Abstract
Aims
This multisite prospective trial, Stress Echocardiography in Menopausal Women At Risk for Coronary Artery Disease (SMART), aimed to evaluate the prognostic value of contrast stress echocardiography (CSE), coronary artery calcification (CAC), and cardiac biomarkers for prediction of cardiovascular events after 2 and 5 years in early menopausal women experiencing chest pain symptoms or risk factors. This report describes the study design, population, and initial test results at study entry.
Methods
From January 2004 through September 2007, 366 early menopausal women (age 54±5 years, Framingham risk score 6.51%±4.4 %, range 1%–27%) referred for stress echocardiography were prospectively enrolled. Image quality was enhanced with an ultrasound contrast agent. Tests for cardiac biomarkers [high-sensitivity C-reactive protein (hsCRP), atrial natriuretic protein (ANP), brain natriuretic protein (BNP), endothelin (ET-1)] and cardiac computed tomography (CT) for CAC were performed.
Results
CSE (76% exercise, 24% dobutamine) was abnormal in 42 women (11.5%), and stress electrocardiogram (ECG) was positive in 22 women (6%). Rest BNP correlated weakly with stress wall motion score index (WMSI) (r=0.189, p<0.001). Neither hsCRP, ANP, endothelin, nor CAC correlated with stress WMSI. Predictors of abnormal CSE were body mass index (BMI), diabetes mellitus, family history of premature coronary artery disease (CAD), and positive stress ECG. Twenty-four women underwent clinically indicated coronary angiography (CA); 5 had obstructive (≥50%), 15 had nonobstructive (10%–49%), and 4 had no epicardial CAD.
Conclusions
The SMART trial is designed to assess the prognostic value of CSE in early menopausal women. Independent predictors of positive CSE were BMI, diabetes mellitus, family history of premature CAD, and positive stress ECG. CAC scores and biomarkers (with the exception of rest BNP) were not correlated with CSE results. We await the follow-up data.
Introduction
Coronary artery disease (CAD) is the leading cause of death in women, exceeding that for men for over two decades, yet remains a challenging diagnosis to establish. In the Women's Ischemia Syndrome Evaluation (WISE) study, nearly 60% of women undergoing coronary angiography (CA) did not have flow-limiting stenosis.1 Functional rather than structural abnormalities of the female coronary circulation appear to account for this paradoxical difference.2
State-of–the-art technology using contrast agents in conjunction with stress echocardiography improves image quality, wall segment visualization, and diagnostic confidence.3 Evolving data suggest the use of the coronary artery calcium (CAC) score and serum biomarkers for detection of CAD in selected women.4 High-sensitivity C-reactive protein (hsCRP) has incremental predictive value over currently established risk factors (RF).5 Brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP) levels are elevated in patients with impaired contractility on stress echocardiography.6 Changes in the level of endothelin (ET-1), a potent vasoconstrictor with growth-promoting and mitogenic properties,7 are associated with stress-induced left ventricular systolic and diastolic dysfunction. This report reviews the Stress Echocardiography in Menopausal Women At Risk for Coronary Artery Disease (SMART) trial objectives and design and the initial results of tests performed at study entry.
Study objectives
The primary objective of this trial is:
To determine the value of contrast stress echocardiography (CSE) as a screening examination in perimenopausal or postmenopausal patients with an intermediate pretest likelihood of CAD in order to identify patients at higher risk of experiencing future cardiac events during 2 and 5 years of follow-up. The primary end point for this trial is a composite of cardiac events obtained at 2 and 5 years after completion of the baseline stress echocardiogram, including (1) hard cardiac events (cardiac death, myocardial infarction [MI], all causes of mortality) and (2) soft cardiac events (hospitalization for chest pain or to rule out MI, development of typical angina, cardiac revascularization [percutaneous translumiral coronary angioplasty, PTCA, or coronary artery bypass graft, CABG]), development of heart failure).
The secondary objectives of this trial are:
To evaluate the associations between each of the clinical and baseline test variables and the result of the stress echocardiogram.
To determine the accuracy of stress echocardiography and exercise electrocardiography (ECG), testing for the detection of epicardial CAD in a subset of patients undergoing clinically indicated CA.
To evaluate the prognostic value of exercise ECG testing for identifying patients with cardiac events during follow-up.
To determine the value of exercise-induced changes in levels of cardiac peptides (ANP and BNP) in identifying patients with cardiac events during follow-up.
To determine the value of CAC score for identifying patients with cardiac events during follow-up in a subset of patients undergoing cardiac computed tomography (CT) for CAC scoring.
Study design
This prospective multicenter study was approved by the Mayo Clinic Institutional Review Board and conducted at the three Mayo Clinic group practice sites: Rochester, MN, Jacksonville, FL, and Scottsdale, AZ. The SMART trial enrolled women referred to the stress echocardiography laboratory for evaluation of the following: atypical chest pain or exertional dyspnea with multiple cardiovascular RF (≥2) typical anginal chest pain with at least 1 cardiovascular RF, or no symptoms but multiple cardiovascular RF (≥3).
Eligibility criteria
Perimenopausal and postmenopausal women aged 40–65 years and characterized by one of the following chest pain symptomatology groups were included: (1) asymptomatic and ≥3 RF for CAD, (2) atypical chest pain or exertional dyspnea and ≥2 RF for CAD, or (3) typical anginal chest pain with ≤1 RF for CAD. Risk factors for CAD included current smoker or quit smoking within past 5 years, diabetes (fasting glucose ≥120 mg/dL, hemoglobin A1c [HbA1c] >6.0) or receiving medication, family history of premature CAD, hypertension (untreated systolic blood pressure [BP] >140 or diastolic BP>90 mm Hg) or receiving medication, dyslipidemia (total cholesterol>200 mg/dL, or low-density lipoprotein cholesterol [LDL-C>130 mg/dL, or high-density lipoprotein cholesterol [HDL-C]<35 mg/dL) or receiving medication for lipid control, and obesity defined as body mass index (BMI)≥30 kg/m2. Perimenopausal status was defined as the absence or irregularity of menstrual periods for 6–12 months; postmenopausal status was defined as >12 months since patient's last menses. Surgical menopause included women who had prior bilateral salpingo-oophorectomy with or without hysterectomy. Patients were excluded if they had previous confirmation of CAD, documented ejection fraction (EF) <50%, acute coronary syndrome, contraindication to exercise or pharmacologic stress testing, or known contraindications to contrast agent.
