Abstract
ERK1/2 (extracellular-signal-regulated kinase 1/2) and their substrates RSKs (p90 ribosomal S6 kinases) phosphorylate different transcription factors, contributing differentially to transcriptomic profiles. In cardiomyocytes ERK1/2 are required for >70% of the transcriptomic response to endothelin-1. In the present study we investigated the role of RSKs in the transcriptomic responses to the Gq-protein-coupled receptor agonists endothelin-1, phenylephrine (a generic α1-adrenergic receptor agonist) and A61603 (α1A-adrenergic receptor selective). Phospho-ERK1/2 and phospho-RSKs appeared in cardiomyocyte nuclei within 2–3 min of stimulation (endothelin-1>A61603≈phenylephrine). All agonists increased nuclear RSK2, but only endothelin-1 increased the nuclear RSK1 content. PD184352 (inhibits ERK1/2 activation) and BI-D1870 (inhibits RSKs) were used to dissect the contribution of RSKs to the endothelin-1-responsive transcriptome. Of the 213 RNAs up-regulated after 1 h, 51% required RSKs for their up-regulation, whereas 29% required ERK1/2 but not RSKs. The transcriptomic response to phenylephrine overlapped with, but was not identical with, endothelin-1. As with endothelin-1, PD184352 inhibited the up-regulation of most phenylephrine-responsive transcripts, but the greater variation in the effects of BI-D1870 suggests that differential RSK signalling influences global gene expression. A61603 induced similar changes in RNA expression in cardiomyocytes as phenylephrine, indicating that the signal was mediated largely through α1A-adrenergic receptors. A61603 also increased expression of immediate early genes in perfused adult rat hearts and, as in cardiomyocytes, up-regulation of the majority of genes was inhibited by PD184352. PD184352 or BI-D1870 prevented the increased surface area induced by endothelin-1 in cardiomyocytes. Thus RSKs play a significant role in regulating cardiomyocyte gene expression and hypertrophy in response to Gq-protein-coupled receptor stimulation.
Keywords: α1-adrenergic receptor, cardiomyocyte, endothelin, mitogen-activated protein kinase (MAPK), p90 ribosomal S6 kinase (p90 RSK), transcriptomics
Abbreviations: AMPKα, AMP-activated protein kinase α; AR, adrenergic receptor; Areg, amphiregulin; Atf3, activating transcription factor 3; BH-MTC, Benjamini and Hochberg multiple testing correction; CREB, cAMP-response-element-binding protein; Dusp, dual-specificity phosphatase; Egr, early growth response; ERK1/2, extracellular-signal-regulated kinase 1/2; ET-1, endothelin-1; FDR, false discovery rate; Fosb, FBJ murine osteosarcoma viral oncogene homologue B; Gapdh, glyceraldehyde 3-phosphate dehydrogenase; GSK3α/β, glycogen synthase kinase 3α/β; Has, hyaluronan synthase; IL11, interleukin 11; Klf, Krüppel-like factor; Lif, leukaemia inhibitory factor; MAPK, mitogen-activated protein kinase; MKK, MAPK kinase; MNK, MAPK-interacting protein kinase; MSK, mitogen- and stress-activated protein kinase; NPE, nuclear protein-enriched; Nr4a, nuclear receptor subfamily 4, group A; Olr1, oxidized low-density lipoprotein (lectin-like) receptor 1; PE, phenylephrine; PKB, protein kinase B; Plk2, polo-like kinase 2; Ptgs2, prostaglandin-endoperoxide synthase 2, qPCR, quantitative PCR; Rgs2, regulator of G-protein signalling 2, 24 kDa; RSK, ribosomal S6 kinase; Sik1, salt-inducible kinase 1; SNK, Student–Newman–Keuls
INTRODUCTION
The ERK1/2 (extracellular-signal-regulated kinase 1/2) cascade plays a major role in the global regulation of gene expression [1]. ERK1/2, the prototypic MAPKs (mitogen-activated protein kinases), are activated by dual phosphorylation of threonine and tyrosine residues within a T-E-Y motif, and they then phosphorylate substrates in both the cytoplasmic and nuclear compartments [2]. In the nucleus ERK1/2 phosphorylate nuclear-localized transcription factors (e.g. Elk1) to regulate their transactivating activities and/or association with other transcription factors, thus directly influencing the transcription of specific genes. ERK1/2 also phosphorylate downstream protein kinases including RSKs (p90 ribosomal S6 kinases) [3,4]. RSKs are ubiquitously expressed and are generally localized in the cytoplasm in unstimulated cells, but mitogenic stimulation results in the nuclear translocation of three of the four RSK isoforms (RSK1, RSK2 and RSK3). Like ERK1/2, RSKs phosphorylate cytoplasmic and nuclear substrates. However, the preferred phosphorylation motifs for ERK1/2 (proline-directed kinases) and RSKs (which phosphorylate serine/threonine residues within an R-X-R-X-X-S/T motif) differ and they phosphorylate different substrates. ERK1/2 also activate MSKs (mitogen- and stress-activated protein kinases), kinases related to RSKs [3]. MSKs are predominantly nuclear-localized and are involved in transcriptional regulation. A third family of downstream kinase substrates of ERK1/2 are MNKs (MAPK-interacting kinases) [3]. Although MNK1/2 may traffic into the nucleus they primarily target components of the translational apparatus.
