Abstract
Purpose
To evaluate the Blood oxygen-level dependent (BOLD) MR imaging findings in kidneys of patients with diabetic nephropathy.
Materials and Methods
BOLD MR imaging of the kidneys (1.5 Tesla, multi-gradient-recalled-echo sequence with 12 echoes) was performed in 20 patients with diabetic nephropathy (moderate to severe chronic kidney disease: n=14; mild chronic kidney disease: n=6), and 7 healthy volunteers. The medullary and cortical R2* values were compared between patients with diabetic nephropathy and healthy volunteers using student t-tests.
Results
The mean medullary R2* values were lower in patients with diabetic nephropathy compared to healthy volunteers (13.8 ± 2.4 sec-1 versus 19.3±1.2 sec-1, p=0.0002). The cortical R2* values were not significantly different between the two groups (11.1±0.9 sec-1 versus 11.5±0.7 sec-1, p=0.7). A multiple logistic regression model using patient age, gender, and degree of chronic kidney disease (none, mild, or moderate to severe) as variables showed that the degree of kidney disease was independently associated with a decrease in medullary R2* values (p=0.005).
Conclusions
The medullary R2* values were lower in patients with diabetic nephropathy compared to healthy volunteers.
Keywords: Magnetic resonance imaging, Blood oxygen-level dependent (BOLD), kidneys, diabetic nephropathy
INTRODUCTION
Diabetic nephropathy is the leading cause of end-stage renal disease in the United States and other western societies. While the exact mechanisms mediating the effect of hyperglycemia on renal function are not fully understood, there is increasing evidence suggesting an association between chronic renal hypoxia and the development and progression of diabetic nephropathy (1-2). In order to understand the renal oxygenation status in diabetic nephropathy and to develop novel treatments that targets renal hypoxia, it is important to be able to monitor renal oxygenation in these patients noninvasively. For example, new compounds that protect organs against hypoxia have been developed and tested in animals (3), and might be an interesting candidate for future therapy in diabetic nephropathy. Imaging tools that can provide surrogate marker of hypoxia will therefore be valuable for monitoring the efficacy of such novel treatment both in preclinical and clinical studies.
Blood oxygen-level dependent (BOLD) MR imaging is a promising technique that has the potential to noninvasively measure intra-renal oxygenation by using deoxyhemoglobin as an endogenous contrast agent. BOLD MR imaging has been used to demonstrate changes in intra-renal oxygenation in several experimental model and human kidney diseases including diabetes (4-17). Two previous BOLD MR imaging studies of rat kidneys showed renal hypoxia as early as 2 days following induction of diabetes with streptozocin (14-15). Two other groups of investigators evaluated the intra-renal oxygenation as measured by BOLD MR imaging in patients with mild diabetes without overt nephropathy (16-17). These investigators did not find any differences in baseline renal oxygenation between the diabetic patients and healthy controls, but did find that water diuresis failed to improve renal medullary oxygenation in the diabetic patients, suggesting early impairment of adaptive vasodilation within the renal medulla. None of the published studies, however, examined intra-renal oxygenation in patients with long-standing diabetes and overt chronic kidney disease. The purpose of our study was to evaluate BOLD MR imaging findings in patients with presumed established diabetic nephropathy.
MATERIALS AND METHODS
Subjects
The prospective study was approved by our institution's Committee on Human Research. Written informed consent was obtained from each study subject. Twenty patients (12 men, 8 women, mean age: 65 years-old, range: 49 to 77 years-old) with chronic kidney disease presumed due to diabetic nephropathy were enrolled in the study. For these patients, the estimated glomerular filtration rate (GFR) within 4 weeks prior to the MR imaging study was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula (18). Fourteen of the 20 patients had estimated GFR < 60 ml/min per 1.72m2 (mean: 37 ml/min per 1.72m2, range: 14 to 57 ml/min per 1.72m2). The other 6 patients had estimated GFR ≥ 60 ml/min per 1.72m2 (mean: 67 ml/min per 1.72m2, range: 64 to 71 ml/min per 1.72m2). All 20 patients had documented micro- or macro-albumin in the urine indicating renal injury. All patients had clinically stable renal function within 4 weeks prior to their MR imaging study. Based on the National Kidney Foundation classification of stages of chronic kidney disease (CKD), 6 of 20 patients had stage 2 CKD (presence of kidney damage including albuminuria, estimated GFR, 60-89 ml/min per 1.72m2), 9 of 20 had stage 3 CKD (estimated GFR, 30-59 ml/min per 1.72m2), 4 of 20 had stage 4 CKD (estimated GFR, 15-29 ml/min per 1.72m2), and 1 of 20 had stage 5 CKD (estimated GFR < 15 ml/min per 1.72m2). Seventeen of 20 patients were on either angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers for the treatment of their kidney disease at the time of the MR imaging studies. The hematocrit level for the 20 patients within 4 weeks of MR imaging was 41.8 ± 2.5 %. In addition to the patients with diabetic nephropathy, 7 healthy volunteers (4 men, 3 women, mean age: 35 years-old, range: 30 to 45 years-old) without history of diabetes or renal disease also enrolled in the study. GFR was not measured in the healthy volunteers. In order to standardize hydration status, all subjects refrained from fluid or food intake for 4 hours prior to the BOLD MR imaging study.
