Abstract
PURPOSE: Ultrasound elastography is a new imaging technique that can be used to assess tissue stiffness. The aim of this study was to investigate the potential of ultrasound elastography for monitoring treatment response of locally advanced breast cancer patients undergoing neoadjuvant therapy. METHODS: Fifteen women receiving neoadjuvant chemotherapy had the affected breast scanned before, 1, 4, and 8 weeks following therapy initiation, and then before surgery. Changes in elastographic parameters related to tissue biomechanical properties were then determined and compared to clinical and pathologic tumor response after mastectomy. RESULTS: Patients who responded to therapy demonstrated a significant decrease (P < .05) in strain ratios and strain differences 4 weeks after treatment initiation compared to non-responding patients. Mean strain ratio and mean strain difference for responders was 81 ± 3% and 1 ± 17% for static regions of interest (ROIs) and 81 ± 3% and 6 ± 18% for dynamic ROIs, respectively. In contrast, these parameters were 102±2%, 110±17%, 101±4%, and 109±30% for non-responding patients, respectively. Strain ratio using static ROIs was found to be the best predictor of treatment response, with 100% sensitivity and 100% specificity obtained 4 weeks after starting treatment. CONCLUSIONS: These results suggest that ultrasound elastography can be potentially used as an early predictor of tumor therapy response in breast cancer patients.
Introduction
Breast cancer is the most frequent type of cancer diagnosed in women, excluding cancer of the skin. An estimated number of 226,870 new cases of invasive breast cancer are expected to occur among women in the United States during 2012 [1]. Approximately 5% to 20% of the cases diagnosed each year will present with locally advanced breast cancer (LABC) [2,3]. LABC is usually defined as stage III or inoperable disease. It typically includes tumors >5 cm and/or involving the skin or chest wall, with or without clinical nodal involvement. LABC is frequently characterized by a much poorer prognosis compared to early-stage breast cancer. In addition to its high rate of its loco-regional recurrence (10–20%), only 55% of LABC patients survive at 5 years because of the high risk for metastatic spread [2].
Current methods for LABC treatment include neoadjuvant chemotherapy followed by mastectomy with axillary nodal clearance. The surgery can be also followed by radiation therapy and possibly Herceptin and/or hormonal manipulation, if indicated [4,5]. Several investigations have reported the importance of clinical/pathologic complete response to neoadjuvant chemotherapy as a factor in improved treatment outcomes, resulting in survival rates up to 70% [6–8]. However, this prognostic factor is assessed at the time of surgery, where the window for optimizing a neoadjuvant treatment is closed. Standard clinical measures based on anatomic information from physical assessment, mammography, and conventional B-mode ultrasound are often unable to objectively evaluate treatment response early during the treatment period since functional changes related to microscopically evident tumor death may precede macroscopic anatomic changes. Recent studies have highlighted the importance of early detection of patients refractory to neoadjuvant chemotherapy, as it has been demonstrated that early salvage treatment for chemotherapy-resistant tumors with radiation and surgery can result in a survival rate of 46% at 5 years [9]. Therefore, imaging for early probing of the extent of therapy response is crucial [10]. An objective low-cost functional imaging method capable of assessing tumor response to guide the personalization and optimization of neoadjuvant therapy would be very useful and permit therapy changes to be made as needed when faced with a lack of tumor response.
Ultrasound elastography is a recently developed dynamic imaging technique that has been established as a promising modality to identify relative tissue stiffness by measuring the degree of strain-related distortion under the application of an external force [11–16]. In this context, elastography consists of applying quasi-static pressure on the examined tissue surface periodically and estimating the induced strain distribution by tracking tissue motion. For this purpose, raw ultrasound data consisting of radiofrequency data are acquired continuously, and elastograms are generated by estimating the strain evident between sequential frames [12,17]. Ultrasound elastography inherits the advantages of ultrasound imaging such as low cost, rapid imaging speed, portability, and high resolution and is finding clinical use.
Ultrasound elastography has been demonstrated as capable of detecting tumors and distinguishing between different types of abnormalities, e.g., benign versus malignant lesions, in breast tissue [14,18], thyroid tissue [13], prostate [19,20], and lymph nodes [21]. One major advantage of ultrasound elastography over other conventional contrast-enhanced imaging modalities is that no contrast agent injection is needed in this modality. This is because of the fact that the differentiable elasticity and stiffness, as main sources of image contrast, are caused by changes in the physical properties of the examined tissue. Ultrasound elastography has also been applied for treatment monitoring of ablative therapies where heating tissue induces a denaturation of proteins, which in turn elevates the stiffness of ablated tissue [20,22,23]. Similarly, other methods of cell death induction such as chemotherapy can substantially alter the biomechanical properties of the malignant tissues during a course of treatment. This is mainly due to the fact that tumor formation and its degeneration in response to treatment exhibits considerable interactions, e.g., fibrosis and inflammation, with stromal cells [24,25] that may change the biomechanical properties with stromal cells. On this basis, ultrasound elastography was investigated here as a method that may potentially provide large contrast between treatment responding and non-responding malignant tissues, early (a few weeks) following the start of neoadjuvant chemotherapy.
