Abstract
Neurobehavioral disorders such as anxiety, autism, and attention deficit hyperactivity disorders are typically influenced by genetic and environmental factors. Although several genetic risk factors have been identified in recent years, little is known about the environmental factors that either cause neurobehavioral disorders or contribute to their progression in genetically predisposed individuals. One environmental factor that has raised concerns is chlorpyrifos, an organophosphate pesticide that is widely used in agriculture and is found ubiquitously in the environment. In the present study, we examined the effects of sub-chronic chlorpyrifos exposure on anxiety-related behavior during development using zebrafish larvae. We found that sub-chronic exposure to 0.01 or 0.1 μM chlorpyrifos during development induces specific behavioral defects in 7-day-old zebrafish larvae. The larvae displayed decreases in swim speed and thigmotaxis, yet no changes in avoidance behavior were seen. Exposure to 0.001 μM chlorpyrifos did not affect swimming, thigmotaxis, or avoidance behavior and exposure to 1 μM chlorpyrifos induced behavioral defects, but also induced defects in larval morphology. Since thigmotaxis, a preference for the edge, is an anxiety-related behavior in zebrafish larvae, we propose that sub-chronic chlorpyrifos exposure interferes with the development of anxiety-related behaviors. The results of this study provide a good starting point for examination of the molecular, cellular, developmental, and neural mechanisms that are affected by environmentally relevant concentrations of organophosphate pesticides. A more detailed understanding of these mechanisms is important for the development of predictive models and refined health policies to prevent toxicant-induced neurobehavioral disorders.
Keywords: Organophosphates, Zebrafish, High-throughput assay, Anxiety behavior, Development
1. Introduction
Organophosphate pesticides (OPs) are environmental toxicants widely used throughout the United States mainly for pest control on food crops. Every year nearly 60 million pounds of OPs are applied to over 60 million acres of agriculture in the USA, used since the 1940s worldwide, making them highly ubiquitous (EPA, 2011). Detectable levels of OPs have been found in air, dust, and food samples from daycare centers and homes, as well as in children’s urine samples (Morgan et al., 2004). Moreover, OPs can be absorbed by ingestion, inhalation, or through the dermis, indicating that there are many potential routes of exposure. Children fed organic diets had four-fold lower (nearly undetectable) urinary levels of two widely used organophosphates (malathion and chlorpyrifos), compared to those of children who consumed conventional foods, suggesting that ingestion of conventionally grown foods is a significant route of exposure to OPs (Lu et al., 2006).
Organophosphates have a considerable number of effects on behavior. In large quantities, OPs have been linked to nausea, dizziness, confusion, increased heart rate, respiratory failure, and even death (CDC, 2011). However, most human exposure occurs from ingestion of foods treated with relatively low levels of OPs (CDC, 2011). There is growing concern that chronic or sub-chronic low-level exposure to OPs may affect neural patterning during embryonic development, and may contribute to various neurobehavioral disorders such as autism, anxiety, depression, and attention deficit hyperactivity disorder (ADHD) (Bouchard et al., 2010; Rauh et al., 2006). While little is known about the underlying causes of these disorders, it is clear that such disorders are increasing in both prevalence and diagnosis (CDC, 2006; Chakrabarti and Fombonne, 2005; Robison et al., 2002), and it is reasonable to question the role that environmental toxicants, such as OPs, play in the development of such disorders. The exposure risks to OPs are not limited to postnatal development. OPs can be passed from mother to child in utero through the umbilical cord (Rauh et al., 2006). Children inadvertently exposed to higher levels of chlorpyrifos in utero or with higher levels of urinary OP metabolites were more likely to exhibit ADHD symptoms (Bouchard et al., 2010; Rauh et al., 2006). Moreover, developing fetuses, infants, and young children are highly susceptible to the adverse effects of toxicants because their immune systems and blood brain barriers are not fully formed (Adinolfi, 1985), and in comparison to adults children have much lower levels of essential enzymes needed to break down OPs (Furlong et al., 2006). Thus, exposure to OPs during critical periods of development can have severe long-term neurobehavioral consequences.
An emerging model organism for the study of toxicants, brain development, and behavior is the zebrafish larvae (Danio rerio) for several reasons. First, embryos can be readily collected in large numbers and immediately exposed to external compounds directly in a dish over different developmental periods. Additionally, zebrafish larvae develop rapidly, hatching from their chorions by 2–3 days post fertilization (dpf), and fully developing all adult organs within their first week. By 4–5 dpf, larvae inflate their swim bladder and become free-swimming, exhibiting a number of behaviors such as avoidance, darting, scoots, and startle response (Colwill and Creton, 2011a). Previously, zebrafish larvae and adults have been utilized in order to study the effects of chlorpyrifos on motor startle response (to a vibrational tap stimulus), swimming activity as measured by the number of segments crossed, and spatial discrimination. Treatment of developing zebrafish with 100 ng/ml of sub-chronic chlorpyrifos decreased the swimming activity of larvae tested at 6 and 9 dpf (Levin et al., 2004) while increasing startle response, severely impairing choice accuracy and causing a biphasic effect on choice latency in adulthood that was dose dependent (Levin et al., 2003; Sledge et al., 2011). Although these studies parallel nicely with the current report, we present a different larval behavioral repertoire using high-throughput data collection and analysis after sub-chronic chlorpyrifos exposure. Both the larvae’s rapid development and their small size make it possible to examine their behavior in a high-throughput fashion.
High-throughput assays have been used to test the acute effects of thousands of pharmaceuticals by analyzing startle and movement of zebrafish larvae (Kokel et al., 2010; Rihel et al., 2010). Additionally, automated imaging systems have been developed for high-throughput analysis of avoidance of visual stimuli and thigmotaxis (edge preference), both considered measures of anxiety, and are readily quantifiable in zebrafish larvae (Champagne et al., 2010; Colwill and Creton, 2011a, 2011b; Pelkowski et al., 2011; Richendrfer et al., 2012). Our results indicate that anxiety-related behaviors, body morphology, and swimming speed are affected by concentrations of chlorpyrifos between 0.01 and 1 μM.
