybridization wash buffer 2 (2x SSC/0.1% NP-40) for 5 minutes at room temperature. Each section was dried at room temperature before using 6 ml of fluorescence 4,6-diamino-2-phenylindole as nuclear counterstain mounting medium.
FISH analysis was performed on one sample of all surgical resections and on all biopsy samples. In IHC-positive cases, the sample with the highest IHC score was chosen. All samples were evaluated with FISH blindly to IHC score.
Immunohistochemical Evaluation
The new scoring system for HER2 assessment in gastric cancer was used [15,20,29]. Complete staining of cell membrane was not required for defining cell positivity, but also incomplete, generally basolateral, staining was accepted (Figure 1, A–C). This scoring system is slightly different if applied to biopsy or surgical specimens and criteria are given as follows:
Figure 1.
HER2 IHC scores (PATHWAY HER2/neu 4B5) in biopsy (A–C) and surgical specimens (D–F) and FISH analysis in surgical specimens (G–I) of gastric/gastroesophageal cancer. (A) IHC score 1+—barely perceptible at low magnification (original magnification, x20). (B) IHC score 2+—faint at low magnification (original magnification, x20). (C) IHC score 3+—easily recognizable at low magnification (original magnification, x10). (D) IHC score 1+ (original magnification, x40). (E) IHC score 2+ (original magnification, x40). (F) IHC score 3+ (original magnification, x40). (G) No amplification by FISH. (H) LGA by FISH. (I) HGA by FISH.
Biopsy sample criteria—0, no reactivity in any tumor cell; 1+, faint/barely perceptible membranous reactivity in at least a cluster of greater than or equal to five cells; 2+, weak to moderate complete or basolateral membranous reactivity in at least a cluster of greater than or equal to five cells; 3+, strong complete or basolateral membranous reactivity in at least a cluster of greater than or equal to five cells.
Surgical specimen criteria—0, no reactivity or membranous reactivity in less than 10% of cells; 1+, faint/barely perceptible membranous reactivity in ≥10% of cells; 2+, weak to moderate complete or basolateral membranous reactivity in ≥10% of tumor cells; 3+, strong complete or basolateral membranous reactivity in ≥10% of tumor cells.
IHC was jointly evaluated by four expert gastrointestinal (GI) pathologists (L.M., M.F., F.G., and P.C.), and any discrepancies were resolved by consensus. During evaluation, the area with the highest IHC positivity in the invasive component was circled so as to facilitate the identification of the area for FISH analysis.
During IHC evaluation, intratumor heterogeneity was also described in surgical samples only and cases were considered homogenous if overexpression was found in more than two thirds of neoplastic cells [22].
FISH Analysis
All cases were evaluated in a blinded fashion by M.C. following the American Society of Clinical Oncology/College of American Pathologists guidelines [17]. Twenty non-overlapping nuclei were counted, excluding from analysis stromal and inflammatory cells. Cases were considered not amplified if the HER2/CEP17 ratio was lower than 2 and amplified if the HER2/CEP17 ratio was equal or higher than 2 as previously described [29]. Borderline cases (HER2/CEP17 ratio between 1.8 and 2.2) were reviewed by L.M. by counting a further 20 tumor nuclei and then assigned to either the amplified or non-amplified category. Amplified cases were further subdivided into cases with low-grade amplification (LGA: HER2/CEP17 ratio between 2 and 4) and cases with high-grade amplification (HGA: HER2/CEP17 ratio >4) (Figure 1, D–F).
Statistical Analysis
Differences of HER2 overexpression in tumors with diverse histologic parameters were analyzed by using the χ2 test.
Differences and correlations between groups were tested with the modified Kruskal-Wallis non-parametric test for trend. The k coefficient was used to check the level of agreement between samples of HER2 staining obtained with 4B5 versus CB11 antibodies and HER2 status established by IHC or FISH. The k values over 0.7 were assumed to indicate a very good agreement. P < .05 were considered statistically significant. The statistical analysis was performed using STATA software (Stata Corporation, College Station, TX).
Fisher exact test and Mann-Whitney U test were used to analyze patient and tumor characteristics according to HER2 IHC and FISH status, as specified in Table 3.
Table 3.
