Abstract
Previous investigations have demonstrated the beneficial effects of mild hypothermia following different types of traumatic brain injury (TBI). In some models, early cooling following TBI has been shown to reduce the frequency of axonal damage, a major consequence of head injury. The purpose of this study was to evaluate the effects of post-traumatic hypothermia in a model that has been shown to be sensitive to temperature manipulations in the early injury setting. Animals underwent moderate parasagittal fluid percussion (FP) brain injury and were then either randomized into normothermic or hypothermic groups. In the hypothermic groups, brain temperature was reduced to either 30°C or 33°C 5 minutes after trauma and maintained for a 3-hour period. Normothermic or sham-operated animals were held under normal temperature conditions. At 3 days after TBI, animals were perfusion-fixed for a quantitative assessment of beta amyloid precursor protein (β-APP) immunohistochemistry and silver staining. Traumatic injury led to a significant increase in the frequency of β-APP immunoreactive profiles within both the corpus callosum, external capsule, and the internal capsule. While early cooling revealed a trend for protection, no significant differences were shown between normothermic and hypothermic animals in terms of the frequency of injured axons at 3 days post-trauma. These results emphasize that axonal pathology is a major consequence of brain injury using this particular model. It is concluded that longer periods of post-traumatic hypothermia may be required to chronically protect axon populations undergoing a progressive injury.
Introduction
Traumatic brain injury (TBI) is a major health problem in the United States, affecting approximately 500,000 people each year (Thurman et al., 1999). Brain trauma as a result of blast injury and high-velocity projectiles have also resulted in a high incidence of brain trauma to our military personnel. Although significant amounts of research have been conducted to evaluate the pathophysiology of TBI (McIntosh et al., 1998; Bramlett and Dietrich, 2004; Raghupathi, 2004), no successful treatments are currently available to treat the devastating effects (Narayan et al., 2002; Langlois et al., 2004). Recent studies have demonstrated the beneficial effects of early cooling in various models of TBI (Dietrich and Bramlett, 2010). In models of diffuse as well as focal brain injury, therapeutic hypothermia has been shown to reduce overall contusion volume, protect vulnerable brain regions from irreversible neuronal damage, and limit altered blood–brain barrier permeability. In several trauma models, early cooling has also been reported to reduce the incidence of axonal pathology demonstrated by various markers of axonal pathology up to 24 hours after injury (Table 1; Marion and White, 1996; Koizumi and Povlishock, 1998; Maxwell et al., 1999; Büki et al., 1999a; Suehiro and Povlishock 2001; Suehiro et al., 2001).
Table 1.
Effects of Hypothermia on Diffuse Axonal Injury
| Study | Year | Species | Model | Time point | Level | Survival | Outcome measure |
|---|---|---|---|---|---|---|---|
| Taft et al. | 1993 | Rat | FP | Pre- and postinjury for 60 min | 30°C | 3 h | MAP 2 |
| Marion and White | 1996 | Rat | CCI | 10, 25 or 40 min postinjury for 4 h | 32°C | 24 h | Neurofilament stain |
| Koizumi and Povlishock | 1998 | Rat | Impact-acceleration | Pre or postinjury for 1 h | 32°C | 24 h | APP |
| Maxwell et al. | 1999 | Guinea Pig | Optic nerve stretch | Poststretch for 4 h | 32°C | 2 or 4 h postinjury | APP |
| Buki et al. | 1999b | Rat | Impact-acceleration | Postinjury for 90 min | 32°C | 180 min | Calpain-mediated spectrin proteolysis, neurofilament |
| Suehiro and Povlishock | 2001 | Rat | Impact-acceleration | Postinjury for 1 h | 32°C | 24 h | APP |
| Suehiro et al. | 2001 | Rat | Impact-acceleration | Postinjury for 1 h | 32°C | 3 h | APP, neurofilament |
| Suzuki et al. | 2004 | Rat | FP | 30 min postinjury for 4 h | 40°C | 72 h | APP |
| Gao et al. | 2010 | Rat | Impact-acceleration | 1 h postinjury for 90 min | 33°C | 4,5 and 6 h | Vascular reactivity and APP |
| Fujita et al. | 2011 | Rat | Impact-acceleration | 1 h postinjury for 60 min | 33°C | 4, 5 and 6 h | Vascular reactivity and APP |
| Oda et al. | 2011 | Rat | FP | 1 h postinjury for 60 min | 33°C | 4,5 and 6 h | Vascular reactivity, APP and BBB breakdown |
APP, amyloid precursor protein; BBB, blood–brain barrier; CCI, controlled cortical impact; FP, fluid percussion; MAP2, microtubule-associated protein 2.
In addition to morphological protection, therapeutic hypothermia reduces many of the pathophysiological mechanisms felt to be associated with irreversible cell damage and long-term functional deficits. In this regard, therapeutic hypothermia has been reported to improve sensorimotor as well as cognitive problems associated with moderate or severe TBI (Bramlett et al., 1995; Dixon et al., 1998). In some clinical studies, early cooling has also been reported to reduce mortality and improve the functional consequences of severe TBI (Marion et al., 1997; Jiang et al., 2000, 2006; Clifton et al., 2002; Shiozaki et al., 2003; Qiu et al., 2007).
Based on the encouraging results from several single-institutional studies, multi-center investigations have now been conducted to evaluate whether therapeutic hypothermia works in a large number of patients with severe TBI (Clifton et al., 2002; 2011; Polderman, 2008). Unfortunately, therapeutic hypothermia in these studies has failed to improve long-term neurological function. Some benefits, including helping to control reactive intracranial pressure (ICP), have been demonstrated. Obviously, more work is needed to determine what specific population of severe TBI patients may most benefit from this experimental therapeutic intervention. In this regard, severe TBI is a heterogenous injury that may require specific therapeutic strategies based on the dominant pathophysiological processes occurring in the early treatment period.
