ABSTRACT
To enhance the research capabilities of investigators interested in Staphylococcus aureus, the Nebraska Center for Staphylococcal Research (CSR) has generated a sequence-defined transposon mutant library consisting of 1,952 strains, each containing a single mutation within a nonessential gene of the epidemic community-associated methicillin-resistant S. aureus (CA-MRSA) isolate USA300. To demonstrate the utility of this library for large-scale screening of phenotypic alterations, we spotted the library on indicator plates to assess hemolytic potential, protease production, pigmentation, and mannitol utilization. As expected, we identified many genes known to function in these processes, thus validating the utility of this approach. Importantly, we also identified genes not previously associated with these phenotypes. In total, 71 mutants displayed differential hemolysis activities, the majority of which were not previously known to influence hemolysin production. Furthermore, 62 mutants were defective in protease activity, with only 14 previously demonstrated to be involved in the production of extracellular proteases. In addition, 38 mutations affected pigment formation, while only 7 influenced mannitol fermentation, underscoring the sensitivity of this approach to identify rare phenotypes. Finally, 579 open reading frames were not interrupted by a transposon, thus providing potentially new essential gene targets for subsequent antibacterial discovery. Overall, the Nebraska Transposon Mutant Library represents a valuable new resource for the research community that should greatly enhance investigations of this important human pathogen.
IMPORTANCE
Infections caused by Staphylococcus aureus cause significant morbidity and mortality in both community and hospital environments. Specific-allelic-replacement mutants are required to study the biology of this organism; however, this process is costly and time-consuming. We describe the construction and validation of a sequence-defined transposon mutant library available for use by the scientific community through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) strain repository. In addition, complementary resources, including a website (http://app1.unmc.edu/fgx/) and genetic tools that expedite the allelic replacement of the transposon in the mutants with useful selectable markers and fluorescent reporter fusions, have been generated. Overall, this library and associated tools will have a significant impact on studies investigating S. aureus pathogenesis and biology and serve as a useful paradigm for the study of other bacterial systems.
Introduction
Staphylococcus aureus is a highly significant pathogen causing multiple types of infections in both the community and the nosocomial environment. Infections caused by this bacterium range from minor pustules and skin and soft tissue infections to biofilm-mediated infections, necrotizing pneumonia, endocarditis, and osteomyelitis. Central to the pathogenicity of S. aureus is the synthesis of many coordinately regulated virulence factors, including superantigens, adherence factors, pore-forming toxins, and immunological mediators (1). We are just beginning to understand the significance of interactions between virulence factors and their relationship to specific types of infections. For example, utilizing a mouse sepsis model, Cheng and colleagues demonstrated that early-phase dissemination of S. aureus is ClfA and ClfB dependent but that IsdA, IsdB, Spa, and SdrD are required for kidney abscess formation (2), suggesting that unique factors are required at distinct stages in the pathogenesis process. Furthermore, factors responsible for foreign-body-mediated biofilm infections are clearly distinct from those responsible for disseminated disease (3). Additional detailed studies are required to fully understand the specific metabolic genes or virulence factors that are required for specific types of infection to promote the development of novel therapeutic modalities, including vaccines.
To support studies aimed at understanding the pathogenesis, colonization, and biology of S. aureus, we developed a sequence-defined bursa aurealis (4) transposon (Tn) library in S. aureus USA300 (5) JE2 called the Nebraska Transposon Mutant Library (NTML), which is deposited in the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) strain repository. bursa aurealis is a mariner-based transposon (6, 7) that has previously been shown to generate random transposon mutations in S. aureus (4). The USA300 (ST8) JE2 genetic background was utilized as a host for the NTML due to its decade-long stability as the most prominent community-associated methicillin-resistant S. aureus (CA-MRSA) lineage in the United States and because its genome sequence is known (5, 8). The NTML will provide investigators with a tool to address specific hypotheses rapidly without undergoing the time-constraining process of creating specific-allelic-replacement mutations. Currently, 1,952 specific bursa aurealis mutants of the USA300 strain JE2 have been deposited in the NARSA strain repository, available to all registered investigators (http://www.narsa.net).
RESULTS AND DISCUSSION
Generation of the Nebraska Transposon Mutant Library.
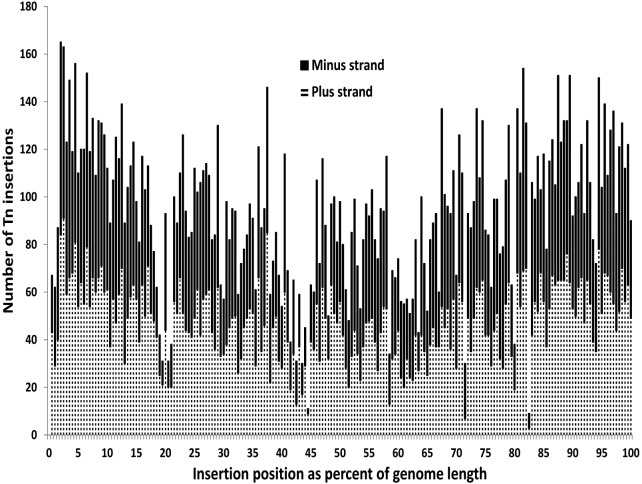
The S. aureus USA300 FPR3757 chromosomal genome sequence (GenBank accession number NC_007793.1) was used to map transpositions of bursa aurealis from the delivery plasmid pBursa into the genome of S. aureus JE2 (see Fig. S1 and S2 in the supplemental material). The Nebraska Transposon Mutant Library (NTML) is a carefully selected subset of 1,952 protein coding sequences (CDS) disrupted by bursa aurealis Tn insertion mutant clones from a larger set of 18,797 Tn insertion clones from which mappable DNA sequencing reads were obtained. The NTML provides the research community with a comprehensive set of Tn insertion mutant clones at sites throughout the JE2 chromosome, with the distribution of hits unbiased by Tn insertion orientation or the length of the AciI-bounded fragments (range, 3 to 8,709 bp; median, 425 bp; interquartile range, 146 to 923 bp) from which pBursa-derived Tn insertions are characterized (Fig. 1 and 2; see also Fig. S3). The average BLASTn alignment obtained when mapping Tn insertion clone-derived Sanger DNA sequencing reads against the FPR3757 genome (which differs from the JE2 genome at only 11 single nucleotide polymorphisms [SNPs] [8]) was 448 bp long (standard deviation = 284 bp), with an expected value of 1e−135 and a percent identity of 97% (see Fig. S2). The cumulative sequencing progress shown in Fig. S4 and S5 indicates that additional sequencing beyond the 18,797 Tn insertion clones sequenced to date is unlikely to increase the number of distinct chromosomal CDS gene hits substantially.
