Abstract
Objective
This study aimed to examine the association of hemiplegic shoulder pain with central hypersensitivity through pressure-pain thresholds (PPT) at healthy, distant tissues.
Design
This study is a cross-sectional study. A total of 40 patients (n=20 hemiplegic shoulder pain (HSP), n=20 stroke without HSP) were enrolled in this study. Pressure-pain thresholds were measured at the affected deltoid and contralateral deltoid and tibialis anterior using a handheld algometer. Differences in PPTs were analyzed by Wilcoxon Rank Sum test and with linear regression analysis controlling for gender, a known confounder of PPTs.
Results
Subjects with hemiplegic shoulder pain had lower local PPTs than stroke control subjects when comparing the painful to dominant shoulders and comparing the non-painful shoulder and tibialis anterior to the non-dominant side controls. Similarly, those with hemiplegic shoulder pain had lower PPTs when comparing to controls in contralesional-to-contralesional comparisons as well as ipsilesional-to-ipsilesional comparisons.
Conclusions
Subjects with hemiplegic shoulder pain have lower local and distal PPTs than subjects without hemiplegic shoulder pain. Our study suggests that chronic shoulder pain may be associated with widespread central hypersensitivity, which has been previously found to be associated with other chronic pain syndromes. This further understanding can then help develop better treatment options for those with this hemiplegic shoulder pain.
Keywords: Hemiplegic Shoulder Pain, PPT, Central Hypersensitivity, Chronic Pain
Hemiplegic shoulder pain (HSP) causes much discomfort and suffering. The typical clinical picture usually includes severe paralysis, glenohumeral subluxation, shoulder pain (occasionally radiating down to the elbow and hand), and localized tenderness over the biceps brachii and supraspinatus tendons.1–3 The patient usually complains of increased pain during passive motion or dependent position of the arm. The most painful and limited shoulder movement is usually external rotation, followed by abduction.1 Hemiplegic shoulder pain is associated with interference of activities of daily living4, a reduced quality-of life5, depression6, and disturbed sleep6. The prevalence of HSP is approximately 22–23% in the general population of stroke survivors and approximately 54–55% among stroke patients in rehabilitation settings.7 Up to a third of patients with HSP exhibit pain refractory to treatment4, a critical problem for those with HSP and for providers who treat stroke survivors.
The etiology of HSP is unknown. Kalichman and Ratmansky performed a literature review to better understand the underlying mechanism of HSP and the factors contributing to its development.7 They categorized HSP based on three underlying aspects: impaired motor control (muscle tone changes), soft-tissue lesions, and altered peripheral and central nervous activity. They concluded that these factors may present separately, may coexist simultaneously, or may evolve during the rehabilitation period by triggering each other’s development. A newer theory is that there is in initial injury to the weak shoulder that causes pain, but maladaptive changes in the peripheral and central nervous system may allow the pain to persist or worsen beyond the initial injury. 8–11 There is evidence that somatosensory changes may be associated with chronic pain from HSP9, 10, 12–15, and peripheral and central hypersensitivity has been implicated in the affected shoulder in those with HSP.9–11 To date, there have been no studies that have identified widespread hyperalgesia, evidence of central hypersensitivity, in those who experience chronic HSP.
Central hypersensitivity is an enhancement in the function of neurons and circuits in nociceptive pathways that can lead to pain from innocuous stimulation or exaggerated perception of pain from low-level painful stimuli. It is a manifestation of the plasticity of the somatosensory nervous system in response to inflammation and neural injury.16 Augmentation in pain perception has been shown to exist in tissues near the site of injury (peripheral or central hypersensitivity) as well as tissues distant to the site of injury (central hypersensitivity).17 Current measurement methods do not allow for discriminating between peripheral and central hypersensitivity; however, hypersensitivity in non-affected tissues distal to the injured tissues suggests central hypersensitivity. Central hypersensitivity has been shown to be associated with multiple other chronic pain syndromes, such as neck pain after whiplash injury18, fibromyalgia19, carpal tunnel syndrome20, osteoarthritis21, tension-type headache 22, temporomandibular joint pain23, and subacromial impingement syndrome24. These studies all showed that patients displayed exaggerated pain perception distal to the site of injury in unaffected, healthy tissues which suggests involvement of a central process.
An association of widespread central hypersensitivity and HSP will provide additional insight into potential causes for this condition and may have implications for treatment. One method of indirectly measuring central hypersensitivity is measuring mechanical hyperalgesia through the pressure-pain threshold (PPT). The PPT is the amount of pressure at which a sensation of pressure first changes to pain. Evidence of reduced pain thresholds has been found in those who are affected by chronic pain syndromes. 19, 23–25 The objective of our study is to examine the pain-pressure thresholds at tissues near the painful shoulder and in non-affected, healthy parts of the body in those with chronic HSP compared to a control group of stroke survivors without chronic pain. We hypothesize that PPTs at non-affected, distal tissues as well as local tissues near the site of injury are lower in subjects with HSP than in stroke survivors without HSP, indirectly indicating central hypersensitivity.
