Abstract
Although inhalation of atmospheric PCBs is the most universal exposure route and has become a substantial concern in urban areas, research is lacking to determine the body burden of inhaled PCBs and consequent health effects. To reflect the Chicago airshed environment and mimic the PCB profile in Chicago air, we generated vapors from a Chicago Air Mixture (CAM). Sprague-Dawley rats were exposed to the CAM vapor for 1.6 hr/day via nose-only inhalation for 4 wks, 520±10 μg/m3. Congener-specific quantification in tissue and air samples was performed by GC/MS/MS. In contrast to the lower-chlorinated congener enriched vapor, body tissues mainly contained tri- to hexachlorobiphenyls. Congener profiles varied between vapor and tissues, and among different organs. The toxic equivalence (TEQ) and neurotoxic equivalence (NEQ) were also investigated for tissue distribution. We evaluated a variety of endpoints to catalog the effects of long-term inhalation exposure, including immune responses, enzyme induction, cellular toxicity and histopathologic abnormalities. GSSG/GSH ratio was increased in blood of exposed animals, accompanied by elevation of hematocrit. This study demonstrated that inhalation contributed to the body burden of mostly tri- to hexachlorobiphenyls and produced a distinct profile of congeners in tissue, yet minimal toxicity was found at this exposure dose estimated at 134 μg/rat.
Keywords: Airborne PCBs, subchronic inhalation exposure, Chicago air, TEQ, NEQ
Introduction
Polychlorinated biphenyls (PCBs) are a family of 209 synthetic organic chemicals (congeners) with varying physicochemical features. The domestic production of most PCBs ceased in 1977, yet the exposure continues as they are still present in environmental media and biota. The most universal exposure and global transport route is atmospheric, where PCBs volatilize from soil and water. People are exposed to airborne PCBs emitted from industrial facilities, waste sites, caulk and light ballasts in buildings and certain paint pigments (1-4). As an area bearing a history of heavy PCB use, Chicago has long been monitored for its airborne PCBs. The average concentrations in Chicago exceed 10 times background levels (5, 6). Along with Chicago, major cities of the Midwest like Milwaukee, Cleveland and Detroit serve as regional PCB sources to the atmosphere and the Great Lakes (7-9).
Concern about higher PCB levels in indoor air than outdoors has been mounting, and regulatory agencies have begun to investigate PCB exposure in contaminated schools (10). However, in contrast to the extensive work devoted to dietary intake of PCBs, research to determine the impact of inhalation is still lacking. There are a number of gaps in our understanding. PCBs exist as mixtures, with lower-chlorinated congeners present at higher levels in air due to their higher vapor pressure. People exposed to elevated airborne PCBs may accumulate higher body burden of those congeners, although information is missing about how their exposure levels in the atmosphere relate to blood and tissue concentrations and how these concentrations induce toxicity over time. We have demonstrated that short-term inhalation of airborne PCBs contributes to the body burden of lower-chlorinated congeners in laboratory animals (11). Inhalation proved efficient for uptake of lower-chlorinated PCBs and the fate of congeners varied significantly due to differences in the extent of uptake and elimination. It appears, therefore, that the accumulated congener spectrum and the consequential toxicological responses in living organisms depend largely on the composition of the exposure mixture. In order to better reflect the environment in the Chicago airshed, we generated PCB vapors from a Chicago Air Mixture (CAM) developed to mimic the average PCB profile recorded from 1996 to 2002 in Chicago (12). By using CAM as the best characterized environmentally-relevant PCB mixture, we sought to investigate the relationship between the exposure mixture and tissue PCB levels, in addition to in vivo toxicity upon subchronic exposure.
PCBs cause a variety of carcinogenic and non-carcinogenic adverse effects including immune, reproductive, neurological, and endocrine toxicities. Well-studied effects of PCBs are the dioxin-like toxicities, characterized by alterations in the levels and activities of cytochrome P450 (CYP) enzymes. Some PCB congeners bind to CYP transcription receptors (e.g. aryl hydrocarbon receptors, Ah receptors) producing bioactivation of toxicants and aberrant cellular processes (13, 14). Likewise, the toxicological evidence for the action of hydroxyl metabolites after CYP-dependent PCB biotransformation is accumulating, exhibited by their downstream production of quinones and semiquinones (15). In response to the generation of those oxidants, glutathione depletion has been found in PCB-exposed rats (16, 17). More recently, mutagenic and tumor-initiating activities of lower-chlorinated congeners and their metabolites in vivo provided evidence (18, 19) to refute the assumption that the readily eliminated lower-chlorinated congeners were the least toxic and that metabolic activation did not contribute significantly to toxicity (20). Yet knowledge about these congeners is limited to a few individual compounds, while the information on inhalation exposure to mixtures is practically absent. Our previous subacute exposure study with Aroclor 1242 showed minimal toxicity in pulmonary immune responses (11). In the present study, we extended the period of exposure to 4 weeks to allow more time for the manifestation of biologic effects. We also evaluated a wider range of biological endpoints to catalog the exposure effects, including immune responses, microsomal enzyme induction, cellular toxicity and histopathologic abnormalities.
Experimental Section
Chemicals
Congeners are designated by their IUPAC identities, numbered PCB1 (monochlorobiphenyl) through PCB209 (decachlorobiphenyl) (21). PCBs for surrogate and internal standards and all other chemicals were obtained from commercial sources or synthesized in our laboratory (see Supporting Information).
Generation of the CAM vapor
Aroclor 1242 and Aroclor 1254 (Electrical Grade, Monsanto Lot KB-05-415 and Lot KB-05-612) were mixed at 65:35 ratio to prepare CAM (12). The mixture was then used as the source material to generate atmospheres using our exposure generation system previously described (11). Briefly, clean dry air (4.0 L/min) was bubbled through the solution in an impinger resting in a precision water bath at 25.0 °C. The PCB vapor-laden air was then diluted and supplied to a radial nose-only exposure chamber (InTox, Inc., Albuquerque, NM) at 10 L/min. A sampling cartridge filled with Amberlite XAD-2 polymeric absorbent resin (Supelco Analytical, Bellefonte, PA) captured the PCBs drawn out of the exposure apparatus and was collected every two days for vapor characterization. The exposure system was held within a 6 m3 secondary containment structure operated at negative pressure. A sham exposure nose-only system for control animals was located in an adjacent lab where no PCBs have ever been deliberately introduced.