Patient and Tumor Characteristics Stratified by HER2 Status Tested by FISH and IHC (PATHWAY HER2/neu 4B5) in Surgical Specimens.
| Clinicopathologic Variable | HER2 FISH- | HER2 FISH+ | P | IIC 0/1+ | IIC 2+/3+ | P |
| Male sex | 52/76 (68) | 23/27 (85) | .051* | 44/66 (67) | 31/37 (84) | .032* |
| Mean age ± SD, years (n = 103) | 69.5 ± 11.2 | 69.5 ± 10.6 | .409† | 70.1 ± 10.2 | 68.5 ± 12.3 | .682† |
| Tumor location | .236* | .162* | ||||
| Distal stomach | 35/76 (46) | 10/27 (37) | 29/66 (44) | 16/37 (43) | ||
| Proximal stomach | 4/76 (5) | 1/27 (4) | 3/66 (5) | 2/37 (6) | ||
| GEJ/distal esophagus | 37/76 (49) | 16/27 (59) | 34/66 (51) | 19/37 (51) | ||
| Mean tumor size ± SD, cm | 5.8 ± 3.2 | 4.7 ± 1.8 | .164† | 5.8 ± 3.2 | 4.9 ± 2.1 | .157† |
| Lauren histotype | .038* | .028* | ||||
| Intestinal | 61/76 (80) | 26/27 (96) | 52/66 (79) | 35/37 (95) | ||
| Diffuse | 15/76 (20) | 1/27 (4) | 14/66 (21) | 2/37 (5) | ||
| Ming histotype | .658* | .834* | ||||
| Expanding | 32/76 (42) | 10/27 (37) | 26/66 (39) | 16/37 (43) | ||
| Infiltrative | 44/76 (58) | 17/27 (63) | 40/66 (61) | 21/37 (57) | ||
| Tumor grade | .059* | .131* | ||||
| Low (G1 or G2) | 45/76 (59) | 22/27 (81) | 39/66 (59) | 28/37 (76) | ||
| High (G3) | 31/76 (41) | 5/27 (19) | 27/66 (41) | 9/37 (24) | ||
| Pathologic T stage | .618* | .115* | ||||
| T1 | 6/76 (8) | 4/27 (15) | 5/66 (8) | 5/37 (14) | ||
| T2 | 14/76 (18) | 5/27 (18.5) | 10/66 (15) | 9/37 (24) | ||
| T3 | 30/76 (40) | 13/27 (48) | 29/66 (44) | 14/37 (38) | ||
| T4 | 26/76 (34) | 5/27 (18.5) | 22/66 (33) | 9/37 (24) | ||
| Pathologic N stage‡ | .806* | .821* | ||||
| N0 | 21/73 (29) | 9/27 (33) | 18/63 (29) | 12/37 (32) | ||
| N1, N2, or N3 | 52/73 (71) | 18/27 (67) | 45/63 (71) | 25/37 (68) | ||
| Pathologic M stage, M1 | 8/73 (11) | 2/27 (7) | .729* | 7/63 (11) | 3/37 (8) | 1* |
| Stage group‡ | 1* | .837* | ||||
| I | 11/73 (15) | 7/27 (26) | 9/63 (14) | 9/37 (24) | ||
| II | 20/73 (27) | 4/27 (15) | 18/63 (29) | 6/37 (16) | ||
| III | 38/73 (52) | 13/27 (48) | 32/63 (51) | 19/37 (52) | ||
| IV | 4/73 (6) | 3/27 (11) | 4/63 (6) | 3/37 (8) |
IIC, IHC score; GEJ, gastroesophageal junction.
Data are given as number/total (percentage).
Fisher exact test.
Mann-Whitney U test.
Missing data are because of lymph node absence in the surgical specimen (pNx).