2. Materials and methods
2.1. Zebrafish
Adult wild type zebrafish were obtained from Carolina Biological Supply and have been housed at Brown University over several generations in multiple 10 and 20 gallon tanks. For breeding purposes, the zebrafish were kept in mixed male and female populations under a 14 hour light/10 hour dark cycle. The fish were fed a combination of TetraMin flake food, frozen brine shrimp, and freeze-dried bloodworms. Embryos were collected from the tanks at the beginning of the light cycle (“dawn”) and were immediately transferred to deep Petri dishes (Fisher 08-752-11Z) containing ‘egg water’ (0.06 g of Instant Ocean and 0.25 mg of methylene blue per liter of deionized water). The embryos were then treated with various dilutions of chlorpyrifos (ChemService F2057), and grown in an incubator at 28.5 °C on a 14 hour light/10 hour dark cycle, and at a density of 60–70 embryos per culture dish.
2.2. Chlorpyrifos exposure
Embryos were exposed to 1 μM, 0.1 μM, 0.01 μM, or 0.001 μM chlorpyrifos (ChemService F2057) immediately following embryo collection throughout behavioral imaging at 7 days post-fertilization (dpf). The low levels of chlorpyrifos chosen for this study were based upon reports indicating that levels of AChE are unimpaired at these levels (Yang et al., 2011) and are within the metabolite ranges typically found in human diets (Lu et al., 2008, 2006). Chlorpyrifos was dissolved in dimethyl sulfoxide (DMSO) as 1000× stocks that were stored at −20 °C. In control groups, embryos were grown in either egg water or in egg water containing 0.1% DMSO. To obtain the desired final concentrations, 50 μl of each 1000× stock was dissolved into 50 ml of egg water in respective culture dishes. Dead embryos were removed daily from culture dishes and all solutions were replaced daily until 7 days post fertilization, when behavioral analysis was conducted.
2.3. Behavioral analyses
2.3.1. Image collection
Using a high-throughput imaging system, a series of images was collected in order to compile behavioral data, using the protocol described by Pelkowski et al. (2011). At 7 dpf, zebrafish larvae, kept in their respective treatment solutions, were placed in 6-well plates (Corning Costar: 3506) which were positioned inside imaging cabinets on top of laptop computer screens, that utilized Microsoft PowerPoint presentations to display visual stimuli to the larvae. Each laptop has a 15.6 in. LCD screen with a brightness set at 220 cd/m2 and 1366×768 pixel resolution. Plastic diffusers (Pendaflex 52345) were placed between the multiwall plates and the laptop screen in order to avoid moiré patterns on the images. Images were taken every 30 s from above the plates using a 15 megapixel Canon EOS Rebel T1i digital camera with an EF-S 55–250 mm f/4.0–5.6 IS zoom lens (www.canon.com) with time-lapse imaging software (see Richendrfer et al., 2012 for details). Two 6-well plates per treatment were used and the experiments were replicated on three different days.
2.3.2. Visual stimuli
Visual stimuli were created in Microsoft Power Point so that a moving red ‘ball’ was at the top of each well while a stationary red ‘ball’ was at the bottom of each well. The ball at the top of the well moved in a continuous pattern from left–right–left at a speed of 1 cm/s. The top and bottom of the well are referred to strictly in the horizontal sense and are not used for larval analysis in a vertical plane (see Richendrfer et al., 2012 for complete details). Therefore, in this study we did not measure the position of the larvae within the vertical plane. The edge vs. center and top vs. bottom of each well are equally matched for area and therefore are expected to have a random 50:50 distribution. The RGB value of the red ball was 255, 0, and 0 respectively so that the red color could be easily removed from the images during analysis.
2.3.3. Five-fish assay
Five larvae were placed in each well of a 6-well plate, and behavioral data were collected over the duration of 1 h (average n=36 wells/180 larvae for each treatment). A time point sampling method was used in order to collect data pertaining to how often larvae were on the bottom or the edge of the well, away from the visual stimulus (one image per 30 s). For the first half-hour, a blank white background was used while the second half-hour utilized the visual stimuli described above.
2.3.4. Swim speed assay
One larva per well was placed in each well of a 6-well plate, while a time point sampling method was used to collect behavioral data for a total of 1 h (one image per 30 s) (average n=36 wells/36 larvae per treatment). In order to assess the baseline swim speed, no visual stimuli were presented during the entire collection of behavioral data.
2.3.5. Interval assay
One larva per well was placed in each well of a 6-well plate, and a time point sampling method was used to collect behavioral data for a total of 1 h (one image per 30 s) (average n=36 wells/36 larvae per treatment). Both a blank white background (no visual stimuli) and visual stimuli were presented to the larvae in intervals of 10 min at a time, so that each sequence (blank–visual stimuli) was displayed three different times during the one hour assay (a within-subjects assay used to further assess avoidance and thigmotaxis behavioral changes throughout the one hour sampling period found to be successful in another zebrafish larval assay (Lovato et al., 2011)).
2.3.6. Statistical analyses
The statistical analyses were performed using SPSS. Excel’s ‘COUNTIFS’ function was used to determine the number of times the larvae were located up or down in the well and how often the larvae are located on the edge or center of the well. The data were analyzed on a per well basis (n=number of wells) in order to assure that the measured values were independent. Average values (± standard error of the mean) were calculated for all wells within an experimental group for each type of assay. Differences in behavior before and during the visual stimuli and between groups were tested for significance using a one way ANOVA to determine the effects of treatment. If the treatments showed significance (p<0.05), Tukey’s multiple comparison test was used to determine any significant differences between treatment groups compared to the DMSO control group. The asterisks in the graphs indicate a significant difference between the chlorpyrifos exposures and the DMSO controls.