MR imaging
BOLD MR imaging was performed on a 1.5 Tesla MR system (Excite, GE Healthcare, Wisconsin) with an eight channel cardiac receiver array coil. A two dimensional multiple gradient-recalled-echo (GRE) sequence was used. The scanning parameters were as follows: 12 echoes, repetition time (TR) 65msec, echo time (TE) 7-53msec, inter-echo spacing, 4.2msec, flip angle 30°, slice thickness 5mm, matrix size 256×256, number of excitation 1, field-of-view 400 × 400 mm, bandwidth 41.67 kHz. Each set of 12 T2*-weighted images, corresponding to 12 different echoes, was acquired during a 15- second breath hold. Three true coronal sections were obtained through the middle of the kidneys. There were no mal-rotated kidneys in the study subjects.
Image Analysis
The images were transferred to a desktop computer (Dell Dimension 4700, Round Rock, TX) for data analysis using a commercially available image analysis software program (MIStar, Apollo Medical Imaging, Melbourne, Australia). R2* (1/T2*, in units of 1/second) maps were calculated and generated by the software automatically by fitting MR signal intensity versus respective echo time to an exponential function on a voxel-by-voxel basis. Regions of interest (ROIs) were placed manually in the renal cortex and medulla, avoiding renal hilar vessels and areas of obvious artifacts such as susceptibility artifacts from adjacent bowel. The ROIs were placed by one author who was blinded to clinical data such as estimated GFR. The ROIs were first placed on the gradient-recalled-echo (GRE) image with an echo time of 7 msec, where the cortico-medullary differentiation was maximized. The ROIs were then copied onto the parametric R2* map. In 3 patients who had advanced chronic kidney disease (all with estimated GFR < 20 ml/min per 1.72m2), it was difficult to visualize the cortico-medullary differentiation on the GRE images. The ROIs were then placed in the expected region of the cortex and medulla in those 3 cases. The average size of the ROIs for all subjects was 0.2cm2. For each kidney (the left or the right kidney), 5-7 ROIs were placed in the cortex, and 5-7 ROIs were placed in the medulla. For each subject, a mean cortical R2* value was obtained by averaging values from both the left and right cortical ROIs, and a mean medullary R2* value was obtained by averaging values from both the left and right medullary ROIs. The left and right kidney ROIs were averaged for each subject for the cortex and medulla after excluding significant left and right differences. In addition to the R2* values, we also calculated the R2* ratios between the medulla and cortex for each subject. This is because R2* values can be influenced by external effects such as magnetic field inhomogeneity, coil positions, and the physiologic status of the subjects (9, 19). Assuming that R2* values in the cortex and medulla are similarly affected by external influences, the R2* ratios between the medulla and cortex will be less impacted by external influences.
Statistical Analysis
Statistical analysis was performed using the software package Stata (version 8.0, Stata Corporation, College Station, TX). The mean cortical and medullary R2* values, and medullary-to-cortical R2* ratios were compared between healthy volunteers and patients with diabetic nephropathy using two sample student t-tests. The patients with diabetic nephropathy were also subdivided into 2 groups: mild disease (estimated GFR ≥ 60 ml/min per 1.72m2), or moderate to severe disease (estimated GFR < 60 ml/min per 1.72m2). The mean cortical and medullary R2* values, and medullary-to-cortical R2* ratios were compared between healthy volunteers and patients with mild disease, and between patients with mild disease and patients with moderate to severe disease, also using two sample student t-tests. To assess factors that were independently associated with a decrease in medullary R2* values, a multiple logistic regression model was employed using patient age, gender, and degree of kidney disease (none, mild, moderate to severe) as independent variables. P-values less than 0.05 were considered significant.