Results
Comparison between Different Anti-HER2 Antibodies (4B5 and CB11)
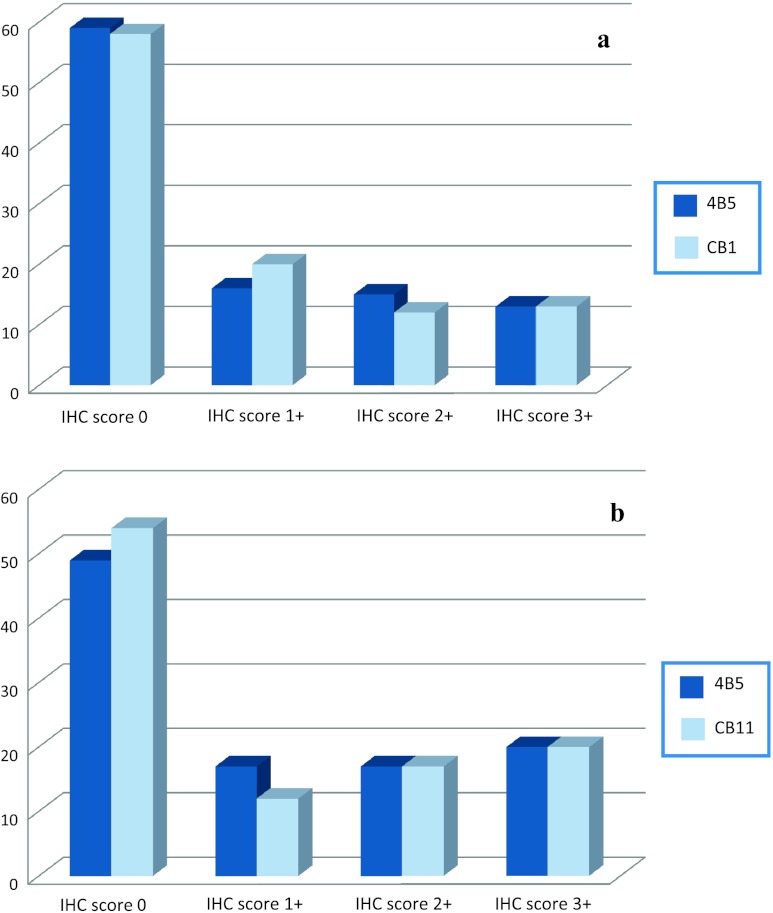
Excellent agreement between the PATHWAY HER2/neu (4B5) and the Oracle HER2 Bond IHC system (CB11) methods (see Figure 2) was observed both in biopsies (agreement 90.3%; k = 0.766; P < .001) and in surgical samples (agreement 90.3%; k = 0.815; P < .001).
Figure 2.
HER2 status tested by IHC (using two different antibodies) in biopsy (A) and surgical material (B). The y-axis corresponds to the number of cases. Note: 4B5, Ventana PATHWAY HER2/neu; CB11, Menarini Oracle HER2 Bond.
Because of the excellent agreement between antibodies, and the already published data concerning the use of 4B5 in gastric cancer [30], only 4B5 anti-HER2 antibody was considered for all further statistical analyses.
With both methods, no or only cytoplasmic staining in normal or metaplastic epithelia, adjacent to neoplasia, was observed; the latter was disregarded as non-specific. It must be mentioned that this type of staining did not create problems in the assessment of cell membrane signal.
Comparison of HER2 Status between Biopsy and Surgical Samples
Comparison of HER2 status between biopsy and surgical samples was performed with both IHC and FISH analysis (see Tables 1 and 2).
Table 1.
Concordance of HER2 Status Tested by IHC (PATHWAY HER2/neu 4B5) in Biopsy and Surgical Samples.
| Biopsy Samples | Surgical Samples | NPV (%) | PPV (%) | ||
| IHC 0 to 1+ | IHC 2+ to 3+ | Total | |||
| IHC 0 to 1+ | 60 | 15 | 75 | 80 | - |
| IHC 2+ to 3+ | 6 | 22 | 28 | - | 78.6 |
| Total | 66 | 37 | 103 | ||
| Concordance rate (%) | 90.9 | 59.5 | 80% | ||
IHC, IHC staining performed with PATHWAY HER2/neu (4B5); NPV, negative predictive value; PPV, positive predictive value.
Table 2.
Concordance of HER2 Status Tested by FISH in Biopsy and Surgical Samples.
| Biopsy Samples | Surgical Samples | NPV (%) | PPV (%) | ||
| FISH NA | FISH A | Total | |||
| FISH NA | 75 | 4 | 79 | 94.9 | - |
| FISH A | 1 | 23 | 24 | - | 95.8 |
| Total | 76 | 27 | 103 | ||
| Concordance rate (%) | 98.7 | 85.2 | 95% | ||
FISH NA, percentage of cases that were not amplified on FISH; FISH A, percentage of cases that were amplified on FISH; NPV, negative predictive value; PPV, positive predictive value.