FIG 1 .
Distribution of Nebraska Transposon Mutant Library Transposon insertion sites in the S. aureus USA300 LAC JE2 genome.
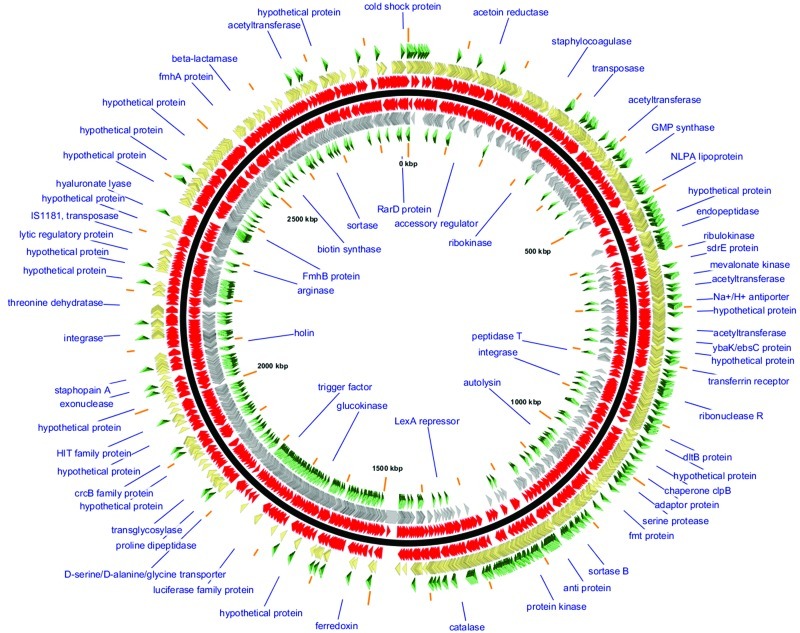
FIG 2 .
GView visualization of the mapping of bursa aurealis transposon insertion mutants of the Nebraska Transposon Mutant Library (NTML). GView (44) software was used to visualize the entire NTML using a custom GView style sheet and a version of the S. aureus USA300 FPR3757 RefSeq genome GenBank sequence (accession number NC_007793.1). The map’s backbone (central solid black line) distinguishes the given (positive) strand of the GenBank sequence (shown outside the backbone) from the reverse complement strand (shown inside the backbone). Tn insertions (red arrowheads, closest to the backbone and so dense that they often merge at the resolution shown) and CDS genes for which Tn insertion mutants were not obtained (green arrowheads, furthest from the backbone) are also shown on either strand. Annotated GenBank genes are shown as arrowheads (gold or silver on either strand, as indicated).
Table 1 provides an overview of the annotated features in the RefSeq DNA sequence for the USA300 FPR3757 chromosomal genome. The 1,952 NTML clones all disrupt the CDS, whereas 27% of the 16,845 “extra” Tn insertion mutations map to the 16% of the chromosomal genome that is intergenic (Tables 1 and 2). Over two-thirds (68%) of the NTML Tn mutant clones were selected because of the presence of Tn insertions in the first third of the length of the affected gene. Another 24% of the clones contained Tn insertions between the first one-third and two-thirds of the affected genes’ lengths, and only 7% of the mutant clones had insertions beyond two-thirds of the affected gene. No mutant clones with insertions beyond 90% of the genes’ lengths were included in the NTML. Thus, it is predicted that the function of each gene/protein will be lost due to the insertion of the transposon in each NTML mutant, maximizing the utility of the library for functional genomic analyses.
TABLE 1 .
GenBank RefSeq feature table entries for the USA300 FPR3757 genomea
|
Staphylococcus aureus subsp. aureus USA300 FPR3757 GenBank RefSeq feature |
Count | % | No. of bp (total) | % |
|---|---|---|---|---|
| Entire genome | 2,872,769 | 100.00 | ||
| All genes | 2,648 | 100.00 | 2,408,457 | 83.84 |
| CDS genes | 2,560 | 96.68 | 2,359,416 | 82.13 |
| rRNA genes | 16 | 0.60 | 23,080 | 0.80 |
| tRNA genes | 53 | 2.00 | 4,099 | 0.14 |
| Pseudogenes | 18 | 0.68 | 21,503 | 0.75 |
| Other genes, including ssrA (SAUSA300_0766; 10Sa RNA→tmRNA) |
1 | 0.04 | 359 | 0.01 |
| Intergenic sequences | 464,312 | 16.16 | ||
| Nongene features (SCCmecIV, ACME, vSAα, SaPI5, ϕSA2USA, vSAβ, ϕSA3USA) |
7 | 224,465 | 7.81 |
tmRNA, transfer-messenger RNA; SCCmecIV, staphylococcal cassette mec chromosome IV.
TABLE 2 .