MATERIALS AND METHODS
Subjects
This study was approved by the institutional review board of the authors’ local institution. Subjects were recruited from an outpatient stroke clinic including physician and allied health services of an urban, academic hospital. After obtaining informed consent and establishing eligibility, baseline information was collected. Inclusion criteria for those with HSP included: 1) shoulder pain at rest, with passive abduction, or with active abduction; 2) age ≥ 21 years; 3) duration of shoulder pain > 6 months; 4) severity of shoulder pain ≥ 4 on a 0 to 10 scale at its worst in the last week; and 5) the pain occurred after stroke or was exacerbated by stroke; 6) stroke was unilateral and shoulder pain presented on opposite side of brain lesion Exclusion criteria for those with HSP included: 1) evidence of joint or overlying skin infection; 2) any other chronic pain syndrome; 3) prior shoulder surgery (affected limb); 4) cognitive or communicative impairments that prevent participation such as hemi-neglect, aphasia, cognitive impairments. Inclusion criteria for pain-free stroke subjects included: 1) age ≥ 21 years; 2) evidence of a prior stroke. Exclusion criteria included: 1) worst pain in last week > 3 on a 0 to 10 scale in any location; 2) pain in a single location at least 16 days of the last 30; 3) evidence of infection or injury at areas of PPT testing; and 4) cognitive or communicative impairments that prevent participation such as severe hemi-neglect, aphasia, or cognitive impairments.
Pressure-Pain Threshold Assessment
The PPT is defined as the minimal amount of pressure where a sense of pressure first changes to pain.26 Pressure algometry has been shown to reliably evaluate deep somatic tissue sensitivity within and between raters.27, 28 Two assessors obtained PPT measurements in subjects with HSP and controls. The assessors underwent training prior to the study to standardize measurement methods including subject positioning, assessor blinding to pressure reading, and rate of pressure application. Pressure-pain thresholds were measured with a hand-held Wagner Instruments FPIX Pain Test Algometer (Wagner Instruments, Greenwich, CT) with 0.785 cm2 rubber tip by applying pressure at a rate of 1 kg/cm2/sec 27 at three locations. Subjects were seated with arms at their sides. A PPT measurement was obtained in the same order for all subjects. The first measurement was at the mid-belly of the deltoid of the painful shoulder (or non-dominant deltoid for controls), followed by areas distant from the painful shoulder, the mid-belly of the deltoid muscle of the non-painful shoulder(or dominant shoulder for controls), and, the mid-belly of the tibialis anterior muscle of the non-painful side (or non-dominant leg for controls). A PPT was measured three times per patient on each site and the average was calculated and used for analysis.
Statistical Analysis
Differences in demographic variables between the two groups were analyzed with a chi-square test, or Fisher’s exact tests for small cell size (< 5) for categorical variables. Continuous variables were analyzed with the Wilcoxon Rank Sum test.
The comparisons to detect differences in PPTs were carried out in two ways. First, comparisons were made between the painful side of those with HSP and the dominant side of the control group. In this analysis, specific comparisons were between subjects with HSP and controls at the painful and dominant shoulders, non-painful and non-dominant shoulders, and at the tibialis anterior muscle of non-painful side and non-dominant sides, respectively. Second, to control for potential differences related to sensory changes due to the stroke, comparisons were made between the HSP and control subjects within contralesional and ipsilesional sides. In this analysis, the painful, contralesional shoulder of those with HSP is compared to the contralesional shoulder of controls, and the non-painful, ipsilesional shoulder of those with HSP is compared to the ipsilesional shoulder of controls. A comparison between ipsilesional tibialis anterior muscles of those with HSP and controls was also conducted.
Differences in the PPT between groups were analyzed by Wilcoxon Rank Sum test. To estimate the difference in PPTs between those with HSP and controls while controlling for gender we predicted PPTs from linear regression models with the covariate of gender added to the model. The 95% confidence intervals were calculated by boot-strapping the estimates (1000 iterations) and finding the 2.5 and 97.5 percentiles. Due to non-normality of the distributions of the local deltoid, non-affected deltoid and tibialis anterior PPTs, these distributions were transformed to their square root29 prior to the regression analysis and predicted values were back-transformed to the original scale before calculation of PPT differences.
RESULTS
A total of 40 patients (n=20 HSP, n=20 stroke without HSP) were enrolled in this study from March through September, 2011. Table 1 contains the demographic information collected on subjects in each group. There was significant difference between groups in allocation by gender (75% women in those with HSP vs. 40% for controls, p=0.02). There were no significant differences by age, race/ethnicity, type of stroke, hemorrhagic/ischemic stroke, history of neglect, history of abnormal sensation, history of aphasia, or medication use.
