ABSTRACT
Uropathogenic Escherichia coli (UPEC) is the most common causative agent of community-acquired urinary tract infection (UTI). In order to cause UTI, UPEC must endure stresses ranging from nutrient limitation to host immune components. RpoS (σS), the general stress response sigma factor, directs gene expression under a variety of inhibitory conditions. Our study of rpoS in UPEC strain CFT073 began after we discovered an rpoS-frameshift mutation in one of our laboratory stocks of “wild-type” CFT073. We demonstrate that an rpoS-deletion mutation in CFT073 leads to a colonization defect during UTI of CBA/J mice at 48 hours postinfection (hpi). There is no difference between the growth rates of CFT073 and CFT073 rpoS in urine. This indicates that rpoS is needed for replication and survival in the host rather than being needed to address limitations imposed by urine nutrients. Consistent with previous observations in E. coli K-12, CFT073 rpoS is more sensitive to oxidative stress than the wild type. We demonstrate that peroxide levels are elevated in voided urine from CFT073-infected mice compared to urine from mock-infected mice, which supports the notion that oxidative stress is generated by the host in response to UPEC. In mice that lack phagocyte oxidase, the enzyme complex expressed by phagocytes that produces superoxide, the competitive defect of CFT073 rpoS in bladder colonization is lost. These results demonstrate that σS is important for UPEC survival under conditions of phagocyte oxidase-generated stress during UTI. Though σS affects the pathogenesis of other bacterial species, this is the first work that directly implicates σS as important for UPEC pathogenesis.
IMPORTANCE
UPEC must cope with a variety of stressful conditions in the urinary tract during infection. RpoS (σS), the general stress response sigma factor, is known to direct the expression of many genes under a variety of stressful conditions in laboratory-adapted E. coli K-12. Here, we show that σS is needed by the model UPEC strain CFT073 to cope with oxidative stress provided by phagocytes during infection. These findings represent the first report that implicates σS in the fitness of UPEC during infection and support the idea of the need for a better understanding of the effects of this global regulator of gene expression during UTI.
Introduction
Urinary tract infections (UTIs) are among the most common human bacterial infections. UTIs cause significant discomfort, malaise, and lethargy, frequently require antibiotic treatment, and can become life threatening. It is estimated that 150 million cases of UTI occur per year, resulting in global health care costs totaling over 6 billion U.S. dollars (1). Forty percent of women will have a UTI in their lifetimes, and 25% of this population will suffer recurrent UTIs, with subsequent infections usually occurring within 6 to 12 months of the previous occurrence (2, 3). Additionally, 12% of men, with a large proportion of those being elderly, will experience a UTI (2, 3). Though many microbes are known to cause UTI (4), Escherichia coli is the most common causative agent, accounting for 70 to 95% of all reported cases (1). Uropathogenic E. coli (UPEC) strains are residents of the gut microbiota and can gain access to the urinary tract through an ascending route (4). This route of infection involves the colonization of the periurethral area and ascension through the urethra to the bladder, where the bacteria cause cystitis. If cystitis is left untreated, the bacteria can ascend into the ureters and kidneys, where they cause pyelonephritis.
Neutrophils have been shown to be critical for the clearance of UPEC during infection (5). Neutrophils produce many antimicrobial factors, a primary component being reactive oxygen species (ROS) (6). These ROS originate from superoxide (O2−), which is produced by phagocyte oxidase, a multisubunit enzyme complex expressed by phagocytic cells (7–9). ROS have pleiotropic effects on bacterial cells, where they react with thiols, lipids, metal centers, nucleic acids, and tyrosine residues (8, 10, 11). Indeed, E. coli is capable of coping with oxidative stress by a variety of mechanisms, and it is thought that resistance to ROS may be important for UPEC pathogenesis (12).
Transcription of genes in E. coli is catalyzed by the RNA polymerase holoenzyme, which is composed of core RNA polymerase (α2ββ′ω) and a dissociable sigma factor (σ). E. coli has one housekeeping sigma factor (σ70) and six other “alternative” σ factors (13). In response to stimuli, each of these σ factors facilitates transcription from a variety of different promoters when bound to core polymerase. The best studied of the alternative sigma factors, σS, positively regulates ~500 genes involved in stationary-phase survival and in resistance to a variety of stresses, including oxidative stress in E. coli K-12 (13–17). Insight into the role of σS in uropathogenesis is lacking, despite an appreciated role in the pathogenesis of other bacterial species and epidemiological data suggesting that a loss of rpoS function may be detrimental to E. coli during human extraintestinal pathogenic E. coli (ExPEC) infections (18, 19).
Here, we show that σS allows UPEC strain CFT073 to cope with phagocyte-mediated oxidative stress during urinary tract infection. These data support the hypothesis that σS is needed for efficient bladder colonization and that σS-regulated genes play a significant role during UTI in tolerating oxidative stress provided by the host innate immune response.