RESULTS
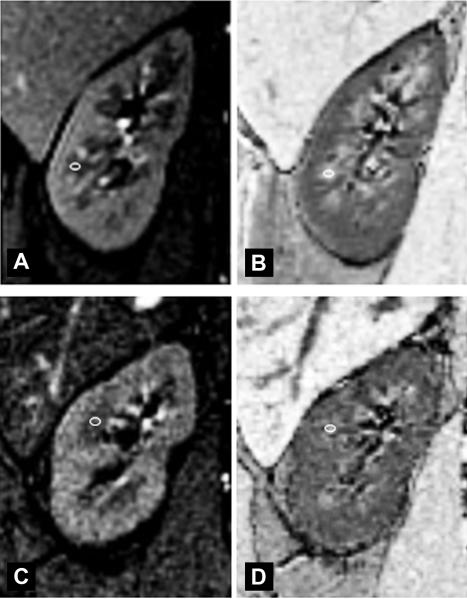
Figure 1 shows examples of GRE T1-weighted anatomical images and the corresponding R2* maps of kidneys in healthy volunteers and patients with diabetic nephropathy. The corticomedullary differentiation was decreased in the kidneys with diabetic nephropathy compared to healthy volunteers.
Fig. 1.
a) Coronal gradient-recalled-echo (GRE) T1-weighted (TE = 7msec) image and b) the corresponding R2* map of the right kidney in a 45 year-old healthy volunteer without history of diabetes or kidney disease. Note the excellent corticomedullary differentiation both on the GRE T1-weighted anatomical image and the R2* map. The small white circle shows an example of region-of-interest (ROI) placement in the medulla. The ROI was placed on the anatomical image first, and then copied onto the corresponding R2* map. c) Coronal GRE T1-weighted (TE = 7msec) image and d) the corresponding R2* map of the right kidney in a 61 year-old patient with diabetic nephropathy and an estimated glomerular filtration rate of 36 ml/min per 1.72m2 (stage 3 chronic kidney disease). Note the visible but reduced corticomedullary differentiation on the GRE T1-weighted anatomical image compared to the healthy volunteer. The corticomedullary differentiation was even less apparent on the corresponding R2* map. The small white circle shows an example of region-of-interest (ROI) placement in the medulla. The ROI was placed on the anatomical image first, and then copied onto the corresponding R2* map.
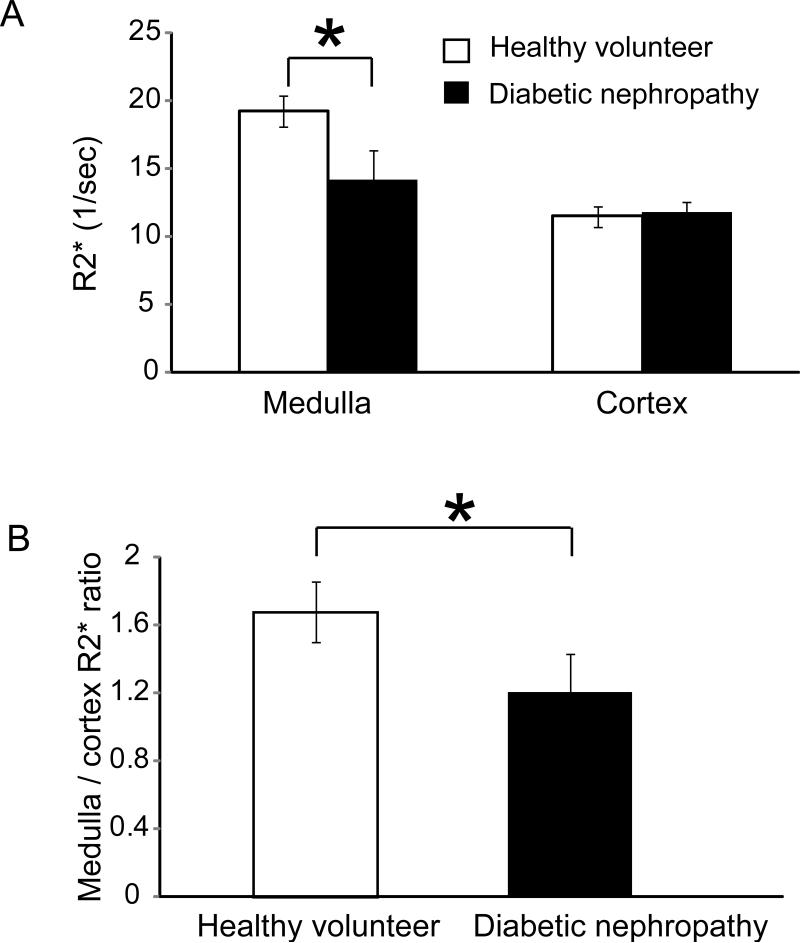
The mean medullary R2* values were significantly lower in patients with diabetic nephropathy compared to healthy volunteers (13.8 ± 2.4 sec-1 versus 19.3±1.2 sec -1, p = 0.0002) (Fig 2a). The cortical R2* values were not significantly different between the two groups (11.1±0.9 sec -1 versus 11.5±0.7 sec -1, p = 0.7) (Fig 2a). The medullary-to-cortical R2* ratios, which were felt to be less affected by external factors, were also significantly lower in patients with diabetic nephropathy compared to healthy volunteers (1.2±0.3 versus 1.7±0.2, p = 0.0006) (Fig 2b).