3. Results
During the first 3 days of exposure, visual inspection of the larvae indicated that the defects in movement and morphology were not observable until 3–4 dpf. Zebrafish embryos and larvae exposed to the highest tested dose of chlorpyrifos (1 μM) showed little to no movement by 7 dpf. At this dose, the larvae exhibited twitching behavior but could not swim or move normally; therefore, they were not used for behavioral analysis. At this concentration, zebrafish larvae exhibited tails that curled upward and shorter than normal body lengths, compared to both DMSO and to the next lower dose of chlorpyrifos (0.1 μM) (Fig. 1). Both egg water (EW) and DMSO were not significantly different for any measure in any of the assays used (data not shown); therefore only DMSO is illustrated in a graphical form for Figs. 2–4. The results are given as percentages of the average number of larvae in a given location and these were averaged over the time course of the assay. There were no significant changes in behavior over time for any assay in the current study consistent with previous studies in our lab (Pelkowski et al., 2011); however, longer recordings could give interesting results and may indicate learning over a longer time course.
Fig. 1.
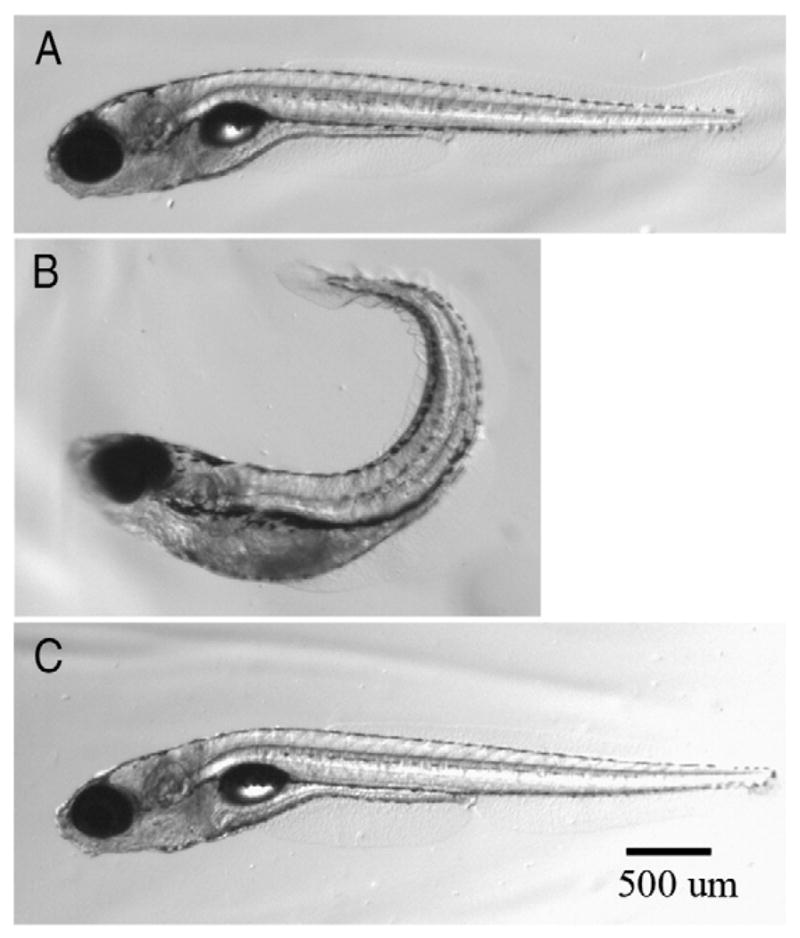
Morphology of 7 dpf zebrafish larvae. Zebrafish larvae at 7 dpf exposed daily with (A) DMSO, and (B) 1 μM and (C) 0.1 μM chlorpyrifos beginning at 2 hours post fertilization (hpf). Larvae treated with either DMSO or 0.1 μM chlorpyrifos have straight elongated tails while the larvae exposed to 1 μM chlorpyrifos exhibited shortened body lengths and tails that curled upward.
Fig. 2.
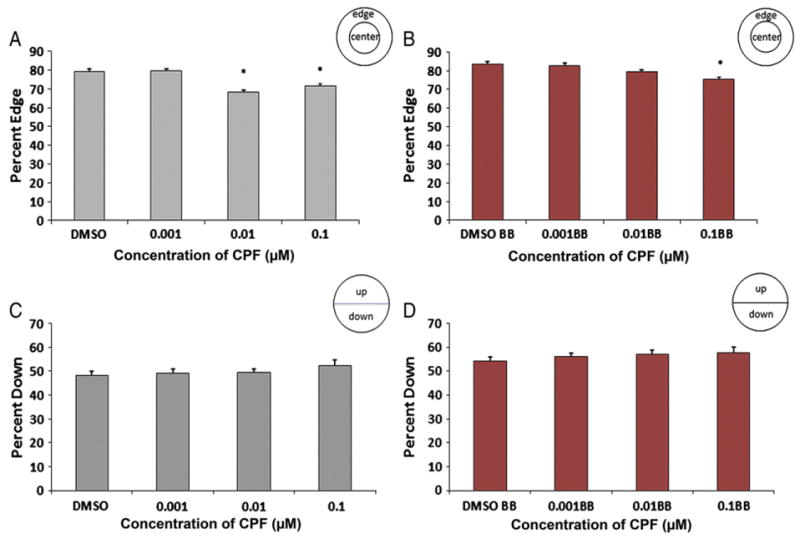
Five fish assay. Percentage of zebrafish larvae on the edge of the well without (A) and with (B) visual stimuli after exposure to DMSO and three concentrations of chlorpyrifos. “BB” indicates the data from the times that the groups were shown the “bouncing ball” visual stimulus. Larvae exposed to 0.1 μM and 0.01 μM of chlorpyrifos were on the edge of the well significantly less compared to DMSO controls without a visual stimulus (p<0.001 for both) whereas larvae treated with 0.1 μM of chlorpyrifos were on the edge significantly less with a visual stimulus (p=0.001). The percentage of zebrafish larvae down in the dish did not differ between groups without (C) or with (D) a visual stimulus (n=36 wells/180 larvae per treatment).
Fig. 4.