The concordance rate between IHC and FISH was 99% in surgical samples and 89.9% in biopsies after exclusion of the IHC score 2+ group, which is known to be “equivocal” [17].
Eighty percent of cases had complete concordance between IHC results on biopsy and surgical samples (60 IHC 0/1+ cases and 22 IHC 2/3+ cases). Discordance was seen in 20% of cases and specifically related to 15 cases with IHC 0/1+ on biopsy and IHC 2/3+ on surgical sample, whereas six cases showed the opposite.
Ninety-five percent of cases analyzed had complete concordance between FISH results on biopsy and surgical samples (71.5% not amplified and 23.5% amplified). Conversely, 5% (five cases) showed discordance in FISH results between biopsy and surgical specimens. In detail, four cases not amplified on biopsy showed amplification (three LGA and one HGA) on surgical samples, whereas one case showed the opposite (HGA on biopsy and no amplification on surgical sample).
Comprehensive Comparison of IHC and FISH Data between Biopsy and Surgical Samples
A comprehensive comparison was performed including all data on IHC and FISH analysis in both types of samples, biopsy and surgical.
In 75 cases (73%), the combined results for IHC and FISH on biopsy material were on the whole negative (FISH not amplified and IHC score 0, 1+, 2+) and all were confirmed on surgical material. On the basis of these results, such patients would not have been candidates for anti-HER2 treatment.
However, 16 cases (16%) were identified in biopsy samples as being IHC score 3+ (12 cases) or IHC score 2+ and FISH amplified (4 cases) and confirmed as such on surgical material. These patients would have been candidates for treatment had we considered biopsy material only.
- The remaining 12 cases (11%) showed variable inconsistencies between results on endoscopic biopsies and surgical samples or between IHC and FISH and were classified as follows:
- Seven cases, IHC score 0 (three cases) or score 1+ (four cases), were FISH amplified on biopsy material and this finding was confirmed on the surgical specimen that showed both IHC positivity (2+, 3+) and amplification by FISH.
- Three cases were FISH non-amplified on biopsy samples and IHC staining ranged between 0 and score 2+; however, all three cases were amplified on surgical samples (all LGA) and IHC positive (score 2+ or 3+).
- One case was IHC score 0 but LGA by FISH on biopsy material; similarly, surgical sample evaluation showed LGA by FISH in the absence of immunoreactivity with IHC.
- One case was FISH amplified (HGA) on biopsy material with IHC score of 3+; however, there was no immunoreactivity or FISH amplification on either block of the surgical specimen.
Intratumor Heterogeneity in HER2 Staining
In surgical samples, 49 cases were completely negative (score 0) for HER2. Of the 54 cases that showed immunostaining (IHC score 1+, 2+, or 3+), only 13 (24%) showed homogenous expression and all of these scored 3+. Given the known intratumor heterogeneity [19], differences in two separate surgical paraffin blocks were also checked. Complete correspondence between blocks was seen in 87 cases (84.5%) of which 66 were negative (scores 0 or 1+), 12 were highly positive (score 3+), and 9 scored 2+. In the remaining 16 cases (15.5%), comparison between blocks provided different results. Particularly, 11 (10.7%) cases resulted positive (scores 2+ or 3+) in one block and negative (scores 0 or 1+) in the other.
HER2 versus Clinicopathologic Variables
Clinicopathologic correlations are summarized in Table 3. No significant difference was noted in HER2 overexpression or amplification when compared to tumor site, mean tumor size, differentiation, growth pattern according to Ming, or stage. Statistically significant differences were observed only for Lauren classification.
Discussion
In this study, we focused on the comparative evaluation of biopsy and surgical samples of GC and GEJC in the determination of HER2 status. The majority of previous studies have evaluated the predictive value of IHC compared to the gold standard FISH, but few authors have considered identifying the predictive value of biopsy material by using matched biopsy/surgical samples. The four major studies [19,22–24] on this topic, dating from 2006 to 2012, report a concordance rate between biopsy and surgical specimens ranging from 88.5% to 96.2% in series composed of matched samples (from 12 to 200 cases in different case series). These rates of concordance are on the whole similar to our study. However, several major methodological differences with our study must be highlighted: 1) only intestinal type gastric cancers or only gastric cancers were considered [19,24]; 2) the IHC evaluation score used had not yet been modified for gastric cancer [24]; 3) FISH analysis on matched biopsies was applied only to a subset of cases that had demonstrated amplification on the surgical specimens [24]; 4) no focus was made on the predictive role of biopsy in HER2 status evaluation [19,23].