RESULTS
CFT073 rpoS is attenuated for colonization relative to wild type in CBA/J mouse bladders at 48 hpi.
To assess if rpoS is important for E. coli CFT073 during urinary tract infection, we utilized the murine model of UTI. When coinoculated with wild type into CBA/J mice, CFT073 rpoS was recovered from bladder homogenates at approximately 50-fold-lower levels at 48 hours postinfection (hpi) (Fig. 1A). This competitive disadvantage was lost when a functional copy of rpoS was reintroduced into its native location in the rpoS mutant by ϕEB49-mediated allelic transductional repair (Fig. 1A).
FIG 1 .
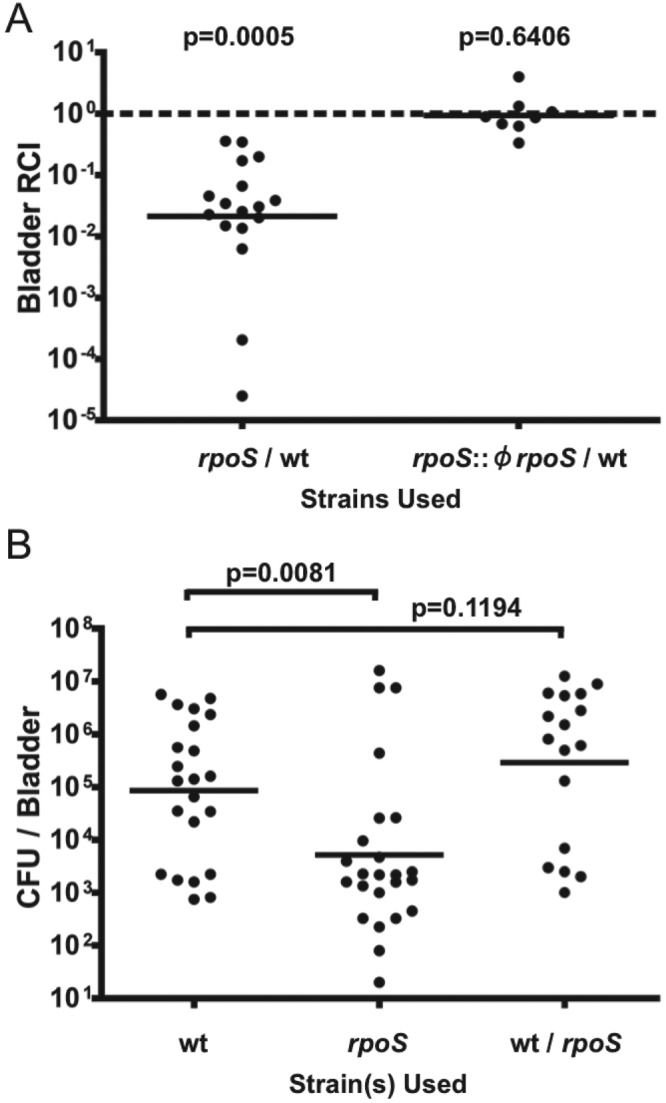
CFT073 rpoS has a colonization defect at 48 hpi in CBA/J murine model UTI. (A) CFT073 rpoS and CFT073 lacZYA were coinoculated at a 1:1 ratio into CBA/J mice (n = 17). A functional copy of rpoS was reintroduced into CFT073 rpoS via generalized transduction with ϕEB49. This transductant (rpoS::ϕrpoS) was also coinoculated with the wild type (wt) into CBA/J mice (n = 8). Mice were sacrificed at 48 hpi. Bacteria from bladder homogenates were enumerated on MacConkey’s medium plus lactose. Lines are drawn at the geometric mean relative competitive index (RCI). Statistical significance was assessed by a Mann-Whitney U test relative to a hypothetical RCI of 1. (B) Manipulations were carried out as described for panel A except that single strains were used (wt, n = 22; rpoS, n = 24). Lines are drawn at the geometric mean CFU/bladder. Total burdens of animals coinfected with the CFT073 wild-type and rpoS strains at 48 hpi (from panel A) are included for comparison. Statistical significance was assessed by the Mann-Whitney U test.
To further investigate the defect shown by a CFT073 rpoS mutant during experimental UTI, we infected CBA/J mice with single strains, either wild type CFT073 or the rpoS mutant. Animals were sacrificed at 48 hpi, and bacterial burdens were assessed. The rpoS mutant was recovered at significantly lower levels than wild type at 48 hpi (Fig. 1B). We assessed several phenotypes known to influence colonization in the murine UTI model. There was no statistically significant difference between mannose-sensitive guinea pig erythrocyte agglutination results nor was there a difference in motility on Adler motility agar (data not shown). Furthermore, the growth kinetics of these two strains in human urine were identical (data not shown).
CFT073 rpoS is more sensitive to oxidative stress than wild type.