Animal Treatment
All protocols were approved by the Institutional Animal Care and Use Committee and animals were housed in our on-site vivarium with food and water provided ad libitum. Female Sprague-Dawley rats (Harlan, Inc., Indianapolis, IN) (212.7±2.3 g) were exposed at the same time to either the CAM vapor (n=12), or HEPA- and activated charcoal-filtered laboratory air (n=8). The animals were exposed to the CAM vapor only through a nasal inhalation chamber. In this manner, dermal or oral exposure due to deposition of PCBs onto fur and subsequent grooming were prevented. Sentinel rats (n=4) were maintained in our vivarium for health surveillance. The exposures lasted 2 hr/day in the first week and 1.5 hr the next three weeks, averaging 1.6 hr/day for a total of 32.5 hr in 4 wks. Animals were euthanized 30 min after the final exposure by overdose with isoflurane followed by cervical dislocation. Whole blood was collected via cardiac puncture. Bronchoalveolar lavage (BAL) fluid was collected and major organs were excised.
Toxicity Assessment
From half of the animals, BAL fluid was processed to enumerate total and differential cells and for analysis of total protein, lactate dehydrogenase (LDH) activity and cytokine levels as previously described (22). Total protein was determined using the Bradford assay with bovine serum albumin as the standard (Bio-Rad Laboratories, Hercules, CA). LDH activity released from the cytosol of damaged cells was measured spectrophotometrically (Roche Diagnostics, Indianapolis, IN). Cytokine assays were performed using a multiplex, suspension array system (BioRad, Hercules, CA) including 10 selected cytokines (Invitrogen Corp., Carlsbad, CA). Concentrations below the lowest standard were assigned a value imputed from the lower limit of detection divided by √2. Rat carcasses were decapitated and the upper respiratory tracts were decalcified and trimmed for histologic evaluation. The left lateral lobe of liver, the accessory lobe of right lung, thymus, spleen, kidney and thyroid were collected and embedded in paraffin. Sections were stained with hematoxylin and eosin and evaluated by a certified veterinary pathologist. The right lateral lobe of liver, the rest of lung + trachea, brain and adipose tissue from the peritoneal fat surrounding the intestines were excised for PCB analysis.
The caudal lobe of the right lung and the left lateral lobe of the liver from each remaining rat were collected to prepare microsomes for CYP activity determination. CYP 1A1, 1A2, 2B1 and 2B2 activities were then estimated by the O-dealkylation of the ethyl-, methyl-, pentyl- and benzyl- ethers of phenoxazone (EROD, MROD, PROD and BROD) respectively (17, 23) (see Supporting Information).
The right lateral lobe of liver and left lung from those animals were perfused with saline and immediately homogenized in 5% 5-sulfosalicylic acid (w/v). Glutathione (GSH) and glutathione disulfide (GSSG) levels were then measured in serum, liver and lung using the GSH-5,5′-dithio-bis-(2-nitro-benzoic acid) recycling assay (24) (see Supporting Information).
Blood samples were collected for hematological testing analyzed in capillary mode using an automatic hematology analyzer (Sysmex XT-2000i, Kobe, Japan).
PCB Analysis from Tissue and XAD Samples
PCBs were extracted from homogenized tissues (liver lobe, lung lobes + trachea, brain, adipose tissue), serum and XAD resins using pressurized liquid extraction (ASE 200, Dionex, Sunnyvale, CA) (see Supporting Information). For blood and tissue, cleanup was performed before gas chromatography with tandem mass spectrometry (GC/MS/MS). Lipid content in tissue samples was determined gravimetrically for normalization of PCB concentration. Serum lipid was determined by measuring total cholesterol and total triglycerides (11). Extracted PCBs were analyzed by GC/MS/MS modified from EPA method 1668A (21), using an Agilent 7683 series gas chromatograph coupled to a Waters Micromass Quattro micro GC-MS (Milford, MA)(11). Congener concentrations were corrected for surrogate recoveries. Quality assurance measures are further described in Supporting Information.
Statistical Analyses
Data from PCB-exposed and sham-exposed rats were compared using t-tests for equal and unequal variance. A p-value less than 0.05 was considered significant (SAS Ver. 9.2, SAS Institute Inc., Cary, NC).
Results
Characterization of PCB atmospheres
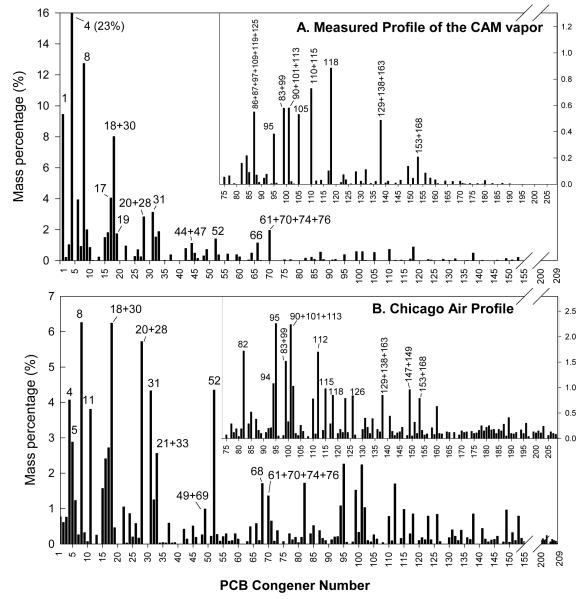
We generated a vapor mixture from the synthetic CAM at a mass flow rate of 5.39±0.14 μg/min, resulting in an average concentration of 520±10 μg/m3 total PCBs. Over 110 congeners were detected in the vapor ranging mostly from mono- to heptachlorobiphenyls (Figure 1A). Many leading congeners in Chicago air (2) (Figure 1B), like PCBs 8, 16, 17, 18+30 and 61+70+74+76 (the + indicates potential coelutions that are quantified as the sum of the congeners listed), were at similar mass percentages in our generation. However, a few were clearly overrepresented, namely PCBs 1, 4 and 8, accounting for 45% of the total PCB mass, while several tri- and tetrachlorobiphenyls (PCBs 20+28, 21+33 and 52) were slightly underrepresented. One particular dichlorobiphenyl, PCB11, one of the most widely distributed and concentrated congeners in Chicago air due to pigment manufacturing (25), is not present in Aroclors, and thus was absent in our generation. On the other end of the profile, most of the relatively abundant congeners in Chicago airshed were prevalent although generated at lower percentages in our study (PCBs 95, 83+99, 90+101+113, 110+115, 118, 129+138+163 and 153+168). Overall, the generated vapor was enriched in mono- and dichlorobiphenyls while penta-, hexa- and heptachlorobiphenyls were reduced in percentages (Table 1).