Mammalian cardiomyocytes, the contractile cells of the heart, are terminally differentiated, withdrawing from the cell cycle around the time of birth. In response to a demand for increased power output (e.g. following the death of adjacent cells as a result of myocardial infarction or in hypertensive states), cardiomyocytes increase in size and myofibrillar content, adapting the components of the contractile apparatus and modulating their metabolism (i.e. they undergo hypertrophic growth). ERK1/2 signalling is associated with cardiomyocyte hypertrophy. Thus hypertrophic stimuli, such as ET-1 (endothelin-1) and α1-AR (adrenergic receptor) agonists, activate the ERK1/2 cascade in cardiomyocytes, and overexpression of constitutively activated components of the cascade induce aspects of the response in isolated cardiomyocytes [5]. Furthermore, small-molecule or genetic inhibition of the cascade prevents hypertrophy [6–8]. In vivo studies in genetically modified mice highlight further the importance of ERK1/2 signalling in the heart [9–11]. However, none of these studies clearly establishes how ERK1/2 elicit their effects.
Biochemical studies in neonatal rat cardiomyocytes, place dually phosphorylated (i.e. activated) ERK1/2 in the nucleus within 2 min of stimulation with ET-1 [12]. ERK1/2 phosphorylate DNA-binding transcription factors, including GATA4 (GATA-binding protein 4) and Elk1 [5], consistent with a role in direct transcriptional regulation [2]. Small-molecule inhibitors of ERK1/2 signalling inhibit the increases in expression of >70% of the mRNAs up-regulated by ET-1 [13–15]. Presumably, activation of other signalling pathways by ET-1 (e.g. c-Jun N-terminal kinases and p38 MAPKs [2]) contributes to the transcriptional changes, but the data suggest that the ERK1/2 cascade plays a major role in regulating cardiomyocyte gene expression [2,5,12–15]. ET-1 is particularly potent at activating ERK1/2. Other stimuli such as PE (phenylephrine; an α1-AR agonist) do not activate ERK1/2 to the same degree [16] and also activate other MAPKs [2]. It is unclear whether (although they signal through a similar Gq-protein-coupled receptor system [17]) ERK1/2 play as significant a role in this context. Even if they do, since different degrees/duration of ERK1/2 signalling can elicit profoundly different cellular responses in other cells, the transcriptional responses to PE compared with ET-1 may still be qualitatively different. In cardiomyocytes, as in other cells, activated ERK1/2 in the cytoplasm phosphorylate and activate RSKs, but the importance of RSKs in regulating cardiomyocyte gene expression has not been explored. In the present study we demonstrate that RSKs contribute to the changes in expression of >50% of the RNAs up-regulated by ET-1 within 1 h (when most changes in immediate early gene expression are detected [14]), whereas 29% required ERK1/2, but not RSKs, for up-regulation. We also demonstrate that the gene expression response to α1-AR stimulation (signalling primarily through α1A-ARs) is not identical with that of ET-1, although the signal is still predominantly mediated by the ERK1/2 cascade. The contribution of RSKs differs according to stimulus and this probably reflects differential signalling to specific RSK isoforms.