In our study, matched biopsy/surgical samples show a better concordance rate for FISH amplification (95%) with respect to IHC (80%). The lower concordance rate in IHC is mostly dependent on a relevant number of patients (15 cases) who were underscored on biopsy samples (IHC score 0/1+) versus surgical sample (IHC score 2+/3+). For therapeutic purposes, these patients (with IHC score 0/1+) should not be tested further with FISH and are excluded from anti-HER2 therapy assignment. However, when evaluating their surgical specimens, they turned IHC score 2+/3+ and therefore would have undergone further evaluation by FISH (score 2+) or would have been treated directly (score 3+). The percentage of these cases that we can identify by applying FISH analysis on all biopsies corresponds to 8.5% of IHC score 0 and 25% of IHC score 1+: this latter value is not dissimilar to IHC score 2+ biopsies with amplification (33%). Similar results were also shown in the ToGA screening program, where 23% of IHC score 0/1+ and 26% of IHC score 2+ were amplified [31]. Whereas IHC evaluation has been modified in GC/GEJC [20], the flowchart to FISH analysis of IHC score 2+ cases remains unvaried with respect to breast cancer, in which 36% of IHC score 2 “equivocal” cases are FISH amplified [15,32]. However, ToGA trial [31] post-hoc analysis has shown that trastuzumab plus chemotherapy substantially improved overall survival in patients with high expression of HER2 (i.e., IHC score 2+ and amplification or IHC score 3+) compared with patients with low expression of HER2 protein (i.e., IHC score 0 or 1+ with FISH amplification). In this post-hoc analysis, cases were subdivided on the basis of IHC scores following the flowchart used for breast cancer (IHC score 0 or 1+ considered as “negative” and IHC score 2+ or 3+ considered as “positive”). Our findings on biopsy samples suggest that both IHC score 2+ and 1+ should be considered “equivocal” and both may benefit from FISH analysis. Indeed, further demonstration of the similarities between these two groups is that both show analogous types of amplification (three LGA and one HGA in both groups). Patients with IHC score 1+ and FISH amplification may not differ so substantially from patients with IHC score 2+ and FISH amplification in terms of therapy response. At the moment, this is still a gray zone that will need to be addressed by specifically designed prospective trials. Indeed, the different overall survivals demonstrated by ToGA trial for subgroups with low and high levels of HER2 could be explained by a leading effect of cases with IHC score 0 and IHC score 3+ on IHC score 1+ and 2+ cases. Conversely, when FISH was applied to IHC score 0/1+ surgical specimens, where a greater quantity of neoplastic cells are available, only 1 of 66 cases showed amplification (LGA).
Similarly to ToGA [31], which reports an 87.5% concordance rate between IHC and FISH on biopsies, we found 89.9% concordance rate. In contrast, concordance on surgical specimen was much higher (99%), similarly to results reported by Yang et al. [19]. If we exclude from the concordance evaluation on biopsies both IHC score 2+ and 1+, then the concordance rate increases to 93.2%. This further substantiates the concept that IHC score 1+ on biopsies should be considered as “equivocal.”
The most probable reason for discordance is intratumor heterogeneity of HER2 status in GC/GEJC [20,33,34]. Many studies have commented on this finding; however, few have given a precise definition. We evaluated heterogeneity as described by Lee et al. [23] and further investigated the differences in HER2 expression between different blocks of the same surgical specimen. In our study, only 24% of IHC-positive cases (that is, all IHC score 3+) showed fully homogenous expression. All other IHC-positive cases (score 1+ and score 2+) showed heterogeneous expression with leopard-spot positivity irrespective of site (superficial vs deep tumor part). These findings have also been described by Kim et al. [35]. Extensive biopsy sampling could minimize this problem. However, as underlined by Lee et al. [23], no formal recommendations on the number of endoscopic biopsies required for HER2 testing have been as yet proposed, but endoscopists should be encouraged to sample extensively especially in advanced GC/GEJC.