Based on previous clinical and experimental data, we performed a study to evaluate whether early cooling in an established model of TBI would protect against axonal pathology at either 30°C or 33°C. We determined at 3 days after injury whether the early cooling decreased the frequency of beta amyloid precursor protein (β-APP) expression and other indicators of injured axons, including silver staining (Bramlett et al., 1997; Hall et al., 2008; Shitaka et al., 2011; Garman et al., 2011). The β-APP immunocytochemical approach has previously been shown to be sensitive to visualizing damaged axons in the present model (Bramlett et al., 1997). Quantitative data summarized in this study show that this model produced a high frequency of damaged axons in several white matter tracts. However, while cooling provided a strong trend for protection, the specific cooling protocol investigated in this study failed to significantly reduce the frequency of these damaged processes at 3 days post-trauma.
Materials and Methods
Animals
Male Sprague-Dawley rats (n=29) each weighing approximately 250 g (obtained from Charles River Breeders) were used for the experiments. Animal care was in accordance with the guidelines set forth by the University of Miami Animal Care and Use Committee adhering to the guidelines for experimental animals at the National Institutes of Health. Animals were kept at a constant-temperature-controlled room (72°F) for at least 7 days before the study and exposed to a 12-hour light–dark cycle. Rats were allowed free access to water, but food was withheld overnight before surgery.
Traumatic brain injury
Animals were prepared for parasagittal fluid percussion (FP) injury as previously described (Dietrich et al., 1994; Lotocki et al., 2009; Atkins et al., 2010). Rats were initially anesthetized with 3% halothane, 70% N2O, and 30% O2 and received a 4.8-mm craniotomy (3.8 mm posterior to bregma, 2.5 mm lateral to the midline) to anchor the modified plastic 18-gauge syringe hub (8 mm length, precision guide needle; Becton Dickenson) over the exposed dura of the right parietal cortex. Twenty-four hours after the craniotomy, animals were anesthetized with 3% halothane, 70% N2O, and 30% O2 and then intubated and mechanically ventilated (Harvard Apparatus) with 0.5% halothane, 70% N2O, and 30% O2. To facilitate mechanical ventilation, Pancuronium bromide (0.5 mg/kg, iv) was administered through the femoral artery. To ensure consistent physiological responses among animals, the femoral artery was cannulated to monitor blood gases (PO2 and PCO2), pH, and mean arterial pressure, which were maintained within normal physiological ranges at 15 minutes before TBI and up to 4 hours after TBI. Sham and TBI animals were attached to the FP device (Dixon et al., 1987), and the TBI animals received a moderate FP pulse (1.90–2.21 atm) delivered to the right parietal cortex. The sham animals underwent all surgical procedures except for the FP pulse.
Temperature manipulation
Rectal and temporalis muscle thermistors measured core and brain temperatures, respectively, using self-adjusting feedback warming lamps (Suzuki et al., 2003). The post-TBI brain temperature was maintained for 3 hours at normothermic (TBI-N, n=9), mild (33°C) hypothermic (TBI-33, n=9), or moderate (30°C) hypothermic (TBI-30, n=11) temperature. Post-traumatic brain hypothermia was achieved within 10 minutes following FP injury by blowing cooled air directly onto the skull with a small fan. Normothermic animals were maintained at a brain temperature of 36.6°C to 37.2°C for 3 hours as well. At the end of the cooling period, the animals were rewarmed to normothermic temperatures within 15 minutes and awoke within 1 hour post-temperature manipulation.
Histopathology
At 3 days after TBI or sham procedures, animals were anesthetized using 3% halothane, 70% NO2, and 30% O2 and transcardially perfused with saline followed by a fixative (FAM, a mixture of 40% formaldehyde, glacial acetic acid, and absolute ethanol; 1:1:8 by volume). The paraffin-embedded brains were sectioned every 300 μm by taking two consecutive tissue sections (10 μm thick). The sections were rehydrated and placed in 6% H2O2 to block the endogenous peroxidase acitivty. Tissue was rinsed and placed in a citrate buffer and microwaved. Sections were then dipped in phosphate-buffered saline (PBS) and incubated with normal horse serum. A primary antibody (Boehringer Mannheim, clone 22C11, β-amyloid precursor protein; dilution 1:500) was applied and the slides were placed in the refrigerator overnight. To test for nonspecific staining, the negative controls were conducted where the primary antibody was omitted during the tissue processing. Further rinsing was done with PBS and a secondary antibody was applied. Avidin-Biotin (Vector) complex was used for antibody detection along with diaminobenzidine (DAB) to increase the staining intensity. Slides were washed in 0.5% Triton X-100, followed by 1% cupric sulfate to further intensify staining. Counterstaining was done with hematoxylin and tissue was then dehydrated and coverslipped.
Silver staining
Adjacent serial sections were stained using the Sevier-Munger method (Sevier and Munger, 1965). Sections were mounted, deparaffinized, and then washed with distilled water. Slides were placed in a 20% silver nitrate solution in a water bath (65°C) and then rinsed. Following rinsing, an ammoniacal silver mixture was used to fully develop the stain. The sections were then washed with tap water followed by sodium thiosulfate, washed again, and then dehydrated and mounted. Sections were quantified by counting the retraction balls or densely stained neurons within the following structures: cortex, subthalamic radiation (STR), thalamus, internal capsule (IC), and external capsule (EC) (level 3.8 posterior from bregma).
β-APP regional quantification
β-APP profiles were identified by their dark brown appearance and elongated or circular shape. These reactive profiles appear to be retraction balls/bulbs or reactive axonal processes. Profiles were counted per high microscope field at 400×for the following structures at various coronal levels according to Paxinos and Watson (1982): dorsolateral striatum (0.8 posterior to bregma), IC, STR, and cerebral cortex (3.8 posterior to bregma). Counts were also performed in the EC at 3 coronal levels (0.8, 3.8 and 6.3 posterior to bregma). Averages were computed for each animal from two serial sections per coronal levels per structure.
Statistical analysis
Histopathological data were expressed as mean + standard error (+SEM). Group differences were assessed using one-way analysis of variance followed by posthoc analysis using Fisher's Least Significant Difference (LSD).