Summary of NTML and extra Tn insertion mutants in the S. aureus USA300 LAC JE2 genome, based on mapping software results using the USA300 FPR3757 GenBank genome
| Type of insertion | NTML clones |
Extra insertion mutants |
||
|---|---|---|---|---|
| Count | % | Count | % | |
| Transposon insertions between and within genes | ||||
| All insertions | 1,952 | 100 | 16,845 | 100 |
| Insertions between genes | 0 | 0 | 4,632 | 27 |
| Insertions affecting ≥1 genea | 1,956 | 100 | 12,228 | 73 |
| Insertions affecting 2 genesa | 4 | 15 | ||
| Insertions affecting >2 genesa | 0 | 0 | ||
| Transposon insertions within CDS genes | ||||
| All insertions | 1,954 | 100 | 12,034 | 100 |
| Within the first third of gene length | 1,336 | 68 | 3,889 | 32 |
| Within the second third of gene length | 472 | 24 | 3,934 | 33 |
| Within the last third of gene length | 146 | 7 | 4,211 | 35 |
| Distinct CDS genes | 2,560 | 100 | 2,560 | 100 |
| Distinct CDS with 0 insertions | 615 | 24 | 661 | 26 |
| Distinct CDS with ≥1 insertion | 1,945 | 76 | 1,899 | 74 |
| Within the first third of gene length | 1,332 | 1,194 | ||
| Within the second third of gene length | 472 | 1,336 | ||
| Within the last third of gene length | 146 | 1,456 | ||
Gene = CDS, rRNA, tRNA, pseudogene, or other gene.
Phenotypic analysis of the Nebraska Transposon Mutant Library.
To demonstrate the utility of the NTML in the identification of genes involved in various processes important to S. aureus pathogenesis and metabolism, we screened all 1,952 mutants in agar plate assays for hemolysin production, protease activity, carotenoid pigment formation, and mannitol fermentation (see Table S1 in the supplemental material). It is important to note that we did not perform studies to assess the possibility of polar effects. However, many of the mutants exhibiting phenotypes described below have Tn insertion phenotypes that stand alone and are not associated with other upstream or downstream Tn insertions that produced the same phenotypes, suggesting the absence of polar effects. Regardless, the phenotype associated with each mutant in the library must be confirmed using standard genetic techniques, including phage backcross, complementation, and possibly specific allelic replacement.
Hemolysis.
To assess the impact of mutations on α-hemolysin production, we spotted each mutant onto rabbit blood agar medium and examined the sizes of the zones of hemolysis produced by each strain. The observed hemolytic activity was found to be dependent solely on α-hemolysin, since the insertion in hla (SAUSA300_1058) was completely nonhemolytic and none of the other hemolysin mutants exhibited altered hemolytic activity in this assay. As shown in Table S1 in the supplemental material, we identified 71 mutants that exhibited alterations in the amount of α-hemolysin produced. Sixty-four of these demonstrated reduced hemolysin production, while seven exhibited increased production. As expected, mutations in hla, agr (agrA, agrB, and agrC), and saeR resulted in reduced hemolysis (9, 10), while σB (rsbU, rsbV, rsbW, and rpoF) and codY mutations caused increased hemolysis (11, 12). Interestingly, we also identified several genes encoding metabolic enzymes, including ureF (encoding urease accessory protein), moeA (encoding molybdopterin biosynthesis protein A), brnQ (encoding branched-chain amino acid transport system II carrier protein), fba (encoding fructose bisphosphate aldolase), and sucC (encoding succinyl-coenzyme A [CoA] synthetase). Interestingly, the ureF gene is part of a large operon yet is the only gene of this operon whose disruption produces this phenotype. Besides CodY and the σB system, only two other mutant proteins resulted in increased hemolytic activity, SAUSA300_2026 and SAUSA300_0014, a PemK family and a DHH subfamily phosphodiesterase, respectively. We did not, however, identify altered hemolytic activity during this screen of mutants containing Tn insertions within several known regulators of α-hemolysin, including SarA (13), SarT (14), Rot (15), and ArlSR (16), likely due to the well-documented strain-dependent regulation of the hla gene (13). Interestingly, we found that an insertion into the gene encoding Stk1 (also called PknB) had decreased activity but that a previous mutant of strain Newman had the opposite phenotype (17).
Protease activity.
Protease activity produced by S. aureus has also been a widely studied trait with a variety of known roles in pathogenesis (18, 19). Although S. aureus produces an abundance of different proteases, casein agar plates were used to assess genes that function specifically in the hydrolysis of casein. As shown in Table S1 in the supplemental material, 62 mutants exhibited differences in protease activity in this assay. Fifty produced lower levels of protease activity, while 12 produced higher levels. Of those that produced lower protease levels, only two contained genes that encoded known secreted proteases, scpA (encoding staphopain A) and aur (encoding aureolysin). Tn insertions within other secreted-protease genes within the USA300 genome, including the spl operon (encoding serine proteases) and the V8 protease gene sspA, did not result in altered protease activity in comparison to that in JE2. Interestingly, 12 known regulators were also found to affect protease production. Six of these, agrA, agrB, agrC, ccpA, purR, and clpP, caused decreased protease activity consistent with previous reports (20–22), while six, sarA, rsbU, rsbV, rsbW, rpoF, and saeR, caused increased protease levels, as previously observed (23, 24). By far the most common class of genes found to affect protease production consisted of those encoding metabolic enzymes (Table S1), possibly reflecting the role of proteases in adapting to changing physiological conditions.
Pigment formation.
The well-recognized carotenoid pigment staphyloxanthin produced by S. aureus has been shown to be a key pathogenic factor important in the resistance of the bacteria to the oxidative burst elicited upon engulfment by immune cells (25, 26). The biosynthetic pathway leading to staphyloxanthin has been characterized, and the associated genes have been identified (27, 28). There are five genes in the crt operon (crtO, crtP, crtQ, crtM, and crtN) encoding the staphyloxanthin biosynthesis enzymes, all of which were found to eliminate pigment production (Fig. 3). Other mutants defective in earlier enzymes in this pathway also showed differences in pigment production. For example, the SAUSA300_1470 gene, encoding geranyltranstransferase, is involved in the biosynthesis of farnesyl diphosphate, an important precursor for carotenoid pigment and heptaprenyl diphosphate biosynthesis. Interestingly, a mutation affecting SAUSA300_1359, encoding polyprenyl synthetase, which converts farnesyl diphosphate to heptaprenyl diphosphate, results in dramatically increased carotenoid pigment production, suggesting that blocking this pathway provides more precursor for carotenoid pigment biosynthesis. In total, mutations in 38 genes, including sarA, agr (agrA, agrB, agrC), σB (rsbU, rsbV, rsbW, rpoF) (29), purA, purB, clpP, and argR, resulted in the loss of pigment production (see Table S1 in the supplemental material).