Table 1.
Demographic information
| HSP n=20 | Stroke control n=20 | p-value | |
|---|---|---|---|
| Age (median, interquartile range) | 57.5 (54.0 – 68.5) | 52.0 (45.3 – 60.0) | p=0.06 |
| Female (%) | 75 | 40 | p=0.02 |
| Ethnicity | p=1.0 | ||
| Caucasian (%) | 50 | 55 | |
| African-American (%) | 40 | 35 | |
| Hispanic (%) | 10 | 10 | |
| Type of stroke | p=0.16 | ||
| Hemorrhagic (%) | 20 | 45 | |
| Ischemic (%) | 70 | 40 | |
| Unknown (%) | 10 | 15 | |
| Left Hemispheric Stroke (%) | 40 | 60 | p=0.20 |
| Abnormal Sensation (%) | 45 | 45 | p=1.0 |
| Aphasia (%) | 40 | 35 | p=1.0 |
| Neglect (%) | 30 | 20 | p=0.72 |
| Medication Use | |||
| Aspirin (%) | 60 | 55 | p=0.8 |
| NSAID (%) | 15 | 5 | p=0.6 |
| Anti-Epileptic (%) | 25 | 10 | p=0.4 |
| Non-opiate analgesic (%) | 15 | 15 | p=1.0 |
| Anti-depressant (%) | 45 | 15 | p=0.08 |
| Opiate (%) | 25 | 5 | p=0.2 |
Abbreviations: HSP = hemiplegic shoulder pain; NSAID= non-steroidal anti-inflammatory drug
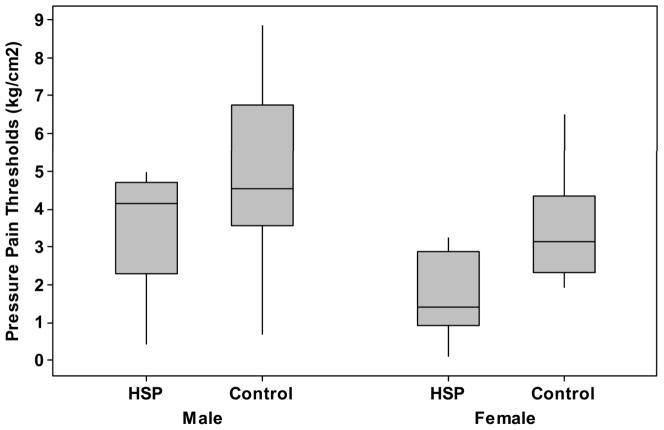
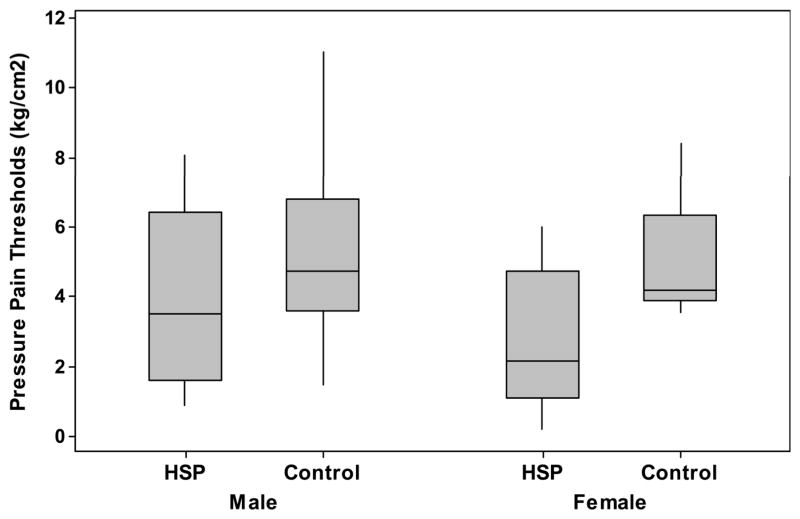
When comparing painful to dominant sides, and non-painful to non-dominant sides, subjects with HSP had lower PPTs than stroke control subjects at the painful/dominant shoulders (2.2 kg/cm2 (+/−1.5kg/cm2) vs. 4.3 kg/cm2 (+/−2.1 kg/cm2), p = 0.001), and lower distal PPTs than stroke control subjects at the non-painful/non-dominant shoulders (3.0 kg/cm2 (+/− 1.6 kg/cm2) vs. 4.6 kg/cm2(+/− 2.4 kg/cm2), p=0.02) and tibialis anterior muscle at the non-painful/non-dominant sides (3.0 kg/cm2 (+/− 2.0 kg/cm2) vs. 5.1 kg/cm2(+/− 2.1 kg/cm2), p = 0.004), see Table 2. Men consistently had higher thresholds than women at all testing locations, see Figures 1–2.