Figure 1.
Average congener distribution profiles in the CAM vapor during 4 wk exposure (A) and in Chicago air (B), adapted from Hu et al. 2010 (6) (with permission), with inset plot showing congeners from PCB 75 to PCB 209.
Table 1.
Homologue composition of PCBs in the vapor generated from CAM and PCBs found in rats after subchronic inhalation exposure to the vapor. The composition of sampled Chicago air (6) and the source material CAM (12) are also included. Values are presented in mass percentage (%) of total PCB mass.
| PCB Homologue |
Airborne exposure source |
Exposed body tissue |
||||||
|---|---|---|---|---|---|---|---|---|
| CAM vapor | Chicago air | CAM | Blood | Lung | Liver | Brain | Adipose | |
| Mono | 11 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Di | 45 | 21 | 15 | 4 | 7 | 7 | 7 | 2 |
| Tri | 28 | 29 | 23 | 23 | 45 | 28 | 56 | 25 |
| Tetra | 10 | 15 | 33 | 26 | 19 | 19 | 9 | 20 |
| Penta | 5 | 20 | 21 | 36 | 20 | 34 | 25 | 39 |
| Hexa | 1 | 8 | 5 | 12 | 10 | 10 | 2 | 13 |
| Hepta | 0 | 4 | 0 | 0 | 0 | 1 | 0 | 1 |
| Octa | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nona-deca | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Uptake and distribution of PCBs in rat tissue
Exposed rats had significantly higher total PCB concentrations than sham-exposed animals in all five tissues of lung, blood, liver, brain and adipose tissue, from 5-fold (brain) to 20-fold (adipose tissue) (Table 2). Assuming breathing at 95 breaths/min, tidal volume of 1.5 mL/breath, and complete uptake of inhaled PCBs, we estimated that each rat from the PCB-exposed group received a nominal dosage of 134 μg PCBs. The detectable PCB levels in sham-exposed animals were comparable to sentinels, indicating exposure from diet and indoor air.
Table 2.
Mean ± standard error of total PCBs (∑PCB), toxic equivalency (TEQ) and neurotoxic equivalency (NEQ) in body tissues from rats and after subchronic inhalation exposure to the generated CAM vapor.
| Sentinels n=2 |
Sham-exposed n=4 |
CAM-exposed n=6 |
||||
|---|---|---|---|---|---|---|
| ∑PCB | ∑PCB | ∑PCB | TEQ | NEQ | ||
| Tissue Concentration (ng/g lipid weight) |
Lung | 210.2 | 219.3±35.3 | 1506±147.0*** | (5.2±0.5)×10−3 ** | 380±36.0 |
| Blood | 497.1 | 294±71.1 | 3065±377.5*** | (2.0±0.3)×10−2 ** | 869±0.1 | |
| Liver | 221.1 | 157.9±16.8 | 1233±106.3*** | 1.6±0.1*** | 349±29.5 | |
| Brain | 18.0 | 14.9±3.4 | 210.8±44.6*** | (6.5±1.7)×10−2 * | 57.4±13.0 | |
| Adipose | 58.0 | 181.5±43.3 | 3665±668.2* (n=4) |
3.4±1.4 | 974±184 | |
|
| ||||||
| CAM Vapor (μg/m3) | 520±10 | (2.1±0.1)×10−3 | 165±4.6 | |||
Tissue PCBs were significantly greater in CAM-exposed versus sham-exposed rats;
p < 0.05
p<0.01
p < 0.001 (t-test for unequal variances).
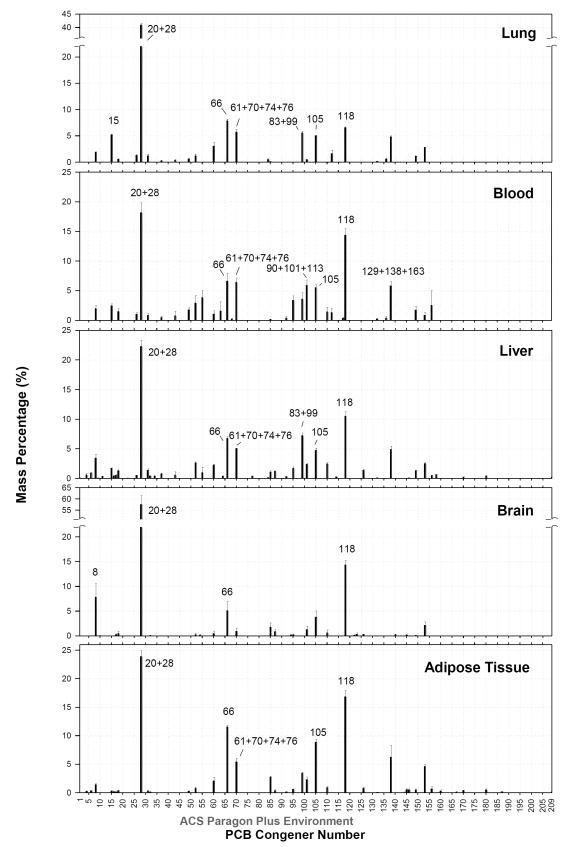
The congener profiles were determined for each tissue (Figure 2). Fifty-nine of the 115 peaks were detected in liver tissue, 47 were found in adipose tissue, 31 in blood, and 26 to 27 in lung and brain. The profiles in blood, liver and adipose tissue shared similar percentages of major congeners ranging from 5% to 25%, whereas the same principal congeners in lung and brain accounted for higher proportions. For example, PCB20+28 accounted for 41% of total PCB congeners (∑PCB) in lung. The load in brain was dominated by PCBs 20+28 (57%), 118 (14%) and 8 (8%). It seemed clear that many PCB congeners were not retained in the lung even though it was the route of entry. Rather, they were distributed to liver for metabolism and adipose tissue for storage. However, it was noteworthy that a few congeners were particularly retained in lung (PCB15) and brain tissue (PCB8) (Figure 2 and 3).