FIG 3 .
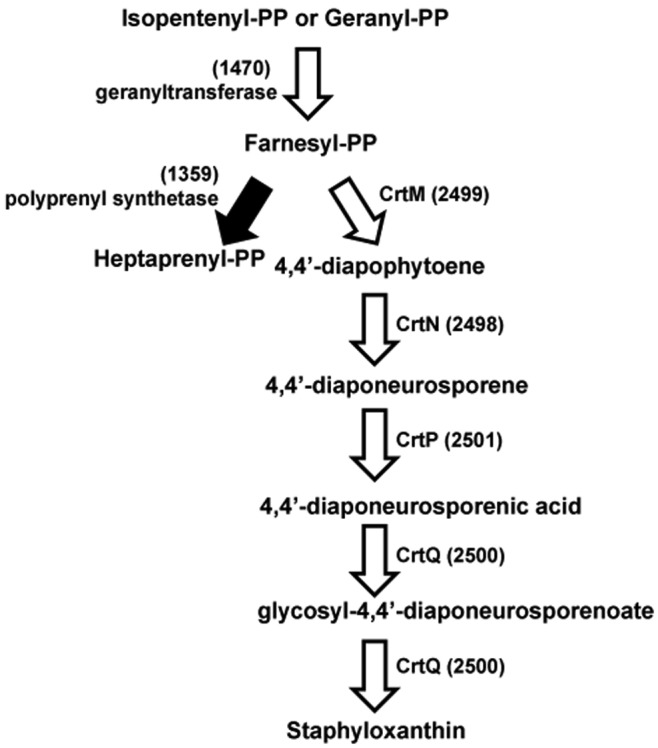
Staphyloxanthin biosynthesis. White arrows denote mutants with loss of pigmentation, and the black arrow denotes a mutant with increased pigmentations. Numbers in parentheses refer to the SAUSA300 ORF numbers encoding these proteins. PP, pyrophosphate.
Mannitol utilization.
We also tested the library for mutants defective in fundamental physiological functions, such as carbohydrate metabolism. Given the importance of mannitol fermentation as a diagnostic indicator and the ease of testing for this in a plate assay, we screened the library on mannitol agar media, which assesses mannitol utilization as a function of the acids produced in agar medium in which mannitol is used as the sole carbohydrate source. Following transport via a phosphoenolpyruvate (PEP)-dependent phosphotransferase system (PTS) system, mannitol-1-phosphate is converted to fructose-6-phosphate by mannitol-1-phosphate 5-dehydrogenase and ultimately to pyruvate through the activity of pyruvate kinase. Only 7 of the 1,952 mutants in the library revealed differences in mannitol utilization (Fig. 4). One of the mutations resulting in an inability to utilize mannitol was in SAUSA300_0984 (ptsI), annotated as encoding PEP phosphotransferase, which is involved in carbohydrate transport and the conversion of PEP into pyruvate. A second mutation was in SAUSA300_1644 (pyk), which encodes pyruvate kinase, the enzyme that converts PEP to pyruvate. The five additional mutations identified during the screen were all found in the mtl operon (Fig. 4). Two of these mutants, NE929 (mtlA) and NE1737 (mtlF), contained insertions within the two components of the mannitol-specific PTS system (30). The two remaining transposon insertions were located in the mtl gene cluster: a putative transcriptional regulator (SAUSA300_2106) and mannitol-1-phosphate 5-dehydrogenase (SAUSA300_2108). The paucity of genes involved in mannitol utilization not only suggests that relatively few genes function to control mannitol catabolism but also demonstrates the utility of the NTML in identifying genes resulting in rare phenotypes.
FIG 4 .
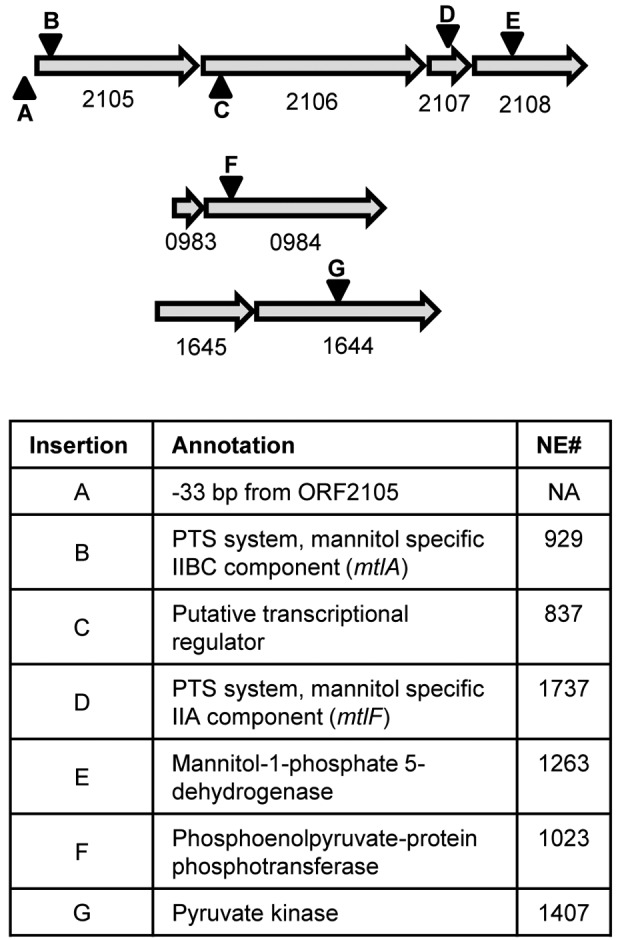
Mutants unable to utilize mannitol. Numbers below arrows are the SAUSA300 ORF numbers. Inverted triangles indicate locations of transposon insertions. All arrows are to scale. The NTML does not have mutants of 1645 or 0983. NE# is the identifying number for the corresponding strains in the NTML; NA, not applicable.