It was previously demonstrated in E. coli K-12 that many σS-regulated genes are involved in detoxifying ROS or repairing damage caused by ROS. To show that rpoS contributes to oxidative stress resistance in CFT073, we utilized a hydrogen peroxide killing assay (modified from reference 20). CFT073 rpoS is killed more readily than wild type by treatment with 5mM hydrogen peroxide (Fig. 2).
FIG 2 .
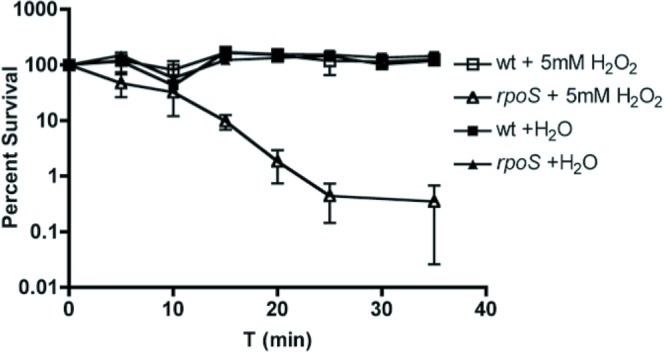
CFT073 rpoS is more sensitive to oxidative stress in vitro than wild type. CFT073 and CFT073 rpoS were grown overnight in LB medium and diluted 1:1,000 into PBS–5 mM H2O2 or an equivalent volume of distilled water (dH2O). Cells were then incubated at 37°C and were enumerated every 5 min by plating onto LB agar. Data from four independent experiments are represented as mean percent survival ± standard error of the mean (SEM) relative to bacterial counts at t = 0.
Mice infected with CFT073 or CFT073 rpoS have elevated levels of peroxide in urine.
To demonstrate that the infected bladder environment provides oxidative stress to bacteria, we measured the levels of H2O2, a relatively stable byproduct of superoxide, in infected CBA/J mouse urine at 24 hpi and 48 hpi. Urine was collected from anesthetized mice that were infected with either strain and subjected to a Xylenol Orange-based Pierce quantitative peroxide assay (n = 12 each). The fold changes in urine peroxide levels were calculated relative to phosphate-buffered saline (PBS) mock-infected control levels. CFT073 rpoS-infected animals did not have increased levels of H2O2 in their urine relative to wild-type-infected animals at either of these time points. From 24 hpi to 48 hpi, however, the concentration of peroxide in all infected animals increased significantly (Fig. 3).
FIG 3 .
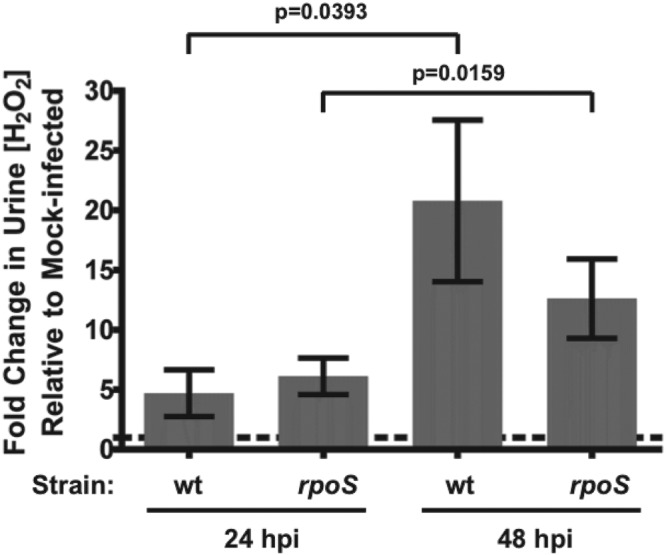
Levels of peroxide in urine are elevated in urine from infected CBA/J mice. CBA/J mice were infected with either CFT073 or CFT073 rpoS (n = 12 each). At 24 and 48 hpi, urine was collected from these animals and H2O2 concentrations were measured using a Xylenol Orange-based quantitative peroxide assay kit (Pierce). Data are represented as mean fold change in [H2O2] ± SEM relative to PBS mock-infected control animals. Mean fold changes in [H2O2] did not differ between mice infected with CFT073 and mice infected with CFT073 rpoS at 24 or 48 hpi, but there was a significant change in [H2O2] in infected animals from 24 to 48 hpi, as assessed by an unpaired t test and a paired t test, respectively.
The competitive defect shown by CFT073 rpoS in bladders at 48 hpi is diminished in mice that lack functional phagocyte oxidase.
During infection, inflammatory cells produce a variety of antimicrobial factors. A primary antimicrobial agent in this repertoire is superoxide, which reacts with other molecules to form ROS. The primary source of bactericidal ROS during infection is provided by phagocyte oxidase. To assess the contribution of phagocyte oxidase to the colonization defect of CFT073 rpoS, we utilized C57BL/6 mice and a congenic strain lacking the p47 subunit of this enzyme complex. p47phox knockout mice lack functional phagocyte oxidase (21, 22). As seen in Fig. 4, the competitive disadvantage seen in C57BL/6 mice was significantly diminished in the mice lacking functional phagocyte oxidase.