Figure 2.
Average distribution profiles of all congeners in lung, blood, liver, brain and adipose tissue after subchronic inhalation exposure to the CAM vapor. Values are expressed as mean mass percent ± standard error (n=4 for adipose tissue, n=6 for the remaining tissues).
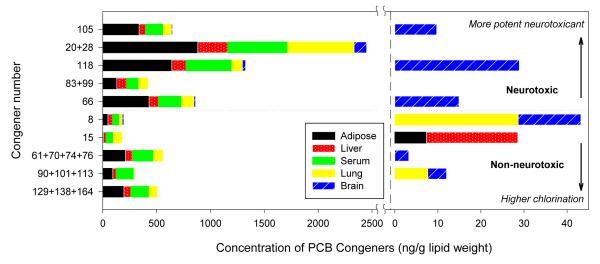
Figure 3.
The concentrations of major congeners (congeners that accounted for over 5% of ∑PCB in any tissue) found in each tissue type after subchronic inhalation exposure to the CAM vapor. The right-hand plot shows the congeners that had concentrations below 30 ng/g lipid weight. Neurotoxic congeners are sorted by NEF values in the top half of the plot. Non-neurotoxic congeners are sorted by their degree of chlorination in the bottom.
For most major congeners, those accounting for more than 5% of ∑PCB in at least one tissue type, the highest deposition occur in adipose tissue (Figure 3). The blood levels of PCBs 8, 61+70+74+76, 83+99, 91+101+113 and 129+138+164 were comparable to the adipose tissue. PCB15 was the lone exception – the lung has the highest concentration of this congener. Although we have seen fewer congeners in the lung compared to the liver (Figure 2), most of the major congeners had similar or higher levels in the lung (Figure 3). A number of these congeners have been reported to exhibit neurotoxicity (26). Neurotoxic PCBs such as PCBs 105, 28, 118 and 66 were found at significant concentrations in brain.
Toxic equivalency
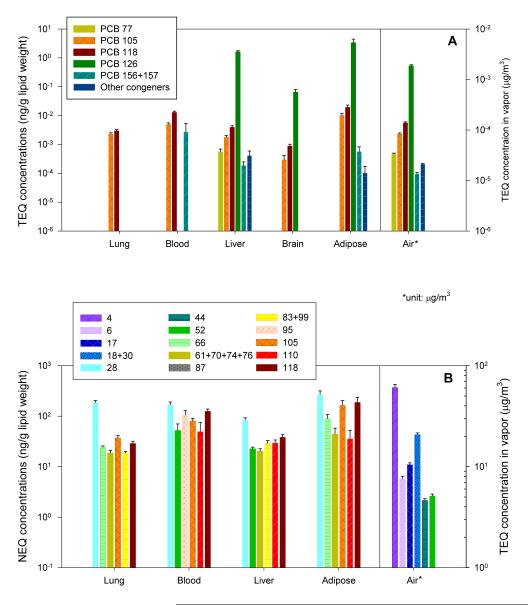
To estimate dioxin-like activity we calculated toxic equivalency (TEQ) concentration according to the reevaluated toxic equivalency factors (TEF) (27). Exposed to a relatively low TEQ value at 2.1 ng/m3, the animal tissues contained TEQ concentrations in descending order of adipose tissue > liver > brain > blood > lung (Table 2). The distribution of congeners that have TEF values also varied between exposure vapor and tissues, and among different tissue types (Figure 4A). Their contribution of TEQ is calculated based on the detected concentrations and any congener below the detection limit is considered not present. The most abundant congener with a TEF value in the CAM vapor and Chicago air was PCB118 yet the major contributor was PCB126 due to its high potency. PCB126 was also a major concern in liver, brain and adipose tissue, although it was found at much lower concentration than other congeners (Figure 2). No PCB126 was detected in the lung or blood, thus the TEQ concentrations in these organs were driven by PCBs 105 and 118 instead. The liver tissue contained the broadest range of dioxin-like toxic congeners. For instance, PCB77, another potent Ah-receptor agonist was only found in the liver.
Figure 4.
The concentrations of TEQ for major dioxin-like congeners (A) and NEQ for the six largest contributors (B) in the CAM vapor and in each tissue after subchronic inhalation exposure. NEQ was distributed between up to 38 congeners in tissue although only the six largest contributors are listed. Values are expressed in the units of ng/g lipid weight for the tissue and in μg/m3 for the air. Y-axes are plotted on logarithmic scale.
Neurotoxic effects are associated with many congeners that exert weak or no effects on Ah receptors. Neurotoxic equivalency factors (NEFs) have been proposed to represent congener-specific neurotoxicity (26) and were applied in our calculations. The NEQ concentrations in body tissues were generally high with lower variances than TEQ, ordered as: adipose tissue > blood > lung ≈ liver > brain (Table 2). NEQ was distributed among up to 38 congeners in tissue although only the six largest contributors of the total NEQ are shown in Figure 4B, with PCBs 20+28 and 118 contributing greatest in every tissue. NEQ was contributed most significantly by tri- to pentachlorobiphenyls in tissue. These distribution profiles differed significantly from that found in the air for which lower chlorinated congeners dominated.