Essential genes.
Several previous studies have addressed gene essentiality in S. aureus (31–33). In particular, Chaudhuri et al. recently utilized a transposon-mediated differential hybridization approach to identify 351 essential genes in S. aureus NCTC 8325 (33). As shown in Table S2 in the supplemental material, excluding the rRNA and tRNA genes, 579 open reading frames (ORFs) were not interrupted by a transposon in JE2 and may potentially be considered essential; however, these data clearly need to be confirmed using further gene essentiality studies. Two hundred ninety-two of 351 (83.2%) essential genes as identified by Chaudhuri et al. were also potentially essential in JE2. Of the 59 remaining essential genes in NCTC 8325, where a Tn insertion was identified in JE2, 23 (39.0%) had the Tn insertion in ≥90% of the total ORF length, suggesting the synthesis of a functional protein with the transposon inserted into the ORF (Table S3). Of the remaining 287 genes not interrupted in JE2 (Table S2), many are not predicted to be essential or are very small ORFs, suggesting that certain areas of the chromosome may not be amenable to bursa aurealis insertion and/or may have incomplete Tn saturation. However, it is possible that specific, unique genes are essential in JE2 under the growth conditions used in this assay. Supporting this hypothesis, 15/287 additional essential genes in JE2 (Table S2) are considered essential in B. subtilis (33) but not in NCTC 8325.
In conclusion, we have generated a sequence-defined Tn mutant library with S. aureus USA300 JE2, from which registered NARSA users can rapidly acquire at no cost mutants of interest to further the study of this bacterium. To assist in the analysis of this resource, we have also developed a website (http://app1.unmc.edu/fgx/) that provides an interactive and user-friendly tool for the evaluation of the mutants available in this collection. The phenotypic screens performed in this study strongly suggest that the library is robust and carries the potential to uncover novel, previously unidentified, gene functions. Furthermore, to enhance the usefulness of the library, specific genetic tools have been developed to rapidly replace the erythromycin (Erm) resistance cassette within bursa aurealis with a tetM (tetracycline [Tet]/minocycline resistance), aphA-3 (kanamycin resistance), or aad9 (spectinomycin resistance) cassette (34). Other tools allow for transcriptional fusions with codon-optimized enhanced cyan fluorescent protein (ECFP), enhanced yellow fluorescent protein (EYFP), DsRed.T3(DNT), superfolder green fluorescent protein (sGFP), or eqFP650 (34). Overall, the availability of this unique combination of resources should greatly enhance the genetic analysis of this important pathogen.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Staphylococcus aureus strains used in this study were derived from a USA300 strain isolated from a skin and soft tissue infection in a detainee from the Los Angeles County Jail (LAC) (35). S. aureus strain LAC contains two plasmids, p01 (a 3.1-kb cryptic plasmid) and p03 (a 27-kb plasmid conferring resistance to erythromycin) (36). Both p01 and p03 were removed from LAC for two reasons. First, curing of these two plasmids eliminated potential plasmid incompatibility concerns with pFA545 and pBursa (see below), and second, curing ensured that the bursa aurealis transposon did not insert preferentially into plasmid DNA. For removal of p03, strain LAC was grown overnight in Tryptic soy broth (TSB) with shaking at 37°C without antibiotics. The next day, serial dilutions were made and the cells were plated on Trypic soy agar (TSA) plates without any antibiotics. Colonies arising after overnight incubation at 37°C were then replica plated onto plates with and without erythromycin (5 µg/ml). Those colonies unable to grow on erythromycin were chosen, and the loss of the plasmid was confirmed by performing plasmid analysis (Promega, Madison, WI). After confirmation that no major genomic rearrangements had occurred by pulsed-field gel electrophoresis (PFGE) (37), one colony, designated LAC-13C, was selected for subsequent manipulation.
We next cured p01 from LAC-13C utilizing a plasmid incompatibility strategy. First, a chimeric plasmid in which pCL84 (conferring tetracycline resistance) (38) and p01 were ligated together at their unique EcoRI sites was created. This plasmid, designated pJE06, was then electroporated into Escherichia coli DH5α, purified, and subsequently electroporated into S. aureus RN4220 (39). pJE06 was then transduced from RN4220 using bacteriophage ϕ11 (40) into LAC-13C using methods as described by McNamara (41). Plasmid preparations of the resulting tetracycline-resistant transductants were screened for the loss of p01 due to plasmid incompatibility (42). The resulting strains were then cured of pJE06 by following the process described above for removal of pUSA03 except that the colonies were screened for tetracycline sensitivity. Multiple tetracycline-susceptible derivatives were examined by PFGE to ensure that no chromosomal rearrangements were detected; one strain was selected for subsequent use and designated JE2.
Transposon mutagenesis.
The mariner-based transposon (Tn) bursa aurealis was used to generate random Tn insertion mutations in S. aureus strain JE2 essentially as described by Bae et al. (4, 43). First, bacteriophage ϕ11 was used to transduce the bursa aurealis delivery plasmid pBursa into JE2 containing the transposase-encoding plasmid pFA545, with selection on TSA medium containing chloramphenicol (Cm) (10 µg/ml) and Tet (5 µg/ml). After growth for 48 h at 30°C to allow for transposition events, one colony was resuspended in 100 µl of prewarmed 45°C water and then 10 µl was plated onto TSA plates containing erythromycin (Erm) (25 µg/ml) and grown at 45°C for 12 to 24 h. Resulting colonies, irrespective of colony size, were then screened for loss of the temperature-sensitive plasmids pBursa and pFA545 by patching them on TSA-Erm (25 µg/ml), TSA-Cm (10 µg/ml), and TSA-Tet (5 µg/ml). Those colonies that were Cm and Tet susceptible but resistant to Erm were arrayed into 1-ml deep-well plates containing 400 µl of TSB-Erm (5 µg/ml) and grown at 37°C overnight. The next day, 400 µl of 50% glycerol was added to each well and the plates were stored in a −80°C freezer.