This study investigated for the first time, the application of ultrasound elastography in monitoring chemotherapy response for cancer treatment. A clinical evaluation was carried out to evaluate the efficacy of ultrasound elastography parameters for distinguishing between clinically and pathologically responding and non-responding patients, early after treatment initiation. Fifteen patients with LABC received neoadjuvant chemotherapy, as “up-front“ treatment, followed by mastectomy and axillary nodal clearance. Data collection consisted of acquiring tumor ultrasound images and elastography data before neoadjuvant treatment onset and then at four times during treatment (weeks 1, 4, and 8, and preoperatively). Clinical examinations were also conducted at each follow-up time, where the tumor size was measured. Pathology examinations were performed on resected specimens after mastectomy using three-dimensional whole-mount histopathology [26], and data on size, grade, histologic subtype, and tumor response were recorded. Relative changes in the ratio of tumor to normal tissue stiffness were calculated and monitored following treatment onset, compared to the pretreatment data. Obtained results demonstrate the potential of ultrasound elastography techniques for predicting chemotherapy response, as early as 4 weeks after treatment initiation.
Materials and Methods
Study Protocol
The study included 15 patients with LABC and was carried out in accordance with research ethics guidelines. Before treatment administration, all patients were investigated using standard clinical imaging and disease biopsied to confirm a cancer diagnosis. Ultrasound B-mode and elastography data were acquired from the affected breast at the following times: before treatment, 1 week, 4 weeks, and 8 weeks following the initiation of treatment, and before surgery. Clinical examinations were also carried out before each imaging session, in addition to regular patient visits. As part of clinical care, tumor magnetic resonance imaging (MRI) using a 1.0-Tesla device (GE Healthcare, Waukesha, WI) was conducted at the time of diagnosis and immediately before surgery to measure tumor size. Following surgery, patient mastectomy specimens were examined by a board-certified pathologist using wholemount [26] 5 in. x 7 in. pathology slides digitized using a confocal scanner (TISSUEscope; Huron Technologies, Waterloo, ON) at 2-µm resolution. Information regarding tumor grade, histologic subtype, size, and treatment response was recorded. Patients were categorized as having either a poor pathologic response or a good response. As defined in previous studies [27–29], a patient was considered a good responder if they had a 50% or more decrease in the tumor size in comparison to pretreatment size along with a tumor cellularity decrease. A poor responder meant a <50% reduction in tumor size or unchanged disease or an enlargement in tumor size compared to the size before treatment.
Instrument
Conventional B-Mode and elastography ultrasound data were acquired using a Sonix RP System (Ultrasonix, Vancouver, Canada) using a 6-cm-wide L14-5 transducer pulsed at 10 MHz at a rate of 12 frames per second. All the ultrasound data in this study were collected by the same sonographer following standardized protocols for data acquisition. For each patient, the transducer focus was set at a tumor center depth and kept consistent throughout the study. Ultrasound B-mode and elastography scan planes of the tumor were acquired in 1-cm increments under the guidance of a physician. On average, five scan planes were acquired at a given evaluation. The resulting elastography images were displayed using a 256-color map of strain using a scale from red (highest strain, soft) to blue (low strain, hard). Raw data were saved digitally for data analysis.
Data Analysis and Statistics
For each scan, the tumor region was identified using B-mode images under the guidance of a physician and two types of tumor region of interest (ROI) were defined: static and dynamic. Static ROIs were chosen to be rectangular in shape, encompassing the majority of the tumor area in cross-section images. The size of these ROIs remained constant throughout the study for a given patient. Dynamic ROIs were selected to conform to the tumor by using a low-pass filtered threshold method to detect the tumor edge in the pretreatment scan and they were adjusted for subsequent scans depending on the shape and size of the tumor. For each scan, static and dynamic B-mode ROIs were also chosen for normal tissue as a comparison. These were located at the same depth as their corresponding tumor ROIs. B-mode ROIs were co-registered with the elastography maps. Mean strains within the tumor ROIs and its surrounding normal tissue ROIs were calculated by averaging data in selected areas for static and dynamic ROI analyses. Strain ratios were obtained by dividing the normal tissue strain by the tumor strain. These ratios were subsequently averaged for each patient's tumor at a given assessment time to obtain the strain ratio of each scan. Similarity, strain differences (by subtraction) between the tumor and its surrounding normal tissue were obtained for each scan.
Changes in the values from baseline (pretreatment) between responders and non-responders at each time point for each elastography parameter were compared independently. Statistical analysis using a t test (two-sided, 95% confidence) was carried out to assess if patients showing statistically significant changes in elastography parameters at weeks 1 and 4 correlated to patient population demonstrating treatment response. Discriminant analysis (PASW Statistics 18; SPSS, Inc, Chicago, IL) was used to determine which elastography parameter discriminate between responders and non-responders at weeks 1 and 4. The changes in the values of strain ratio and strain difference were used as predictors in the analysis, which examined the best separation between the two groups. Sensitivity and specificity were calculated to measure the performance of the classification method in terms of discriminating responders from non-responders. These were calculated for both static and dynamic ROI methods of data analysis.