Fig. 2.
a) Bar graph of medullary and cortical R2* values (mean ± standard deviation) in healthy volunteers and patients with diabetic nephropathy. The medullary R2* values were lower in patients with diabetic nephropathy compared to healthy volunteers (13.8 ± 2.4 sec-1 versus 19.3±1.2 sec -1, p = 0.0002). The cortical R2* values were not significantly different between the two groups (11.1±0.9 sec -1 versus 11.5±0.7 sec -1, p = 0.7). b) Bar graph of medullary-to-cortical R2* ratios in healthy volunteers and patients with diabetic nephropathy. The medullary-to-cortical R2* ratios were lower in patients with diabetic nephropathy compared to healthy volunteers (1.2±0.3 versus 1.7±0.2, p = 0.0006). * indicates significant p values.
A subgroup analysis was performed after dividing the patients with diabetic nephropathy into 2 groups: those with mild disease (estimated GFR ≥ 60 ml/min per 1.72m2, n = 6), and those with moderate to severe disease (estimated GFR < 60 ml/min per 1.72m2, n = 14). The mean medullary R2* values were significantly higher in healthy volunteers than patients with mild disease (19.3±1.2 sec -1 versus 15.9±1.8 sec -1, p = 0.02), which were in turn significantly higher than patients with moderate to severe disease (15.9±1.8 sec -1 versus 12.9±1.3 sec -1, p = 0.005). There were no significant differences in cortical R2* values among the 3 groups (11.5±0.7 sec -1 versus 11.3±1.1 sec -1 versus 11.0±0.7 sec -1, p values > 0.3).
A multiple logistic regression model using patient age, gender, and the degree of kidney disease (none, mild, or moderate to severe) as independent variables showed that the degree of kidney disease was independently associated with a decrease in medullary R2* values (p = 0.005). Patient age and gender were not independently associated with a decrease in medullary R2* values (p=0.8 and 0.7, respectively). In other words, the medullary R2* values were affected by the degree of kidney disease, but not affected by age nor gender in our study.
DISCUSSION
We showed decreased medullary R2* values in patients with diabetic nephropathy compared to healthy volunteers. Our results differ from those in several other BOLD MR imaging studies of diabetes in animal models and in patients. Both Santos et al (15) and Ries et al (14) found increased R2* values corresponding to decreased oxygenation in the rat kidneys in the first several days following the induction of diabetes. Epstein et al (16) and Economides et al (17) did not find any differences in baseline renal medullary R2* values between patients with diabetes but without overt nephropathy and healthy control. In contrast, we found lower medullary R2* values suggesting higher oxygenation in our patients with diabetic nephropathy. We believe the discrepancies may be explained in part by the differences in the stages of diabetic renal injury that were investigated. In the two rat BOLD MR studies, the kidneys were studied within a few days of induction of diabetes, and likely exhibited glomerular hyperfiltration which is known to occur early on in the kidneys due to diabetes (20). While the exact causes of renal hypoxia at this stage of diabetes are uncertain, it has been suggested that different factors may contribute toward the observed hypoxia in animal model of diabetes. One such factor is the increased oxygen consumption to support sodium reabsorption during the hyperfiltration stage, and the other is oxidative stress with augmented oxygen consumption (21-22). It is possible that the observed renal hypoxia in the two rat BOLD MR studies may be in part due to the glomerular hyperfiltration during early stage of diabetes. In the human studies by Epstein et al (16) and Economides et al (17), the patients had mean duration of diabetes greater than 5 years but had not developed overt nephropathy. The glomerular filtration rate in those cases was normal or close to normal. In contrast, the patients in our study had already developed presumed diabetic nephropathy with reduced glomerular filtration rate and protein in the urine. The associated decrease in tubular sodium reabsorption may therefore have contributed to the reduced oxygen consumption and the apparent increase in oxygenation in the renal medulla in our study. The relationship between tubular sodium reabsorption and oxygen consumption has been evaluated in previous study which showed improvement in renal medullary oxygenation following administration of furosemide which reduces tubular sodium reabsorption (19).