To identify the locations of the bursa aurealis transposon insertions, 400 µl of TSB-Erm (5 µg/ml) was inoculated into 96-well plates using a 96-prong replicator. After overnight growth, the Wizard genomic DNA purification kit (Promega) was used to isolate genomic DNA from the cultures with the following modifications. Briefly, after centrifugation at 4,100 rpm for 5 min in a Sorvall (Newtown, CT) Legend tabletop centrifuge, supernatants were removed, the content of each well was resuspended in 110 µl of 50 mM EDTA (pH 8.0), and 5 µl of 10-mg/ml lysostaphin was added. After incubation at 37°C for 60 min, 600 µl of Nuclei Lysis solution was added and the genomic DNA was collected according to the manufacturer’s instructions. After resuspension in Tris-EDTA (TE) buffer, approximately 2 µg of genomic DNA was digested with 10 units of AciI (New England Biolabs) at 37°C for 4 h. AciI was then heat inactivated at 65°C for 30 min; T4 DNA ligase (200 U) (Monserate Biotechnologies, San Diego, CA) was then added to each sample and ligated overnight at 4°C, followed by heat inactivation at 65°C for 30 min. DNA fragments spanning the bursa aurealis insertion sites in each sample were amplified using the Buster (5′ GCTTTTTCTAAATGTTTTTTAAGTAAATCAAGTACC 3′) and Martn-ermR (5′ AAACTGATTTTTAGTAAACAGTTGACGATATTC 3′) primer set. PCR conditions included 30 cycles with an annealing temperature of 63°C and an extension time of 3 min. Once amplified, samples of the DNA products were separated in a 1% agarose gel by electrophoresis, and the remainder was purified for sequencing using Exo-SAP-IT (GE Healthcare) according to the manufacturer’s instructions. Finally, determination of the nucleotide sequences of the genomic DNA flanking the transposons was achieved using the Buster primer at the DNA Microarray and Sequencing Core Facility at the University of Nebraska Medical Center.
Phenotype screens.
The NTML was screened for phenotypes on Handke mannitol medium (per liter, 10 g Bacto protease peptone, 1 g Bacto beef extract, 10 g d-mannitol, 5 g NaCl, 10 g Bacto yeast extract, and 2.5 mg phenol red, pH 7.4), TSA containing 1.5% nonfat dry milk as an indicator of protease activity, and TSA supplemented with 5% defibrinated rabbit blood (Becton, Dickinson, Franklin Lakes, NJ) for hemolysis activity and pigmentation changes. For the hemolysis tests, mutants were grown overnight at 37°C in deep-well 96-well plates with shaking. A 96-well solid-pin replica blotter was dipped into the cultures and then stamped onto each medium type and incubated at 37°C. Following 8 h of incubation, the plates were examined and phenotypes identified by visual inspection of zones of clearing around each colony. Any mutant with either a larger or smaller zone of clearing around the colony than that of the wild type were considered to have an altered activity. During the screen, care was taken to account for colony size differences. Likewise, protease activity and pigmentation alterations were identified by visual inspection of plates after a 24-h incubation at 37°C. The ability to ferment mannitol was viewed by a change in medium color from red to yellow around the colony. All mutants with phenotypes were arrayed into new 96-well plates with JE2 spaced intermittently as a control. These plates were then rescreened twice for observed phenotypes. Phenotypes that were observed in at least 2 out of the 3 examinations were considered valid.
SUPPLEMENTAL MATERIAL
Relevant landmarks in the DNA sequence of the mini-mariner bursa aurealis transposon (Tn) region of the delivery vector pBursa. The circular pBursa plasmid is 7,383 bp long. The 3,237-bp bursa aurealis region is between pBursa map coordinates 7383 and 4147, which include the flanking terminal inverted repeats. (a) Region of bursa aurealis that becomes incorporated into the S. aureus USA300 LAC JE2 genome of the Nebraska Transposon Mutant Library (NTML) clones. (b) Subregion of bursa aurealis between the ermB gene’s AciI site and the ermB Tn insertion terminus. We isolated DNA from Tn insertion clones and cleaved it with Acil. Then, genomic DNA beyond the ermB bursa aurealis terminus sequence (CCTGTTA) up to the nearest AciI site in the S. aureus USA300 LAC JE2 genome was isolated by circularization and amplified for DNA sequencing by inverse PCR (b and c) using the indicated reverse primer and either of the two forward primers shown (in most cases, the 42F forward primer was used). Map coordinates and DNA sequence landmarks are from the pBursa GenBank sequence (accession number AY672109.3) and are drawn to scale. The indicated strand orientation of bursa aurealis is shown because all mapped Tn insertion clones in the NTML are oriented with respect to transcription of the gfp and ermB genes, which are on the reverse complement strand of the pBursa GenBank sequence. Download
Alignment-based determination of coordinates and orientation for mapping of Tn insertion clone DNA sequencing reads to the S. aureus USA300 FPR3757 genome. This genome was used for mapping because it differs from the USA300 LAC JE2 genome at only 11 nucleotide positions. Download
Distribution of AciI fragment lengths in the S. aureus USA300 FPR3757 genome. Download
Saturation of CDS genes with Nebraska Transposon Mutant Library Tn insertions in the S. aureus USA300 LAC JE2 genome. Download
Influence of CDS gene length on Nebraska Transposon Mutant Library Tn insertion frequency. Download
Identified phenotypes.
Genes lacking Tn insertions.