Results
All physiological variables except for post-TBI brain temperature were within normal ranges before and following the traumatic insult (Table 2). TBI hypothermic animals at 33°C were significantly different from the other two groups on a few pre- and post-TBI measurements, all the values were within the normal range. The normothermic brain temperature was 36.7+0.1 and the two hypothermic groups were 33.1±0.1 and 30.1±0.1 postinjury. Following the hypothermic period, the temperature was normalized over a course of 15 minutes to a normothermic level.
Table 2.
Physiologic Variables (Mean±Standard Error of Mean)
| Normothermia | Hypothermia (30°C) | Hypothermia (33°C) | |
|---|---|---|---|
| Pretrauma | |||
| pH | 7.43±0.01 | 7.46±0.01 | 7.44±0.01 |
| pCO2 | 38.9±0.8 | 39.4±0.7 | 41.7±0.6a,b |
| pO2 | 152.1+7.5 | 138.1±7.6 | 140.4±7.7 |
| MABP | 112.0±5.1 | 119.2±2.0 | 126.0±2.4a |
| Brain temperature | 36.7±0.1 | 36.7±0.1 | 36.6±0.1 |
| Post-trauma | |||
| pH | 7.43±0.01 | 7.43±0.01 | 7.43±0.01 |
| pCO2 | 38.0±1.1 | 40.2±1.0 | 41.5±1.6 |
| pO2 | 151.3±10.8 | 152.2±6.6 | 144.4±6.5 |
| MABP | 105.2±7.9 | 116.4±2.4 | 122.5±2.1a |
| Brain temperature | 36.6±0.1 | 30.1±0.1a | 33.1±0.1a,b |
All physiological variables were within normal range throughout the experiment.
p<0.05 compared with normothermia
p<0.05 compared with hypothermia (30°C)
MABP, mean arterial blood pressure.
Qualitative histopathology
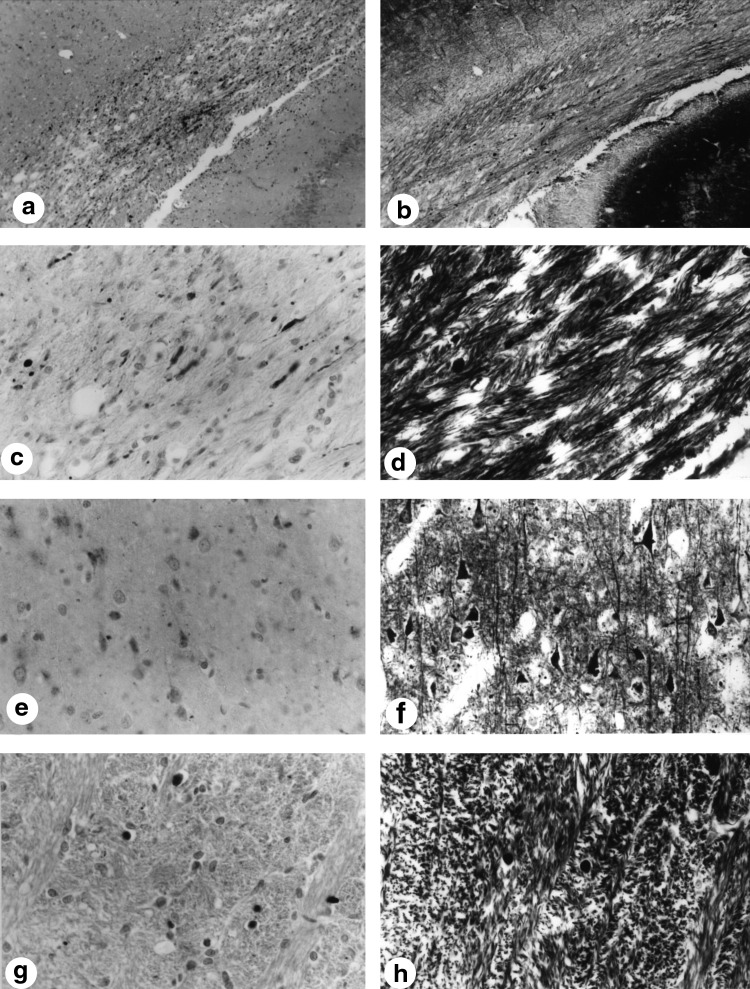
β-APP staining was evident in both hypothermic and normothermic groups. There was no apparent difference in the pattern of staining between groups. Figure 1 shows a β-APP stained section of the EC at the epicenter of the trauma. In addition, a silver stained section is shown in Figure 1 showing again the presence of axonal pathology using this classic technique. Note the retraction balls in both sections at higher magnification (Fig. 1). β-APP also appears to accumulate in neurons (Fig. 1e) with corresponding accumulation using the classical silver stain (Fig. 1f). Subcortical structures, such as the IC, also exhibited both β-APP reactive profiles (Fig. 1g) and silver stained retraction balls (Fig. 1h). Although it appears that the silver stained sections contained a smaller number of profiles compared to the β-APP staining within most structures, there was no obvious difference between any temperature groups.
FIG. 1.
β-APP immunoreactivity and silver staining after moderate TBI. (a) β-APP immunoreactive profiles within the lateral external capsule after TBI (280×). (b) Silver-stained retraction balls within the lateral external capsule after TBI (280×). (c) Higher magnification of immunoreactive profiles within the lateral external capsule (1,120×). (d) Similar staining patterns are seen with the silver staining at higher magnification of the lateral external capsule (1,120×). (e) High magnification of cortical neuronal perikarya flooded with β-APP immunoreactivity (1,120×). (f) Numerous cortical neurons displayed silver staining at high magnification (1,120×). (g) High magnification of β-APP immunoreactive profiles within the internal capsule (1,120×). (h) Retraction balls are also present within the internal capsule using a silver stain (1,120×). β-APP, β-amyloid precursor protein; TBI, traumatic brain injury.