Study procedures
Contrast stress echocardiography
The study protocol flow chart is shown in Figure 1. All women underwent CSE for left ventricular opacification (LVO) using the perfluorocarbon ultrasound contrast agent (Definity®, Lantheus Medical Imaging, Billerica, MA) regardless of the baseline image quality, in order to provide optimal endocardial definition.3 One vial of Definity was diluted with 0.9% saline (total volume 10 mL) and was given as boluses of 0.5–1.0 mL, followed by slow 3–5 mL saline flush. Exercise or dobutamine-CSE was performed according to standard protocols as previously described.8 Imaging was performed on commercially available ultrasound systems: iE33 (Philips Medical Systems, Bothell, WA), Sequoia 512 (Siemens Acuson, Mountain View, CA), or Vivid 7 (GE Healthcare, Princeton, NJ). Baseline echocardiographic images were obtained from parasternal and apical windows before contrast injection. LVO images were acquired using low mechanical index (0.2–0.3) contrast-specific imaging using vendor presets in the apical 4-chamber, 2-chamber, and 3-chamber and apical short axis views at all stages of dobutamine-CSE and at rest and immediate postexercise-CSE stages. Machine settings were adjusted to optimize LVO images. All studies were recorded on videotape and digitized for storage. Twelve-lead ECG was monitored continuously, and BP was measured at each stage of CSE until heart rate recovery was achieved. Monitoring and electronic documentation of adverse events were performed.
FIG. 1.

Flow chart of the study protocol. Serum biomarkers included: high-sensitivity C-reactive protein (hsCRP), endothelin (ET-1), brain natriuretic peptide (BNP), and atrial natriuretic peptide (ANP). The contrast agent used during stress echocardiography was Definity®. CT, computed tomography; ECG, electrocardiogram.
Echocardiographic studies were analyzed off-line by two blinded readers (S.S.A., M.B) and by a third (S.L.M) for consensus as necessary. Rest and stress images were reviewed side-by-side using a 17-segment model according to established criteria.8 Wall motion score index (WMSI) and biplane Simpson's EF were calculated. Abnormal CSE was defined as development of new or worsening of existing wall motion abnormalities during peak stress. Positive stress ECG was defined as ≥1 mm horizontal or downsloping ST segment depression or ST segment elevation 80 msec after the J-point during exercise or recovery.
Serum biomarkers
All patients underwent serologic assessment of BNP, ANP, and ET-1 at rest and 5 minutes post-CSE; hsCRP was assessed at rest only. All serum assays were performed according to the Mayo Clinic Laboratory procedure and reference guidelines. The difference between stress and rest values was defined as Δ (stress-induced change). hsCRP distribution was stratified on previously defined thresholds as low (1.0 mg/dL), intermediate (1.0–3.0 mg/dL), and high (>3.0 mg/dL) risk. If an hsCRP level>10 mg/L was identified, consistent with acute inflammation or infection, the result was excluded.5
Coronary artery calcium scores
A subset of patients consented to additional cardiac imaging with noncontrast chest CT to determine CAC score. CT was performed within 11±25 days of CSE according to previously described methodology9 and was interpreted by an experienced radiologist blinded to clinical data CAC score was calculated using commercially available semiautomated computer software (GE Smartscore, GE Healthcare) according to the Agatston method.
Coronary angiography
CA was performed as deemed clinically indicated by primary caregivers in a subset (n=24) of women within 11±10.99 days of CSE (median 5, range 1–41 days). Two independent cardiologists blinded to clinical data performed quantitative assessment of coronary artery diameter and percent stenosis using automated edge detection equipment (Paeion, Inc., NY). Significant obstructive epicardial CAD was defined as ≥50% stenosis in any of the three major epicardial coronary arteries. Nonobstructive epicardial CAD, defined as 10%–49% stenosis, was also reported.
Study analysis
Sample size determination
Sample size was calculated based on the primary objective of the trial. It was assumed that the proportion of the low-risk patients to intermediate-risk patients will be 2:1. The 1-year event rate was assumed to be no more than 1% in the low-risk group and 4% in the intermediate-risk group. The 5-year cumulative event rates were estimated to be 2% and 7.8%, respectively. A sample size of 500 patients provides a statistical power of approximately 90% for a 2-sided Fisher's exact test of comparison of event rates between low-risk and intermediate-risk groups at 5% level of significance. A Cox proportional hazard regression-based analysis provides a statistical power of 85% for the noted comparison, assuming the overall event rate in the study is 4.6%. However, the comparison in 375 patients with the same allocation ratio between risk groups provides an acceptable statistical power of approximately 80%. Thus, because the statistical power of the comparison was at least 80%, the study was stopped once the enrollment reached 400 patients.
Statistical plan for primary analysis of end points (cardiac events at completion of 5 years)
Primary analysis will be based on the Cox proportional hazard model to estimate the hazard ratio (HR) of predictors of cardiac outcomes. Univariate and multivariate Cox proportional hazard models will be calculated using stress WMSI as a predictor variable. Unadjusted and risk-adjusted HR will be reported. The cardiac event rate will be summarized by categorical levels of WMSI score and the difference in WMSI. In addition, the predictive value of stress ECG testing, serum biomarkers, changes in cardiac peptides, and CAC score will be examined.
Statistical plan for secondary analysis of baseline data (initial results)
After collection of the baseline data, we (1) evaluated the associations between each of the clinical and baseline test variables and the result of the CSE (abnormal vs. abnormal) and (2) determined the diagnostic accuracy of stress echocardiography and stress ECG for detection of CAD in the subgroup of patients who underwent CA. Clinical characteristics of patients were reported as numbers and proportions for categorical variables and mean (with standard deviation [SD]) for continuous variables with normal distribution; median and range were used to report skewed data. Comparison between groups with abnormal or normal CSE was performed using a 2-sided Fisher's exact test. Paired t test or nonparametric Wilcoxon paired sample testing was used for assessment of within-subject changes in hemodynamics and cardiac peptides. Univariate logistic regression was performed to evaluate the association between each of the clinical and test variables separately and the outcome (abnormal CSE). Variables with likelihood test p<0.2 in univariate analysis were included in the multivariate logistic regression model and provided adjusted odds ratios (OR) and 95% confidence intervals (95% CI). Diagnostic accuracy for CSE and stress ECG was calculated. All analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC) and used 2-sided testing with significance levels of 5%. In the subset of patients undergoing CA, to evaluate the reliability of CSE, interobserver variability was assessed in 43 randomly assigned patients by two independent observers (S.S.A., S.L.M), and intraobserver variability was assessed on two occasions at least 5 months apart. Variability was reported using kappa coefficient.
Results
Clinical characteristics of enrolled women
From January 2004 to August 2007, 400 women participated in the study across the three Mayo Clinic sites. A total of 377 women completed the study (7.4% perimenopausal, 52.5% postmenopausal, and 46.2% with surgical menopause). Menopausal status was further characterized by follicle-stimulating hormone (FSH) and estradiol levels (56.1±33.1 IU/mL and 67.0±60.9 pg/mL, respectively). Mean age at surgical menopause was 40±9 years. Framingham risk score (FRS) for CAD was 6.51%±4.4% (median 6%, range 1%–27%). Symptoms (atypical chest pain or exertional dyspnea) and≥2 RF for CAD were present in 63.1%; 34% were asymptomatic with≥3 RF for CAD; and 2.2% of women had typical anginal chest pain with≤1 RF for CAD. In addition, 11 women terminated the study early (4 with adverse events [back pain at time of contrast], 4 with consent withdrawal, and 3 for other causes). Thus, 366 (97.1%) women (mean age 54.4±5.5 years, range 40–65) completed CSE and were included in the analysis. Baseline characteristics during CSE are shown in Table 1.
Table 1.