FIG 4 .
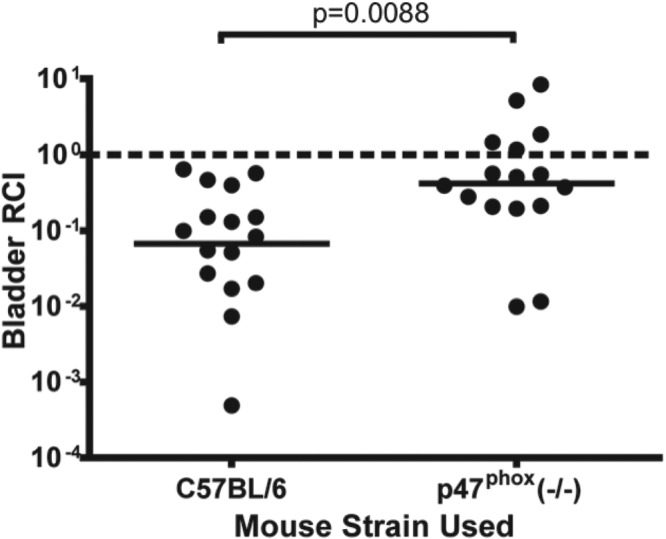
The competitive defect of CFT073 rpoS during UTI is diminished in mice that lack functional phagocyte oxidase. C57BL/6 mice (n = 16) and age-matched congenic mice lacking phagocyte oxidase [p47phox (−/−)] (n = 16) were infected with a 1:1 ratio of CFT073 rpoS and wild-type strains. Animals were infected and sacrificed at 48 hpi, and bacteria were enumerated as described in the Fig. 2 legend. Lines are drawn at the geometric mean relative competitive index (RCI). Statistical significance was assessed by the Mann-Whitney U test.
DISCUSSION
Here we present data showing that rpoS is needed for efficient UTI by UPEC. These studies began after we discovered that the sequenced CFT073 strain (23) has a 5-bp duplication in rpoS, which results in the production of a nonfunctional, truncated σS protein. The wild-type strain used in the present study was obtained from the original CFT073 clinical isolate (Table 1). The nucleotide sequence of the CFT073 wild-type rpoS allele has been reported previously (24) and can be found in GenBank (accession no. AF270497).
TABLE 1 .
Bacterial strains and transducing phage used in this study
| Strain name | Genotype | Source or reference |
|---|---|---|
| WAM 4505 | Escherichia coli CFT073 | Original clinical isolate |
| WAM 4519 | CFT073 rpoS | Our laboratory |
| WAM 4520 | CFT073 lacZYA | Our laboratory |
| WAM 4530 | CFT073 rpoS+ Kan | Our laboratory |
| WAM 4560 | CFT073 rpoS::ϕrpoS+ Kan | Our laboratory |
| ϕEB49 | Generalized transducing phage | 25 |
This report represents the first use of ϕEB49, the uropathogenic E. coli transducing phage (25), to conduct allelic repair of the rpoS null allele. Allelic repair involves the cotransduction of a functional copy of an allele of interest with a closely linked antibiotic resistance marker. Because the concentration of σS within cells and the subsequent influence on σS-dependent genes are regulated at several different levels (17), allelic repair was used rather than more “traditional” methods of genetic complementation (i.e., single-copy complementation in cis or low-copy-number vector complementation in trans) to maintain wild-type regulation of σS levels. The CFT073 rpoS::ϕrpoS repaired strain shown in Fig. 1, as well as other CFT073 rpoS::ϕrpoS transductants tested (n = 10), were shown to have growth kinetics in Luria-Bertani (LB) broth identical to those of wild-type CFT073. Furthermore, transductants that incorporated the kanamycin (Kan) cassette but not the functional rpoS allele (n = 3) and CFT073 rpoS exhibited a small decrease in the final optical density at 600 nm (OD600) in LB broth in late stationary phase (data not shown).
In E. coli K-12, many σS-regulated genes are involved in mitigating the toxic effects of reactive oxygen species by a variety of mechanisms. Although these genes (dps, katE, sodC, xthA, and otsAB, among others) are shared between K-12 and CFT073, their expression during infection and their degree of dependence on σS in CFT073 are not known. Regardless, we show that CFT073 rpoS is killed more readily than wild type by treatment with hydrogen peroxide (Fig. 2), indicating that one or more mechanisms of oxidative stress resistance are regulated by σS in CFT073. The extent of the σS regulon in CFT073 during experimental UTI is a subject of ongoing investigation in our laboratory. A role of σS-regulated genes in resistance to oxidative and pH stress in the model UPEC strain UTI89 has been suggested (26). Our observations provide further evidence that σS regulates the expression of oxidative stress resistance genes in UPEC.