β-APP and silver profile assessment
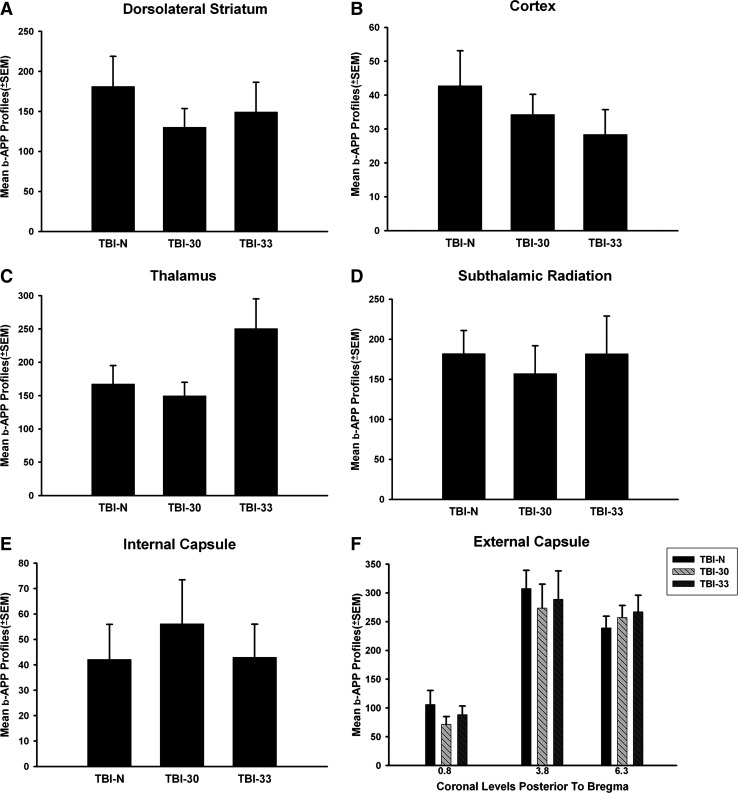
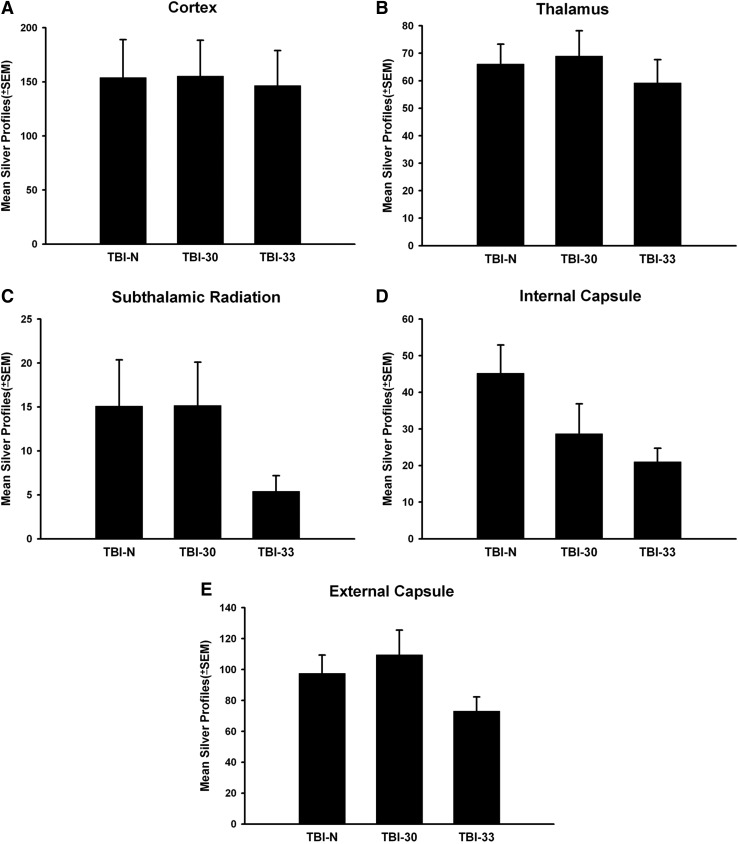
There was no significant difference in the number of either β-APP or silver stained profiles between either the normothermic TBI group or mild/moderate hypothermic groups. β-APP profile counts showed no significant difference between the groups in the striatum (Fig. 2A), cortex (Fig. 2B), thalamus (Fig. 2C), STR (Fig. 2D), IC (Fig. 2E), or EC (Fig. 2F). However, there were some trends for a decrease in the number of silver profiles within specific white matter tracts (IC and STR), but again these were not significant (Fig. 3).
FIG. 2.
Bar graph of mean + SEM numbers of β-APP-positive axonal profiles per microscopic field in gray and white matter structures. (A) β-APP profiles in the dorsolateral striatum. (B) Cortical β-APP profiles within the lateral cortex. (C) β-APP profiles in the thalamus. (D) β-APP immunoreactive profiles in the subthalamic radiation. (E) Reactive profiles within the internal capsule. (F) β-APP profiles in the external capsule with higher levels present at more posterior levels. No effect of mild or moderate hypothermia versus normothermia after TBI was found between groups within any structures analyzed. SEM, standard error of mean.
FIG. 3.
Bar graph of mean + SEM number of silver positive profiles per microscopic field in gray and white matter structures. (A) Cortex. (B) Thalamus. (C) Subthalamic Radiation. (D) Internal Capsule. (E) External Capsule. No significant differences were observed between groups in any structure analyzed.
Discussion
The present data showed that following moderate FP brain injury, a consistent pattern of β-APP immunoreactive and silver stained axonal profiles were seen within vulnerable brain regions throughout the forebrain of the traumatized animal at 3 days after injury. A high frequency of abnormal axons appeared adjacent to the contusion site as well as in some white matter areas more remote from that focal site of damage. These findings are consistent with previously published studies showing that experimental brain injury produces a significant amount of diffuse axonal injury that mimics some of the pathological findings seen with human brain trauma (Adams et al., 1989; Blumbergs et al., 1994; Sherriff et al., 1994; Gentleman et al., 1995; Povlishock and Christman, 1995; Bramlett et al., 1997; Van Den Heuvel et al., 1998). However, in contrast to published studies, we did not show a significant effect of early cooling on the frequency of these immunoreactive profiles. Quantitative assessment showed that early cooling to 30°C or 33°C provided partial protection in several brain regions by reducing the number of β-APP or silver immunoreactive profiles. Interestingly, we observed no additional beneficial effect of reducing the brain temperature to 30°C in this study.