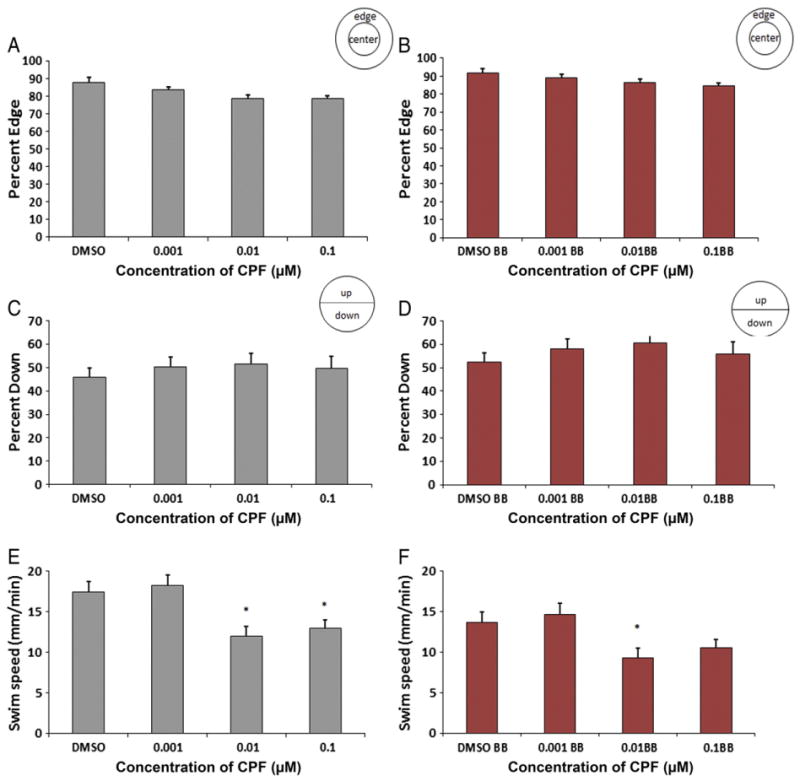
Interval one-fish assay. Zebrafish exposed to any concentration of chlorpyrifos did not differ from controls in their preference for the edge of the well either without a visual stimulus (A) or with a visual stimulus (B) compared to DMSO controls. There were no significant differences in the percentage of larvae showing a preference for being down in the dish compared to DMSO controls either without (C) or with (D) a visual stimulus. Larvae exposed to 0.1 μM and 0.01 μM chlorpyrifos showed a significant decrease in swim speed compared to controls without a visual stimulus (p=0.033 and p=0.005) (E) whereas larvae treated with 0.01 μM chlorpyrifos had a significant decrease in swim speed with a visual stimulus (F) (p=0.007) (n=36 wells/36 larvae per treatment). “BB” indicates the data from the times that the groups were shown the “bouncing ball” visual stimulus.
3.1. Five-fish assay
Exposure of zebrafish larvae to 0.1 μM and 0.01 μM of chlorpyrifos significantly reduced larval preference for the edge of the well (thigmotaxis) in the absence of a visual stimulus whereas those treated with 0.1 μM reduced thigmotaxis with the visual stimulus. Larvae exposed to 0.1 μM chlorpyrifos were on the edge of the well 71±1.4% of the time with the blank background, and 75±1.6% of the time when exposed to the visual stimulus. Larvae in the 0.01 μM exposure group were on the edge of the well 68±1.5% of the time and 79±1.3% of the time with the blank background or visual stimulus, respectively. DMSO control larvae were 79±1.2% on the edge without, and 84± 1.5% on the edge with the visual stimulus (Fig. 2A/B, p<0.001 for both chlorpyrifos groups (0.1 μM and 0.01 μM) compared to DMSO without stimulus and p=0.001 in the 0.1 μM chlorpyrifos group in comparison to the DMSO control with a visual stimulus). The percentage of larvae down in the dish, away from the visual stimulus (moving ball) did not differ significantly from controls, and percent down ranged from 48 to 52% (± 1.5–2.2) without a stimulus (for 0.1 μM and 0.01 μM chlorpyrifos treated groups) and 54–57% (± 1.8–2.4) with visual stimuli (for 0.1 μM and 0.01 μM chlorpyrifos treated groups) (Fig. 2C/D). However, comparisons without visual stimulus vs. with visual stimulus within groups were all significant for percent down in the dish (p<0.05 for each group). Exposure of zebrafish larvae to the lowest dose of chlorpyrifos (0.001 μM) elicited no change in either edge preference (80% ± 1 on edge without a stimulus and 83% ± 1.5 with a visual stimulus) or preference to be down in the well (49% ± 2.1 down without a stimulus and 56% ± 2.4 down with a visual stimulus) when compared to DMSO controls (Fig. 2A–D).
3.2. Swim speed assay (one-fish assay without visual stimuli)
Zebrafish larvae exposed to 0.1 μM and 0.01 μM of chlorpyrifos exhibited a statistically significantly decrease in preference for the edge of the well (71 ± 3% and 69 ± 3% on the edge, respectively) (p=0.005 for 0.01 μM and p=0.035 for 0.1 μM) compared to DMSO controls (82 ± 2.4%), while exposure to 0.001 μM chlorpyrifos did not alter the edge preference compared to controls (80% ± 2.6 edge) (Fig. 3A). There were no significant differences in the percentage of larvae down in the dish (ranging from 46 to 52 ± 3.3 to 4.5% down in the dish) (Fig. 3B). Exposure of zebrafish larvae to 0.1 μM and 0.01 μM of chlorpyrifos significantly reduced larval swimming speed (10–11± 0.9 mm/min) compared to DMSO controls (18 ± 1.1 mm/min) (Fig. 3C, p<0.001 for both), while the swimming speed of larvae exposed to the lowest dose of chlorpyrifos (0.001 μM) did not differ from the control (18 ± 1.3 mm/min, p=0.8).
Fig. 3.