EXPERIMENTAL
Cardiomyocyte cultures
Ventricles were dissected from neonatal (2–4-day-old) Sprague–Dawley rat hearts (Harlan) and the cardiomyocytes were prepared and plated as described previously [15]. After 18 h in medium containing 15% (v/v) fetal bovine serum, the medium was changed to serum-free maintenance medium for a further 24 h. Stock solutions of agonists/inhibitors were prepared at 1000× the working concentration and added directly to the tissue culture medium. PD184352 (Alexis Biochemicals, Enzo Life Sciences) and BI-D1870 (Division of Signal Transduction Therapy Unit, University of Dundee, Dundee, U.K. and Enzo Life Sciences) were prepared in DMSO. ET-1 (Bachem), PE (Sigma–Aldrich) and A61603 (Tocris Bioscience) were dissolved in water.
Adult rat heart perfusions
Male 275–300 g Sprague–Dawley rats were housed and work was undertaken in accordance with local institutional animal care committee procedures and the U.K. Animals (Scientific Procedures) Act 1986. Hearts were perfused retrogradely (37°C at 70 mmHg) as described previously [18]. For the experiments with PD184352, the drug was added to the perfusate during the 15 min equilibration period. A61603 was added at the end of the equilibration period and all perfusions were continued for 1 h. Hearts were ‘freeze-clamped’ between aluminium tongs cooled in liquid nitrogen and were pulverized under liquid nitrogen in a pestle and mortar. The powders were stored at −80°C.
Immunoblotting
Total cardiomyocyte extracts were prepared as described previously [15]. Cytosolic and NPE (nuclear protein-enriched) extracts were prepared as described in [19] with the addition of 4 μM microcystin-LR in all buffers. Samples were stored at −20°C. Proteins were separated by SDS/PAGE (10% gels). For ERK1/2, volumes equivalent to 2×105 or 5.3×105 cells were used for analysis of the cytosolic or NPE fractions respectively. For RSKs, volumes equivalent to 3×105 or 8×105 cells were used for analysis of the cytosolic or NPE fractions respectively. For RSK1 and RSK2, volumes equivalent to 1×106 cells were used for analysis of the NPE fractions. Immunoblotting was performed as described previously [15] (primary antibodies are listed in Supplementary Table S1A at http://www.biochemj.org/bj/450/bj4500351add.htm). Secondary antibodies conjugated to horseradish peroxidase were from Dako (1:5000 dilution). Bands were detected using ECL reagent and X-ray film ECL Prime and an Imagequant 350 system or a LAS4000 mini system (GE Healthcare). Quantification was performed using Imagequant software. To determine relative amounts of total ERK1/2 or total RSKs in the cytosol compared with the NPE fractions, cytosol samples (control and 5 min ET-1 treatment) were analysed on the same blots as the NPE fractions.
Microarray hybridizations, data analysis and qPCR (quantitative PCR) validations
The additions of inhibitors/agonists were staggered and cells were harvested simultaneously. To minimize variation from different cardiomyocyte preparations, equal amounts of RNA from three separate myocyte preparations were pooled to generate a single sample set and three such sets were hybridized to separate microarrays. For changes in RNA expression induced by PE or ET-1 at 2, 4 or 24 h, RNA was extracted, labelled cRNA was prepared and hybridization to Affymetrix rat genome 230 2.0 arrays was performed as described previously [14]. For studies using Affymetrix rat exon 1.0 ST arrays, cardiomyocytes were unstimulated (controls), exposed to 2 μM PD184352 or 10 μM BI-D1870 for 70 min, exposed to PE or ET-1 for 0.5 or 1 h, or exposed to PD184352 or BI-D1870 for 10 min before the addition of ET-1 for a further 1 h. Total RNA was provided to the NASC (Nottingham Arabidopsis Stock Centre) for preparation and hybridization according to their protocols (http://affymetrix.arabidopsis.info). The data were deposited in ArrayExpress (accession numbers E-MIMR-3, E-MIMR-37, E-MEXP-3393, E-MEXP-3394, E-MEXP-3678 and E-MEXP-3679).