Results
Patient and Tumor Characteristics
Patient physical, tumor, and treatment characteristics are given in Table 1. The mean patient age was 45 years (SD = 7; range, 34–55) and 10 patients were premenopausal. The average maximum tumor size was 7.3 cm (SD = 3.0; range, 3–13). Fourteen patients had invasive ductal carcinoma, one had metaplastic carcinoma, eight patients were estrogen (ER)/progestron (PR) positive, seven were Her-2-neu positive, three patients had grade 2 tumors, and six had grade 3 tumors. Patients had a variety of neoadjuvant treatment plans with 80% of patients receiving combined anthracycline and taxane-based chemotherapy. One patient received docetaxel, carboplatin, and trastuzumab, and another received adriamycin and cytoxan with cisplatinum and gemcitabine platinum chemotherapy. One patient received docetaxel and trastuzumab chemotherapy.
Table 1.
Patient Characteristics.
| Patient No. | Age | Menopausal Status | Pretreatment Tumor Dimension (AP x ML x SI) in cm | Histology | Grade | ER/PR | Her-2-neu | Neoadjuvant Treatment |
| 1 | 55 | N/A | 5.4 x 5 x 2.3 | Ductal | N/A | - | + | FEC + paclitaxel, trastuzumab |
| 2 | 53 | N/A | 7.4 x 7 | Ductal | 2 | + | + | Epirubicin, docetaxel |
| 3 | 41 | Premenopausal | 4 | Ductal | 3 | + | + | Docetaxel, carboplatin, trastuzumab |
| 4 | 46 | N/A | 7 x 8 | Ductal | 3 | - | - | AC + paclitaxel |
| 5 | 33 | Premenopausal | 5.4 x 5 x 8 | Ductal | N/A | + | + | AC + docetaxel, paclitaxel, trastuzumab |
| 6 | 48 | Postmenopausal | 4.9 x 4.9 x 4.1 and 3.2 x 1.3 x 2.9 | Ductal | 2 | + | - | AC + docetaxel |
| 7 | 36 | Premenopausal | 4.4 x 3.9 x 5.8 | Ductal | N/A | + | - | AC + paclitaxel |
| 8 | 40 | Premenopausal | 4.4 x 3.4 | Ductal | 3 | - | - | AC + paclitaxel |
| 9 | 38 | Premenopausal | 7.5 x 4.9 x 9.2 | Ductal | 2 | + | - | AC + paclitaxel |
| 10 | 53 | N/A | 8.4 x 9.4 x 12.7 | Metaplastic | 3 | - | - | AC + cisplatinum, gemcitabine platinum |
| 11 | 50 | Premenopausal | 13 x 11 | Ductal | N/A | - | - | AC + paclitaxel |
| 12 | 49 | Premenopausal | 7.1 x 5.5 x 8.9 | Ductal | N/A | - | + | Docetaxel, trastuzumab |
| 13 | 40 | Premenopausal | 3 x 2.4 x 3 | Ductal | 3 | + | + | AC + paclitaxel, trastuzumab |
| 14 | 52 | Premenopausal | 4.1 x 3 x 2.5 | Ductal | N/A | + | - | AC + paclitaxel, docetaxel |
| 15 | 47 | Premenopausal | 6.3 x 4.1 x 7.4 | Ductal | 3 | - | + | AC + paclitaxel, trastuzumab |
AC indicates adriamycin and cytoxan; FEC, 5-fluorouracil, epirubicin and cyclophosphamide.
Clinical and Pathologic Assessment of Tumor Response
LABC pathology specimens were examined in the same manner by a board-certified pathologist (J.Z.) to determine tumor response (Table 2). Patients 1, 5, 6, 8, 11, 12, 13, 14, and 15 were classified as good responders because of the absence of tumor or presence of only minimal invasive disease. Patients 3, 4, 7, 9, and 10 had a poor pathologic response. Their tumor sizes slightly changed compared to their pretreatment size. Patient 2 was an exceptional case in that pretreatment MRI indicated an invasive ductal carcinoma measuring 7.4 [anterior-posterior (AP)] x 7 [medial-lateral (ML)] cm. After treatment, the pathologic tumor size slightly decreased and measured 7 (AP) x 5 (ML) x 3 [superior-inferior (SI)]. However, the residual mass was composed of very large pools of extracellular mucin. Tumor cells were very infrequent on examination, and thus tumor cellularity was very low. Although the remaining pathologic tumor size was large, the appearance of decreased tumor cellularity indicated a good response.
Table 2.