Table 2.
Pressure-pain thresholds for those with hemiplegic shoulder pain and stroke controls.
| HSP | Controls | p-value | |
|---|---|---|---|
| P/D Shoulder cm/kg2 (std) | 1.7 (+/− 1.2) | 3.4 (+/− 1.7) | 0.01 |
| Men | 2.9 (+/− 1.4) | 3.8 (+/− 1.9) | |
| Women | 1.3 (+/− 0.8) | 2.8 (+/− 1.2) | |
| NP/ND Shoulder cm/kg2 (std) | 2.3 (+/− 1.3) | 3.6 (+/− 1.9) | 0.02 |
| Men | 3.5 (+/− 1.5) | 4.1 (+/− 2.2) | |
| Women | 1.9 (+/− 1.0) | 2.8 (+/1.0) | |
| NP/ND Leg cm/kg2 (std) | 2.4 (+/− 1.6) | 4.1 (+/− 1.7) | 0.004 |
| Men | 3.1 (+/− 2.1) | 4.2 (+/− 1.9) | |
| Women | 2.1 (+/− 1.4) | 3.9 (+/− 1.4) | |
| CL/CL Shoulder cm/kg2 (std) | 1.7 (+/− 1.2) | 3.3 (+/− 1.6) | 0.002 |
| Men | 2.9 (+/− 1.4) | 3.7 (+/− 1.8) | |
| Women | 1.3 (+/− 0.8) | 2.7 (+/− 1.2) | |
| IL/IL Shoulder cm/kg2 (std) | 2.3 (+/− 1.3) | 3.7 (+/− 1.9) | 0.002 |
| Men | 3.5 (+/− 1.5) | 4.2 (+/− 2.3) | |
| Women | 2.0 (+/− 1.0) | 2.8 (+/1.0) | |
| IL/IL Leg cm/kg2 (std) | 2.4 (+/− 1.6) | 4.3 (+/− 1.9) | 0.007 |
| Men | 3.1 (+/− 2.1) | 4.3 (+/− 2.3) | |
| Women | 2.1 (+/− 1.4) | 4.1 (+/− 1.5) |
Abbreviations: HSP = hemiplegic shoulder pain, std= standard deviation; P=painful side in HSP; NP= non-painful side in HSP; D= dominant side in controls; ND- non-dominant side in controls; CL=contralesional side (HSP and controls); IL= ipsilesional side (HSP and controls)
Figure 1.
Pressure-pain thresholds (kg/cm2) of those with HSP (Pain) and pain-free stroke survivors (Control) of the, A) painful shoulder in HSP and dominant shoulder in controls; B) non-painful shoulder in HSP and non-dominant shoulder in controls; and, C) tibialis anterior muscle of non-painful side in HSP and non-dominant side in controls.
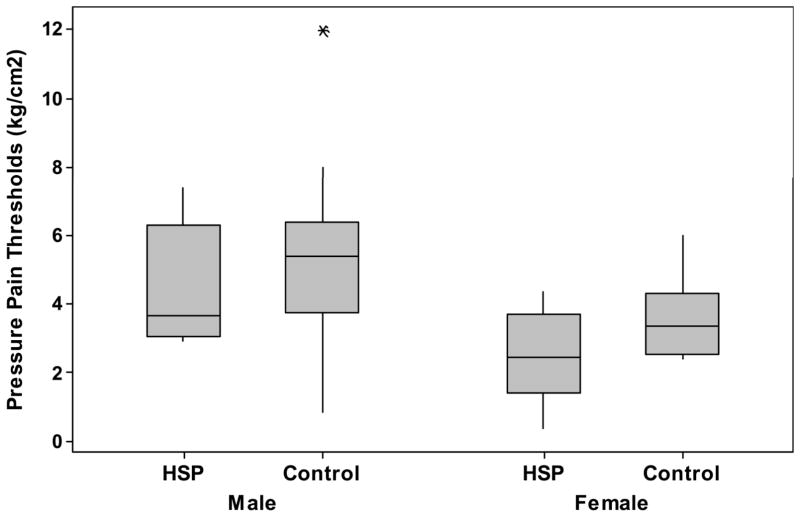
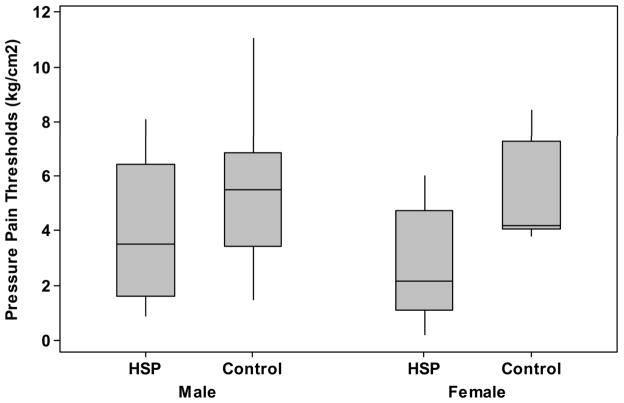
Figure 2.