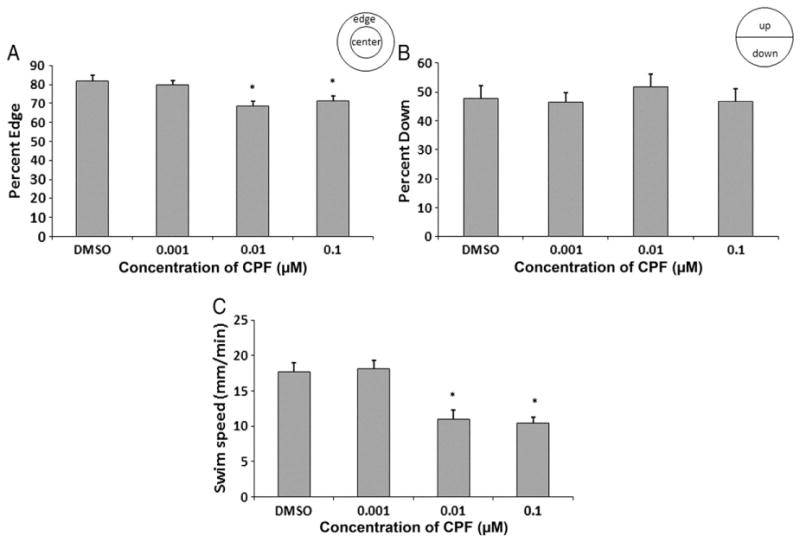
One fish assay without visual stimuli. Zebrafish exposed to 0.1 μM and 0.01 μM chlorpyrifos were on the edge significantly less compared to DMSO controls (p=0.035 and p=0.005). There were no differences between groups for the percentage of zebrafish larvae down in the dish. Zebrafish exposed to 0.1 μM and 0.01 μM chlorpyrifos swam significantly slower than the DMSO controls (p<0.001 for both) (n=36 wells/36 larvae per treatment).
3.3. Interval assay (one-fish assay with intervals of no visual and visual stimuli)
Exposure of zebrafish larvae to any concentration of chlorpyrifos did not reveal any difference from controls in larval preference for the edge as shown in Fig. 4A/B (ranges of 79–88 ± 3% without and 84–92 ± 2.9% with visual stimulus). For all concentrations of chlorpyrifos, the percentage of larvae down in the dish did not differ significantly from those in the DMSO control group (ranges of 45–51 ± 4.0–\5.1% without and 52–60 ± 4.3–5.2% with a visual stimulus) (Fig. 4C/D). The swimming speed of larvae treated with 0.1 μM and 0.01 μM of chlorpyrifos was significantly decreased without a visual stimulus (12–13 ± 1.0 mm/min; p=0.033 and p=0.005 respectively) while the group treated with 0.01 μM was significantly decreased with visual stimuli (9 ± 0.9 mm/min, p=0.007), compared to those treated with DMSO (17 ± 1.3 mm/min without and 14 ± 1.1 mm/min with visual stimuli). The group of larvae treated with 0.001 μM of chlorpyrifos, however, did not show a statistically significant difference in swim speed compared with the DMSO control group (18 ± 1.3 mm/min without and 15 ± 1.1 mm/min with visual stimuli) (Fig. 4E/F).
4. Discussion
The present study assessed the effects of exposure to four sub-chronic doses of chlorpyrifos on body morphology, swimming speed, thigmotaxis behavior (% on the edge), and avoidance behavior (% down in the well) in 7 dpf zebrafish larvae. The results of the current study indicate that the administration of sub-chronic dose of 1 μM chlorpyrifos from 0 to 7 dpf significantly impacted body morphology. Sub-chronic administration of 0.1 and 0.01 μM chlorpyrifos significantly impacted the swimming speed and thigmotaxis behavior in zebrafish larvae, but the avoidance behavior remained unaffected by either dose. In a previous study by Colwill and Creton (2011b), zebrafish larvae were analyzed for locomotion and edge preference during development. These parameters did not change between 6 and 7 dpf indicating that a decrease in locomotion speed and thigmotaxis behavior cannot be attributed to a developmental delay. The results of the present study show that even extremely low sub-chronic doses of chlorpyrifos administered induced specific behavioral defects or morphological deformities at concentrations that correspond with those found in epidemiologic and animal studies (Bouchard et al., 2010; Kienle et al., 2009; Lu et al., 2006; Morgan et al., 2004; Ton et al., 2006) and in similar doses that mimic the levels found in the general population (Lu et al., 2008, 2006).
The highest dose of chlorpyrifos administered, 1 μM, had a highly toxic effect on movement, body morphology, and body size; these changes began around 3–4 dpf. At this dose, the larvae exhibited twitching behaviors, with tails curling upward with abnormal swimming behavior. These observed morphological defects secondary to sub-chronic chlorpyrifos exposure after 3–4 dpf have also been previously documented in zebrafish larvae in which motor movement, body morphology, and activity levels were altered (Kienle et al., 2009).
Previous studies in zebrafish indicate that larvae may be unable to break down chlorpyrifos into its more harmful metabolites until after 3 dpf, because they do not possess sufficient quantities of necessary enzymes until after 3 dpf (Yang et al., 2011). If correct, this would explain the delayed onset of motor and morphological defects. When exposed to levels of chlorpyrifos less than or equal to 0.1 μM, 7 dpf larvae did not differ morphologically from their DMSO control groups.
Although the larvae exposed to the two higher levels of chlorpyrifos in our study (0.01 and 0.1 μM) exhibited reduced thigmotaxis and decreased swimming speeds, there were no significant differences with respect to the percentage of larvae down in the well across treatments (each treatment group of larvae showed a significant response to the moving ball by moving down in the well consistent with previous studies in our laboratory (Pelkowski et al., 2011; Richendrfer et al., 2012)). These data indicate that while higher levels of chlorpyrifos interfere with the speed of locomotion, the larvae are still able to detect the visual stimuli and respond to these cues by exhibiting a clear preference to be on the edge, though no difference in preference for being down in the dish when exposed to a visual stimulus was observed, compared to controls. This suggests that any motor deficits that may be present are not the cause of the decreased edge preference.