Study Population Characterized by Contrast Stress Echocardiography Results
| Variable | Overall (N=366) | Abnormal CSE (N=42) | Normal CSE (N=324) | p value |
|---|---|---|---|---|
| Age, years | 54.4±5.24 | 54.79±5.52 (range 40–65) | 54.31±5.20 (range 40–65) | 0.598 |
| BMI, kg/m2 | 31.4±6.68 | 33.09±6.17 (range 23–51) | 31.19±6.72 (range 15–62) | 0.068 |
| CSE, n (%) | ||||
| Exercise | 278 (75.96) | 29 (69.1) | 249 (76.9) | 0.256 |
| Dobutamine | 88 (24.04) | 13 (30.9) | 75 (23.2) | |
| Race, n (%) | ||||
| White | 349 (95.4) | 39 (92.86) | 310 (95.7) | 0.112 |
| Black | 4 (1.1) | 0 (0) | 4 (1.23) | |
| Asian/Pacific Islander | 5 (1.4) | 0 (0) | 5 (1.54) | |
| Hispanic | 4 (1.1) | 1 (2.38) | 3 (0.93) | |
| American Indian/Alaskan | 3 (0.82) | 2 (4.76) | 1 (0.31) | |
| Native | 1 (0.27) | 0 (0) | 1 (0.31) | |
| CAD risk factors, n (%) | ||||
| Smoking history | 92 (25.14) | 11 (26.2) | 81 (25.0) | 0.852 |
| Diabetes mellitus | 56 (15.3) | 13 (30.95) | 43 (13.3) | 0.006 |
| Family history of CAD | 222 (60.7) | 29 (69.1) | 193 (59.6) | 0.314 |
| Hypertension | 231 (63.1) | 29 (69.1) | 202 (62.4) | 0.497 |
| Dyslipidemia | 292 (79.8) | 29 (69.1) | 263 (81.2) | 0.099 |
| BMI≥30 kg/m2 | 205 (56.0) | 30 (71.4) | 175 (54.0) | 0.046 |
| Hormone therapy | 103 (28.1) | 11 (26.2) | 92 (28.4) | 0.857 |
| Chest pain groups, n (%) | ||||
| Asymptomatic | 125 (34.2) | 16 (38.1) | 109 (33.6) | 0.605 |
| Atypical chest pain | 233 (63.7) | 25 (59.5) | 208 (64.2) | 0.609 |
| Typical chest pain | 8 (2.2) | 1 (2.4) | 7 (2.2) | 1.00 |
| Medications, n. (%) | ||||
| ACE inhibitor | 64 (17.5) | 12 (28.6) | 52 (16.1) | 0.053 |
| Alpha blocker | 5 (1.37) | 1 (2.4) | 4 (1.23) | 0.458 |
| Beta blocker | 89 (24.3) | 5 (11.9) | 84 (25.9) | 0.055 |
| Antiplatelet/ASA | 110 (30.1) | 15 (35.7) | 95 (29.3) | 0.474 |
| Anticoagulant | 10 (2.7) | 3 (7.1) | 7 (2.2) | 0.095 |
| A-II receptor blocker | 49 (13.4) | 7 (16.7) | 42 (12.9) | 0.475 |
| Calcium channel blocker | 31 (8.5) | 5 (11.9) | 26 (8.0) | 0.379 |
| Lipid-lowering agent | 147 (40.2) | 16 (38.1) | 131 (40.4) | 0.868 |
Mean±standard deviation (SD) FSH and estradiol levels were 56.1±33.1 IU/mL and 67.0±60.9 pg/mL. Mean±SD years of hormone therapy use was 2.74±5.04 years. Types of hormone therapy included estradiol (oral and transdermal), conjugated equine estrogen (Premarin), alone or combined with medroxyprogesterone acetate (Prempro), and natural progesterone (Prometrium).
ACE, angiotensin-converting enzyme; ASA, acetylsalicylic acid; BMI, body mass index; CAD, coronary artery disease.
Contrast stress echocardiography and ECG
CSE was interpretable in all 366 women and was abnormal in 42 (11.5%). Hemodynamic changes during stress testing are summarized in Table 2. Mean WMSI and EF were 1.02±0.12 and 61%±6% at rest, and 1.06±0.18 and 70%±7% at peak stress (p<0.001). Stress ECG results were available in all women; 22 (6.4%) were positive and 23 (6.2%) were nondiagnostic: resting ST/T wave abnormalities due to left bundle branch block (LBBB) or pacing in 18 (4.79%), uninterpretable tracing due to artifact in 2 (0.5%), and inadequate in 3 (0.8%). Mean Duke treadmill score was 6.9±3.1 (median 7.0, range −17–13.0), with risk distribution high 15 (5.4%), intermediate 44 (15.8%), and low 219 (78.9%). After excluding the nondiagnostic ECG tracings, a total of 343 women had complete stress ECG and CSE data. CSE was abnormal in 41 (12%), and stress ECG was positive in 22 (6.4%). In 7 (2%) women, both CSE and stress ECG were abnormal/positive, whereas in 287 (83.7%), both were normal/negative. There was significant (p=0.009) discordance in 49 women, 34 abnormal CSE with negative stress ECG and 15 normal CSE with positive stress ECG.
Table 2.
Hemodynamic Changes During Contrast Stress Echocardiography
| Variable | Exercise (N=278) | Dobutamine (N=88) |
|---|---|---|
| Mean±SD duration of test (minutes) | 8.0±1.9 | 13.5±2.6 |
| (median, range) | (8, 2.9-14) | (14, 5-19) |
| Δ HR (SE), beats/minute* | 77.62 (1.04) | 68.65 (1.46) |
| Δ rate pressure product (SE), mm Hg/minute* | 16592 (257.2) | 10928 (380.8) |
p<0.0001.
Δ stress-rest values; HR, heart rate; SE, standard error.
Predictors of abnormal CSE
All variables were considered as potential predictors of abnormal CSE in univariate modeling. The significant adjusted OR for the final multivariate model included BMI, diabetes mellitus, family history of premature CAD, and positive stress ECG (Table 3).
Table 3.
Univariate and Multivariate Predictors of Abnormal Contrast Stress Echocardiography
| |
Univariatea |
Multivariateb |
||||
|---|---|---|---|---|---|---|
| Clinical variable | Chi-square | OR (95% CI) | p | Chi-square | OR (95% CI) | p |
| Age | 0.309 | 0.982 (0.92-1.05) | 0.578 | |||
| BMI, kg/m2 (per 1 unit increase) | 2.986 | 0.960 (0.92-1.01) | 0.084 | 4.18 | 1.06 (1.01-1.12) | 0.04 |
| Smoking | 0.028 | 1.07 (0.51-2.21) | 0.867 | |||
| Diabetes mellitus | 8.359 | 2.93 (1.41-6.07) | 0.004 | 8.73 | 3.6 (1.5-8.3) | 0.003 |
| Family history of CAD | 1.384 | 1.51 (0.76-3.02) | 0.239 | 4.66 | 2.5 (1.1-5.6) | 0.031 |
| Hypertension | 0.713 | 1.34 (0.68-2.69) | 0.398 | |||
| Dyslipidemia | 3.29 | 0.517 (0.25-1.05) | 0.069 | |||
| Hormone therapy | 0.89 | 0.895 (0.43-1.86) | 0.765 | |||
| Positive stress ECG | 8.31 | 4.12 (1.57-10.79) | 0.004 | 7.18 | 5.6 (1.6-20) | 0.007 |
| Coronary calcium score (per 1-unit increase) | 0.103 | 1.00 (0.95-1.01) | 0.748 | |||
If variable had p value<0.20 in unadjusted (univariate) analysis, it was considered in final (multivariate) model.