With regard to performance between different antibodies, we found no substantial differences between the PATHWAY HER2/neu (4B5) and the Oracle HER2 Bond IHC system (CB11). Similar findings have been reported by a recent paper, in which four different antibodies were compared [36], even though this group found a lower sensitivity for CB11 compared with the other tested antibodies.
In conclusion, the present study shows that the predictive value of IHC in biopsies with regard to HER2 assessment is high but probably dependent on HER2 heterogeneity in GC/GEJC. Our findings support the concept that FISH analysis should be considered for both IHC score 2+ and 1+ biopsy cases, and such an approach also solves the problem of no easy distinction between “faint” and “weak” staining in practice. Even with this recommendation, approximately 8% of cases will not be accurately predicted (both underestimation and overestimation) by biopsy evaluation.
Acknowledgments
We thank Alice Pernigotti for her contribution in selecting cases. We also thank Simona Pigozzi and Vincenza Guzzardo for their technical support in IHC and Margherita Colombara for her organizing support. M.F. is a paid consultant for Menarini Diagnostics S.r.l. for matters of diagnostic testing. The other authors have no competing interests to declare.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Ohtsu A. Chemotherapy for metastatic gastric cancer: past, present, and future. J Gastroenterol. 2008;43:256–264. doi: 10.1007/s00535-008-2177-6. [DOI] [PubMed] [Google Scholar]
- 3.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 4.Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27–39. doi: 10.1097/01.sla.0000149300.28588.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fassan M, Mastracci L, Grillo F, Zagonel V, Bruno S, Battaglia G, Pitto F, Nitti D, Celiento T, Zaninotto G, et al. Early HER2 dysregulation in gastric and esophageal carcinogenesis. Histopathology. 2012;61:769–776. doi: 10.1111/j.1365-2559.2012.04272.x. [DOI] [PubMed] [Google Scholar]
- 6.Normanno N, Bianco C, Strizzi L, Mancino M, Maiello MR, De Luca A, Caponigro F, Salomon DS. The ErbB receptors and their ligands in cancer: an overview. Current Drug Targets. 2005;6:243–257. doi: 10.2174/1389450053765879. [DOI] [PubMed] [Google Scholar]
- 7.Ross JS, Fletcher JA. HER-2/neu (c-erb-B2) gene and protein in breast cancer. Am J Clin Pathol. 1999;112:S53–S67. [PubMed] [Google Scholar]
- 8.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 9.Fukushige S, Matsubara K, Yoshida M, Sasaki M, Suzuki T, Semba K, Toyoshima K, Tamamoto T. Localization of a novel v-erbB-related gene, c-erb B-2, on human chromosome 17 and its amplification in a gastric cancer cell line. Mol Cell Biol. 1986;6:955–958. doi: 10.1128/mcb.6.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, Matsuda M, Sakaguchi T, Hirao T, Nakano H. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894–1902. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 11.Joergensen JT, Hersom M. HER2 as a prognostic marker in gastric cancer—a systematic analysis of data from the literature. J Cancer. 2012;3:137–144. doi: 10.7150/jca.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joergensen JT. Targeted HER2 treatment in advanced gastric cancer. Oncology. 2010;78:26–33. doi: 10.1159/000288295. [DOI] [PubMed] [Google Scholar]
- 13.Reichelt U, Duesedau P, Tsourlakis MCh, Quaas A, Link BC, Schurr PG, Kaifi JT, Gros SJ, Yekebas EF, Marx A, et al. Frequent homogeneous HER-2 amplification in primary and metastatic adenocarcinoma of the esophagus. Mod Pathol. 2007;20:120–129. doi: 10.1038/modpathol.3800712. [DOI] [PubMed] [Google Scholar]
- 14.Langer R, Von Rahden BH, Nahrig J, Von Weyhern C, Reiter R, Feith M, Stein HJ, Siewert JR, Höfler H, Sarbia M. Prognostic significance of expression patterns of c-erb B-2, p53, p16INK4A, p27KIP1, cyclin D1 and epidermal growth factor receptor in oesophageal adenocarcinoma: a tissue microarray study. J Clin Pathol. 2006;59:631–634. doi: 10.1136/jcp.2005.034298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 16.EMEA, author. European Medicines Agency Opinion. 2009. Available at: www.emea.europa.eu/pdfs/human/opinion/Herceptin_82246709en.pdf.