Complex alteration in renal oxygenation through different stages of renal injury has been documented by invasive means in other form of chronic renal disease. In an experimental model of chronic tubulointerstitial disease induced by ischemia-reflow injury, both the hypoxic marker pimonidazole and the hypoxia inducible factor (HIF) expression level were higher in the kidneys with moderate tubulointerstitial damage when compared to more severely damaged kidneys, indicating less renal hypoxia with more severe renal damage (23). This paradoxical improvement in hypoxia with severe renal injury may be related to a more pronounced reduction of glomerular filtration rate in the more severely injured kidneys, with subsequent lower oxygen consumption from tubular sodium reabsorption (23). The results from this experimental model of chronic kidney disease lend support to our findings of the apparent increase in renal medullary oxygenation with long standing diabetic nephropathy, possibly due to the diminished tubular sodium reabsorption and reduced oxygen consumption.
In addition to the alteration of tubular reabsorption being a possible contributing factor to the apparent increase in medullary oxygenation in our patients with diabetic nephropathy, another potential contributing factor may be related to the BOLD MR imaging technique. It is important to note that although changes in tissue oxygenation can be reflected as changes in R2* values, changes in R2* values do not necessarily correlate with changes in tissue oxygenation. BOLD MR imaging indirectly measures tissue oxygenation by measuring changes in the deoxyhemoglobin concentration in the adjacent capillaries. R2* values are a measurement of capillary deoxyhemoglobin concentration, which is thought to be in equilibrium with that of the surrounding tissue under normal circumstances. However, the kidneys in long standing diabetic nephropathy as well as in other forms of chronic kidney disease are frequently affected by tubulointerstitial fibrosis and depletion of peritubular capillaries (24-25). The tubulointerstitial fibrosis characterized by the deposition of extracellular matrix will likely interfere with oxygen diffusion from the capillary to the surrounding tissue (24). The depletion of peritubular capillaries will likely further impair oxygen delivery from blood to tissue. This may lead to reduced oxygen extraction by the tissue, and possibly non-equilibrium between capillary and tissue oxygenation. Therefore, the R2* values, which is a measurement of capillary deoxyhemoglobin concentration, may be decreased (corresponding to increased capillary oxygenation) in the face of renal tissue hypoxia because of the reduced oxygen extraction from the capillaries.
The apparent increase in renal medullary oxygenation observed in our patients has also been reported in other forms of chronic kidney disease studied by BOLD MR imaging. In a study of patients with renal artery stenosis, the investigators reported reduced medullary R2* values suggesting increased oxygenation in the atrophic and non-functioning kidneys distal to the stenosed renal artery compared with normal kidneys without renal artery stenosis or to functioning kidneys downstream from mild renal artery stenosis (11). In a study of renal transplants, the investigators noted reduced R2* values corresponding to an apparent increase in oxygenation in transplants with chronic allograft nephropathy compared with normal functioning transplants (5). In yet another study, the investigators reported decreased medullary R2* values corresponding to an apparent increase in oxygenation in patients with chronic tubulointerstitial nephropathy compared to healthy control(13). The diseased kidneys in these studies likely had features common to most forms of chronic kidney disease including reduced tubular reabsorption function and tubulointerstitial fibrosis. We speculate that these factors may have also contributed towards the apparent increase in renal medullary oxygenation as measured by BOLD MR imaging in these studies.