Final model was adjusted for known predictors of cardiovascular disease, including age, BMI, hypertension, diabetes, family history of CAD, hormone therapy, and dyslipidemia.
CI, confidence interval; ECG, electrocardiogram; OR, odds ratio.
Accuracy of CSE and stress ECG compared to CA
As part of clinical care, 24 women underwent CA within 41 days of CSE: 4 (16.7%) had normal CA, and 20 (83.3%) had CAD, 5 (21%) obstructive and 15 (62.5%) nonobstructive. Of those 20 with CAD, CSE was positive in 15 (75%), and ECG was positive in 4 (20%). For obstructive CAD (≥50%), the sensitivity of CSE was higher than stress ECG (80% vs. 40%), although specificity was lower (37% vs. 79%). CSE was positive in 11 (73.3%) patients with nonobstructive CAD and negative in 4 (26.7%). In all 4 patients with angiographically normal coronary arteries, CSE was positive in the left anterior descending artery (LAD) territory, and the ECG was negative. Table 4 presents the CA, CSE, and stress ECG results for each patient undergoing CA.
Table 4.
Coronary Angiographic Results (n=24)
| Patient number | Quantitative coronary angiography results | Stress echocardiography type | Stress echocardiography results | Stress ECG results |
|---|---|---|---|---|
| 1 | Mid-LAD 30% | DSE | Positive for ischemia (LAD territory) | Negative for ischemia |
| 2 | Proximal LAD 20%, mid-LAD 30%, distal LAD 30%, OM 50%, proximal RCA 30%, mid-RCA 50% | EXCE | Positive for ischemia (RCA and CX territories) | Negative for ischemia |
| 3 | Proximal LAD 20%, mid-LAD 20%, 1st diagonal 20%, | EXCE | Positive for ischemia (LAD territory) | Negative for ischemia |
| 4 | Proximal LAD 30%, mid-LAD 20%, 1st diagonal 30%, proximal RCA 20% | EXCE | Positive for ischemia (LAD territory) | Negative for ischemia |
| 5 | Proximal LAD 20 % | EXCE | Positive for ischemia (CX territory) | Negative for ischemia |
| 6 | LM 20%, proximal LAD 30%, mid-LAD 30%, 1st diagonal 30%, proximal CX 20%, proximal RCA 30%, mid-RCA 20% | DSE | Positive for ischemia (LAD and RCA territories) | Negative for ischemia |
| 7 | Normal coronaries | EXCE | Positive for ischemia (LAD territory) | Negative for ischemia |
| 8 | Normal coronaries | EXCE | Positive for ischemia (LAD territory) | Negative for ischemia |
| 9 | LM 20%, proximal LAD 20%, 1st diagonal 30% | DSE | Positive for ischemia (LAD and CX territories) | Negative for ischemia |
| 10 | Ramus Intermedius 70%, 1st diagonal 20% | EXCE | Positive for ischemia (LAD, CX, and RCA territories) | Negative for ischemia |
| 11 | Normal coronaries | EXCE | Positive for ischemia (LAD territory) | Negative for ischemia |
| 12 | Mid-LAD 10%, distal RCA 10% | EXCE | Positive for ischemia (RCA territory) | Negative for ischemia |
| 13 | Ramus intermedius 70%, proximal LAD 70%, 1st diagonal 70%, OM 60%, proximal RCA 80% | DSE | Positive for ischemia (LAD, CX, and RCA territories) | Positive for ischemia |
| 14 | LM 50%, mid-LAD 30%, distal CX 20% | DSE | Positive for ischemia (LAD, CX, and RCA territories) | Positive for ischemia |
| 15 | 1st diagonal 20%, mid-RCA 20% | DSE | Negative for ischemia | Negative for ischemia |
| 16 | Proximal LAD 20%, distal LAD 30, distal CX 30, OM 30, mid-RCA 20 | DSE | Positive for ischemia (LAD territory) | Negative for ischemia |
| 17 | 1st diagonal 30%, OM 20%, mid-RCA 20% | EXCE | Positive for ischemia (LAD territory) | Negative for ischemia |
| 18 | LM 30%, proximal LAD 30%, mid-LAD 20%, proximal CX 40%, mid-RCA 20% | EXCE | Positive for ischemia (LAD territory) | Positive for ischemia |
| 19 | Normal coronaries | EXCE | Positive for ischemia (LAD territory) | Negative for ischemia |
| 20 | Mid-LAD20% | DSE | Negative for ischemia | Positive for ischemia |
| 21 | Proximal LAD 60%, proximal RCA 30% | EXCE | Negative for ischemia | Negative for ischemia |
| 22 | OM 30%, posterolateral artery 20% | EXCE | Negative for ischemia | Positive for ischemia |
| 23 | LM 20%, proximal LAD 30%, mid-LAD 20% | EXCE | Positive for ischemia (LAD territory) | Positive for ischemia |
| 24 | LM 20% | EXCE | Negative for ischemia | Negative for ischemia |
CX, circumflex artery; DSE, dobutamine stress echocardiography; EXCE, exercise echocardiography; LAD, left anterior descending artery; LM, left main; OM, obtuse marginal; RCA, right coronary artery.
Intraobserver and interobserver variability
Total agreement was 88% (κ 0.685 [0.13]) for intraobserver and 87% (κ 0.754 [0.16]) for interobserver variability for echocardiographic wall motion assessment.
Contrast-related side effects
Adverse events related to contrast were reported in 6 (1.6%) women, backache in 3 (0.8%), headache and backache in 1 (0.27 %), shortness of breath in 1 (0.27%), and urticaria or hives in 1 (0.27%).
Serum biomarkers
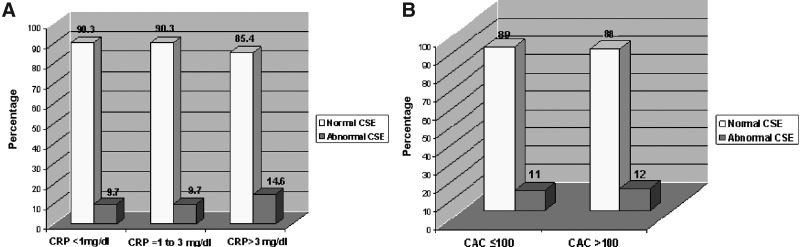
Serum biomarkers were available in 86% of women (Table 5). There was weak but significant correlation between rest BNP and peak stress WMSI (r=0.189, p<0.001), whereas the correlation between Δ BNP and peak stress WMSI was not significant (r=0.091, p=0.102). There were no significant correlations between peak WMSI and rest ANP (r=− 0.002, p=0.968), Δ ANP (r=− 0.008, p=0.906), rest ET-1 (r=0.015, p=0.792), or Δ ET-1 (r=0.025, p=0.666). hsCRP at rest (mean 3.1±2.6 mg/dL, median 2.3, range 0.1–10 mg/dL), was low in 72, intermediate in 113, and high in 124 women; 57 women were excluded from analysis (hsCRP >10 mg/dL in 40, not available in 17). hsCRP was higher in women with abnormal vs. normal CSE, although the difference was not significant (3.51±2.64 vs. 3.034±2.53 mg/dL, p=0.317), and there was no correlation with stress WMSI (r=0.019, p=0.669). By hsCRP risk categories, abnormal CSE trended with increasing hsCRP levels (p=0.423) (Fig. 2A).