Interestingly, there was a significant increase in the concentration of peroxide in urine from 24 hpi to 48 hpi in all infected animals (Fig. 2). This increase in peroxide concentration correlates with a significant change in the bladder relative competitive index (RCI) but not in total bacterial loads in animals coinfected with the wild-type and rpoS strains (data not shown). These observations support the notion that CFT073 rpoS is more sensitive than wild type to oxidative stress in vivo. Indeed, when the source of phagocyte-mediated oxidative stress was removed, CFT073 rpoS no longer showed a pronounced colonization defect (Fig. 4).
Our findings that rpoS is needed during UTI conflict with previously reported results from Culham et al. (24), where an assessment of σS and σS-dependent osmotic stress resistance mechanisms suggested that rpoS was not important during infection. We surmise that the use of outbred mice as in the previous study does not control for host genetic polymorphism and may have helped lead to a conclusion that underestimates the importance of rpoS during UTI. Furthermore, the CFT073 variant used by Culham et al. expresses σS but not the σS-regulated catalase KatE, whereas the original clinical isolate of CFT073 used in this study expresses KatE (reference 24 and data not shown). We established that several research groups have variants of CFT073 with respect to rpoS status, and this observation suggests that there may be further variations in what a particular research group refers to as “wild-type” CFT073. Mutations in rpoS have been demonstrated in accordance with the transfer of E. coli strains between laboratories, which is likely due to the growth advantage in stationary phase (GASP) afforded by loss-of-function mutations in rpoS (27–29).
Oxidative stress resistance by UPEC during urinary tract infection was explored previously in the context of OxyR, a transcriptional activator responsible for expression of antioxidant genes under conditions of oxidative stress in E. coli K-12 (30–32). Johnson et al. showed that although the deletion of oxyR results in a bladder colonization disadvantage for UPEC strain Ec1a in C57BL/6 mice during experimental UTI, this competitive defect is not diminished in mice lacking phagocyte oxidase (33). These findings, coupled with the decreased ability of Ec1a oxyR to grow in human urine, suggest that the OxyR regulon is needed to cope with stresses present in urine alone and that there may be genes independent of oxyR in UPEC involved in resistance to phagocyte-mediated oxidative stress during UTI. We report here that the growth kinetics of CFT073 rpoS are indistinguishable from those of wild type in filter-sterilized human urine (data not shown). This lends support to our assertion that the observed defect in colonization by CFT073 rpoS is due to phagocyte oxidase-mediated stress during infection rather than to limiting nutrients or growth inhibitors that may be present in uninfected urine.
It is apparent that E. coli strains capable of causing urinary tract infections do not have a single virulence factor that distinguishes them from non-UPEC strains. Although much work has characterized the roles of adhesion, motility, and nutrient acquisition in UPEC pathogenesis, the ability to cope with host stress during UTI is also important. For example, it is known that the SOS response (34), DNA mismatch repair (35), and components of the PhoP regulon (36) are needed during experimental UTI. Our laboratory demonstrated that DegS and DegP, elements of the RpoE (σE) regulon, the extracytoplasmic stress sigma factor, are critical for successful CFT073 colonization of the murine urinary tract (37, 38). We hypothesize that differences in the regulation of stress response systems may determine if a particular pathovar of E. coli is able to cause disease in its niche and host. Indeed, it is known that σS levels are elevated during log-phase growth in CFT073 and HU734, two independently isolated UPEC strains (24). This observation contrasts with the well-established stationary-phase induction of σS levels in laboratory-adapted E. coli strains (17).
We demonstrate that rpoS is needed to cope with phagocyte oxidase-mediated stress during infection. However, we cannot discount the possibility that this global regulator may be important for directing the expression of other genes important during UTI. With a better understanding of σS-regulated genes and the regulation of cellular σS levels in UPEC, we expect to garner a greater understanding of how UPEC causes UTIs.
MATERIALS AND METHODS
Strains.
Strains used in this study are listed in Table 1. In-frame deletion mutants of E. coli CFT073 were constructed using the lambda Red mutagenesis protocol (39), which was modified to incorporate a generalized transduction step using ϕEB49 prior to pCP20-mediated antibiotic resistance cassette removal (25). Allelic repair of the rpoS null allele was carried out by ϕEB49-mediated cotransduction of a functional rpoS allele linked to a kanamycin resistance cassette (see Fig. S1 in the supplemental material).
Media and bacterial growth conditions.