- 17.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 18.Sui W, Ou M, Chen J, Wan Y, Peng H, Qi M, Huang H, Dai Y. Comparison of immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) assessment for HER-2 status in breast cancer. World J Surg Oncol. 2009;7:83. doi: 10.1186/1477-7819-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Luo H, Li Y, Li J, Cai Z, Su X, Dai D, Du W, Chen T, Chen M. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem Biophys. 2012;62:221–228. doi: 10.1007/s12013-011-9286-1. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, Ochiai A, Ruschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 21.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 22.Yan B, Yau EX, Bte Omar SS, Ong CW, Pang B, Yeoh KG, Salto-Tellez M. A study of HER2 gene amplification and protein expression in gastric cancer. J Clin Pathol. 2010;63:839–842. doi: 10.1136/jcp.2010.076570. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, de Boer WB, Fermoyle S, Platten M, Kumarasinghe MP. Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology. 2011;59:832–840. doi: 10.1111/j.1365-2559.2011.04017.x. [DOI] [PubMed] [Google Scholar]
- 24.Yano T, Doi T, Ohtsu A, Boku N, Hashizume K, Nakanishi M, Ochiai A. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep. 2006;15:65–71. [PubMed] [Google Scholar]
- 25.Bosman F, Carniero F, Hruban R, Thiese N. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC Lyon Press; 2010. [Google Scholar]
- 26.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 27.Ming SC. Gastric carcinoma. A pathobiological classification. Cancer. 1977;39:2475–2485. doi: 10.1002/1097-0142(197706)39:6<2475::aid-cncr2820390626>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 28.Sobin L, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours. 7th ed. Oxford, UK: Blackwell Publishing Ltd; 2009. [Google Scholar]
- 29.Ruschoff J, Dietel M, Baretton G, Arbogast S, Walch A, Monges G, Chenard MP, Penault-Llorca F, Nagelmeier I, Schlake W, et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010;457:299–307. doi: 10.1007/s00428-010-0952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boers JE, Meeuwissen H, Methorst N. HER2 status in gastro-oesophageal adenocarcinomas assessed by two rabbit monoclonal antibodies (SP3 and 4B5) and two in situ hybridization methods (FISH and SISH) Histopathology. 2011;58:383–394. doi: 10.1111/j.1365-2559.2011.03760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bang YH, Chung H, Xu J, Lordick F, Sawaki A, Al-Sakaff N, Lipatov O, See C, Ruschoff J, Van Cutsem E. Pathological features of advanced gastric cancer (GC): relationship to human epidermal growth factor receptor 2 (HER2) positivity in the global screening program of the ToGA trial. J Clin Oncol. 2009;27:15s. [Google Scholar]
- 32.Ridolfi RL, Jamehdor MR, Arber JM. HER-2/neu testing in breast carcinoma: a combined immunohistochemical and fluorescence in situ hybridization approach. Mod Pathol. 2000;13:866–873. doi: 10.1038/modpathol.3880154. [DOI] [PubMed] [Google Scholar]
- 33.Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W. HER2 expression in gastric cancer: rare, heterogeneous and of no prognostic value—conclusions from 924 cases of two independent series. Cell Oncol. 2010;32:57–65. doi: 10.3233/CLO-2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim MA, Lee HJ, Yang HK, Bang YJ, Kim WH. Heterogeneous amplification of ERBB2 in primary lesions is responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology. 2011;59:822–831. doi: 10.1111/j.1365-2559.2011.04012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KC, Koh YW, Chang HM, Kim TH, Yook JH, Kim BS, Jang SJ, Park YS. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18:2833–2840. doi: 10.1245/s10434-011-1695-2. [DOI] [PubMed] [Google Scholar]
- 36.Cho EY, Srivastava A, Park K, Kim J, Lee MH, Do I, Lee J, Kim KM, Sohn TS, Kang WK, et al. Comparison of four immunohistochemical tests and FISH for measuring HER2 expression in gastric carcinomas. Pathology. 2012;44:216–220. doi: 10.1097/PAT.0b013e3283513e8b. [DOI] [PubMed] [Google Scholar]