Table 5.
Serum Biomarkers
| BNP, pg/mL | Median, range |
|---|---|
| Rest BNP | 22.5, 0.6–414.8 |
| Peak BNP | 25.6, 4.0–542.0 |
| Δ BNP* | 3.3, 392.7–208 |
| ANP, pg/mL | |
| Rest ANP | 731, 14.3–9131 |
| Peak ANP | 784, 174–4327 |
| Δ ANP | 38, −8155.0–1496.8 |
| Endothelin, pg/mL | |
| Rest endothelin | 1.1, 0.3–3.7 |
| Peak endothelin | 0.9, 0.31–7.5 |
| Δ endothelin | −0.14, −1.6–6.9 |
p<0.0001.
ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide.
FIG. 2.
The prevalence of abnormal CSE studies categorized according to (A) hsCRP and (B) coronary artery calcium score (CAC).
Coronary artery calcium score
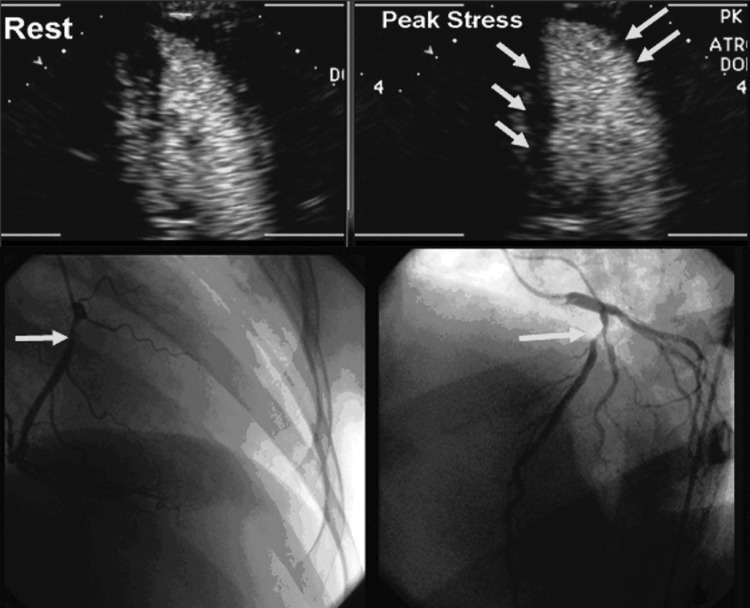
Cardiac CT for coronary calcium was performed in 124 (33.9%) women, mean CAC score 39.7±84.0 (range 0–431), excluding a single patient with an outlier value of 1026; 107 (86%) women had a CAC score ≤100, whereas in 17 (14%), it was >100. CAC was localized to the LAD in 51 (13.9%), right coronary artery (RCA) in 23 (6.3%), and circumflex artery (CX) in 18 (4.9%); 65 women (52.4%) had a CAC score of zero. CSE was abnormal in 14 (11.3%), and CAC was present in 6 (42.9%), while 110 women had normal CSE, and CAC was present in 53 (48.2%). Mean CAC score was not different in women with abnormal vs. normal CSE (37.29±74.28 vs. 48.44±126.98, p=0.636). When stratified according to CAC score categories (≤100 or >100), the majority of women in each group had normal CSE (Fig. 2B). There was no correlation between CAC score and peak stress WMSI (r=0.149, p=0.098). Figure 3 shows multimodality imaging results in a 43-year-old woman with inducible ischemia in the distribution of RCA on dobutamine-CSE, positive stress ECG (1–1.4 mm horizontal inferior ST segment depression), multivessel CAD (90% RCA and 60% LAD stenoses) yet no detectable CAC on cardiac CT.
FIG. 3.
Representative imaging studies from a 43-year-old women with (Top) rest and of stress views inducible ischemia in RCA and LAD distribution on dobutamine-CSE (arrows, lack of inward motion of inferior and anterior walls at end-systole compared to rest image) with positive stress ECG (1–1.4 mm horizontal S-T depression) in inferior leads, yet no detectable CAC. (Bottom) Coronary angiography: 90% proximal RCA and 60% proximal LAD stenoses (arrows).
Discussion
In women with low to intermediate risk of CAD (range 1%–27%), diagnostic detection strategies remain controversial. To our knowledge, this is the largest prospective cohort study in early to mid-postmenopausal women at low to intermediate risk for CAD (FRS 6.5%±4.4%) to address the relationship among CSE, stress ECG, CAC scoring, and cardiac biomarkers. In this initial report, we describe the baseline population of women entered into this multicenter, prospective study, who are representative of the female patients we evaluate daily in clinical practice. The majority (63.8%) of women had atypical chest pain or exertional dyspnea or both and ≥2 RF for CAD. The main findings included (1) BMI, diabetes mellitus, family history of premature CAD, and positive stress ECG were independently associated with abnormal CSE, (2) CAC score and hsCRP were not significantly correlated with CSE, (3) only rest BNP levels correlated with peak WMSI, (4) CSE detected obstructive epicardial CAD twice as often as stress ECG but was thrice as often abnormal in the presence of nonobstructive CAD.
Challenges to diagnose ischemic heart disease in women are based on the discrepancies between chest pain symptomatology and the relatively low prevalence of underlying obstructive epicardial coronary diseases on CA. Detecting MI by novel noninvasive imaging modalities, even at subclinical disease level is of diagnostic importance and has been shown to be a predictor of cardiac events. Furthermore, it has been reported that women with abnormal stress testing should be treated as aggressively as men.10–12
The use of contrast agents is increasing in light of substantial data confirming the safety and added benefit of increased accuracy of echocardiographic interpretation during stress by improving reader confidence and reducing interobserver variability.3,13 Existing guidelines recommend the use of contrast agents for rest and stress echocardiograms when≥2 segments are not visualized in any one view,3 based on data that show that up to 33% of patients (frequently due to large body habitus or chronic lung disease) have suboptimal endocardial border definition.14 In our high-volume stress echocardiography laboratory, despite the use of tissue harmonic imaging, we have reported on the rate of contrast agent use routinely during clinically indicated stress ECG (approximately 25%)15 and that contrast use improves image quality in>90% of stress ECG studies. In the SMART population, 56% of women had a BMI≥30 kg/m2. The rationale for administering contrast agents to all study subjects in this trial was to employ a standardized approach using validated methodology to provide optimal wall motion interpretation, both at rest and after stress.