Essential gene comparisons.
ACKNOWLEDGMENTS
This study was supported by Department of Defense grant W911NF-09-1-0164 and NIH P01 grant 3453012054304 to P.D.F. and K.W.B.
We would also like to thank Emergent Biosolutions Inc. for funding the development of the website.
Footnotes
Citation Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4(1):e00537-12. doi:10.1128/mBio.00537-12.
REFERENCES
- 1. Nizet V. 2007. Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J. Allergy Clin. Immunol. 120:13–22 [DOI] [PubMed] [Google Scholar]
- 2. Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. 2009. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 23:3393–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boles BR, Horswill AR. 2011. Staphylococcal biofilm disassembly. Trends Microbiol. 19:449–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bae T, Banger AK, Wallace A, Glass EM, Aslund F, Schneewind O, Missiakas DM. 2004. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc. Natl. Acad. Sci. U. S. A. 101:12312–12317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367:731–739 [DOI] [PubMed] [Google Scholar]
- 6. Lampe DJ, Churchill ME, Robertson HM. 1996. A purified mariner transposase is sufficient to mediate transposition in vitro. EMBO J. 15:5470–5479 [PMC free article] [PubMed] [Google Scholar]
- 7. Robertson HM, Lampe DJ. 1995. Recent horizontal transfer of a mariner transposable element among and between Diptera and Neuroptera. Mol. Biol. Evol. 12:850–862 [DOI] [PubMed] [Google Scholar]
- 8. Kennedy AD, Otto M, Braughton KR, Whitney AR, Chen L, Mathema B, Mediavilla JR, Byrne KA, Parkins LD, Tenover FC, Kreiswirth BN, Musser JM, DeLeo FR. 2008. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc. Natl. Acad. Sci. U. S. A. 105:1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mainiero M, Goerke C, Geiger T, Gonser C, Herbert S, Wolz C. 2010. Differential target gene activation by the Staphylococcus aureus two-component system saeRS. J. Bacteriol. 192:613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Recsei P, Kreiswirth B, O’Reilly M, Schlievert P, Gruss A, Novick RP. 1986. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol. Gen. Genet. 202:58–61 [DOI] [PubMed] [Google Scholar]
- 11. Bischoff M, Dunman P, Kormanec J, Macapagal D, Murphy E, Mounts W, Berger-Bächi B, Projan S. 2004. Microarray-based analysis of the Staphylococcus aureus sigmaB regulon. J. Bacteriol. 186:4085–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Majerczyk CD, Sadykov MR, Luong TT, Lee C, Somerville GA, Sonenshein AL. 2008. Staphylococcus aureus CodY negatively regulates virulence gene expression. J. Bacteriol. 190:2257–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zielinska AK, Beenken KE, Joo HS, Mrak LN, Griffin LM, Luong TT, Lee CY, Otto M, Shaw LN, Smeltzer MS. 2011. Defining the strain-dependent impact of the staphylococcal accessory regulator (sarA) on the alpha-toxin phenotype of Staphylococcus aureus. J. Bacteriol. 193:2948–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidt KA, Manna AC, Gill S, Cheung AL. 2001. SarT, a repressor of alpha-hemolysin in Staphylococcus aureus. Infect. Immun. 69:4749–4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li D, Cheung A. 2008. Repression of hla by rot is dependent on sae in Staphylococcus aureus. Infect. Immun. 76:1068–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fournier B, Klier A, Rapoport G. 2001. The two-component system ArlS-ArlR is a regulator of virulence gene expression in Staphylococcus aureus. Mol. Microbiol. 41:247–261 [DOI] [PubMed] [Google Scholar]
- 17. Burnside K, Lembo A, de Los Reyes M, Iliuk A, Binhtran NT, Connelly JE, Lin WJ, Schmidt BZ, Richardson AR, Fang FC, Tao WA, Rajagopal L. 2010. Regulation of hemolysin expression and virulence of Staphylococcus aureus by a serine/threonine kinase and phosphatase. PLoS One 5:e11071 http://dx.doi.org/10.1371/journal.pone.0011071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kantyka T, Shaw LN, Potempa J. 2011. Papain-like proteases of Staphylococcus aureus. Adv. Exp. Med. Biol. 712:1–14 [DOI] [PubMed] [Google Scholar]
- 19. Koziel J, Potempa J. 2012. Protease-armed bacteria in the skin. Cell Tissue Res. http://dx.doi.org/10.1007/s00441-012-1355-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Novick RP. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 48:1429–1449 [DOI] [PubMed] [Google Scholar]
- 21. Seidl K, Stucki M, Ruegg M, Goerke C, Wolz C, Harris L, Berger-Bächi B, Bischoff M. 2006. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 50:1183–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michel A, Agerer F, Hauck CR, Herrmann M, Ullrich J, Hacker J, Ohlsen K. 2006. Global regulatory impact of ClpP protease of Staphylococcus aureus on regulons involved in virulence, oxidative stress response, autolysis, and DNA repair. J. Bacteriol. 188:5783–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nygaard TK, Pallister KB, Ruzevich P, Griffith S, Vuong C, Voyich JM. 2010. SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J. Infect. Dis. 201:241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karlsson A, Arvidson S. 2002. Variation in extracellular protease production among clinical isolates of Staphylococcus aureus due to different levels of expression of the protease repressor sarA. Infect. Immun. 70:4239–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clauditz A, Resch A, Wieland KP, Peschel A, Götz F. 2006. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect. Immun. 74:4950–4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, Nizet V. 2005. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 202:209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu CI, Liu GY, Song Y, Yin F, Hensler ME, Jeng WY, Nizet V, Wang AH, Oldfield E. 2008. A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence. Science 319:1391–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pandit J, Danley DE, Schulte GK, Mazzalupo S, Pauly TA, Hayward CM, Hamanaka ES, Thompson JF, Harwood HJ. 2000. Crystal structure of human squalene synthase. A key enzyme in cholesterol biosynthesis. J. Biol. Chem. 275:30610–30617 [DOI] [PubMed] [Google Scholar]