Patient Pathologic Response Results.
| Patient No. | Posttreatment Tumor Dimensions (AP x ML x SI) in cm | Notes | Pathologic Response |
| 1 | N/A | Complete pathological response | Good |
| 2 | 7 x 5 x 3 | Carcinoma with mucinous features; very low cellularity | Good |
| 3 | 2.7 x 2.5 x 2.4 | Tumor cellularity remains very high | Poor |
| 4 | 6.4 x 3.5 x 3 | Large tumor volume remaining | Poor |
| 5 | N/A | Complete pathological response | Good |
| 6 | 1.4 x 1 x 1 | Small volume of invasive tumor remaining | Good |
| 7 | 11.4 | Extensive residual disease | Poor |
| 8 | N/A | No residual carcinoma | Good |
| 9 | 6.5 x 3 x 7.3 | Invasive ductal carcinoma remaining | Poor |
| 10 | all the breast | Residual tumor took up all the breast; no response | Poor |
| 11 | 4 | Good response | Good |
| 12 | 2 x 1.5 x 1 | Complete response with only in situ disease | Good |
| 13 | N/A | No residual invasive carcinoma | Good |
| 14 | N/A | No residual invasive carcinoma | Good |
| 15 | N/A | No residual invasive carcinoma | Good |
Ultrasound Elastography Assessment of Treatment Response
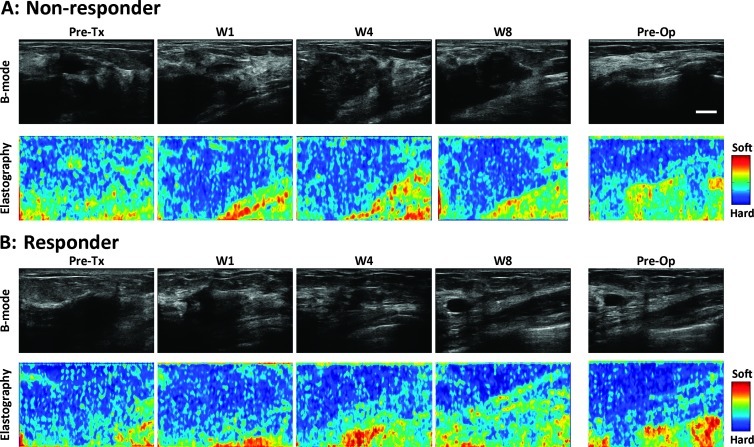
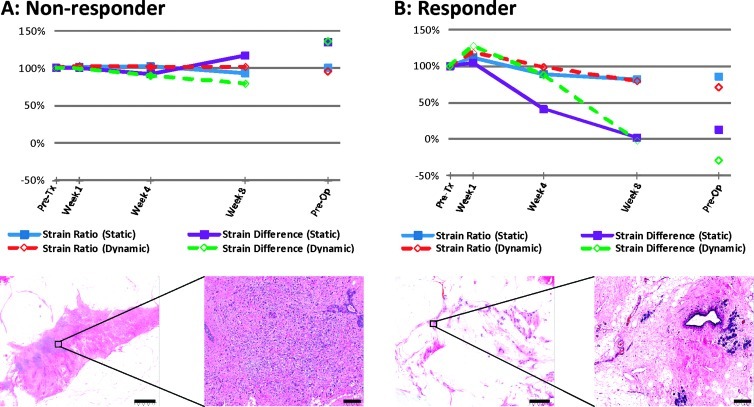
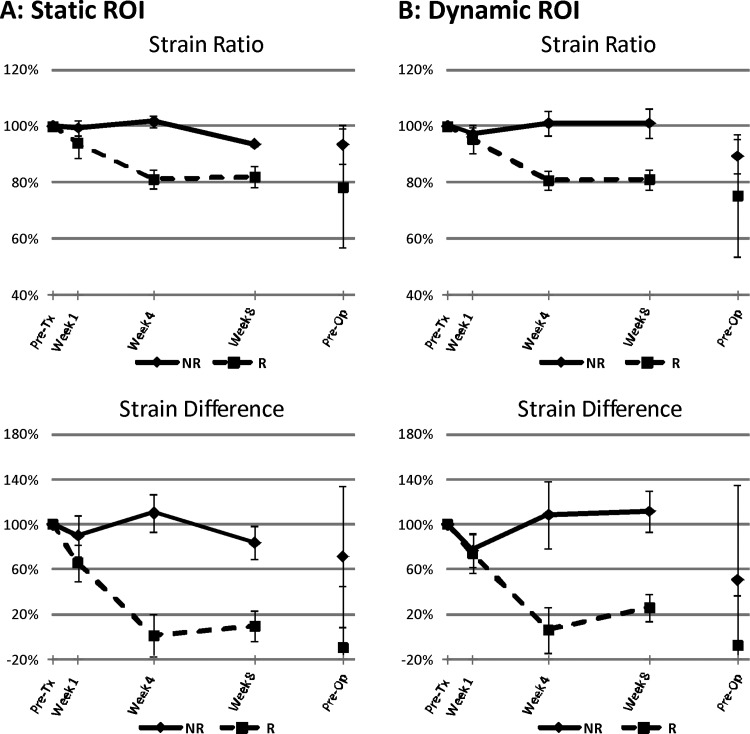
Tumors were easily identified from ROI placement on the basis of their hypoechogenicity and lack of normal typical breast structure. Before starting treatment, responders and non-responders demonstrated similar tumor stiffness, with average strain ratios of 1.29 ± 0.04 and 1.26 ± 0.11, respectively. Non-responders typically revealed no change in tumor elasticity during treatment for several weeks (average strain ratios of 1.25 ± 0.11, 1.28 ± 0.09, and 1.23 ± 0.11 at weeks 1, 4, and 8, respectively). In contrast, responders indicated visibly detectable changes in tumor stiffness (average strain ratios of 1.19 ± 0.04, 1.04 ± 0.04, and 1.04 ± 0.05 at weeks 1, 4, and 8, respectively). Non-tumor breast tissue showed no changes in elasticity during treatment. Representative ultrasound B-mode and elastography images for a non-responder and a responder acquired before treatment, at week 1, week 4, week 8, and preoperatively are presented in Figure 1. Representative quantitative data for the same patients and whole-mount histopathology are presented in Figure 2. In the non-responding patient's pathology, a large mass of residual carcinoma was present at low and high magnifications. In the responding patient, minimal residual disease was detected with areas demonstrating low cellularity. Figure 3 demonstrates average changes in strain ratios and strain differences between non-responders and responders using both static and dynamic ROIs. Table 3 presents averaged strain ratios and averaged strain differences 1 and 4 weeks after treatment initiation. Averaged strain ratios and strain differences for non-responders and responders using static ROIs were extremely (P < .001) and highly (P = .001) significant 4 weeks after treatment initiation, respectively. There was no significance at 1 week after the start of treatment. Non-responders and responders were found to be highly significantly different 4 weeks after treatment initiation for averaged strain ratios and strain differences using dynamic ROIs (P = .002 and P = .008), respectively. There was no significance at 1 week after the start of treatment. Sensitivity and specificity analysis results are also presented in Table 3. The best predictor of treatment response was strain ratio using static ROIs, where 100% for both sensitivity and specificity were achieved 4 weeks after treatment initiation. This was followed by the strain difference using static ROIs and the strain ratio using dynamic ROIs (sensitivity, 89%; specificity, 100%). Strain difference using dynamic ROIs was also found to be a good predictor of therapeutic response (sensitivity, 89%; specificity, 80%) 4 weeks after treatment initiation.