We explored the association of cardiac serum biomarkers and coronary calcification imaging with CSE. Indeed, hsCRP has been shown to be a predictor of ischemic events, independent of other known RF.16 Only few observational studies, however, primarily in men with CAD, have reported variable correlations with severity of ischemia on stress testing.17,18 We found no significant correlation between hsCRP and CSE results. Similarly, cardiac peptides increase during stress-induced myocardial ischemia; BNP has been proposed as a mean of increasing the diagnostic accuracy of exercise testing, although findings have been inconsistent.6,19 In our study, concentrations of BNP and ANP increased during peak stress from baseline, although this increase was marginally significant only for BNP. Similar to others, we found that ET-1 did not increase with myocardial ischemia.20
An increasing body of literature confirmed the importance of CAC as an effective tool for improving the diagnostic assessment and risk stratification of patients with suspected CAD.21,22 We previously found, in a retrospective study of an unselected population of mostly men, that CAC score was predictive of abnormal exercise WMSI.23 This was not the case in the current study, however, in which we studied only relatively young women with low to intermediate risk for subsequent CAD and found that most of those with elevated CAC scores had normal WMSI, and, conversely, a few with low CAC scores (<100) had abnormal CSE. A possible explanation of this observation could be derived from the difference between the two tests (functional vs. anatomic imaging), which may reflect very different aspects of the atherosclerotic process. Development of myocardial ischemia results from an imbalance in myocardial oxygen supply and demand. In the typical ischemic cascade, abnormal myocardial perfusion (within seconds) occurs initially, followed by the development of regional wall motion abnormalities (within 10–20 seconds) and, subsequently, ECG changes and chest pain.24
Stress echocardiography aims to detect regional wall motion abnormalities under ischemic conditions (functional imaging modality) rather than assessing atherosclerosis burden by direct visualization of the coronary arteries (anatomic imaging modality) and, thus, allows diagnosis at an earlier stage of the cascade than would be achieved by detection of ECG changes and symptoms. CT detection of CAC is used clinically as a marker of the atherosclerotic burden for determination of risk stratification, but it is not directly correlative with the degree of epicardial coronary stenosis.25 Additionally, studies that have compared CAC scores with hemodynamically significant coronary artery stenosis as assessed by myocardial perfusion stress imaging reported mixed results,26,27 suggesting that the functional significance of atherosclerotic burden may not be accurately represented. It is also possible that women in our study had noncalcified plaques that were not detected by cardiac CT; it has been reported recently that women tend to have more exclusively noncalcified plaque and are less likely to have calcified or mixed plaques compared to men.28
Sex-specific differences have been reported in numerous CA studies, supporting unique features of atherosclerotic pathophysiology in women. Moreover, the noninvasive detection of CAD is more complex in women because of the lower prevalence of CAD in women than men at similar chronologic stages of life, gender differences in referral patterns to CA after stress testing,29,30 and less angiographically significant disease in women. In the SMART population, 6.6% women were referred for CA at the discretion of their cardiologist. The prevalence of epicardial CAD by the gold standard CA was 20.8%. These findings support the recently published and evolving observations supporting the concept of gender differences in ischemic heart disease pathophysiology.31 The significance of chest pain symptomatology in women associated with no or nonobstructive angiographic CAD is evolving, and emerging evidence suggests significant prognostic implications. A study of 42 women with de novo angina, and normal or near normal coronary angiograms demonstrated that 30% of these women developed obstructive CAD during a 10-year follow-up period.32 A combined report from the WISE study, enrolling symptomatic women referred for clinically indicated CA and followed up for a mean of 5.2 years, and the St. James Women Take Heart (WTH) project, enrolling asymptomatic, community-based women with no history of heart disease and followed up for 10 years, reported 5-year annualized cardiac event rates in WISE women of 16.0% in those having mild epicardial CAD (stenosis 10%–49%) and 7.9% in those with no epicardial CAD, while in WTH women, it was 2.4% after adjustment of baseline cardiac risk factors.33 These findings suggest that the traditional concept of symptomatic angina being associated with fixed epicardial CAD may not be applicable to women and that, alternatively, a new paradigm in ischemic heart disease in women whereby atherosclerosis-related endothelial dysfunction in the absence of significant fixed obstructive epicardial CAD is predictive of increased risk of later adverse cardiac events.2
In this small subset of women referred to CA, we performed within-patient comparison between CSE and stress ECG and reported similar sensitivity and specificity for ischemic changes on stress ECG in comparison to prior studies in women that showed a wide range for sensitivity and specificity (from 31% to 71% and 66% to 86%, respectively).34 Although specificity was in favor of stress ECG for detection of significant obstructive CAD, the positive predictive value of both techniques was similar (stress ECG 33% vs. CSE 25%). Based on the bayesian theorem, noninvasive imaging modalities will usually show a decrease in positive predictive value when applied to lower prevalence groups.35 These observed results may reflect the combined effect of intrinsic test performance, cutoff for percent stenosis, and referral pattern. We also reported a lower specificity of stress ECG in our study in comparison to others, who reported a specificity of 80%–86% for the diagnosis of obstructive CAD (≥50%). This can be explained by the small number referred for CA and low to intermediate risk for CAD (mean FRS 6.5%) in our population in comparison to other studies that involved mainly high/intermediate risk for CAD or those with prior documented Q wave MI.36,37 Our study involved a higher number of “false positive” CSE tests that contributed to lower specificity, which may have resulted from detection of nonobstructive ischemic heart disease associated with microvascular disease, or subclinical cardiomyopathy, including hypertensive response to stress, or transient stress-test induced cardiomyopathy.38
A published study showed that large BMI or obesity may play a role in false positive studies on noninvasive stress testing, resulting in a sensitivity of 75% and specificity of 39%.39 In the present study, 56% of women had a BMI≥30, and BMI was predictive of an abnormal stress ECG. Despite the lower specificity of CSE in our study, it was able to identify 73% (11 of 15 women) of those having nonobstructive CAD and 80% (4 of 5 women) of those having obstructive CAD. These findings support the recently published and evolving observations supporting the concept of gender differences in ischemic heart disease pathophysiology.31 The significance of chest pain symptomatology in women associated with no or nonobstructive angiographic CAD is evolving, and emerging evidence suggests significant prognostic implications.
A recent study40 evaluated the outcomes of patients with false positive findings on stress ECG. Younger age, female, absence of diabetes, absence of hypertension, absence of CAD history, and negative results on stress ECG were identified as the predictive characteristics for discordance between the abnormal stress ECG and the absence of significant stenosis (≥50% stenosis) on CA. During a 2.4-year follow-up, the outcomes of patients with false positive results were similar to those of patients with true positive results, suggesting that patients with false positive results on stress ECG should still receive careful clinical follow-up.