Toxicological responses
The growth rate of the rats exposed to the CAM vapor did not differ significantly from that of sham-exposed rats, although the PCB-exposed group averaged 5.0±0.6 g heavier throughout the study (Figure S1 of Supporting Information). Average normalized thymus weight (g/g body weight) was lower in the PCB-exposed group (0.14±0.01 versus 0.16±0.01, p = 0.055). The number of macrophages, neutrophils and lymphocytes in BAL fluid were not significantly changed. Neither the total protein nor LDH activity was significantly different between groups (Table S5 of Supporting Information). The cytokine levels in BAL fluid were low and similar between groups (Table S6 of Supporting Information). Histologic evaluation of the nasal passages and trachea showed minimal cellular infiltrates and mild degenerative changes including mineralization and extramedullary hematopoesis in one case. Small foci of hepatic mononuclear cells and very mild focal tubular degeneration with casts and epithelial hyperplasia in kidney were observed. These findings are common background lesions in rats and not attributed to treatment. Hematocrit was elevated in exposed group (62.0 ± 0.5% versus 48.9 ± 0.6%), without any change in hemogloblin level, red blood cell counts or other blood cell populations (Table S7 of Supporting Information). No increase in any CYP1A (EROD and MROD) or CYP2B (BROD and PROD) activity was detected in either lung or liver samples (Figure S2 of Supporting Information).
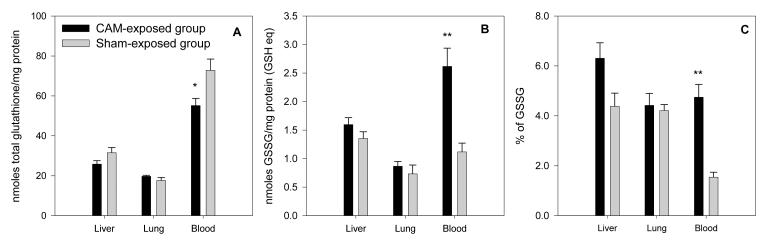
Total glutathione level in blood from PCB-exposed animals was decreased 24% relative to the sham group (p = 0.028, Figure 5A) and was accompanied by a 2-fold higher GSSG (p = 0.006, Figure 5B) and a 27% decrease in GSH (p = 0.018). This reflected a more oxidized environment in blood circulation as indicated by a significantly higher GSSG/GSH ratio (p = 0.001, Figure 5C). No significant difference was found in lung or liver, although hepatic glutathione showed a decrease in total glutathione and GSSG/GSH ratio was slightly higher.
Figure 5.
Total glutathione (A), glutathione disulfide (GSSG) (B) and percentage of GSSG in total glutathione (C) in liver, lung and blood exposed to the CAM vapor. Values are expressed as mean ± standard error. Asterisks indicate a significant increase from control group, * = p < 0.05; ** = p < 0.01.
Discussion
Congener profiles in Chicago ambient air correlated strongly with a mixture of Aroclor 1242 and 1254 in solution (9). CAM, a synthetic mixture (Aroclor 1242 &1254, 65:35, wt%:wt%) was used in our study to better reflect PCBs in the Chicago airshed. Compared to our previous generation of Aroclor 1242 vapor, CAM produced a wider spectrum of congeners (11) with larger representation of higher-chlorinated congeners. However, it is noteworthy that although the distribution of CAM in solution was a good approximation of Chicago air profiles, our CAM vapor was still enriched with lower-chlorinated congeners due to their high volatilities and depletion from the Chicago airshed over time. The Chicago airshed also contained a small fraction of very highly-chlorinated congeners that were missing in Aroclor 1242 and 1254 (Table 1 and Figure 1). In fact, as a result of their low vapor pressures, we were unable to generate vapor-phase hexa- to deca-chlorianted PCBs from any Aroclor mixture. This fraction in Chicago air is likely due to heterogeneity of the surfaces in the city. For example, volatilization of PCBs from water – determined by Henry’s law air/water equilibrium, does not favor low-chlorinated congeners as much as from pure PCB liquid – determined by vapor pressure (28), and therefore may release a relatively higher portion of highly-chlorinated PCBs than our laboratory apparatus.
The observations are consistent with our previous finding (11) that compared to the vapor profile, fewer congeners were detected in the animal body after the exposure and the majority was shifted to higher chlorination (Table 1). The differences in the profiles could be attributed to the following factors. First, the uptake of congeners in pulmonary alveoli surfaces: PCBs are likely to be absorbed differently via inhalation, due to their octanol-air partition coefficients (28). The congener profile in body tissue upon the termination of a 2-hr exposure to Aroclor 1242 vapor supported the selective uptake (data from (11)). Second, the metabolic elimination within organ/tissue: The metabolic attack by CYP has been shown to proceed faster for non-dioxin-like congeners with adjacent meta and para positions open (29). It explains that the congeners accumulated in the body were mostly mono-ortho- and para-substituted on one or both rings (Table S4 of Supporting Information). Assuming all congeners followed first-order elimination within the organ, we predicted congener concentrations in each type of tissue after 20 doses of exposure given at a time interval of 24 hr. The calculations were conducted by using the biological half-lives obtained over a 12-hr period post exposure (11) and the dosage information in both studies. The predicted concentrations of some congeners agreed well with the actual levels detected in most types of tissues, while the concentrations of most persistent congeners (e.g., PCBs 20+28, 66, 105 and 118) were underestimated significantly. This indicates that the differential elimination rates could only explain part for the large variation in congener levels. Third, the redistribution from adipose tissue to other tissues: The detected levels of many persistent congeners in adipose tissue were much lower than the values predicted according to their accumulation rates in adipose tissue. We believe that the redistribution from adipose tissue to other tissues played an important role for persistent congeners like PCBs 20+28, 66, 105 and 118. For example, PCBs 28 and 31, two congeners with very similar physio-chemical properties and almost equal concentrations in air (Figure 1), had large (20- to 300-fold) differences in their tissue levels (Figure 2). The para occupied PCB28 (2,4,4′-diclorobiphenyl) had twice as long half-lives as PCB31 (2,4′,5-dichlorobiphenyl) in non-adipose tissues. While PCB31was eliminated fast, PCB28 accumulated after 4 wks even though elimination was seen within 12 hr post exposure (11).