There are several potential reasons why early hypothermia using the present experimental procedure did not have a significant effect on protecting axons from damage. In contrast to other published studies, we used a moderate parasagittal FP brain injury that produces severe white matter damage in the brain regions adjacent to areas of evolving contusion formation and focal blood–brain barrier disruption (Dietrich et al., 1994; Lotocki et al., 2009). While previous studies have identified axonal damage to result from primary axotomy or tissue tears in human specimens (Bramlett et al., 1997; Graham et al., 2000), other studies have also emphasized secondary axonal pathology that is more gradual and progressive in maturation (Povlishock, 1992). In this regard, alterations in axolemmal integrity, activation of calpain, disruption of microtubules, and axonal transport as well as changes in the neurofilament structure and phosphorylation status and mitochondrial damage have been documented (Povlishock and Christman, 1995; Saatman et al., 1996; Maxwell et al., 1997; Okonkwo et al., 1998; Büki et al., 1999a, 1999b).
Specific populations of damaged axons may therefore be more severely injured in the current model compared to what was previously evaluated in the models of more diffuse axonal injury. Indeed, studies by Povlishock and colleagues (1983) have concentrated on axon perturbations occurring in the brain stem levels remote from overt damage including contusion formation or hemorrhagic damage (Koizumi and Povlishock, 1998; Büki et al., 1999a). In this regard, differences between animal models including the lateral FP brain injury and impact injury have been suggested to underlie variations seen with drug treatments targeting axonal damage (Fujita et al., 2011). Thus, the degree of axonal injury may be too severe for therapeutic hypothermia in the present paradigm to provide long-lasting benefits. In the current study, we also used a silver staining approach to identify patterns of axonal damage. Recently, Brody and colleagues (Shitaka et al., 2011) have emphasized different patterns of axonal damage that can be visualized using APP and silver methods. Silver staining appears to be more widespread and intense than APP immunohistochemistry. It would be important in future studies targeting axonal protection to utilize multiple markers of axonal pathology.
Studies using a stretch injury model to optic nerve axons have also reported that hypothermia reduced the number of axons labeled with antibodies against β-APP (Saatman et al., 1996; Maxwell et al., 1999). In one study, morphometric evidence that post-traumatic hypothermia reduced the loss of axonal microtubules and compaction of neurofilaments at the acute stages of injury was evaluated (Maxwell et al., 1999). Hypothermia may therefore improve the outcome following brain and other injuries by reducing the degree of cytoskeletal pathology generally observed after an axonal injury (Okonkwo et al., 1998). In this regard, Taft and colleagues (1993) have reported that post-traumatic hypothermia inhibits the trauma-induced reductions and hippocampal microtubule-associated protein 2 (MAP2). Hypothermia may protect in specific situations where progressive cytoskeletal pathologies underlie the axonal vulnerability. In contrast, under more severe conditions, pharmacological approaches targeting cytoskeletal damage may be required in addition to early cooling.
There are several factors that are known to be important in whether early cooling leads to benefits in protection against structural damage to neurons, oligodendrocytes, blood vessels, and cell processes (Dietrich and Bramlett, 2010). One factor that is critical is the duration of cooling. Early studies with cerebral ischemia, for example, showed that while restrictive periods of cooling provided some degree of protection early after injury (Dietrich et al., 1993), long-term protection was only seen when more prolonged cooling periods were evaluated (Colbourne et al., 1997). In the present study, we only cooled for 3 hours immediately after the traumatic insult. It may therefore be important in future studies to test whether a longer period of cooling leads to axonal protection at clinically relevant post-traumatic time periods. Interestingly, few studies have evaluated the beneficial effects of therapeutic hypothermia past 24 hours after TBI (Table 1). The present 3-day data therefore emphasize a need for more chronic survival studies when evaluating treatments to target axonal injury in the future.
Another factor that may influence the degree of protection seen with post-traumatic hypothermia is the temporal profile of the rewarming period. A slow rewarming period after the hypothermic treatment has been used to maximize the chances of demonstrating axonal and neuronal cell protection (Matsushita et al., 2001; Suehiro and Povlishock, 2001a). Importantly, Povlishock and colleagues (Shiozaki et al., 2003) have reported that rapid rewarming after hypothermia negates the beneficial effects of the therapeutic treatment in terms of axonal pathology. The type of anesthetic that is used in an injury paradigm may also influence the injury cascades that may affect the ability of a therapeutic intervention to be protective. In this study, we utilized halothane, which is known to be a vasodilator (Staunton et al., 2000). However, work from our laboratory has previously shown that hypothermia is protective in the current model in terms of various morphological and behavioral outcome measures using halothane. Thus, in the present study our rewarming procedure as well as the choice of halothane may have been suboptimal to demonstrate significant axonal protection.
Hypothermia alone may not be potent enough to target the severe axonal pathology seen in the present TBI model. Importantly, studies from several laboratories have assessed the effects of cooling plus pharmacological treatments on axonal protection and functional recovery (Dietrich et al., 1995; Suehiro et al., 2001; Fujita et al., 2011). To this end, Marion and White (1996) showed that post-traumatic hypothermia delayed up to 24 hours after a cortical impact injury significantly decreased the frequency of immunoreactive damaged axons when 21 aminosteroids were combined with the cooling protocol. In a recent study by Suehiro and colleagues (2001), postinjury cooling combined with cyclosporine A also enhanced the protection seen in terms of damaged axons. Thus, there is a need in future studies to evaluate combination therapies, including hypothermia plus drugs on long-term axonal pathology targeting specific pathophysiological mechanisms underlying axonal vulnerability after TBI (Okonkwo and Povlishock, 1999; Margulies et al., 2009).