All strains were grown in Luria-Bertain (LB) broth, on LB agar, on MacConkey’s agar containing lactose (Difco), or in filter-sterilized human urine samples. Urine was collected and pooled from healthy human volunteers (n = 3) with no recent history of antibiotic use. Inocula for murine model UTI were prepared as described previously (38). For in vitro growth analysis, bacteria were grown overnight at 37°C with aeration, washed twice in phosphate-buffered saline (PBS), and added to fresh growth medium to reach an OD600 = 0.01. Bacterial growth was measured by OD600 readings over time in a Synergy HT plate reader (Bio-Tek). Antibiotic selection (kanamycin [50 µg/ml] and chloramphenicol [20 µg/ml]) was used when applicable.
Murine model UTI.
Six-to-nine-week-old mice were used for all experiments described here. CBA/J mice were purchased from Harlan Laboratories (Indianapolis, IN). C57/BL6 mice and age-matched, congenic p47phox(−/−) mice were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were inoculated with either a single strain (single infection) or equal numbers of two strains (competitive infection) as described previously (38) and sacrificed at 48 hours postinfection (hpi). Bladders were homogenized in PBS–0.0025% Triton X-100, and 10-fold serial dilutions in PBS were plated onto MacConkey’s agar containing lactose. In competitive infections, CFT073 lacZYA was used to facilitate enumeration of wild type/mutant ratios. It has been shown previously that CFT073 lacZYA colonizes the murine urinary tract as well as wild-type CFT073 (37). Competitive infection data are reported as a relative competitive index (RCI), which is defined as the ratio of mutant to wild type recovered normalized to the ratio of mutant to wild type in the inoculum.
Sensitivity to hydrogen peroxide.
Bacteria were grown overnight at 37°C in LB broth with aeration and diluted 1:1,000 in PBS. Diluted cultures were incubated at 37°C, and hydrogen peroxide (Fisher Scientific) was added to reach a final concentration of 5 mM (20). After the addition of peroxide, samples of cells were plated at various time points for viable counts on LB agar and compared to samples where hydrogen peroxide was not added.
Fold change in levels of peroxides in infected urine samples.
A Pierce quantitative peroxide colorimetric assay kit (Thermo Scientific) was used according to the manufacturer’s protocol, and a Synergy HT plate reader (Bio-Tek) was used to measure peroxide concentration in infected and PBS mock-infected mouse urine samples.
Assays for motility and expression of type 1 pili.
The motility of single-strain inocula containing CFT073 or CFT073 rpoS was assessed on Adler motility agar as described previously (40). The diameter of the growth area was measured after 22 h of incubation at room temperature. Semiquantitative mannose-sensitive guinea pig erythrocyte agglutination was carried out to assess the presence of type 1 pili as described previously (37). For each assay, the OD600 of the inocula was normalized by dilution in PBS where necessary and viable counts on LB agar were used to confirm that there was no significant difference in numbers of bacterial cells used in the assays.
Statistical analyses.
All statistical analyses were carried out using Prism 4.0 (GraphPad, Inc.). Statistical significance was determined by the Mann-Whitney U test for log distributed data and was determined by paired or unpaired t tests for normally distributed data where applicable. P values ≤ 0.05 represent statistically significant differences between data sets.
SUPPLEMENTAL MATERIAL
Basic characteristics of rpoS and rpoS+ strains constructed via ϕEB49-mediated allelic repair. The ygbN-nlpD loci of CFT073, CFT073 rpoS, rpoS+ transductants, and rpoS transductants are indicated. Red bars denote known transduced regions. Since no other selection was done, cotransduction of additional DNA (up to the total amount packaged into ϕEB49, ~50 kB) may have occurred in addition to that in the known region. As such, X5′ + X3′ ≈ 46.5 kB and Y5′ + Y3′ ≈ 48.5 kB. Download
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant R01 DK063250-07.
Harry L. T. Mobley generously provided our laboratory with the wild-type CFT073 strain from the original patient isolate. We thank Eric Battaglioli, Justin Lemke, and Kevin Schwartz for constructive comments.
Footnotes
Citation Hryckowian AJ, Welch RA. 2013. RpoS contributes to resistance to phagocyte oxidase-mediated stress during urinary tract infection by Escherichia coli CFT073. mBio 4(1):e00023-13. doi:10.1128/mBio.00023-13.