Differences in the mass percentages of principal compounds were found in different type of tissues, although they shared similarity in profiles. The interesting phenomenon of PCB15 retention in lung and PCB8 in brain tissue (Figure 2 and 3) is consistent with our prior inhalation study (11). Given their short biological half-lives (<7 hr), our elimination kinetic model suggested that the level of PCB8 was likely a reflection of the last exposure, while the level of PCB15 is higher than the predicted value. Other disposition processes such as redistribution may allow greater uptake of PCB15 in spite of its high turnover rate. A few other congeners (PCBs 105 and 138) have been reported to accumulate more in the lung compared to the animal carcass after intraperitoneal injection of Aroclor 1254 (30). A methyl sulfone metabolite of PCB77 was retained predominantly in lung after intraperitoneal and intravenous injection. Further studies confirmed that this metabolite binds to an uteroglobin-like macromolecule in Clara cells (31). Overall, we think the higher levels of highly-chlorinated congeners reflect the accumulation of these congeners as they have long biological half lives (>24 hr). The levels of most low-chlorinated congeners are determined mainly by the uptake during the last exposure. The redistribution from adipose tissue to other tissues contributes significantly to the high levels of many persistent congeners and may contribute to the selective retention of congener in certain type of tissue as well.
The unequal and selective disposition of dioxin-like congeners was also seen in tissues, implying that the potential effects of PCB exposure may be organ-specific. Hepatic sequestration of PCB126 by CYP1A2 (32) may explain the susceptibility of liver to dioxin-like congener disposition. While PCBs 105 and 118 were two congeners with a TEF value that were consistently found in every tissue, other more potent Ah-receptor agonists were found mainly in liver and adipose tissue. However, it should be noted that, due to the striking potency of PCBs 126 and 169, TEQs in lung and blood would be significantly affected with the presence of undetectable trace amounts of PCB126/169 despite of their low detection limit (Table S1.1 and S1.2 of Supporting Information). Also, many higher-chlorinated potent congeners were underrepresented in the CAM vapor, including PCB 126, the major TEQ contributor in Chicago air (6). Thus, these congeners might contribute more in the Chicago environment than what we have observed in our study.
Atmospheric PCBs comprise mostly non-dioxin-like congeners, featured by ortho-substituted non-coplanar structure. Many interfere with Ca2+ homeostasis by inducing the activation of Ryanodine receptors (RyRs) and contribute to short- and long-term neurotoxic events (33). Congeners with two or more ortho-substitutions, favorably with lateral substitutions are most active, including a wide range of tetra- and pentachlorinated congeners such as PCBs 44, 52, 95, 110 (34). Based on the relative potency of each congener, NEQ scheme has been developed and applied in risk assessment. In our study, the CAM vapor is a good approximation of the Chicago atmosphere in that they share similar major NEQ contributors including PCBs 4, 17, 18+33, 20+28 and 52 (6). Compared to the airborne mixture, the leading contributors in body tissue were shifted to higher-chlorinated congeners. A few potent neurotoxicants that prevail in Chicago air (PCBs 52, 95 and 110) remained significant contributors to NEQ in body tissues. The most abundant neurotoxicants found in Chicago air (PCBs 4, 6, 16, 17, 18+30) (6) were not detected or were found at minimal levels, while many congeners contributing less than 2% in Chicago became the major contributors in the body, such as PCBs 66, 61+70+74+76, 87, 83+99, 105 and 112. It is thus important to bear in mind that the congener profiles accumulated in the body can be very different from the ambient air environment, as the biological effects exhibited by parent PCBs are more likely to be associated with congeners absorbed and distributed in body tissue rather than those predominate in the ambient air environment.
CYP1A1 and CYP1A2 induction mediated by Ah-receptor is a typical marker for dioxin-like congeners. Given the relatively low TEQ concentrations of airborne PCBs, the induction of CYP1A was expected to be minor. Induction was found at much higher dosage, by non-ortho and mono-ortho substituted congeners (PCBs 126 and 118), as well as Aroclor 1242 and 1254, after a 7-day gavage dosing at 4 to 32 mg/kg/day (PCB126: 2.5 to 40 μg/kg/day) (35). The induction of CYP2B mediated by constitutively active receptor (CAR) is another mode of toxic action caused by non-dioxin like congeners, preferably those with two or more ortho substitutions. The CAM vapor mixture contained a wide range of di-ortho-substituted congeners. Some most potent agonists (e.g. PCBs 99, 101 and 153) shown by the structure-activity relationship were detected at pronounced levels in exposed tissues. Yet our dosage level (26 μg/kg total PCBs) is much lower than in other induction studies (35), or the minimal threshold dose (0.5 mg/kg) for many potent inducers and Aroclors in female SD rats (14). Nevertheless, we observed different activity levels of CYP isoforms in rat lung compared to liver, in agreement with previous report (36). Collectively, our study indicated that CYP1A/2B activities may not be a sensitive biomarker of exposure to atmospheric PCBs at the single exposure dose used in this study.
The importance of intracellular GSH in detoxification of xenobiotics and defense against oxidant injury is well recognized. Its protective action in scavenging oxygen and radicals results in an increased formation of GSSG and protein-mixed disulfides. The redox status of blood glutathione may reflect the stress in less accessible tissues and a shift to a more oxidized state has been observed repeatedly in many toxicities and pathologies. In our study, the rise of blood GSSG may have mainly resulted from erythrocyte excretion of excess intracellular GSSG, yet could also be attributed to the excretion from liver into blood (37). Hepatic GSH is the major determinant of plasma GSH (37), thus the fall in blood GSH was likely due to a decreased efflux of GSH from liver. The slight increase of GSSG and decrease of GSH or total glutathione in PCB-exposed liver, if any, were minor compared to the enormous amount of intrahepatic glutathione. Similar findings showing no change in hepatic GSH/GSSG level were reported after intraperitoneal injection of Aroclor 1254 or individual congeners (16, 38) except PCB126 at a high dose of 326 g/kg (17). Although there were minimal toxicity-related effects observed in general, blood GSSG-to-GSH ratio provides an index of intracellular redox status.