Pressure-pain thresholds (kg/cm2) of those with HSP (Pain) and pain-free stroke survivors (Control) of the, A) contralesional shoulders; B) ipsilesional shoulders; and C) ipsilesional tibialis anterior muscles.
Comparisons between contralesional/contralesional sides, and ipsilesional/ipsilesional sides, of subjects with HSP and controls had similar results. Note, the number of measurements for ipsilesional tibialis anterior muscles of the HSP group is 14 of the 20 subjects due to study design. The number of shoulder comparisons are 20 in both the contralesional and ipsilesional comparisons. Subjects with HSP had lower PPTs than stroke control subjects at the at the contralesional shoulders (2.2 kg/cm2 (+/− 1.5 kg/cm2) vs. 4.2 kg/cm2 (+/−2.1 kg/cm2), p = 0.002). At sites distal from the painful shoulder of those with HSP, the PPTs were lower for those with HSP than stroke control subjects at the ipsilesional shoulder (3.0 kg/cm2 (+/− 1.6 kg/cm2) vs. 4.7 kg/cm2(+/− 2.5 kg/cm2), p=0.02) and tibialis anterior at ipsilesional side (3.0 kg/cm2 (+/− 2.0 kg/cm2) vs. 5.4 kg/cm2(+/− 2.5 kg/cm2), p = 0.007), see Table 2.
The model for predicting the difference in PPT between the painful shoulder of the HSP group and dominant shoulder of the controls when controlling for gender was significant (adjusted r2= 0.33, F(1, 39) = 10.8, p=0.0002) and the predicted difference of 2.1 kg/cm2 (95% CI 1.8 – 2.4 kg/cm2) lower for those with HSP than controls. The model for the difference in PPT at the non-painful shoulder compared to non-dominant shoulder was significant (adjusted r2=0.24, F(1, 39)=7.0, p=0.003) and those with HSP had a predicted 1.5 kg/cm2 (95% CI 1.2 – 1.9 kg/cm2) lower PPT than controls. Similarly, the model for the tibialis anterior muscle on the non-painful and non-dominant side was significant (adjusted r2=0.22, F(1, 39) = 6.6, p=0.003) and a lower predicted PPT for those with HSP than controls of 2.3 kg/cm2 (95% CI 2.2 – 2.5 kg/cm2).
The model for predicting the difference in PPT between the contralesional shoulders of the HSP group and control group when controlling for gender was significant (adjusted r2= 0.32, F(1, 39) = 10.2, p=0.0003) and the predicted difference of 2.1 kg/cm2 (95% CI 1.7 – 2.3 kg/cm2) lower for those with HSP than controls. The model for the difference in PPT at the ipsilesional shoulders was significant (adjusted r2=0.25, F(1, 39)=7.6, p=0.002) and those with HSP had a predicted 1.6 kg/cm2 (95% CI 1.2 – 2.0 kg/cm2) lower PPT than controls. Similarly, the model for the tibialis anterior muscle on the ipsilesional side was significant (adjusted r2=0.21, F(1, 33) = 5.4, p=0.01) and a lower predicted PPT for those with HSP than controls of 2.5 kg/cm2 (95% CI 2.3 – 2.7 kg/cm2).
DISCUSSION
Chronic HSP affects many stroke survivors and is often difficult to treat. The lack of knowledge of the pathogenesis of chronic HSP contributes to the challenge of developing an effective treatment. One of the factors hypothesized to play a role in chronic HSP is central sensitivity, and recent studies have provided initial evidence that those experiencing HSP have somatosensory abnormalities.9–11 This study provides evidence of central hypersensitivity through widespread, reduced pressure-pain thresholds, or a lower threshold for the experience of pain, in those with chronic HSP.
This study indicates that subjects with chronic HSP not only have lower PPTs at their painful shoulder than pain-free control subjects, but also have lower PPTs at distal, pain-free sites, which provides evidence of widespread, central hypersensitivity to pain. If the finding were limited to the painful shoulder it would not be possible to discern between peripheral hypersensitivity, central hypersensitivity, or sensory abnormalities due to a spinothalamocortical lesion. The finding that distal, pain-free sites experience pain at lower pressure levels supports that a central process may affect the perception of pain over the whole body of those with chronic HSP. Evidence of central hypersensitivity in chronic HSP suggests that new targets for treatment should be explored, those that are not attempting to ameliorate the painful response to a noxious stimulus (nociceptive pain) but instead address changes within the nervous system that result in central hypersensitivity. It has been hypothesized that electrical stimulation could be a neuromodulatory treatment that works in this manner8, 30, though this has not yet been shown in clinical trials.