Figure 1.
Representative ultrasound B-mode and elastography images for (A) non-responder and (B) responder patients acquired before treatment, at week 1, week 4, week 8, and preoperatively. The color bar shows tissue softness. Scale bar indicates 1 cm.
Figure 2.
Representative quantitative data for patients presented in Figure 1 ((A) non-responder; (B) responder) whole-mount histopathology. The graphs illustrate the percentage change frompretreatment in elastographic parameters plotted over time. In the non-responding patient's pathology, a largemass of residual carcinoma is present at low and high magnifications. In the responding patient, minimal residual disease is visualized with areas demonstrating low cellularity. Scale bars indicate 5 and 0.2 mm in the low and high magnifications, respectively.
Figure 3.
Changes in strain ratio and strain difference measured in non-responders (NR) and responders (R) using (A) static ROI and (B) dynamic ROI methods. The graphs illustrate elastography parameters plotted as a percentage change from pretreatment for nonresponders versus responders over time. All differences were statistically significant (P < .05) at 4 weeks after treatment initiation. Error bars represent ±1 SE.
Table 3.
Discriminant Analysis at (A) 1 Week and (B) 4 Weeks after Treatment Initiation.
| Parameter | Non-responders Mean ± SE (%) | Responders Mean ± SE (%) | P value | Sensitivity (%) | Specificity (%) |
| (A) | |||||
| Strain ratio (static) | 99 ± 3 | 94 ± 5 | .4 | 44 | 60 |
| Strain difference (static) | 90 ± 18 | 65 ± 15 | .3 | 44 | 60 |
| Strain ratio (dynamic) | 97 ± 2 | 95 ± 5 | .8 | 56 | 60 |
| Strain difference (dynamic) | 77 ± 15 | 74 ± 16 | .9 | 44 | 60 |
| (B) | |||||
| Strain ratio (static) | 102 ± 2 | 81 ± 3 | .0003* | 100 | 100 |
| Strain difference (static) | 110 ± 17 | 1 ± 17 | .001† | 89 | 100 |
| Strain ratio (dynamic) | 101 ± 4 | 81 ± 3 | .002† | 89 | 100 |
| Strain difference (dynamic) | 109 ± 30 | 6 ± 18 | .008† | 89 | 80 |
Percentages show the current values of parameters relative to the pretreatment. All strain ratios and strain differences for non-responders and responders were statistically significant 4 weeks after treatment initiation. There was no statistical significance at 1 week after the start of treatment.
Extremely significant (P < .001).
Statistically highly significant (P < .01).
Discussion
Ultrasound elastography is an emerging imaging modality that provides information regarding tissue stiffness. It is non-invasive, portable, and less expensive than other imaging modalities such asMRI or computed tomography (CT). It does not involve the use of exogenous contrast agents, which makes it ideal for multiple scans typically required for treatment monitoring. Previous studies have demonstrated that this modality may be used to monitor the treatment response of ablative therapies [20,23]. Ultrasound-based elastography was shown to accurately depict thermal lesions after radiofrequency ablation therapy in porcine liver in vivo [23]. Specifically, in a study of 29 patients with prostate cancer, elastography was successfully used to visualize thermal lesions of the prostate in vivo, during and after high-intensity focused ultrasound (HIFU) treatment [20]. In these patients, the prostate appeared stiffer after treatment and a 40% to 60% decrease in average strain was obtained compared to pretreatment measurements.