To approach the environmental human exposure, we chose a dose level (134 μg for 4 wks) that is much lower than other in vivo studies reporting toxicity on our investigated endpoints. Assuming an adult breathing 5500 pg/m3 (6) PCBs for 24 hr/day at a breathing volume of 8 L/min, the person is exposed to about 63 ng/day, or 120 μg for five years, if not more from contaminated indoor air. Whether a longer term or a higher level of inhalation exposure will cause more toxicity on these endpoints remains to be addressed.
Supplementary Material
Acknowledgement
This work was supported by NIEHS through grants NIH P42 ES013661 and NIH P30 ES005605. The authors thank Dr. Ian Lai for Cytochrome P450 activity assay, Dr. Nukhet Aykin-Burns and Dr. Yueming Zhu for help with glutathione assay, Dr. Izabela Kania-Korwal for advice in PCB extraction, and Dr. Wanda Haschek-Hock for evaluating tissue for pathology.
Abbreviations
- Ah
aryl hydrocarbon
- BAL
bronchoalveolar lavage
- BROD
benzyloxyresorufin-O-dealkylation
- CAM
Chicago Air Mixture
- CYP
cytochrome P450 enzymes
- EROD
ethoxyresorufin-O-dealkylation
- GC
gas chromatography, gas chromatograph
- GSH
reductive glutathione
- GSSG
glutathione disulfide
- LDH
lactate dehydrogenase
- NEF
neurotoxic equivalency factors
- NEQ
neurotoxic equivalency
- MROD
methoxyresorufin-O-dealkylation
- MS
mass spectrometry
- PCB
polychlorinated biphenyl
- PROD
pentoxyresorufin-O-dealkylation
- TEF
toxic equivalency factors
- TEQ
toxic equivalency
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- ∑PCB
sum of total PCB congeners
Footnotes
Supporting Information Available: Chemicals, toxicity assay, extraction procedure and quality control; tables for quality assurance measures, prevailing congener mass, and immune and hematology response in subchronically exposed rat tissue; and figures for rat weight gain and CYP activities in exposed rat liver and lung.
Literature Cited
- 1.Herrick RF, McClean MD, Meeker JD, Baxter LK, Weymouth GA. An unrecognized source of PCB contamination in schools and other buildings. Environ. Health Perspect. 2004;112:1051–1053. doi: 10.1289/ehp.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu D, Martinez A, Hornbuckle KC. Discovery of non-aroclor PCB (3,3′-dichlorobiphenyl) in Chicago air. Environ. Sci. Technol. 2008;42:7873–7877. doi: 10.1021/es801823r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez A, Wang K, Hornbuckle KC. Fate of PCB congeners in an industrial harbor of Lake Michigan. Environ. Sci. Technol. 2010;44:2803–2808. doi: 10.1021/es902911a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodenburg LA, Guo J, Du S, Cavallo GJ. Evidence for unique and ubiquitous environmental sources of 3,3′-dichlorobiphenyl (PCB 11) Environ. Sci. Technol. 2010;44:2816–2821. doi: 10.1021/es901155h. [DOI] [PubMed] [Google Scholar]
- 5.Sun P, Basu I, Blanchard P, Brice KA, Hites RA. Temporal and spatial trends of atmospheric polychlorinated biphenyl concentrations near the great lakes. Environ. Sci. Technol. 2007;41:1131–1136. doi: 10.1021/es061116j. [DOI] [PubMed] [Google Scholar]
- 6.Hu D, Lehmler H, Martinez A, Wang K, Hornbuckle KC. Atmospheric PCB congeners across Chicago. Atmos. Environ. 2010;44:1550–1557. doi: 10.1016/j.atmosenv.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wethington DM, Hornbuckle KC. Milwaukee, WI, as a Source of Atmospheric PCBs to Lake Michigan. Environ. Sci. Technol. 2005;39:57–63. doi: 10.1021/es048902d. [DOI] [PubMed] [Google Scholar]
- 8.Hornbuckle KC, Carlson DL, Swackhamer DL, Baker JE, Eisenreich SJ. PCBs in the Great Lakes. In: Hites RA, editor. The Handbook in Environmental Chemistry. Springer-Verlag; Heidelberg: 2006. pp. 33–95. [Google Scholar]
- 9.Persoon C, Peters TM, Kumar N, Hornbuckle KC. Spatial distribution of airborne polychlorinated biphenyls in Cleveland, Ohio and Chicago, Illinois. Environ. Sci. Technol. 2010;44:2797–2802. doi: 10.1021/es901691s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Environmental Protection Agency Public Health Levels for PCBs in Indoor School Air 2011. Available at http://www. epa. gov/pcbsincaulk.