Recently, post hoc analysis of the National Acute Brain Injury Study: Hypothermia II was reported (Clifton et al., 2011). Interestingly, although outcome was not significantly affected by early hypothermic treatment following severe TBI in this patient population, it appeared from a subgroup analysis that there was a significant interaction between the hypothermic treatment and presence of surgically removed hematomas compared to the diffuse brain injury group. When the treatment was assessed in each subgroup, hypothermia patients who underwent surgical removal of intracranial hematomas had significantly fewer poor outcomes than patients with normothermia. In contrast, evidence was also reported that patients with diffuse brain injury who were treated with hypothermia had poorer outcomes compared to normothermia. Thus, it might be hypothesized that hypothermia may work best in patients where decompression surgeries are performed under hypothermic conditions to reduce reperfusion injury (Qiu et al., 2007; Yokobori et al., 2011). If this is the case, then perhaps hypothermia may benefit some subpopulations of severely injured patients with focal brain injuries that can be surgically manipulated with early surgical procedures. Although this preliminary analysis does not suggest that hypothermia cannot protect against diffuse axonal injury, it is an interesting clinical observation that needs to be pursued in future experimental and clinical programs. Alternatively, hypothermia may not be protective in all forms of axonal injury across the wide spectrum of injury severity.
In summary, we demonstrated that a significant amount of diffuse axonal injury occurs in vulnerable brain regions after a moderate parasagittal FP brain injury in rats. In contrast to other trauma models, this animal model produces a significant amount of focal and acute vascular and neuronal damage. Early cooling followed by a 3-hour period of hypothermia failed to significantly reduce the frequency of β-APP immunoreactive and silver-stained axons in several brain regions. This lack of efficacy may result from the use of a focal TBI model, a relatively short duration of cooling, or a nonoptimal rewarming phase. In future studies, longer cooling periods and the possible addition of pharmacological agents that target specific pathophysiological mechanisms underlying axonal damage may yield better findings. Finally, additional studies are required in the field to identify therapeutic strategies including cooling protocols that chronically protect against axonal vulnerability.
Acknowledgments
The authors wish to thank Jeremy Lytle for editorial support. This study was supported by NIH Grants NS030291 and NS042133.
Disclosure Statement
No competing financial interests exist.
References
- Adams JH. Doyle D. Ford I. Gennarelli TA. Graham DI. McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Atkins CM. Truettner JS. Lotocki G. Sanchez-Molano J. Kang Y. Alonso OF. Dietrich WD. Bramlett HM. Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur J Neurosci. 2010;32:1912–1920. doi: 10.1111/j.1460-9568.2010.07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumbergs PC. Scott G. Manavis J. Wainwright H. Simpson DA. McLean AJ. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055–1056. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- Bramlett HM. Green EJ. Dietrich WD. Busto R. Globus MY-T. Ginsberg MD. Post-traumatic brain hypothermia provides protection from sensorimotor and cognitive behavioral deficits. J Neurotrauma. 1995;12:289–298. doi: 10.1089/neu.1995.12.289. [DOI] [PubMed] [Google Scholar]
- Bramlett HM. Kraydieh S. Green EJ. Dietrich WD. Temporal and regional patterns of axonal damage following traumatic brain injury: a beta-amyloid precursor protein immunocytochemical study in rats. J Neuropathol Exp Neurol. 1997;56:1132–1141. doi: 10.1097/00005072-199710000-00007. [DOI] [PubMed] [Google Scholar]
- Bramlett HM. Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- Büki A. Siman R. Trojanowski JQ. Povlishock JT. The role of calpain-mediated spectrin proteolysis in traumatically induced axonal injury. J Neuropathol Exp Neurol. 1999a;58:365–375. doi: 10.1097/00005072-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Büki A. Koizumi H. Povlishock JT. Moderate posttraumatic hypothermia decreases early calpain-mediated proteolysis and concomitant cytoskeletal compromise in traumatic axonal injury. Exp Neurol. 1999b;159:319–328. doi: 10.1006/exnr.1999.7139. [DOI] [PubMed] [Google Scholar]
- Clifton GL. Miller ER. Choi SC. Levin HS. McCauley S. Smith KR., Jr Muizelaar JP. Marion DW. Luerssen TG. Hypothermia on admission in patients with severe brain injury. J Neurotrauma. 2002;19:293–301. doi: 10.1089/089771502753594864. [DOI] [PubMed] [Google Scholar]
- Clifton GL. Valadka A. Zygun D. Coffey CS. Drever P. Fourwinds S. Janis LS. Wilde E. Taylor P. Harshman K. Conley A. Puccio A. Levin HS. McCauley SR. Bucholz RD. Smith KR. Schmidt JH. Scott JN. Yonas H. Okonkwo DO. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol. 2011;10:131–139. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne F. Sutherland G. Corbett D. Postischemic hypothermia. A critical appraisal with implications for clinical treatment. Mol Neurobiol. 1997;14:171–201. doi: 10.1007/BF02740655. [DOI] [PubMed] [Google Scholar]
- Dietrich WD. Alonso O. Halley M. Early microvascular and neuronal consequences of traumatic brain injury: a light and electron microscopic study in rats. J Neurotrauma. 1994;11:289–301. doi: 10.1089/neu.1994.11.289. [DOI] [PubMed] [Google Scholar]