Our study is the first to show reduced PPTs in distal, non-affected tissues of those with chronic HSP compared to pain-free stroke controls. Roosink, et al.10 surmise a role of central hypersensitivity in HSP through observed somatosensory differences between those with HSP, pain-free stroke survivors, and a healthy population; however, no differences were found in those with HSP and pain-free stroke survivors in electrical- or pressure -pain thresholds at the affected or unaffected shoulders. Greater allodynia to cold and sharpness were found when comparing ratios of affected to unaffected arms of those with HSP and pain-free stroke survivors; however, it is not discernable whether these differences are related to a spinothalamocortical lesion, peripheral hypersensitivity, or central hypersensitivity.9, 10 It is not clear why Roosink, et al. did not find evidence of widespread hyperalgesia to pressure-pain as we did in this study. Inclusion and exclusion criteria were similar, although there are a few methodological differences. Similar to our study, Roosink10 analyzes side-to-side comparisons to detect abnormal pressure-pain perception, although limited the examination sites to the shoulders, whereas our study also included the tibialis anterior muscle. Roosink measured 3 separate sites on each deltoid muscle and recorded the average, whereas we performed 3 measurements at the same site of each deltoid muscle.
Other differences exist in study design and interpretation of findings differ from our approach. In this study we compare painful/non-painful to dominant/non-dominant, respectively, as well as compare between contralesional sides and ipsilesional sides, and infer evidence of central hypersensitivity in the presence of hyperalgesia in distal, healthy tissues of those with HSP compared to controls. In addition to direct comparison of site-specific PPT values, Roosink utilizes intraindividual side-to-side comparisons to detect differences in pressure-pain thresholds and compares proportions of abnormal individuals between groups. Recommendations for using intraindividual side-to-side comparisons on the basis that they are more sensitive to detection of sensory abnormalities31 may not be ideal for detecting widespread hyperalgesia that is seen in central hypersensitivity. This may be more of a problem in the stroke population given the high prevalence of unilateral sensory abnormalities.7, 12
There are limitations that should be kept in mind in this study. One main limitation of our study is the small sample size. Future studies should include larger samples as important differences may exist between subgroups that could provide further information about central hypersensitivity and HSP. A further limitation is that PPTs are indirect and subjective measures of central hypersensitivity. While other objective, indirect measures of central hypersensitivity have been studied in other populations 32, 33, at this time there are no direct measures available to researchers or clinicians. We feel that relative PPT values can serve as a proxy for central hypersensitivity and the association with chronic pain. The evaluators in this study were also not blinded to group and, with that knowledge, have potential for introducing bias to the results. We attempted to diminish such bias by having the evaluators perform the PPT testing while being blinded to the pressure reading. There is evidence that psychological measures may affect pain perception, however we did not gather psychological information of subjects in this study. However, this may not be as problematic for this study given results of a recent study that showed PPTs were not correlated with psychological factors in women.34 Other factors that could affect PPTs, such as painless diabetic neuropathy or severity of motor impairment, cannot be ruled out as potentially influencing results. Finally, this study provides no information about causality. One longitudinal study has shown lowered PPT thresholds after onset of chronic pain35 though no study has replicated those results. There is also evidence that pain thresholds can predict development of chronic pain36, so the direction of the relationship is not known at this time.
Further studies need to be conducted to determine appropriate interpretation of the phenomena observed here, such as a longitudinal analysis of PPTs in local and distal, healthy tissues in a population of stroke survivors to observe changes in PPTs relative to development and treatment of HSP. It is also important to determine ideal methods for detecting central hypersensitivity in the stroke population and within individuals. Ideally, a single test would be able to determine if an individual was experiencing central hypersensitivity
CONCLUSION
Evidence that widespread central hypersensitivity is associated with chronic HSP is evidenced by the presence of lower pain thresholds (hypersensitivity to pressure pain) at distal, healthy tissues when compared to control subjects. Further exploration into the role central hypersensitivity in HSP should be undertaken. The results of this study may be useful creating more effective and easy to use treatments for HSP.
Acknowledgments
Secure data storage was made possible through grants M01 RR00080 and UL1 RR024989 from NCRR/NIH. Material support and salary support (Chae, Wilson) was provided through K24HD054600 from NICHD/NIH.
We thank Steven M. Sidik, affiliated with (1) Cleveland FES Center (Staff Statistician), and (2) Department of Statistics, Case Western Reserve University (Lecturer) for assistance in statistical analyses.