At present, despite these compelling findings, many women with angina (typical or atypical) are told that they have no significant heart disease after angiographic demonstration of normal or near-normal coronary arteries and are usually offered no specific treatment beyond reassurance. This frequently results in repeated hospitalizations and further invasive procedures in response to refractory symptoms.32 There is a definite need for studies of noninvasive testing of chest pain syndromes and their appropriate use, as determined by clinical outcomes analysis and cost-effectiveness, in women. A multicenter, prospective randomized study similarly evaluating the use of nuclear techniques for stress testing in women is in progress.41 Although the 2-year and 5-year outcomes results of our study are yet pending, the noninvasive strategy offered by stress ECG may have distinct advantages with respect to overall efficiency, portability, cost-effectiveness, and lack of incremental risk from ionizing radiation.3
Study limitations
In the SMART trial design, we sought to include women of racial/ethnic minority groups. This study attempted to recruit at least 10% of minority women, but recruitment fell short in the area of minority participation, with total of 17 (4.6%) women only. The results of this trial may not necessarily be applicable to minority women. We recruited women who were in the early phases of menopause, referred for stress ECG testing, and these women, not surprisingly were at low to intermediate likelihood of CAD. Alternatively, we might have restricted the study to an older age range (>55years) to simplify the verification of postmenopausal status and to enhance the pretest likelihood of CAD. However, we were interested in studying the outcomes of a cohort of recently menopausal women who frequently are referred for CAD assessment.
Our study was designed to be representative of patients in the selected demographic who are actually referred to our stress ECG laboratory and, as a result, included primarily low-risk to intermediate-risk women. Not surprisingly, abnormal CSE was present in only 11%, which may impact the statistical power in the analysis and the generalization of our conclusions. The study end points were defined by clinical outcomes, rather than angiography. However, we have reported this information in the small subset (n=24) of women undergoing CA at the discretion of their referring caregiver. The comparison between CSE and CA is subject to posttest verification bias, with implications regarding accuracy determinations. As the angiographic correlate was not the primary purpose of the study, we did not intend to further characterize patients by coronary physiologic testing using adenosine or acetylcholine to ascertain the presence or absence of coronary microvascular dysfunction, especially in those patients with angiographically normal coronary arteries, although that information would likely be very insightful.
Contrast agents were used in all study subjects enrolled in this trial. With currently available image optimization presets on cardiac ultrasound equipment optimized, LVO was routinely obtained; no studies were reported as having deterioration of image quality after contrast administration. Before the advent of optimized ultrasound imaging technology, there were isolated reports of possible decrease in accuracy of interpretation if contrast was given to subjects with good image quality.42 However; these studies used different harmonic and high mechanical index contrast settings that are not relevant or applicable to our methodology. The use of myocardial contrast echocardiography to assess myocardial perfusion was not part of the trial design because of the logistics of the multicenter design.
Conclusions
The SMART trial is a prospective trial of early menopausal women at low to intermediate risk for CAD with cardiovascular symptoms or RF or both. This initial report presents the results of the study design, population characteristics, and baseline test results. BMI, diabetes mellitus, family history of premature CAD, and positive stress ECG were independent predictors of abnormal CSE. CAC scores and cardiac biomarkers were not correlated with CSE results. CSE detected obstructive CAD twice as often as did stress ECG but was less specific because of enhanced detection of nonobstructive CAD. These initial clinical findings support the evolving concept of sex-based differences in the development and continuum of the atherosclerotic disease process and the need for further elucidation of optimal strategies in the diagnosis and treatment of women at risk for ischemic heart disease. We eagerly await the 2-year and 5-year outcomes data for the determination of prognostic value of CSE in prediction of cardiovascular events.
Acknowledgments
This publication was in-part supported by grant number 1 KL2 RR024151-01 from the National Center for Research Resources (NCRR), a component of National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
The SMART trial (clinicalTrial.gov identifier NCT00162370) was funded solely by Lantheus Medical Imaging (North Billerica, MA). Lantheus Medical Imaging provided the contrast agent (Definity) and additional research funding under grant DMP 115-407.
Disclosure Statement
S.L.M. has received research grants from Lantheus Medical Imaging and Astellas Pharma Inc. and has been a member of the Advisory Board for GE HealthCare. There are no conflicts of interest to disclose for all other authors.
References
- 1.Lerman A. Sopko G. Women and cardiovascular heart disease: Clinical implications from the Women's Ischemia Syndrome Evaluation (WISE) Study. Are we smarter? J Am Coll Cardiol. 2006;47:S59–62. doi: 10.1016/j.jacc.2004.10.083. [DOI] [PubMed] [Google Scholar]
- 2.Shaw LJ. Bugiardini R. Merz CN. Women and ischemic heart disease: Evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulvagh SL. Rakowski H. Vannan MA, et al. American Society of Echocardiography consensus statement on the clinical applications of ultrasonic contrast agents in echocardiography. J Am Soc Echocardiogr. 2008;21:1179–1201. doi: 10.1016/j.echo.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Greenland P. Alpert JS. Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 5.Pearson TA. Mensah GA. Alexander RW, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 6.Bergeron S. Moller JE. Bailey KR. Chen HH. Burnett JC. Pellikka PA. Exertional changes in circulating cardiac natriuretic peptides in patients with suggested coronary artery disease. J Am Soc Echocardiogr. 2006;19:772–776. doi: 10.1016/j.echo.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Gossl M. Lerman A. Endothelin: Beyond a vasoconstrictor. Circulation. 2006;113:1156–1158. doi: 10.1161/CIRCULATIONAHA.105.609271. [DOI] [PubMed] [Google Scholar]
- 8.Pellikka PA. Nagueh SF. Elhendy AA. Kuehl CA. Sawada SG. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–1041. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Jayachandran M. Litwiller RD. Owen WG, et al. Characterization of blood borne microparticles as markers of premature coronary calcification in newly menopausal women. Am J Physiol Heart Circ Physiol. 2008;295:H931–H938. doi: 10.1152/ajpheart.00193.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortigiani L. Dodi C. Paolini EA. Bernardi D. Bruno G. Nannini E. Prognostic value of pharmacological stress echocardiography in women with chest pain and unknown coronary artery disease. J Am Coll Cardiol. 1998;32:1975–1981. doi: 10.1016/s0735-1097(98)00477-x. [DOI] [PubMed] [Google Scholar]
- 11.Arruda-Olson AM. Juracan EM. Mahoney DW. McCully RB. Roger VL. Pellikka PA. Prognostic value of exercise echocardiography in 5,798 patients: Is there a gender difference? J Am Coll Cardiol. 2002;39:625–631. doi: 10.1016/s0735-1097(01)01801-0. [DOI] [PubMed] [Google Scholar]