When we subdivided the patients with diabetic nephropathy into those with mild disease (estimated GFR ≥ 60 ml/min per 1.72m2) and those with moderate to severe disease (estimated GFR < 60 ml/min per 1.72m2), we found lower medullary R2* values with worsening chronic kidney disease. The results from this subgroup analysis should be considered preliminary given the small sample size. Also because of the small sample size, we were not able to correlate the medullary R2* values with individual stages of chronic kidney disease (National Kidney Foundation 5 stages of chronic kidney disease) or with individual estimated GFR.
We performed a multivariate logistic regression model analysis which revealed significant independent association between a decrease in medullary R2* values and the degree of kidney disease categorized as none, mild, or moderate to severe. We did not find the age of the subject to be independently associated with a decrease in medullary R2* values, even though the mean age of the healthy volunteers was significantly younger than that of patients with diabetic nephropathy. This is in agreement with a previous study which showed no significant differences in baseline renal medullary R2* values between healthy young and healthy elderly subjects (26).
We did not find any significant differences in R2* values in the renal cortex between patients with diabetic nephropathy and healthy volunteers. A potential explanation is that unlike the renal medulla which functions in relative hypoxia even under physiologic conditions, the renal cortex is better oxygenated and may be less susceptible to injury caused by diabetes (27). Another potential explanation relates to the property of the hemoglobin dissociation curve. Because the renal cortex functions at an oxygen tension greater than 50 mm Hg under normal physiologic condition, the hemoglobin in the cortex is on a shallow portion of its dissociation curve (28). Hence a small change in oxygenation in the cortex does not affect the hemoglobin saturation to the same degree as in the medulla, and may not be detectable with BOLD MR imaging.
Our study has several limitations. First, the sample size was relatively small. Despite the small number of subjects, however, there were significant differences in the renal medullary R2* values between patients with diabetic nephropathy and healthy control. Of note, both the medullary and cortical R2* values in the healthy volunteers in our study were in close agreement with what had been reported previously (16, 29). Second, we did not have kidney biopsy data to prove that the underlying cause of CKD was indeed classic diabetic glomerulosclerosis / nephropathy in all of our patients. But the diagnosis of diabetic nephropathy is often made on clinical grounds without a renal biopsy. It is possible that other co-morbidities such as nephrosclerosis may have also contributed to the underlying renal parenchymal disease and the BOLD MR imaging findings in our study. As discussed previously, many forms of chronic kidney disease, such as those from diabetes, hypertension and renal vascular disease, share similar features including reduced glomerular filtration rate and tubulo-interstitial fibrosis. Therefore, it is not likely that the potential effect of other co-morbidities on the kidneys would significantly alter our results. Third, the potential effect on R2* values by ACE inhibitors or angiotensin receptor blockers use in our patients with diabetic nephropathy was not investigated. These drugs have been shown in animal model of renal injury to reduce oxygen consumption, probably by reducing oxidative stress and improving the efficiency of oxygen utilization for sodium reabsorption (30). Deng et al demonstrated that angiotensin II blockade normalized renal oxygen consumption in the remnant kidney model of chronic kidney disease in rats which had markedly increased oxygen consumption before treatment (30). However, in a BOLD MRI study of renal allograft with chronic allograft nephropathy, the use of angiotensin receptor blocker did not alter the renal R2* value (5). In our study, it is possible that the apparent increase in renal medullary oxygenation in the diabetic patients may be in part due to the use of ACE inhibitors or angiotensin receptor blockers. It is, however, unlikely that the use of these drugs alone will correct the medullary oxygenation to a level that is higher in diabetic nephropathy than in healthy control. Future BOLD MR imaging studies are needed to assess the effect of these drugs on renal oxygenation in patients with diabetic nephropathy and other forms of chronic kidney disease by obtaining scans prior to and after the initiation of these drug therapies. Fourth, in the 3 patients with very advanced kidney disease (GFR < 20 ml/min per 1.72m2) and poor visualization of corticomedullary differentiation on the T1-weighted reference images, it is possible that volume averaging between the cortex and medulla may have occurred due to less precise ROI placement. This could underestimate the R2* values in the medulla. Diminished corticomedullary differentiation on T1-weighted images in patients with renal insufficiency has been described previously, and was thought to be primarily related to an increased T1 relaxation time of the cortex (31). In that study, visualization of corticomedullary differentiation was poor when GFR was < 20 ml/min. In general, differentiation of the renal cortex from medulla may be limited in cases of advanced renal disease with very low GFR. In our study, however, we were able to visualize sufficient, albeit reduced, cortiomedullary differentiation in 17 of 20 patients on the GRE T1-weighted anatomical reference images. We believe our results were unlikely to be changed significantly by the few cases where there was poor corticomedullary differentiation. Fifth, another potential factor that may alter BOLD MR signal is the hematocrit level. Because BOLD MR technique depends on the presence of hemoglobin, the R2* values may be affected by abnormally low hematocrit level where there may be reduced sensitivity of the MR signal. However, the mean hematocrit level of 41% in our patients with diabetic nephropathy would fall under the normal range for hematocrit, and therefore is unlikely to have contributed significantly to our observation.