- 29. Palma M, Cheung AL. 2001. Sigma(B) activity in Staphylococcus aureus is controlled by RsbU and an additional factor(s) during bacterial growth. Infect. Immun. 69:7858–7865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fischer R, Hengstenberg W. 1992. Mannitol-specific enzyme II of the phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus carnosus. Sequence and expression in Escherichia coli and structural comparison with the enzyme IImannitol of Escherichia coli. Eur. J. Biochem. 204:963–969 [DOI] [PubMed] [Google Scholar]
- 31. Forsyth RA, Haselbeck RJ, Ohlsen KL, Yamamoto RT, Xu H, Trawick JD, Wall D, Wang L, Brown-Driver V, Froelich JM, C KG, King P, McCarthy M, Malone C, Misiner B, Robbins D, Tan Z, Zhu Zy ZY, Carr G, Mosca DA, Zamudio C, Foulkes JG, Zyskind JW. 2002. A genome-wide strategy for the identification of essential genes in Staphylococcus aureus. Mol. Microbiol. 43:1387–1400 [DOI] [PubMed] [Google Scholar]
- 32. Ji Y, Zhang B, Van SF, Horn, Warren P, Woodnutt G, Burnham MK, Rosenberg M. 2001. Identification of critical staphylococcal genes using conditional phenotypes generated by antisense RNA. Science 293:2266–2269 [DOI] [PubMed] [Google Scholar]
- 33. Chaudhuri RR, Allen AG, Owen PJ, Shalom G, Stone K, Harrison M, Burgis TA, Lockyer M, Garcia-Lara J, Foster SJ, Pleasance SJ, Peters SE, Maskell DJ, Charles IG. 2009. Comprehensive identification of essential Staphylococcus aureus genes using transposon-mediated differential hybridisation (TMDH). BMC Genomics 10:291 http://dx.doi.org/10.1186/1471-2164-10-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bose JL, Fey PD, Bayles KW. 25 January 2013 Genetic tools to enhance the study of gene function and regulation in Staphylococcus aureus. Appl. Environ. Microbiol. http://dx.doi.org/10.1128/AEM.00136-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Saïd-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, DeLeo FR. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175:3907–3919 [DOI] [PubMed] [Google Scholar]
- 36. Kennedy AD, Porcella SF, Martens C, Whitney AR, Braughton KR, Chen L, Craig CT, Tenover FC, Kreiswirth BN, Musser JM, DeLeo FR. 2010. Complete nucleotide sequence analysis of plasmids in strains of Staphylococcus aureus clone USA300 reveals a high level of identity among isolates with closely related core genome sequences. J. Clin. Microbiol. 48:4504–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee CY, Buranen SL, Ye ZH. 1991. Construction of single-copy integration vectors for Staphylococcus aureus. Gene 103:101–105 [DOI] [PubMed] [Google Scholar]
- 39. Kreiswirth BN, Löfdahl S, Betley MJ, O’Reilly M, Schlievert PM, Bergdoll MS, Novick RP. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712 [DOI] [PubMed] [Google Scholar]
- 40. Novick R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155–166 [DOI] [PubMed] [Google Scholar]
- 41. McNamara PJ. 2008. Genetic manipulation of Staphylococcus aureus, p 89–129 In Lindsay JA, Staphylococcus molecular genetics. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 42. Novick RP. 1987. Plasmid incompatibility. Microbiol. Rev. 51:381–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bae T, Glass EM, Schneewind O, Missiakas D. 2008. Generating a collection of insertion mutations in the Staphylococcus aureus genome using bursa aurealis. Methods Mol. Biol. 416:103–116 [DOI] [PubMed] [Google Scholar]
- 44. Petkau A, Stuart-Edwards M, Stothard P, Van Domselaar G. 2010. Interactive microbial genome visualization with GView. Bioinformatics 26:3125–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relevant landmarks in the DNA sequence of the mini-mariner bursa aurealis transposon (Tn) region of the delivery vector pBursa. The circular pBursa plasmid is 7,383 bp long. The 3,237-bp bursa aurealis region is between pBursa map coordinates 7383 and 4147, which include the flanking terminal inverted repeats. (a) Region of bursa aurealis that becomes incorporated into the S. aureus USA300 LAC JE2 genome of the Nebraska Transposon Mutant Library (NTML) clones. (b) Subregion of bursa aurealis between the ermB gene’s AciI site and the ermB Tn insertion terminus. We isolated DNA from Tn insertion clones and cleaved it with Acil. Then, genomic DNA beyond the ermB bursa aurealis terminus sequence (CCTGTTA) up to the nearest AciI site in the S. aureus USA300 LAC JE2 genome was isolated by circularization and amplified for DNA sequencing by inverse PCR (b and c) using the indicated reverse primer and either of the two forward primers shown (in most cases, the 42F forward primer was used). Map coordinates and DNA sequence landmarks are from the pBursa GenBank sequence (accession number AY672109.3) and are drawn to scale. The indicated strand orientation of bursa aurealis is shown because all mapped Tn insertion clones in the NTML are oriented with respect to transcription of the gfp and ermB genes, which are on the reverse complement strand of the pBursa GenBank sequence. Download
Alignment-based determination of coordinates and orientation for mapping of Tn insertion clone DNA sequencing reads to the S. aureus USA300 FPR3757 genome. This genome was used for mapping because it differs from the USA300 LAC JE2 genome at only 11 nucleotide positions. Download
Distribution of AciI fragment lengths in the S. aureus USA300 FPR3757 genome. Download
Saturation of CDS genes with Nebraska Transposon Mutant Library Tn insertions in the S. aureus USA300 LAC JE2 genome. Download
Influence of CDS gene length on Nebraska Transposon Mutant Library Tn insertion frequency. Download
Identified phenotypes.
Genes lacking Tn insertions.
Essential gene comparisons.