While the 0.01 and 0.1 μM doses of chlorpyrifos significantly decreased swimming speed, the larvae swam and moved adequately enough for behavior to be assessed. The lowest dose of chlorpyrifos administered, 0.001 μM, did not affect the swim speed of the 7 dpf larvae. Previous studies in zebrafish larvae treated only up to 72 hpf with levels of chlorpyrifos less than 1 μM showed that touch-induced swimming was not affected (Yang et al., 2011); however, this is likely due to the fact that swimming was assessed prior to the time point at which sufficient quantities of necessary enzymes are present to break down chlorpyrifos into its metabolites. Considered together with the present study, which extended the time period of treatment with chlorpyrifos until 7 dpf, these findings suggest that the crucial time point of toxicity in zebrafish larvae happens after 72 hpf (or 3 dpf). In line with this argument, sub-chronic exposure to levels of chlorpyrifos less than 0.25 mg/l significantly decreased motor activity after 5 dpf in zebrafish larvae (Kienle et al., 2009) and in 6 and 9 dpf larvae after sub-chronic exposure of 100 ng/ml (but not 10 ng/ml) chlorpyrifos between 1 and 5 dpf (Levin et al., 2004). Interestingly, the same treatment in larvae (100 ng/ml) promoted hyperactivity in adult zebrafish whereas the 10 ng/ml treatment promoted hypoactivity in adults (Levin et al., 2003). Similarly, chlorpyrifos metabolites greater than 0.1 μM slowed motor neuron growth by 3 dpf (Yang et al., 2011) which may be a contributing factor to variations in swimming speed. In the current report, we were able to accurately measure changes in activity levels at even lower doses of chlorpyrifos than those in previous studies and have extended the time period of chlorpyrifos exposure. Therefore, the timing and concentrations of chlorpyrifos treatments appear to be very sensitive, as treatment during different stages of development produces very distinct responses with regard to swimming and motor movement, which are likely due to levels of enzymatic activity necessary for chlorpyrifos metabolism.
It is clear from the results of this study that exposure to 0.01 and 0.1 μM chlorpyrifos significantly reduces the percentage of larvae on the edge of the well (decreased thigmotaxis), while the lowest dose in our study (0.001 μM) had no effect on thigmotaxis. Thigmotaxis is commonly used as a measure of anxiety-related behavior (Champagne et al., 2010; Colwill and Creton, 2011b; Miller et al., 2010), and increased thigmotaxis is directly correlated with higher levels of anxiety. Thus, the results of the present study indicate that zebrafish larvae treated with sub-chronic doses of chlorpyrifos equal to or greater than 0.1 μM present less anxiety-related behavior when they are shown a visual stimulus in comparison to controls or to the lowest dose of chlorpyrifos administered (0.001 μM). The changes in manifestations of anxiety-related behavior after exposure to chlorpyrifos could pose a potential threat to the survival of zebrafish larvae in the instance of predator avoidance and food capture.
In addition to zebrafish, thigmotaxis following exposure to chlorpyrifos has been measured in other animal models, but some of the results are conflicting. Adult female mice exhibited increased thigmotaxis behavior after prenatal chlorpyrifos exposure, as evidenced by their spending more time in the dark side of a tunnel (Venerosi et al., 2010). However, utilizing the paradigm of an elevated plus-maze to measure anxiety in male and female mice perinatally exposed to chlorpyrifos yielded conflicting reports (Braquenier et al., 2009; Ricceri et al., 2006). Again, it appears as though timing of chlorpyrifos treatment during development is crucial. Further, when differing treatment time points are combined with different methodologies for measuring behavior, varying results pertaining to neurobehavioral disorders, including anxiety-related behaviors may be observed.
It is possible that decreased measures of anxiety after developmental chlorpyrifos exposure may reflect a permanent change in neural patterning. A previous study which evaluated the effects of zebrafish larval exposure to levels of chlorpyrifos very similar to those utilized in our study demonstrated that AChE activity was not affected (Yang et al., 2011), suggesting that the behavioral changes observed in our study were not a result of AChE inhibition. Low levels of acute and sub-chronic chlorpyrifos (≤1 μM) and its oxon form (≤1 nM) elicited neurotoxicity without affecting AChE and modify axonal and dendritic outgrowth in neuronal cultures, which is concentration dependent (Howard et al., 2005). Along similar lines, chlorpyrifos administration (at levels not inhibiting AChE) in rodent models indicates that anxiety behavior was modified, yet results were conflicting (Chen et al., 2011; Ricceri et al., 2006; Venerosi et al., 2010). In the present study, observed changes in anxiety-related behavior suggest that chlorpyrifos promotes neurotoxicity, thereby damaging neural patterning and circuitry in early brain development, at doses that do not alter AChE levels. Such abnormalities could be the result of aberrations in mitosis, cell signaling, and neuronal apoptosis (Sişman, 2010; Slotkin, 1999). Additionally, evidence exists that changes in serotonergic and dopaminergic function and metabolism may occur after exposure to chlorpyrifos. In fact, 5-HT turnover was significantly increased in adult rats perinatally exposed to low levels of chlorpyrifos (Aldridge et al., 2005). The persisting effects of chlorpyrifos on neurotransmitter levels in adult zebrafish larvae from exposure during development are also a cause for concern. Treatment of larvae with 100 ng/ml of chlorpyrifos between 1 and 5 dpf decreased the levels of serotonin and dopamine while increasing their turnover in zebrafish larvae; the decreased dopamine levels continued into adulthood (Sledge et al., 2011). Moreover, prenatal exposure of mice to chlorpyrifos prevents changes in forced swimming behavior after administration of the drug fluvoxamine (a selective serotonin reuptake inhibitor) in adulthood, when compared to vehicle controls (Venerosi et al., 2010). In line with the results of the current study, acute exposure of larvae to the anxiolytic diazepam significantly reduced the percentage of larvae on the edge of the dish, both in the absence and presence of a visual stimulus (Richendrfer et al., 2012). Because diazepam exerts its effects by binding to neuronal GABA receptors, there is also the potential that chlorpyrifos may modify GABAergic neurons, or GABA levels. Overall, the observed behavioral defects following chlorpyrifos exposure are concerning, especially since long-term behavioral changes induced by fetal exposure to stress, drugs, and environmental toxicants, including chlorpyrifos, have been documented in other species, including humans (Beer et al., 2004; Berkowitz et al., 2004; Dufour-Rainfray et al., 2011; Lazinski et al., 2008; Senger et al., 2011).