In conclusion, we found decreased medullary R2* values in patients with diabetic nephropathy compared to healthy volunteers. The etiology for the decrease in medullary R2* values corresponding to an apparent increase in medullary oxygenation in diabetic nephropathy is not clear. We speculate that it may be related to an alteration in oxygen consumption or possibly to factors inherent to BOLD MR imaging techniques. Future studies with correlative measurement of tissue oxygenation by invasive means are needed to better understand the relationship between the BOLD MR imaging findings and intra-renal oxygenation in a chronic kidney disease model.
Acknowledgments
This study was supported in part by the Radiological Society of North America (RSNA) Fellow Research Grant (ZJW)
ZJW was supported by an NIBIB training grant (1T32 EB001631)
REFERENCES
- 1.Palm F. Intrarenal oxygen in diabetes and a possible link to diabetic nephropathy. Clin Exp Pharmacol Physiol. 2006;33(10):997–1001. doi: 10.1111/j.1440-1681.2006.04473.x. [DOI] [PubMed] [Google Scholar]
- 2.Heyman SN, Khamaisi M, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am J Nephrol. 2008;28(6):998–1006. doi: 10.1159/000146075. [DOI] [PubMed] [Google Scholar]
- 3.Nangaku M, Izuhara Y, Takizawa S, et al. A novel class of prolyl hydroxylase inhibitors induces angiogenesis and exerts organ protection against ischemia. Arterioscler Thromb Vasc Biol. 2007;27(12):2548–54. doi: 10.1161/ATVBAHA.107.148551. [DOI] [PubMed] [Google Scholar]
- 4.Alford SK, Sadowski EA, Unal O, et al. Detection of acute renal ischemia in swine using blood oxygen level-dependent magnetic resonance imaging. J Magn Reson Imaging. 2005;22(3):347–53. doi: 10.1002/jmri.20389. [DOI] [PubMed] [Google Scholar]
- 5.Djamali A, Sadowski EA, Muehrer RJ, et al. BOLD-MRI assessment of intrarenal oxygenation and oxidative stress in patients with chronic kidney allograft dysfunction. Am J Physiol Renal Physiol. 2007;292(2):F513–22. doi: 10.1152/ajprenal.00222.2006. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann L, Simon-Zoula S, Nowak A, et al. BOLD-MRI for the assessment of renal oxygenation in humans: acute effect of nephrotoxic xenobiotics. Kidney Int. 2006;70(1):144–50. doi: 10.1038/sj.ki.5000418. [DOI] [PubMed] [Google Scholar]
- 7.Juillard L, Lerman LO, Kruger DG, et al. Blood oxygen level-dependent measurement of acute intra-renal ischemia. Kidney Int. 2004;65(3):944–50. doi: 10.1111/j.1523-1755.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Storey P, Kim D, Li W, Prasad P. Kidneys in hypertensive rats show reduced response to nitric oxide synthase inhibition as evaluated by BOLD MRI. J Magn Reson Imaging. 2003;17(6):671–5. doi: 10.1002/jmri.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen M, Dissing TH, Morkenborg J, et al. Validation of quantitative BOLD MRI measurements in kidney: application to unilateral ureteral obstruction. Kidney Int. 2005;67(6):2305–12. doi: 10.1111/j.1523-1755.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 10.Sadowski EA, Fain SB, Alford SK, et al. Assessment of acute renal transplant rejection with blood oxygen level-dependent MR imaging: initial experience. Radiology. 2005;236(3):911–9. doi: 10.1148/radiol.2363041080. [DOI] [PubMed] [Google Scholar]
- 11.Textor SC, Glockner JF, Lerman LO, et al. The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol. 2008;19(4):780–8. doi: 10.1681/ASN.2007040420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thoeny HC, Kessler TM, Simon-Zoula S, et al. Renal oxygenation changes during acute unilateral ureteral obstruction: assessment with blood oxygen level-dependent mr imaging--initial experience. Radiology. 2008;247(3):754–61. doi: 10.1148/radiol.2473070877. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Cao J, Wang X. Diffusion-weighted and Blood Oxygen Level-dependent MRI in Renal Tubulointerstitial Nephropathy: Initial Experience.. Proceedings of the 18th Annual Meeting of International Society of Magnetic Resonance in Medicine (ISMRM); Stockholm, Sweden. 2010. [Google Scholar]