In this study, we report for the first time the results of 15 patients with LABC receiving neoadjuvant chemotherapy whose tumor responses were monitored using ultrasound elastography. Strain ratios and strain differences in responders exhibited a decrease during the first weeks of the treatment (Figures 1–3). Using a static ROI analysis, the mean strain ratio and strain difference for responders was 81 ± 3% and 1 ± 17% versus non-responders who exhibited values of 102 ± 2% and 110 ± 17% at 4 weeks after treatment initiation, respectively. These differences between responders and non-responders were found to be statistically significant (P < .05). The use dynamic ROIs led to similar findings; the mean strain ratio and strain difference for responders was 81 ± 3% and 6 ± 18% versus non-responders of 101 ± 4% and 109 ± 30%, respectively. These differences between responders and non-responders were also found to be statistically significant (P < .05) and provide further evidence on the use of ultrasound elastography for monitoring treatment response in LABC patients. As the tumor begins to respond to the treatment in responding patients, it begins to become less stiff as a result of the changes in its structure and biomechanical properties. This then leads to an increase in the tumor strain and, hence, a decrease in the strain ratio and strain difference.
Patient 2 was an exceptional case and a data outlier. This 53-year-old female was diagnosed with ductal carcinoma measuring 7.4 cm x 7 cm (AP x ML). She was treated with epirubicin and docetaxel chemotherapy. After surgery, her residual mass measured 7 cm x 5 cm x 3 cm (AP x ML x SI) but only had mucinous features remaining with very low tumor cellularity and this patient's clinical response was pathologically rated as good. However, the analysis of the elastography data showed similar trends to those of non-responding patients. This implies that the tumor strain remained unchanged during the treatment. This finding is not surprising because, for this particular patient, the biomechanical properties of the tumor remained unaffected due to the abundance of extracellular mucin. For this tumor subtype, ultrasound elastography may not be sensitive or specific. However, this will not likely cause any major limitation to this imaging modality because mucinous tumors are rare, representing 1% to 4% of all breast cancers [30].
The use of static ROIs for strain estimation was found to be superior to using dynamic ROIs because it permitted the distinction between responders and non-responders as early as 4 weeks after treatment initiation when using strain ratio (estimated sensitivity, 100%; estimated specificity, 100%) and strain difference (estimated sensitivity, 89%; estimated specificity, 100%) as predictors of treatment response (Table 3). Static ROIs were rectangular with constant size and included peritumoral regions in addition to the tumor, contrary to dynamic ROIs that only included tumor areas. The concurrent changes in the peritumor and tumor stiffness as a result of the neoadjuvant treatment has likely contributed to the increased sensitivity of this technique when using static ROIs in comparison with dynamic ones. Another advantage of static ROIs is that they only need to be defined once for a given patient after the initial scan and they can be further used in subsequent scans without the need for tumor segmentation. This makes the ultrasound elastography technique readily feasible for breast cancer treatment monitoring.
Other methods have been devised for therapy response monitoring including optical spectroscopy [27–29], new MRI methods for cell death detection [31,32], and spectroscopic ultrasound for cell death detection [33] and are reviewed elsewhere [34,35]. Such methods are not generally used clinically. Shear wave imaging is similar to elastography in that tissue stiffness measurement can be carried out quantitatively, which estimate the Young's modulus of tissue [36,37]. Another method, acoustic radiation force impulse imaging, provides an alternative method of estimating tissue stiffness [38,39]. Both potentially can be used for therapy response monitoring.
In conclusion, this study demonstrates for the first time that ultrasound elastography technique may be used to discriminate between clinically responding or non-responding patients early during the course of neoadjuvant treatment. Results reported in this study indicate that changes in ultrasound elastography parameters of treated breast tumors strongly correlated with the clinical and pathologic response of patients. These findings pave the way for establishing protocols for the clinical applications of ultrasound elastography techniques in therapy response monitoring of breast cancer patients. Such protocols are required for the enhancement of personalized cancer therapy in which patients are switched from ineffective therapies to more effective ones early after treatment initiation.
Acknowledgments
The authors thank Joris Nofiele for his technical support.
Footnotes
This study was funded, in part, by the Canadian Breast Cancer Foundation - Ontario Region through a research grant to G.J.C. and through fellowships to O.F. and A.S.N. Funding for this project was also provided by the Terry Fox Foundation and the Natural Sciences and Engineering Research Council of Canada. This work was also supported through a Cancer Care Ontario Research Chair in experimental therapeutics and imaging awarded to G.J.C.