Results from the current study revealed that larval avoidance (another measure of anxiety (Ahmed et al., 2011; Maximino et al., 2010)) of the moving ball was not affected by exposure to low doses of chlorpyrifos. There are a few possibilities that may explain the discrepancies in the results of our study. One possibility is that avoidance behavior may involve different brain regions and neural networks than thigmotaxis behavior (Jesuthasan, 2011), and these brain areas may not be affected by chlorpyrifos exposure. A second possibility is that the timing (period of development) and length of treatment, and specific behavioral test administered are also likely to produce varying avoidance behavior results because of differences in the sensitivity of the measures themselves. In rats acutely treated with chlorpyrifos during adulthood, latency of inhibitory avoidance in the elevated T-maze significantly decreased after 5 days of treatment, compared to controls, although these differences were not observed 2 days after treatment (Selderslaghs et al., 2010). It is possible that analysis of zebrafish larvae treated with chlorpyrifos at different developmental time points could yield significantly different avoidance behavior responses to visual stimuli.
5. Conclusions
The present study reports behavioral defects associated with low doses of chlorpyrifos administered during development. We show that sub-chronic doses of 0.01 or 0.1 μM chlorpyrifos decreases anxiety-related behavior (thigmotaxis) and swimming speed in zebrafish that are evaluated at 7 days post fertilization. Higher doses (1 μM) altered body morphology whereas the lowest dose administered (0.001 μM) elicited no changes in morphology, anxiety, or swimming speed. To test this we used a novel high-throughput assay that utilized time-lapse imaging. Because zebrafish embryos are transparent, future studies examining toxicant-induced neural patterning defects using live imaging of the developing brain will increase our understanding of neurodevelopmental disorders and may assist in the transformation of dietary guidelines and risk prevention strategies.
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, R01HD060647) and the National Institute of Environmental Health Sciences (NIEHS, R03ES017755).
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Contributor Information
Holly Richendrfer, Email: Holly_Richendrfer@brown.edu.
Sean D. Pelkowski, Email: Sean_Pelkowski@brown.edu.
Ruth M. Colwill, Email: Ruth_Colwill@brown.edu.
Robbert Créton, Email: Robbert_Creton@brown.edu.
References
- Adinolfi M. The development of the human blood–CSF–brain barrier. Dev Med Child Neurol. 1985;27:532–7. doi: 10.1111/j.1469-8749.1985.tb04581.x. [DOI] [PubMed] [Google Scholar]
- Ahmed O, Seguin D, Gerlai R. An automated predator avoidance task in zebrafish. Behav Brain Res. 2011;216:166–71. doi: 10.1016/j.bbr.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect. 2005;113:1027–31. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer A, Slotkin TA, Seidler FJ, Aldridge JE, Yanai J. Nicotine therapy in adulthood reverses the synaptic and behavioral deficits elicited by prenatal exposure to phenobarbital. Neuropsychopharmacology. 2004;30:156–65. doi: 10.1038/sj.npp.1300582. [DOI] [PubMed] [Google Scholar]
- Berkowitz G, Wetmur J, Birman-Deych E, Obel J, Lapinski R, Godbold J, et al. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ Health Perspect. 2004;112:388–91. doi: 10.1289/ehp.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 2010;125:e1270–7. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braquenier J-B, Quertemont E, Tirelli E, Plumier J-C. Anxiety in adult female mice following perinatal exposure to chlorpyrifos. Neurotoxicol Teratol. 2009;32:234–9. doi: 10.1016/j.ntt.2009.08.008. [DOI] [PubMed] [Google Scholar]
- CDC. Mental health in the United States: parental report of diagnosed autism in children aged 4–17 years—United States, 2003–2004. MMWR Morb Mortal Wkly Rep. 2006;55:481–6. [PubMed] [Google Scholar]
- CDC. Nerve agent and organophosphate pesticide poisoning. http://emergency.cdc.gov/agent/nerve/tsd.asp2011.
- Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatry. 2005;162:1133–41. doi: 10.1176/appi.ajp.162.6.1133. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Hoefnagels CCM, de Kloet RE, Richardson MK. Translating rodent behavioral repertoire to zebrafish (Danio rerio): relevance for stress research. Behav Brain Res. 2010;214:332–42. doi: 10.1016/j.bbr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Chen J, Huang C, Zheng L, Simonich M, Bai C, Tanguay R, et al. Trimethyltin chloride (TMT) neurobehavioral toxicity in embryonic zebrafish. Neurotoxicol Teratol. 2011;33:721–6. doi: 10.1016/j.ntt.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill RM, Creton R. Imaging escape and avoidance behavior in zebrafish larvae. Rev Neurosci. 2011a;22:63–73. doi: 10.1515/RNS.2011.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill RM, Creton R. Locomotor behaviors in zebrafish (Danio rerio) larvae. Behav Processes. 2011b;86:222–9. doi: 10.1016/j.beproc.2010.12.003. (NIHMS264983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour-Rainfray D, Vourc’h P, Tourlet S, Guilloteau D, Chalon S, Andres CR. Fetal exposure to teratogens: evidence of genes involved in autism. Neurosci Biobehav Rev. 2011;35:1254–65. doi: 10.1016/j.neubiorev.2010.12.013. [DOI] [PubMed] [Google Scholar]
- EPA. Organophosphate pesticides in food — a primer on reassessment of residue limits. http://www.epa.gov/pesticides/2011.