- Dietrich WD. Busto R. Alonso O. Globus MY. Ginsberg MD. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993;13:541–549. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- Dietrich WD. Lin B. Globus MY-T. Green EJ. Ginsberg MD. Busto R. Effect of delayed MK-801 (dizocilpine) treatment with or without immediate postischemic hypothermia on chronic neuronal survival after global forebrain ischemia in rats. J Cereb Blood Flow Metabol. 1995;15:960–968. doi: 10.1038/jcbfm.1995.122. [DOI] [PubMed] [Google Scholar]
- Dietrich WD. Bramlett HM. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics. 2010;7:43–50. doi: 10.1016/j.nurt.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE. Lyeth BG. Povlishock JT. Findling RL. Hamm RJ. Marmarou A. Young HF. Hayes RL. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Dixon CE. Markgraf CG. Angileri F. Pike BR. Wolfson B. Newcomb JK. Bismar MM. Blanco AJ. Clifton GL. Hayes RL. Protective effects of moderate hypothermia on behavioral deficits but not necrotic cavitation following cortical impact injury in the rat. J Neurotrauma. 1998;15:95–103. doi: 10.1089/neu.1998.15.95. [DOI] [PubMed] [Google Scholar]
- Fujita M. Oda Y. Wei EP. Povlishock JT. The combination of either tempol or FK506 with delayed hypothermia: implications for traumatically induced microvascular and axonal protection. J Neurotrauma. 2011;28:1209–1218. doi: 10.1089/neu.2011.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Oda Y. Wei EP. Povlishock JT. The adverse pial arteriolar and axonal consequences of traumatic brain injury complicated by hypoxia and their therapeutic modulation with hypothermia in rat. J Cereb Blood Flow Metab. 2010;30:628–637. doi: 10.1038/jcbfm.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman RH. Jenkins LW. Switzer RC. Bauman RA. Tong LC. Swauger PV. Parks SA. Ritzel DV. Dixon CE. Clark RSB. Bayir H. Kagan V. Jackson EK. Kochanek PM. Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J Neurotrauma. 2011;28:947–959. doi: 10.1089/neu.2010.1540. [DOI] [PubMed] [Google Scholar]
- Gentleman SM. Roberts GW. Gennarelli TA. Maxwell WL. Adams JH. Kerr S. Graham DI. Axonal injury: a universal consequence of fatal closed head injury? Acta Neuropathol. 1995;89:537–543. doi: 10.1007/BF00571509. [DOI] [PubMed] [Google Scholar]
- Graham DI. Raghupathi R. Saatman KE. Meaney D. McIntosh TK. Tissue tears in the white matter after lateral fluid percussion brain injury in the rat: relevance to human brain injury. Acta Neuropathol. 2000;99:117–124. doi: 10.1007/pl00007414. [DOI] [PubMed] [Google Scholar]
- Hall ED. Bryant YD. Cho W. Sullivan PG. Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J Neurotrauma. 2008;25:235–247. doi: 10.1089/neu.2007.0383. [DOI] [PubMed] [Google Scholar]
- Jiang J. Yu M. Zhu C. Effect of long-term mild hypothermia therapy in patients with severe traumatic brain injury: 1-year follow-up review of 87 cases. J Neurosurg. 2000;93:546–549. doi: 10.3171/jns.2000.93.4.0546. [DOI] [PubMed] [Google Scholar]
- Jiang JY. Xu W. Li WP. Gao GY. Bao YH. Liang YM. Luo QZ. Effect of long-term mild hypothermia or short-term mild hypothermia on outcome of patients with severe traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:771–776. doi: 10.1038/sj.jcbfm.9600253. [DOI] [PubMed] [Google Scholar]
- Koizumi H. Povlishock JT. Posttraumatic hypothermia in the treatment of axonal damage in an animal model of traumatic axonal injury. J Neurosurg. 1998;89:303–309. doi: 10.3171/jns.1998.89.2.0303. [DOI] [PubMed] [Google Scholar]
- Langlois JA. Rutland-Brown W. Thomas KE. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Centers for Disease Control and Prevention. Atlanta, GA: National Center for Injury Prevention and Control; 2004. pp. 1–68. [Google Scholar]
- Lotocki G. de Rivero Vaccari JP. Sanchez-Molano J. Furones-Alonso O. Bramlett HM. Dietrich WD. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: effects of therapeutic hypothermia. J Neurotrauma. 2009;29:1251–1261. doi: 10.1089/neu.2008.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies S. Hicks R Combination Therapies for Traumatic Brain Injury Workshop Leaders. Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion DW. Penrod LE. Kelsey SF. Obrist WD. Kochanek PM. Palmer AM. Wisniewski SR. DeKosky ST. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336:540–546. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- Marion DW. White MJ. Treatment of experimental brain injury with moderate hypothermia and 21-aminosteroids. J Neurotrauma. 1996;13:139–147. doi: 10.1089/neu.1996.13.139. [DOI] [PubMed] [Google Scholar]
- Matsushita Y. Bramlett HH. Alonso O. Dietrich WD. Posttraumatic hypothermia is neuroprotective in a model of traumatic brain injury complicated by a secondary hypoxic insult. Crit Care Med. 2001;29:2060–2066. doi: 10.1097/00003246-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Maxwell WL. Povlishock JT. Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma. 1997;14:419–440. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- Maxwell WL. Donnelly S. Sun X. Fenton T. Puri N. Graham DI. Axonal cytoskeletal responses to nondisruptive axonal injury and the short-term effects of posttraumatic hypothermia. J Neurotrauma. 1999;16:1225–1234. doi: 10.1089/neu.1999.16.1225. [DOI] [PubMed] [Google Scholar]
- McIntosh TK. Saatman KE. Raghupathi R. Graham DI. Smith DH. Lee VM. Trojanowski JQ. The Dorothy Russell Memorial Lecture. The molecular and cellular sequelae of experimental traumatic brain injury: pathogenetic mechanisms. Neuropathol Appl Neurobiol. 1998;24:251–267. doi: 10.1046/j.1365-2990.1998.00121.x. [DOI] [PubMed] [Google Scholar]