- 14.Ries M, Basseau F, Tyndal B, et al. Renal diffusion and BOLD MRI in experimental diabetic nephropathy. Blood oxygen level-dependent. J Magn Reson Imaging. 2003;17(1):104–13. doi: 10.1002/jmri.10224. [DOI] [PubMed] [Google Scholar]
- 15.dos Santos EA, Li LP, Ji L, Prasad PV. Early changes with diabetes in renal medullary hemodynamics as evaluated by fiberoptic probes and BOLD magnetic resonance imaging. Invest Radiol. 2007;42(3):157–62. doi: 10.1097/01.rli.0000252492.96709.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein FH, Veves A, Prasad PV. Effect of diabetes on renal medullary oxygenation during water diuresis. Diabetes Care. 2002;25(3):575–8. doi: 10.2337/diacare.25.3.575. [DOI] [PubMed] [Google Scholar]
- 17.Economides PA, Caselli A, Zuo CS, et al. Kidney oxygenation during water diuresis and endothelial function in patients with type 2 diabetes and subjects at risk to develop diabetes. Metabolism. 2004;53(2):222–7. doi: 10.1016/j.metabol.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li LP, Storey P, Pierchala L, Li W, Polzin J, Prasad P. Evaluation of the reproducibility of intrarenal R2* and DeltaR2* measurements following administration of furosemide and during waterload. J Magn Reson Imaging. 2004;19(5):610–6. doi: 10.1002/jmri.20043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palm F, Liss P, Fasching A, Hansell P, Carlsson PO. Transient glomerular hyperfiltration in the streptozotocin-diabetic Wistar Furth rat. Ups J Med Sci. 2001;106(3):175–82. doi: 10.3109/2000-1967-147. [DOI] [PubMed] [Google Scholar]
- 21.Prabhakar SS. Role of nitric oxide in diabetic nephropathy. Semin Nephrol. 2004;24(4):333–44. doi: 10.1016/j.semnephrol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia. 2003;46(8):1153–60. doi: 10.1007/s00125-003-1155-z. [DOI] [PubMed] [Google Scholar]
- 23.Goldfarb M, Rosenberger C, Abassi Z, et al. Acute-on-chronic renal failure in the rat: functional compensation and hypoxia tolerance. Am J Nephrol. 2006;26(1):22–33. doi: 10.1159/000091783. [DOI] [PubMed] [Google Scholar]
- 24.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281(5):F887–99. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 25.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14(5):1358–73. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 26.Prasad PV, Epstein FH. Changes in renal medullary pO2 during water diuresis as evaluated by blood oxygenation level-dependent magnetic resonance imaging: effects of aging and cyclooxygenase inhibition. Kidney Int. 1999;55(1):294–8. doi: 10.1046/j.1523-1755.1999.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brezis M, Rosen S. Hypoxia of the renal medulla--its implications for disease. N Engl J Med. 1995;332(10):647–55. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 28.Jandl J. Blood: Textbook of hematology, 2nd Edition. 2nd ed. Lippincott Williams & Wilkins; Philadelphia: 1996. [Google Scholar]
- 29.Simon-Zoula SC, Hofmann L, Giger A, et al. Non-invasive monitoring of renal oxygenation using BOLD-MRI: a reproducibility study. NMR Biomed. 2006;19(1):84–9. doi: 10.1002/nbm.1004. [DOI] [PubMed] [Google Scholar]
- 30.Deng A, Tang T, Singh P, et al. Regulation of oxygen utilization by angiotensin II in chronic kidney disease. Kidney Int. 2009;75(2):197–204. doi: 10.1038/ki.2008.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee VS, Kaur M, Bokacheva L, et al. What causes diminished corticomedullary differentiation in renal insufficiency? J Magn Reson Imaging. 2007;25(4):790–5. doi: 10.1002/jmri.20878. [DOI] [PubMed] [Google Scholar]