Footnotes
Disclosures:
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
References
- 1.Griffin JW. Hemiplegic shoulder pain. Physical therapy. 1986;66(12):1884–93. doi: 10.1093/ptj/66.12.1884. [DOI] [PubMed] [Google Scholar]
- 2.Jensen EM. The hemiplegic shoulder. Scandinavian journal of rehabilitation medicine Supplement. 1980;7:113–9. [PubMed] [Google Scholar]
- 3.Najenson T, Pikielny SS. Malalignment of the Gleno-Humeral Joint Following Hemiplegia. A Review of 500 Cases. Annals of physical medicine. 1965;8:96–9. doi: 10.1093/rheumatology/8.3.96. [DOI] [PubMed] [Google Scholar]
- 4.Wanklyn P, Forster A, Young J. Hemiplegic shoulder pain (HSP): natural history and investigation of associated features. Disability and rehabilitation. 1996;18(10):497–501. doi: 10.3109/09638289609166035. [DOI] [PubMed] [Google Scholar]
- 5.Chae J, Mascarenhas D, Yu DT, Kirsteins A, Elovic EP, Flanagan SR, et al. Poststroke shoulder pain: its relationship to motor impairment, activity limitation, and quality of life. Archives of physical medicine and rehabilitation. 2007;88(3):298–301. doi: 10.1016/j.apmr.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Savage R, Robertson L. Relationship between adult hemiplegic shoulder pain and depression. Physiother Can. 1982;34:86–90. [Google Scholar]
- 7.Kalichman L, Ratmansky M. Underlying pathology and associated factors of hemiplegic shoulder pain. American journal of physical medicine & rehabilitation/Association of Academic Physiatrists. 2011;90(9):768–80. doi: 10.1097/PHM.0b013e318214e976. [DOI] [PubMed] [Google Scholar]
- 8.Yu DT, Chae J, Walker ME, Kirsteins A, Elovic EP, Flanagan SR, et al. Intramuscular neuromuscular electric stimulation for poststroke shoulder pain: a multicenter randomized clinical trial. Archives of physical medicine and rehabilitation. 2004;85(5):695–704. doi: 10.1016/j.apmr.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Roosink M, Renzenbrink GJ, Buitenweg JR, Van Dongen RT, Geurts AC, MJIJ Persistent shoulder pain in the first 6 months after stroke: results of a prospective cohort study. Archives of physical medicine and rehabilitation. 2011;92(7):1139–45. doi: 10.1016/j.apmr.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Roosink M, Renzenbrink GJ, Buitenweg JR, van Dongen RT, Geurts AC, Ijzerman MJ. Somatosensory symptoms and signs and conditioned pain modulation in chronic post-stroke shoulder pain. The journal of pain: official journal of the American Pain Society. 2011;12(4):476–85. doi: 10.1016/j.jpain.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Roosink M, Buitenweg JR, Renzenbrink GJ, Geurts AC, Ijzerman MJ. Altered cortical somatosensory processing in chronic stroke: A relationship with post-stroke shoulder pain. NeuroRehabilitation. 2011;28(4):331–44. doi: 10.3233/NRE-2011-0661. [DOI] [PubMed] [Google Scholar]
- 12.Lindgren I, Jonsson AC, Norrving B, Lindgren A. Shoulder pain after stroke: a prospective population-based study. Stroke; a journal of cerebral circulation. 2007;38(2):343–8. doi: 10.1161/01.STR.0000254598.16739.4e. [DOI] [PubMed] [Google Scholar]
- 13.Gamble GE, Barberan E, Laasch HU, Bowsher D, Tyrrell PJ, Jones AK. Poststroke shoulder pain: a prospective study of the association and risk factors in 152 patients from a consecutive cohort of 205 patients presenting with stroke. European journal of pain. 2002;6(6):467–74. doi: 10.1016/s1090-3801(02)00055-1. [DOI] [PubMed] [Google Scholar]
- 14.Gamble GE, Barberan E, Bowsher D, Tyrrell PJ, Jones AK. Post stroke shoulder pain: more common than previously realized. European journal of pain. 2000;4(3):313–5. doi: 10.1053/eujp.2000.0192. [DOI] [PubMed] [Google Scholar]
- 15.Widar M, Samuelsson L, Karlsson-Tivenius S, Ahlstrom G. Long-term pain conditions after a stroke. Journal of rehabilitation medicine: official journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2002;34(4):165–70. doi: 10.1080/16501970213237. [DOI] [PubMed] [Google Scholar]