REFERENCES
- 1. Kucheria R, Dasgupta P, Sacks SH, Khan MS, Sheerin NS. 2005. Urinary tract infections: new insights into a common problem. Postgrad. Med. J. 81:83–86 http://dx.doi.org/10.1136/pgmj.2004.023036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Foxman B. 1990. Recurring urinary tract infection: incidence and risk factors. Am. J. Public Health 80:331–333 http://dx.doi.org/10.2105/AJPH.80.3.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O’Hanley P. 1996. Prospects for urinary tract infection vaccines, p 405–425 In Mobley HL, Warren JW, Urinary tract infections: molecular pathogenesis and clinical management. ASM Press, Washington, DC [Google Scholar]
- 4. Foxman B. 2010. The epidemiology of urinary tract infection. Nat. Rev. Urol. 7:653–660 http://dx.doi.org/10.1038/nrurol.2010.190 [DOI] [PubMed] [Google Scholar]
- 5. Haraoka M, Hang L, Frendéus B, Godaly G, Burdick M, Strieter R, Svanborg C. 1999. Neutrophil recruitment and resistance to urinary tract infection. J. Infect. Dis. 180:1220–1229 http://dx.doi.org/10.1086/315006 [DOI] [PubMed] [Google Scholar]
- 6. Babior BM. 2000. Phagocytes and oxidative stress. Am. J. Med. 109:33–44 http://dx.doi.org/10.1016/S0002-9343(00)00481-2 [DOI] [PubMed] [Google Scholar]
- 7. Yang HC, Cheng ML, Ho HY, Chiu DT. 2011. The microbicidal and cytoregulatory roles of NADPH oxidases. Microbes Infect. 13:109–120 [DOI] [PubMed] [Google Scholar]
- 8. Fang FC. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2:820–832 http://dx.doi.org/10.1038/nrmicro1004 [DOI] [PubMed] [Google Scholar]
- 9. Altenhöfer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, Schmidt HH. 2012. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell. Mol. Life Sci. 69:2327–2343 http://dx.doi.org/10.1007/s00018-012-1010-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Groote MA, Fang FC. 1999. Antimicrobial properties of nitric oxide, p 231–261 In Fang FC. (ed.), Nitric oxide and infection. Academic; Plenum Press, New York, NY. [Google Scholar]
- 11. Nathan C, Shiloh MU. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. U. S. A. 97:8841–8848 http://dx.doi.org/10.1073/pnas.97.16.8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rama G, Chhina DK, Chhina RS, Sharma S. 2005. Urinary tract infections—microbial virulence determinants and reactive oxygen species. Comp. Immunol. Microbiol. Infect. Dis. 28:339–349 http://dx.doi.org/10.1016/j.cimid.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 13. Gruber TM, Gross CA. 2003. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu. Rev. Microbiol. 57:441–466 http://dx.doi.org/10.1146/annurev.micro.57.030502.090913 [DOI] [PubMed] [Google Scholar]
- 14. Weber H, Polen T, Heuveling J, Wendisch VF, Hengge R. 2005. Genome-wide analysis of the general stress response network in Escherichia coli: sigmas-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 187:1591–1603 http://dx.doi.org/10.1128/JB.187.5.1591-1603.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eisenstark A, Calcutt MJ, Becker-Hapak M, Ivanova A. 1996. Role of Escherichia coli rpoS and associated genes in defense against oxidative damage. Free Radic. Biol. Med. 21:975–993 http://dx.doi.org/10.1016/S0891-5849(96)00154-2 [DOI] [PubMed] [Google Scholar]
- 16. Lange R, Hengge-Aronis R. 1991. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol. Microbiol. 5:49–59 http://dx.doi.org/10.1111/j.1365-2958.1991.tb01825.x [DOI] [PubMed] [Google Scholar]
- 17. Battesti A, Majdalani N, Gottesman S. 2011. The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 65:189–213 http://dx.doi.org/10.1146/annurev-micro-090110-102946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levert M, Zamfir O, Clermont O, Bouvet O, Lespinats S, Hipeaux MC, Branger C, Picard B, Saint-Ruf C, Norel F, Balliau T, Zivy M, Le Nagard H, Cruveiller S, Chane-Woon-Ming B, Nilsson S, Gudelj I, Phan K, Ferenci T, Tenaillon O, Denamur E, Denamur E. 2010. Molecular and evolutionary bases of within-patient genotypic and phenotypic diversity in Escherichia coli extraintestinal infections. PLoS Pathog. 6:e1001125 http://dx.doi.org/10.1371/journal.ppat.1001125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dong T, Schellhorn HE. 2010. Role of RpoS in virulence of pathogens. Infect. Immun. 78:887–897 http://dx.doi.org/10.1128/IAI.00882-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Demple B, Halbrook J, Linn S. 1983. Escherichia coli xth mutants are hypersensitive to hydrogen peroxide. J. Bacteriol. 153:1079–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang CK, Zhan L, Hannigan MO, Ai Y, Leto TL. 2000. P47(phox)-deficient NADPH oxidase defect in neutrophils of diabetic mouse strains, C57BL/6J-m db/db and db/+. J. Leukoc. Biol. 67:210–215 [DOI] [PubMed] [Google Scholar]