- Narayan RK. Michel ME. Ansell B. Baethmann A. Biegon A. Bracken MB. Bullock MR. Choi SC. Clifton GL. Contant CF. Coplin WM. Dietrich WD. Ghajar J. Grady SM. Grossman RG. Hall ED. Heetderks W. Hovda DA. Jallo J. Katz RL. Knoller N. Kochanek PM. Maas AI. Majde J. Marion DW. Marmarou A. Marshall LF. McIntosh TK. Miller E. Mohberg N. Muizelaar JP. Pitts LH. Quinn P. Riesenfeld G. Robertson CS. Strauss KI. Teasdale G. Temkin N. Tuma R. Wade C. Walker MD. Weinrich M. Whyte J. Wilberger J. Young AB. Yurkewicz L. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y. Gao G. Wei EP. Povlishock JT. Combinational therapy using hypothermia and the immunophilin ligand FK506 to target altered pial arteriolar reactivity, axonal damage, and blood-brain barrier dysfunction after traumatic brain injury in rat. J Cereb Blood Flow Metab. 2011;31:1143–1154. doi: 10.1038/jcbfm.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo DO. Pettus EH. Moroi J. Povlishock JT. Alteration of the neurofilament sidearm and its relation to neurofilament compaction occurring with traumatic axonal injury. Brain Res. 1998;784:1–6. doi: 10.1016/s0006-8993(97)01075-5. [DOI] [PubMed] [Google Scholar]
- Okonkwo DO. Povlishock JT. An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J Cereb Blood Flow Metab. 1999;19:443–451. doi: 10.1097/00004647-199904000-00010. [DOI] [PubMed] [Google Scholar]
- Paxinos G. Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1982. [Google Scholar]
- Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- Povlishock JT. Becker DP. Cheng CL. Vaughan GW. Axonal change in minor head injury. J Neuropathol Exp Neurol. 1983;42:225–242. doi: 10.1097/00005072-198305000-00002. [DOI] [PubMed] [Google Scholar]
- Povlishock JT. Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1–12. [PubMed] [Google Scholar]
- Povlishock JT. Christman CW. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J Neurotrauma. 1995;12:555–564. doi: 10.1089/neu.1995.12.555. [DOI] [PubMed] [Google Scholar]
- Qiu W. Zhang Y. Sheng H. Zhang J. Wang W. Liu W. Chen K. Zhou J. Xu Z. Effects of therapeutic mild hypothermia on patients with severe traumatic brain injury after craniotomy. J Crit Care. 2007;22:229–235. doi: 10.1016/j.jcrc.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman KE. Bozyczko-Coyne D. Marcy V. Siman R. McIntosh TK. Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. J Neuropathol Exp Neurol. 1996;55:850–860. doi: 10.1097/00005072-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Sevier AC. Munger BL. A silver method for paraffin section of neural tissue. J Neuropath Exp Neurol. 1965;24:130–135. doi: 10.1097/00005072-196501000-00012. [DOI] [PubMed] [Google Scholar]
- Sherriff FE. Bridges LR. Gentleman SM. Sivaloganathan S. Wilson S. Markers of axonal injury in post mortem human brain. Acta Neuropathol. 1994;88:433–439. doi: 10.1007/BF00389495. [DOI] [PubMed] [Google Scholar]
- Shiozaki T. Nakajima Y. Taneda M. Tasaki O. Inoue Y. Ikegawa H. Matsushima A. Tanaka H. Shimazu T. Sugimoto H. Efficacy of moderate hypothermia in patients with severe head injury and intracranial hypertension refractory to mild hypothermia. J Neurosurg. 2003;99:47–51. doi: 10.3171/jns.2003.99.1.0047. [DOI] [PubMed] [Google Scholar]
- Shitaka Y. Tran HT. Bennett RE. Sanchez L. Levy MA. Dikranian K. Brody DL. Repetitive close-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol. 2011;70:551–567. doi: 10.1097/NEN.0b013e31821f891f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton M. Drexler C. Schmid PG. Havlik HS. Hudetz AG. Farber NE. Neuronal nitric oxide synthase mediates halothane-induced cerebral microvascular dilation. Anesthesiology. 2000;92:125–132. doi: 10.1097/00000542-200001000-00023. [DOI] [PubMed] [Google Scholar]
- Suehiro E. Povlishock JT. Exacerbation of traumatically induced axonal injury by rapid posthypothermic rewarming and attenuation of axonal change by cyclosporin A. J Neurosurg. 2001;94:493–498. doi: 10.3171/jns.2001.94.3.0493. [DOI] [PubMed] [Google Scholar]
- Suehiro E. Singleton RH. Stone JR. Povlishock JT. The immunophilin ligand FK506 attenuates the axonal damage associated with rapid rewarming following posttraumatic hypothermia. Exp Neurol. 2001;172:199–210. doi: 10.1006/exnr.2001.7765. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Bramlett HM. Dietrich WD. The importance of gender on the beneficial effects of posttraumatic hypothermia. Exp Neurol. 2003;184:1017–1026. doi: 10.1016/S0014-4886(03)00389-3. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Bramlett HM. Dietrich WD. The effects of post-traumatic hyperthermia in female and ovariectomized rats. J Neurotrauma. 2004;21:842–853. doi: 10.1089/0897715041526186. [DOI] [PubMed] [Google Scholar]
- Taft WC. Yang K. Dixon CE. Clifton GL. Hayes RL. Hypothermia attenuates the loss of hippocampal microtubule-associated protein 2 (MAP2) following traumatic brain injury. J Cereb Blood Flow Metab. 1993;13:796–802. doi: 10.1038/jcbfm.1993.101. [DOI] [PubMed] [Google Scholar]
- Thurman DJ. Alverson C. Dunn KA. Guerrero J. Sniezek JE. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel C. Lewis S. Wong M. Manavis J. Finnie J. Blumbergs P. Jones N. Reilly P. Diffuse neuronal perikaryon amyloid precursor protein immunoreactivity in a focal head impact model. Acta neurochi Suppl. 1998;71:209–211. doi: 10.1007/978-3-7091-6475-4_60. [DOI] [PubMed] [Google Scholar]
- Yokobori S. Frantzen J. Bullock R. Gajavelli S. Burks S. Bramlett H. Dietrich WD. The use of therapeutic hypothermia in traumatic ischemic/reperfusional brain injury: review of the literatures. Ther Hypo Temp Management. 2011;1:185–192. doi: 10.1089/ther.2011.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]