References
- 1.American Cancer Society, author. Cancer Facts and Figures. Atlanta, GA: American Cancer Society, Inc; 2011. [Google Scholar]
- 2.Giordano SH. Update on locally advanced breast cancer. Oncologist. 2003;8:521–530. doi: 10.1634/theoncologist.8-6-521. [DOI] [PubMed] [Google Scholar]
- 3.Mankoff DA, Dunnwald LK, Gralow JR, Ellis GK, Drucker MJ, Livingston RB. Monitoring the response of patients with locally advanced breast carcinoma to neoadjuvant chemotherapy using [technetium 99m]-sestamibi scintimammography. Cancer. 1999;85:2410–2423. [PubMed] [Google Scholar]
- 4.Esteva FJ, Hortobagyi GN. Locally advanced breast cancer. Hematol Oncol Clin North Am. 1999;13:457–472vii. doi: 10.1016/s0889-8588(05)70065-4. [DOI] [PubMed] [Google Scholar]
- 5.Hortobagyi GN. Comprehensive management of locally advanced breast cancer. Cancer. 1990;66:1387–1391. doi: 10.1002/1097-0142(19900915)66:14+<1387::aid-cncr2820661414>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Chollet P, Charrier S, Brain E, Curé H, van Praagh I, Feillel V, de Latour M, Dauplat J, Misset JL, Ferrière JP. Clinical and pathological response to primary chemotherapy in operable breast cancer. Eur J Cancer. 1997;33:862–866. doi: 10.1016/s0959-8049(97)00038-5. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 8.Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, Ah-See AK, Eremin O, Walker LG, Sarkar TK, et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002;20:1456–1466. doi: 10.1200/JCO.2002.20.6.1456. [DOI] [PubMed] [Google Scholar]
- 9.Huang E, McNeese MD, Strom EA, Perkins GH, Katz A, Hortobagyi GN, Valero V, Kuerer HM, Singletary SE, Hunt KK, et al. Locoregional treatment outcomes for inoperable anthracycline-resistant breast cancer. Int J Radiat Oncol Biol Phys. 2002;53:1225–1233. doi: 10.1016/s0360-3016(02)02878-x. [DOI] [PubMed] [Google Scholar]
- 10.Esteva FJ, Hortobagyi GN. Can early response assessment guide neoadjuvant chemotherapy in early-stage breast cancer? J Natl Cancer Inst. 2008;100:521–523. doi: 10.1093/jnci/djn098. [DOI] [PubMed] [Google Scholar]
- 11.Maurice RL, Daronat M, Ohayon J, Stoyanova E, Foster FS, Cloutier G. Non-invasive high-frequency vascular ultrasound elastography. Phys Med Biol. 2005).;50:1611–1628. doi: 10.1088/0031-9155/50/7/020. [DOI] [PubMed] [Google Scholar]
- 12.Ophir J, Alam SK, Garra BS, Kallel F, Konofagou EE, Krouskop T, Merritt CRB, Righetti R, Souchon R, Srinivasan S, et al. Elastography: imaging the elastic properties of soft tissues with ultrasound. J Med Ultrason. 2002;29:155–171. doi: 10.1007/BF02480847. [DOI] [PubMed] [Google Scholar]
- 13.Rago T, Santini F, Scutari M, Pinchera A, Vitti P. Elastography: new developments in ultrasound for predicting malignancy in thyroid nodules. J Clin Endocrinol Metab. 2007;92:2917–2922. doi: 10.1210/jc.2007-0641. [DOI] [PubMed] [Google Scholar]
- 14.Satake H, Nishio A, Ikeda M, Ishigaki S, Shimamoto K, Hirano M, Naganawa S. Predictive value for malignancy of suspicious breast masses of BI-RADS categories 4 and 5 using ultrasound elastography and MR diffusion-weighted imaging. AJR Am J Roentgenol. 2011;196:202–209. doi: 10.2214/AJR.09.4108. [DOI] [PubMed] [Google Scholar]
- 15.Taylor LS, Porter BC, Rubens DJ, Parker KJ. Three-dimensional sonoelastography: principles and practices. Phys Med Biol. 2000;45:1477–1494. doi: 10.1088/0031-9155/45/6/306. [DOI] [PubMed] [Google Scholar]
- 16.Varghese T. Quasi-static ultrasound elastography. Ultrasound Clin. 2009;4:323–338. doi: 10.1016/j.cult.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ophir J, Céspedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–134. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 18.Garra BS, Cespedes EI, Ophir J, Spratt SR, Zuurbier RA, Magnant CM, Pennanen MF. Elastography of breast lesions: initial clinical results. Radiology. 1997;202:79–86. doi: 10.1148/radiology.202.1.8988195. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz A, Ermert H, Sommerfeld HJ, Garcia-Schürmann M, Senge T, Philippou S. Ultrasound elastography of the prostate. A new technique for tumor detection. Ultraschall Med. 2000;21:8–15. doi: 10.1055/s-2000-8926. [DOI] [PubMed] [Google Scholar]
- 20.Souchon R, Rouvière O, Gelet A, Detti V, Srinivasan S, Ophir J, Chapelon JY. Visualisation of HIFU lesions using elastography of the human prostate in vivo: preliminary results. Ultrasound Med Biol. 2003;29:1007–1015. doi: 10.1016/s0301-5629(03)00065-6. [DOI] [PubMed] [Google Scholar]