- Furlong CE, Holland N, Richter RJ, Bradman A, Ho A, Eskenazi B. PON1 status of farm-worker mothers and children as a predictor of organophosphate sensitivity. Pharmacogenet Genomics. 2006;16:183–90. doi: 10.1097/01.fpc.0000189796.21770.d3. [DOI] [PubMed] [Google Scholar]
- Howard AS, Bucelli R, Jett DA, Bruun D, Yang D, Lein PJ. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol Appl Pharmacol. 2005;207:112–24. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Jesuthasan S. Fear, anxiety and control in the zebrafish. Dev Neurobiol. 2012;72(3):395–403. doi: 10.1002/dneu.20873. (n/a–n/a) [DOI] [PubMed] [Google Scholar]
- Kienle C, Köhler H-R, Gerhardt A. Behavioural and developmental toxicity of chlorpyrifos and nickel chloride to zebrafish (Danio rerio) embryos and larvae. Ecotoxicol Environ Saf. 2009;72:1740–7. doi: 10.1016/j.ecoenv.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Kokel D, Bryan J, Laggner C, White R, Cheung CYJ, Mateus R, et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol. 2010;6:231–7. doi: 10.1038/nchembio.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazinski M, Shea A, Steiner M. Effects of maternal prenatal stress on offspring development: a commentary. Arch Womens Ment Health. 2008;11:363–75. doi: 10.1007/s00737-008-0035-4. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol Teratol. 2003;25:51–7. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Swain HA, Donerly S, Linney E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol Teratol. 2004;26:719–23. doi: 10.1016/j.ntt.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Lovato A, Pelkowski S, Creton R, Colwill R. Embryonic exposure to environmentally relevant concentrations of polychlorinated biphenyls (PCBs) disrupts avoidance behavior in zebrafish larvae. Washington, DC: Society for Neuroscience; 2011. [Google Scholar]
- Lu C, Barr DB, Pearson MA, Waller LA. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ Health Perspect. 2008;116:537–42. doi: 10.1289/ehp.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Toepel K, Irish R, Fenske RA, Barr DB, Bravo R. Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environ Health Perspect. 2006:114. doi: 10.1289/ehp.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximino C, de Brito TM, da Silva Batista AW, Herculano AM, Morato S, Gouveia A., Jr Measuring anxiety in zebrafish: a critical review. Behav Brain Res. 2010;214:157–71. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Miller BH, Schultz LE, Gulati A, Su AI, Pletcher MT. Phenotypic characterization of a genetically diverse panel of mice for behavioral despair and anxiety. PLoS One. 2010;5:e14458. doi: 10.1371/journal.pone.0014458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MK, Sheldon LS, Croghan CW, Jones PA, Robertson GL, Chuang JC, et al. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J Expo Anal Environ Epidemiol. 2004;15:297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- Pelkowski S, Kapoor M, Richendrfer H, Wang X, Colwill RM, Creton R. A novel high-throughput imaging system for automated analyses of avoidance behavior in zebrafish larvae. Behav Brain Res. 2011;223:135–44. doi: 10.1016/j.bbr.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, et al. Impact of pre-natal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:e1845–59. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricceri L, Venerosi A, Capone F, Cometa MF, Lorenzini P, Fortuna S, et al. Developmental neurotoxicity of organophosphorous pesticides: fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice. Toxicol Sci. 2006;93:105–13. doi: 10.1093/toxsci/kfl032. [DOI] [PubMed] [Google Scholar]
- Richendrfer H, Pelkowski S, Colwill RM, Creton R. On the edge: pharmacological evidence for anxiety-related behavior in zebrafish larvae. Behav Brain Res. 2012;228:99–106. doi: 10.1016/j.bbr.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihel J, Prober DA, Arvanites A, Lam K, Zimmerman S, Jang S, et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science. 2010;327:348–51. doi: 10.1126/science.1183090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison LM, Skaer TL, Sclar DA, Galin RS. Is attention deficit hyperactivity disorder increasing among girls in the US? : trends in diagnosis and the prescribing of stimulants. CNS Drugs. 2002;16:129–37. doi: 10.2165/00023210-200216020-00005. [DOI] [PubMed] [Google Scholar]
- Selderslaghs IWT, Hooyberghs J, De Coen W, Witters HE. Locomotor activity in zebrafish embryos: a new method to assess developmental neurotoxicity. Neurotoxicol Teratol. 2010;32:460–71. doi: 10.1016/j.ntt.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Senger M, Seibt K, Ghisleni G, Dias R, Bogo M, Bonan C. Aluminum exposure alters behavioral parameters and increases acetylcholinesterase activity in zebrafish (Danio rerio) brain. Cell Biol Toxicol. 2011;27:199–205. doi: 10.1007/s10565-011-9181-y. [DOI] [PubMed] [Google Scholar]
- Sişman T. Dichlorvos-induced developmental toxicity in zebrafish. Toxicol Ind Health. 2010;26:567–73. doi: 10.1177/0748233710373089. [DOI] [PubMed] [Google Scholar]
- Sledge D, Yen J, Morton T, Dishaw L, Petro A, Donerly S, et al. Critical duration of exposure for developmental chlorpyrifos-induced neurobehavioral toxicity. Neurotoxicol Teratol. 2011;33:742–51. doi: 10.1016/j.ntt.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect. 1999;107(Suppl 1):71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton C, Lin Y, Willett C. Zebrafish as a model for developmental neurotoxicity testing. Birth Defects Res A Clin Mol Teratol. 2006;76:553–67. doi: 10.1002/bdra.20281. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Ricceri L, Rungi A, Sanghez V, Calamandrei G. Gestational exposure to the organophosphate chlorpyrifos alters social–emotional behaviour and impairs responsiveness to the serotonin transporter inhibitor fluvoxamine in mice. Psychopharmacology. 2010;208:99–107. doi: 10.1007/s00213-009-1713-2. [DOI] [PubMed] [Google Scholar]
- Yang D, Lauridsen H, Buels K, Chi L-H, La Du J, Bruun D, et al. Chlorpyrifosoxon disrupts zebrafish axonal growth and motor behavior. Toxicol Sci. 2011;121(1):146–59. doi: 10.1093/toxsci/kfr028. [DOI] [PMC free article] [PubMed] [Google Scholar]