- 16.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. The journal of pain: official journal of the American Pain Society. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curatolo M, Arendt-Nielsen L, Petersen-Felix S. Central hypersensitivity in chronic pain: mechanisms and clinical implications. Physical medicine and rehabilitation clinics of North America. 2006;17(2):287–302. doi: 10.1016/j.pmr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Scott D, Jull G, Sterling M. Widespread sensory hypersensitivity is a feature of chronic whiplash-associated disorder but not chronic idiopathic neck pain. The Clinical journal of pain. 2005;21(2):175–81. doi: 10.1097/00002508-200503000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, et al. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis and rheumatism. 2003;48(5):1420–9. doi: 10.1002/art.10893. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-de-las-Penas C, de la Llave-Rincon AI, Fernandez-Carnero J, Cuadrado ML, Arendt-Nielsen L, Pareja JA. Bilateral widespread mechanical pain sensitivity in carpal tunnel syndrome: evidence of central processing in unilateral neuropathy. Brain: a journal of neurology. 2009;132(Pt 6):1472–9. doi: 10.1093/brain/awp050. [DOI] [PubMed] [Google Scholar]
- 21.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573–81. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Bendtsen L, Jensen R, Olesen J. Decreased pain detection and tolerance thresholds in chronic tension-type headache. Archives of neurology. 1996;53(4):373–6. doi: 10.1001/archneur.1996.00550040113021. [DOI] [PubMed] [Google Scholar]
- 23.Svensson P, List T, Hector G. Analysis of stimulus-evoked pain in patients with myofascial temporomandibular pain disorders. Pain. 2001;92(3):399–409. doi: 10.1016/S0304-3959(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 24.Hidalgo-Lozano A, Fernandez-de-las-Penas C, Alonso-Blanco C, Ge HY, Arendt-Nielsen L, Arroyo-Morales M. Muscle trigger points and pressure pain hyperalgesia in the shoulder muscles in patients with unilateral shoulder impingement: a blinded, controlled study. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2010;202(4):915–25. doi: 10.1007/s00221-010-2196-4. [DOI] [PubMed] [Google Scholar]
- 25.Schneider GM, Smith AD, Hooper A, Stratford P, Schneider KJ, Westaway MD, et al. Minimizing the source of nociception and its concurrent effect on sensory hypersensitivity: an exploratory study in chronic whiplash patients. BMC musculoskeletal disorders. 2010;11:29. doi: 10.1186/1471-2474-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer AA. Application of pressure algometry in manual medicine. J Manual Medicine. 1990;5:145–50. [Google Scholar]
- 27.Chesterton LS, Sim J, Wright CC, Foster NE. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. The Clinical journal of pain. 2007;23(9):760–6. doi: 10.1097/AJP.0b013e318154b6ae. [DOI] [PubMed] [Google Scholar]
- 28.Nussbaum EL, Downes L. Reliability of clinical pressure-pain algometric measurements obtained on consecutive days. Physical therapy. 1998;78(2):160–9. doi: 10.1093/ptj/78.2.160. [DOI] [PubMed] [Google Scholar]
- 29.Box G, Cox D. An Analysis of Transformations. J Royal Statistics Society. 1964;B(26):211–34. [Google Scholar]
- 30.Yu DT, Friedman AS, Rosenfeld EL. Electrical stimulation for treating chronic poststroke shoulder pain using a fully implanted microstimulator with internal battery. American journal of physical medicine & rehabilitation/Association of Academic Physiatrists. 2010;89(5):423–8. doi: 10.1097/PHM.0b013e3181d8d06f. [DOI] [PubMed] [Google Scholar]
- 31.Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. European journal of pain. 2006;10(1):77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Banic B, Petersen-Felix S, Andersen OK, Radanov BP, Villiger PM, Arendt-Nielsen L, et al. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain. 2004;107(1–2):7–15. doi: 10.1016/j.pain.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: a comparison of different scoring criteria. Pain. 2007;128(3):244–53. doi: 10.1016/j.pain.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallin M, Liedberg G, Borsbo B, Gerdle B. Thermal Detection and Pain Thresholds but Not Pressure Pain Thresholds Are Correlated With Psychological Factors in Women With Chronic Whiplash-associated Pain. The Clinical journal of pain. 2012;28(3):211–21. doi: 10.1097/AJP.0b013e318226c3fd. [DOI] [PubMed] [Google Scholar]
- 35.Buchgreitz L, Lyngberg AC, Bendtsen L, Jensen R. Increased pain sensitivity is not a risk factor but a consequence of frequent headache: a population-based follow-up study. Pain. 2008;137(3):623–30. doi: 10.1016/j.pain.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Lundblad H, Kreicbergs A, Jansson KA. Prediction of persistent pain after total knee replacement for osteoarthritis. The Journal of bone and joint surgery British volume. 2008;90(2):166–71. doi: 10.1302/0301-620X.90B2.19640. [DOI] [PubMed] [Google Scholar]