- 21.Alam F, Naito K, Horiguchi J, Fukuda H, Tachikake T, Ito K. Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: comparison with conventional B-mode sonography. AJR Am J Roentgenol. 2008;191:604–610. doi: 10.2214/AJR.07.3401. [DOI] [PubMed] [Google Scholar]
- 22.Kolokythas O, Gauthier T, Fernandez AT, Xie H, Timm BA, Cuevas C, Dighe MK, Mitsumori LM, Bruce MF, Herzka DA, et al. Ultrasound-based elastography: a novel approach to assess radio frequency ablation of liver masses performed with expandable ablation probes: a feasibility study. J Ultrasound Med. 2008;27:935–946. doi: 10.7863/jum.2008.27.6.935. [DOI] [PubMed] [Google Scholar]
- 23.Varghese T, Zagzebski JA, Lee FT., Jr Elastographic imaging of thermal lesions in the liver in vivo following radiofrequency ablation: preliminary results. Ultrasound Med Biol. 2002;28:1467–1473. doi: 10.1016/s0301-5629(02)00656-7. [DOI] [PubMed] [Google Scholar]
- 24.Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 25.Schedin P, O'Brien J, Rudolph M, Stein T, Borges V. Microenvironment of the involuting mammary gland mediates mammary cancer progression. J Mammary Gland Biol Neoplasia. 2007;12:71–82. doi: 10.1007/s10911-007-9039-3. [DOI] [PubMed] [Google Scholar]
- 26.Clarke GM, Eidt S, Sun L, Mawdsley G, Zubovits JT, Yaffe MJ. Whole-specimen histopathology: a method to produce whole-mount breast serial sections for 3-D digital histopathology imaging. Histopathology. 2007;50:232–242. doi: 10.1111/j.1365-2559.2006.02561.x. [DOI] [PubMed] [Google Scholar]
- 27.Falou O, Soliman H, Sadeghi-Naini A, Iradji S, Lemon-Wong S, Zubovits J, Spayne J, Dent R, Trudeau M, Boileau JF, et al. Diffuse optical spectroscopy evaluation of treatment response in women with locally advanced breast cancer receiving neoadjuvant chemotherapy. Transl Oncol. 2012;5:238–246. doi: 10.1593/tlo.11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roblyer D, Ueda S, Cerussi A, Tanamai W, Durkin A, Mehta R, Hsiang D, Butler JA, McLaren C, Chen WP, et al. Optical imaging of breast cancer oxyhemoglobin flare correlates with neoadjuvant chemotherapy response one day after starting treatment. Proc Natl Acad Sci USA. 2011;108:14626–14631. doi: 10.1073/pnas.1013103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soliman H, Gunasekara A, Rycroft M, Zubovits J, Dent R, Spayne J, Yaffe MJ, Czarnota GJ. Functional imaging using diffuse optical spectroscopy of neoadjuvant chemotherapy response in women with locally advanced breast cancer. Clin Cancer Res. 2010;16:2605–2614. doi: 10.1158/1078-0432.CCR-09-1510. [DOI] [PubMed] [Google Scholar]
- 30.Barkley CR, Ligibel JA, Wong JS, Lipsitz S, Smith BL, Golshan M. Mucinous breast carcinoma: a large contemporary series. Am J Surg. 2008;196:549–551. doi: 10.1016/j.amjsurg.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Bailey C, Desmond KL, Czarnota GJ, Stanisz GJ. Quantitative magnetization transfer studies of apoptotic cell death. Magn Reson Med. 2011;66:264–269. doi: 10.1002/mrm.22820. [DOI] [PubMed] [Google Scholar]
- 32.Bailey C, Giles A, Czarnota GJ, Stanisz GJ. Detection of apoptotic cell death in vitro in the presence of Gd-DTPA-BMA. Magn Reson Med. 2009;62:46–55. doi: 10.1002/mrm.21972. [DOI] [PubMed] [Google Scholar]
- 33.Czarnota GJ, Kolios MC, Abraham J, Portnoy M, Ottensmeyer FP, Hunt JW, Sherar MD. Ultrasound imaging of apoptosis: high-resolution noninvasive monitoring of programmed cell death in vitro, in situ and in vivo. Br J Cancer. 1999;81:520–527. doi: 10.1038/sj.bjc.6690724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadeghi-Naini A, Falou O, Hudson JM, Bailey C, Burns PN, Yaffe MJ, Stanisz GJ, Kolios MC, Czarnota GJ. Imaging innovations for cancer therapy response monitoring. Imaging Med. 2012;4:311–327. [Google Scholar]
- 35.Brindle K. New approaches for imaging tumour responses to treatment. Nat Rev Cancer. 2008;8:94–107. doi: 10.1038/nrc2289. [DOI] [PubMed] [Google Scholar]
- 36.Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396–409. doi: 10.1109/tuffc.2004.1295425. [DOI] [PubMed] [Google Scholar]
- 37.Deffieux T, Montaldo G, Tanter M, Fink M. Shear wave spectroscopy for in vivo quantification of human soft tissues visco-elasticity. IEEE Trans Med Imaging. 2009;28:313–322. doi: 10.1109/TMI.2008.925077. [DOI] [PubMed] [Google Scholar]
- 38.Nightingale K. Acoustic radiation force impulse (ARFI) imaging: a review. Curr Med Imaging Rev. 2011;7:328–339. doi: 10.2174/157340511798038657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nightingale K, Soo MS, Nightingale R, Trahey G. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med Biol. 2002;28:227–235. doi: 10.1016/s0301-5629(01)00499-9. [DOI] [PubMed] [Google Scholar]