- 22. Jackson SH, Gallin JI, Holland SM. 1995. The p47phox mouse knockout model of chronic granulomatous disease. J. Exp. Med. 182:751–758 http://dx.doi.org/10.1084/jem.182.3.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Welch RA, Burland V, Plunkett G, III, Redford P, Roesch P, Rasko D, Buckles EL, Liou SR, Boutin A, Hackett J, Stroud D, Mayhew GF, Rose DJ, Zhou S, Schwartz DC, Perna NT, Mobley HL, Donnenberg MS, Blattner FR. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:17020–17024 http://dx.doi.org/10.1073/pnas.252529799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Culham DE, Lu A, Jishage M, Krogfelt KA, Ishihama A, Wood JM. 2001. The osmotic stress response and virulence in pyelonephritis isolates of Escherichia coli: contributions of RpoS, ProP, ProU and other systems. Microbiology 147:1657–1670 [DOI] [PubMed] [Google Scholar]
- 25. Battaglioli EJ, Baisa GA, Weeks AE, Schroll RA, Hryckowian AJ, Welch RA. 2011. Isolation of generalized transducing bacteriophages for uropathogenic strains of Escherichia coli. Appl. Environ. Microbiol. 77:6630–6635 http://dx.doi.org/10.1128/AEM.05307-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Donovan GT, Norton JP, Bower JM, Mulvey MA. 2013. Adenylate cyclase and the cyclic AMP receptor protein (CRP) modulate the stress resistance and virulence capacity of uropathogenic Escherichia coli. Infect. Immun. 81:249–258 http://dx.doi.org/10.1128/IAI.00796-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spira B, de Almeida Toledo R, Maharjan RP, Ferenci T. 2011. The uncertain consequences of transferring bacterial strains between laboratories—rpoS instability as an example. BMC Microbiol. 11:248 http://dx.doi.org/10.1186/1471-2180-11-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zambrano MM, Siegele DA, Almirón M, Tormo A, Kolter R. 1993. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science 259:1757–1760 http://dx.doi.org/10.1126/science.7681219 [DOI] [PubMed] [Google Scholar]
- 29. Snyder E, Gordon DM, Stoebel DM. 2012. Escherichia coli lacking RpoS are rare in natural populations of non-pathogens. G3 (Bethesda) 2:1341–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mongkolsuk S, Helmann JD. 2002. Regulation of inducible peroxide stress responses. Mol. Microbiol. 45:9–15 http://dx.doi.org/10.1046/j.1365-2958.2002.03015.x [DOI] [PubMed] [Google Scholar]
- 31. Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, Storz G. 2001. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol. 183:4562–4570 http://dx.doi.org/10.1128/JB.183.15.4562-4570.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Storz G, Tartaglia LA, Ames BN. 1990. The OxyR regulon. Antonie Van Leeuwenhoek 58:157–161 http://dx.doi.org/10.1007/BF00548927 [DOI] [PubMed] [Google Scholar]
- 33. Johnson JR, Clabots C, Rosen H. 2006. Effect of inactivation of the global oxidative stress regulator oxyR on the colonization ability of Escherichia coli O1:K1:H7 in a mouse model of ascending urinary tract infection. Infect. Immun. 74:461–468 http://dx.doi.org/10.1128/IAI.74.1.461-468.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li B, Smith P, Horvath DJ, Jr, Romesberg FE, Justice SS. 2010. SOS regulatory elements are essential for UPEC pathogenesis. Microb. Infect. 12:662–668 [DOI] [PubMed] [Google Scholar]
- 35. Labat F, Pradillon O, Garry L, Peuchmaur M, Fantin B, Denamur E. 2005. Mutator phenotype confers advantage in Escherichia coli chronic urinary tract infection pathogenesis. FEMS Immunol. Med. Microbiol. 44:317–321 http://dx.doi.org/10.1016/j.femsim.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 36. Alteri CJ, Lindner JR, Reiss DJ, Smith SN, Mobley HL. 2011. The broadly conserved regulator PhoP links pathogen virulence and membrane potential in Escherichia coli. Mol. Microbiol. 82:145–163 http://dx.doi.org/10.1111/j.1365-2958.2011.07804.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Redford P, Welch RA. 2006. Role of sigma E-regulated genes in Escherichia coli uropathogenesis. Infect. Immun. 74:4030–4038 http://dx.doi.org/10.1128/IAI.01984-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Redford P, Roesch PL, Welch RA. 2003. degS is necessary for virulence and is among extraintestinal Escherichia coli genes induced in murine peritonitis. Infect. Immun. 71:3088–3096 http://dx.doi.org/10.1128/IAI.71.6.3088-3096.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 http://dx.doi.org/10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adler J. 1966. Chemotaxis in Bacteria. Science 153:708–716 http://dx.doi.org/10.1126/science.153.3737.708 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Basic characteristics of rpoS and rpoS+ strains constructed via ϕEB49-mediated allelic repair. The ygbN-nlpD loci of CFT073, CFT073 rpoS, rpoS+ transductants, and rpoS transductants are indicated. Red bars denote known transduced regions. Since no other selection was done, cotransduction of additional DNA (up to the total amount packaged into ϕEB49, ~50 kB) may have occurred in addition to that in the known region. As such, X5′ + X3′ ≈ 46.5 kB and Y5′ + Y3′ ≈ 48.5 kB. Download