- 12.Heupler S. Mehta R. Lobo A. Leung D. Marwick TH. Prognostic implications of exercise echocardiography in women with known or suspected coronary artery disease. J Am Coll Cardiol. 1997;30:414–420. doi: 10.1016/s0735-1097(97)00167-8. [DOI] [PubMed] [Google Scholar]
- 13.Abdelmoneim SS. Bernier M. Scott CG, et al. Safety of contrast agent use during stress echocardiography: A 4-year experience from a single-center cohort study of 26,774 patients. JACC Cardiovase Imaging. 2009;2:1048–1055. doi: 10.1016/j.jcmg.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Olszewski R. Timperley J. Szmigielski C, et al. The clinical applications of contrast echocardiography. Eur J Echocardiogr. 2007;8:S13–23. doi: 10.1016/j.euje.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Moir WS AS. McCully RB. Pellikka PA. Mulvagh SL. Contrast stress echo in the era of contemporary imaging modalities: Rate of usage, safety and impact on test interpretation in a high volume stress laboratory. J Am Soc Echocardiogr. 2006;19:644. [Google Scholar]
- 16.Ridker PM. Buring JE. Shih J. Matias M. Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 17.Beattie MS. Shlipak MG. Liu H. Browner WS. Schiller NB. Whooley MA. C-reactive protein and ischemia in users and nonusers of beta-blockers and statins: Data from the Heart and Soul Study. Circulation. 2003;107:245–250. doi: 10.1161/01.cir.0000044387.23578.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaspardone A. Perino M. Ghini AS, et al. Exercise induced myocardial ischaemia does not cause increase in C-reactive protein concentration. Heart. 2000;84:668A–669. doi: 10.1136/heart.84.6.668a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moller JE. Bergeron S. Jaffe A. Pellikka PA. Influence of left ventricular filling pattern on exercise-induced changes of natriuretic peptides in patients with suspected coronary artery disease. Int J Cardiol. 2008;124:204–210. doi: 10.1016/j.ijcard.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Cherng WJ. Wang CH. Hung MJ. Changes of endothelin-1 and atrial natriuretic peptide during dobutamine stress echocardiography. J Formos Med Assoc. 1998;97:812–818. [PubMed] [Google Scholar]
- 21.Kondos GT. Hoff JA. Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: A 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 22.Shaw LJ. Raggi P. Schisterman E. Berman DS. Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 23.Ramakrishna G. Breen JF. Mulvagh SL. McCully RB. Pellikka PA. Relationship between coronary artery calcification detected by electron-beam computed tomography and abnormal stress echocardiography: Association and prognostic implications. J Am Coll Cardiol. 2006;48:2125–2131. doi: 10.1016/j.jacc.2006.04.105. [DOI] [PubMed] [Google Scholar]
- 24.Nesto RW. Kowalchuk GJ. The ischemic cascade: Temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. J Am Cardiol. 1987;59:23C–30C. doi: 10.1016/0002-9149(87)90192-5. [DOI] [PubMed] [Google Scholar]
- 25.Taylor AJ. Cerqueira M. Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–1894. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Berman DS. Wong ND. Gransar H, et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44:923–930. doi: 10.1016/j.jacc.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 27.Anand DV. Lim E. Raval U. Lipkin D. Lahiri A. Prevalence of silent myocardial ischemia in asymptomatic individuals with subclinical atherosclerosis detected by electron beam tomography. J Nucl Cardiol. 2004;11:450–457. doi: 10.1016/j.nuclcard.2004.06.125. [DOI] [PubMed] [Google Scholar]
- 28.Nasir K. Gopal A. Blankstein R, et al. Noninvasive assessment of gender differences in coronary plaque composition with multidetector computed tomographic angiography. Am J Cardiol. 2010;105:453–458. doi: 10.1016/j.amjcard.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 29.Shaw LJ. Miller DD. Romeis JC. Kargl D. Younis LT. Chaitman BR. Gender differences in the noninvasive evaluation and management of patients with suspected coronary artery disease. Ann Intern Med. 1994;120:559–566. doi: 10.7326/0003-4819-120-7-199404010-00005. [DOI] [PubMed] [Google Scholar]
- 30.Roger VL. Pellikka PA. Bell MR. Chow CW. Bailey KR. Seward JB. Sex and test verification bias. Impact on the diagnostic value of exercise echocardiography. Circulation. 1997;95:405–410. doi: 10.1161/01.cir.95.2.405. [DOI] [PubMed] [Google Scholar]
- 31.Shaw LJ MJ. Hendel RH. Gulati M, et al. Comparative effectiveness of exercise electrocardiography versus exercise electrocardiography plus myocardial perfusion SPECT in women with suspected coronary artery disease: Results From the What's the Optimal Method for Ischemia Evaluation in Women Trial. Circulation. 2010;122:2215–2226. doi: 10.1161/CIRCULATIONAHA.111.029660. [DOI] [PubMed] [Google Scholar]
- 32.Bugiardini R. Bairey Merz CN. Angina with “normal” coronary arteries: A changing philosophy. JAMA. 2005;293:477–484. doi: 10.1001/jama.293.4.477. [DOI] [PubMed] [Google Scholar]
- 33.Gulati M. Cooper-DeHoff RM. McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: A report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–850. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohli P. Gulati M. Exercise stress testing in women: Going back to the basics. Circulation. 2010;122:2570–2580. doi: 10.1161/CIRCULATIONAHA.109.914754. [DOI] [PubMed] [Google Scholar]
- 35.Diamond GA. Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–1358. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 36.Marwick TH. Anderson T. Williams MJ, et al. Exercise echocardiography is an accurate and cost-efficient technique for detection of coronary artery disease in women. J Am Coll Cardiol. 1995;26:335–341. doi: 10.1016/0735-1097(95)80004-z. [DOI] [PubMed] [Google Scholar]
- 37.Williams MJ. Marwick TH. O'Gorman D. Foale RA. Comparison of exercise echocardiography with an exercise score to diagnose coronary artery disease in women. Am J Cardiol. 1994;74:435–438. doi: 10.1016/0002-9149(94)90898-2. [DOI] [PubMed] [Google Scholar]
- 38.Dhoble A. Abdelmoneim SS. Bernier M. Oh JK. Mulvagh SL. Transient left ventricular apical ballooning and exercise induced hypertension during treadmill exercise testing: Is there a common hypersympathetic mechanism? Cardiovasc Ultrasound. 2008;6:37. doi: 10.1186/1476-7120-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNulty PH. Ettinger SM. Field JM, et al. Cardiac catheterization in morbidly obese patients. Catheter Cardiovasc Interv. 2002;56:174–177. doi: 10.1002/ccd.10186. [DOI] [PubMed] [Google Scholar]
- 40.From AM. Kane G. Bruce C. Pellikka PA. Scott C. McCully RB. Characteristics and outcomes of patients with abnormal stress echocardiograms and angiographically mild coronary artery disease (<50% stenoses) or normal coronary arteries. J Am Soc Echocardiogr. 2010;23:207–214. doi: 10.1016/j.echo.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 41.Mieres JH. Shaw LJ. Hendel RC. Heller GV. The WOMEN study: What is the optimal method for ischemia evaluation in women? A multi-center, prospective, randomized study to establish the optimal method for detection of coronary artery disease (CAD) risk in women at an intermediate-high pretest likelihood of CAD: Study design. J Nucl Cardiol. 2009;16:105–112. doi: 10.1007/s12350-008-9002-8. [DOI] [PubMed] [Google Scholar]
- 42.Cohen JL. Cheirif J. Segar DS, et al. Improved left ventricular endocardial border delineation and opacification with OPTISON (FS069), a new echocardiographic contrast agent. Results of a phase III multicenter trial. J Am Coll Cardiol. 1998;32:746–752. doi: 10.1016/s0735-1097(98)00311-8. [DOI] [PubMed] [Google Scholar]