- 11.Hu X, Adamcakova-Dodd A, Lehmler HJ, Hu D, Kania-Korwel I, Hornbuckle KC, Thorne PS. Time course of congener uptake and elimination in rats after short-term inhalation exposure to an airborne polychlorinated biphenyl (PCB) mixture. Environ. Sci. Technol. 2010;44:6893–6900. doi: 10.1021/es101274b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao HX, Adamcakova-Dodd A, Hu D, Hornbuckle KC, Just CL, Robertson LW, Thorne PS, Lehmler HJ. Development of a synthetic PCB mixture resembling the average polychlorinated biphenyl profile in Chicago air. Environ. Int. 2010;36:819–827. doi: 10.1016/j.envint.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit. Rev. Toxicol. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- 14.Connor K, Safe S, Jefcoate CR, Larsen M. Structure-dependent induction of CYP2B by polychlorinated biphenyl congeners in female Sprague-Dawley rats. Biochem. Pharmacol. 1995;50:1913–1920. doi: 10.1016/0006-2952(95)02087-x. [DOI] [PubMed] [Google Scholar]
- 15.McLean MR, Bauer U, Amaro AR, Robertson LW. Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem. Res. Toxicol. 1996;9:158–164. doi: 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- 16.Twaroski TP, O’Brien ML, Robertson LW. Effects of selected polychlorinated biphenyl (PCB) congeners on hepatic glutathione, glutathione-related enzymes, and selenium status: implications for oxidative stress. Biochem. Pharmacol. 2001;62:273–281. doi: 10.1016/s0006-2952(01)00668-2. [DOI] [PubMed] [Google Scholar]
- 17.Lai I, Chai Y, Simmons D, Luthe G, Coleman MC, Spitz D, Haschek WM, Ludewig G, Robertson LW. Acute toxicity of 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) in male Sprague-Dawley rats: effects on hepatic oxidative stress, glutathione and metals status. Environ. Int. 2010;36:918–923. doi: 10.1016/j.envint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espandiari P, Glauert HP, Lehmler HJ, Lee EY, Srinivasan C, Robertson LW. Polychlorinated biphenyls as initiators in liver carcinogenesis: resistant hepatocyte model. Toxicol. Appl. Pharmacol. 2003;186:55–62. doi: 10.1016/s0041-008x(02)00018-2. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann L, L Esch H, A Kirby P, W Robertson L, Ludewig G. 4-monochlorobiphenyl (PCB3) induces mutations in the livers of transgenic Fisher 344 rats. Carcinogenesis. 2007;28:471–478. doi: 10.1093/carcin/bgl157. [DOI] [PubMed] [Google Scholar]
- 20.Safe S. Toxicology, structure-function relationship, and human and environmental health impacts of polychlorinated biphenyls: progress and problems. Environ. Health Perspect. 1993;100:259–268. doi: 10.1289/ehp.93100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Method 1668, Revision A: Chlorinated Biphenyl Congeners in Water, Soil, Sediment, and Tissue by HRGC/HRMS. U.S. Environmental Protection Agency; Washington, DC: 1999. EPA-821-R-00-002. [Google Scholar]
- 22.Thorne PS, Adamcakova-Dodd A, Kelly KM, O’neill ME, Duchaine C. Metalworking fluid with mycobacteria and endotoxin induces hypersensitivity pneumonitis in mice. Am. J. Respir. Crit. Care Med. 2006;173:759–768. doi: 10.1164/rccm.200405-627OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke MD, Thompson S, Elcombe CR, Halpert J, Haaparanta T, Mayer RT. Ethoxy-, pentoxy- and benzyloxyphenoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P-450. Biochem. Pharmacol. 1985;34:3337–3345. doi: 10.1016/0006-2952(85)90355-7. [DOI] [PubMed] [Google Scholar]
- 24.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 25.Hu D, Hornbuckle KC. Inadvertent Polychlorinated Biphenyls in Commercial Paint Pigments (dagger) Environ. Sci. Technol. 2009 doi: 10.1021/es902413k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon T, Britt JK, James RC. Development of a neurotoxic equivalence scheme of relative potency for assessing the risk of PCB mixtures. Regul. Toxicol. Pharmacol. 2007;48:148–170. doi: 10.1016/j.yrtph.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H.e.a. The 2005 World Health Organization Reevaluation of Human and Mammalian Toxic Equivalency Factors for Dioxins and Dioxin-Like Compounds. Toxico. Sci. 2005;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meylan WM, Howard PH. Estimating octanol-air partition coefficients with octanol-water partition coefficients and Henry’s law constants. Chemosphere. 2005;61:640–644. doi: 10.1016/j.chemosphere.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 29.Brown JF., Jr. Determination of PCB Metabolic, Excretion, and Accumulation Rates for Use as Indicators of Biological Response and Relative Risk. Environ. Sci. Technol. 1994;28:2295–2305. doi: 10.1021/es00062a013. [DOI] [PubMed] [Google Scholar]
- 30.Anderson LM, Fox SD, Riggs CW, Issaq HJ. Selective retention of polychlorinated biphenyl congeners in lung and liver after single-dose exposure of infant mice to Aroclor 1254. J. Environ. Pathol. Toxicol. Oncol. 1993;12:3–16. [PubMed] [Google Scholar]
- 31.Lund J, Brandt I, Poellinger L, Bergman A, Klasson-Wehler E, Gustafsson JA. Target cells for the polychlorinated biphenyl metabolite 4,4′-bis(methylsulfonyl)-2,2′,5,5′-tetrachlorobiphenyl. Characterization of high affinity binding in rat and mouse lung cytosol. Mol. Pharmacol. 1985;27:314–323. [PubMed] [Google Scholar]
- 32.DeVito MJ, Ross DG, Dupuy AE, Jr, Ferrario J, McDaniel D, Birnbaum LS. Dose-response relationships for disposition and hepatic sequestration of polyhalogenated dibenzo-p-dioxins, dibenzofurans, and biphenyls following subchronic treatment in mice. Toxicol. Sci. 1998;46:223–234. doi: 10.1006/toxs.1998.2530. [DOI] [PubMed] [Google Scholar]
- 33.Pessah IN, Cherednichenko G, Lein PJ. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol. Ther. 2010;125:260–285. doi: 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kodavanti PR. Neurotoxicity of persistent organic pollutants: possible mode(s) of action and further considerations. Dose Response. 2006;3:273–305. doi: 10.2203/dose-response.003.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin L, Klaassen CD. Differential effects of polychlorinated biphenyl congeners on serum thyroid hormone levels in rats. Toxicol. Sci. 2010;117:36–44. doi: 10.1093/toxsci/kfq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guengerich FP. Purification and characterization of xenobiotic-metabolizing enzymes from lung tissue. Pharmacol. Ther. 1990;45:299–307. doi: 10.1016/0163-7258(90)90068-d. [DOI] [PubMed] [Google Scholar]
- 37.Adams JD, Jr, Lauterburg BH, Mitchell JR. Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J. Pharmacol. Exp. Ther. 1983;227:749–754. [PubMed] [Google Scholar]
- 38.Makary M, Kim HL, Safe S, Womack J, Ivie GW. Constitutive and Aroclor 1254-induced hepatic glutathione S-transferase, peroxidase and reductase activities in genetically inbred mice. Comp. Biochem. Physiol. C. 1988;91:425–429. doi: 10.1016/0742-8413(88)90054-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.