Microarray data (.CEL files) were imported into GeneSpring 12.0 (Agilent Technologies). For the Affymetrix rat genome 230 2.0 microarrays, PE and ET-1 data were imported, normalized and analysed as described previously [15]. Probesets were selected with minimum raw values of 50 in all of any condition, with >1.5-fold change at any time relative to controls and statistically significant changes [FDR (false discovery rate)<0.05] identified by one-way ANOVA with an SNK (Student–Newman–Keuls) post-test, applying a BH-MTC (Benjamini and Hochberg multiple testing correction). The data for PE and ET-1 at 24 h were co-analysed, selecting probesets according to the same criteria. Heatmaps were generated by hierarchical clustering on entities using a Euclidean similarity measure and centroid linkage rule. For the Affymetrix exon 1.0 ST microarrays, the data for PE and ET-1 (0.5 and 1 h) with their corresponding controls were summarized and normalized as described previously [20], selecting probesets according to the criteria listed above. For analysis of the effects of PD184352 or BI-D1870 on RNA responses to ET-1, samples were imported and summarized as for the time course analysis. For the baseline effects of the inhibitors, probesets were selected with >1.5-fold change with PD184352 or BI-D1870 relative to the controls. Statistically significant changes (FDR<0.05) were identified by unpaired Student's t test with a BH-MTC. To identify effects of PD184352 or BI-D1870 on the response to ET-1, probesets were selected with >1.5-fold change with ET-1 relative to the controls (unpaired Student's t test with a BH-MTC). For the up-regulated RNAs, the significant effects of PD184352 or BI-D1870 were then identified by one-way ANOVA with an SNK post-test, applying a BH-MTC. PE data were analysed by k-means clustering of entities using a Euclidean similarity measure.
Validations were performed using different RNA preparations from those used for microarray analysis. Total RNA and cDNAs were prepared and qPCR performed as described previously [15]. The primers designed for qPCR are listed in Supplementary Table S1(B). Values were normalized to Gapdh (glyceraldehyde 3-phosphate dehydrogenase) expression and then to the controls.
Immunostaining and planimetry
Cardiomyocytes were unstimulated (controls) or exposed to inhibitors with and without ET-1 (24 h at 37°C). Immunostaining was performed as described previously [15] using mouse primary monoclonal antibodies to cardiac troponin T. Cardiomyocytes were viewed with a Zeiss Axioskop fluorescence microscope using a ×40 objective. Digital images captured using a Canon PowerShot G3 camera were converted into greyscale using Adobe Photoshop 7.0. Planimetry was performed using ImageJ (http://rsbweb.nih.gov/ij/). Cardiomyocyte surface area was measured for an average of 51 cells per condition. The experiment was performed four times. Mean values were taken for each experiment and used as a single observation.
Data interpretation and statistical analysis
Graphs were constructed and statistical analysis performed with GraphPad Prism 4.0. Unless otherwise stated, statistical testing used one-way ANOVA with an SNK post-test.
RESULTS
Nuclear localization of activated ERK1/2 and RSKs in cardiomyocytes
Activated ERK1/2 and RSKs translocate to the nucleus to modulate transcription [2,4]. To examine the subcellular localization of activated (i.e. dually phosphorylated) ERK1/2 and RSKs, cardiomyocytes were exposed to 100 nM ET-1, 100 μM PE (a generic α1-AR agonist activating α1A-ARs and α1B-ARs, both being expressed in cardiomyocytes [21]) or 50 nM A61603 (selective for α1A-ARs). The concentrations were selected on the basis of the EC50 values for activation of protein kinase Cϵ (acting upstream of the ERK1/2 cascade) in cardiomyocytes (~1 nM and 1 μM for ET-1 and PE respectively [16]) and the EC50 value for activation of ERK1/2 by ET-1 (~10 nM [22]). The reported EC50 value for A61603 stimulation of α1A-ARs and α1B-ARs is ~6 and ~380 nM respectively [23]. We therefore selected 50 nM A61603 for activation of α1A-ARs. Propranolol (20 μM), a β-AR antagonist, did not enhance the stimulation of MKK (MAPK kinase) 1/2 or ERK1/2 by PE, but inhibited the activation of ERK1/2 signalling by 50 μM isoprenaline (Figure 1A). There is, therefore, no inhibitory input from β-AR signalling into the activation of ERK1/2 by PE.
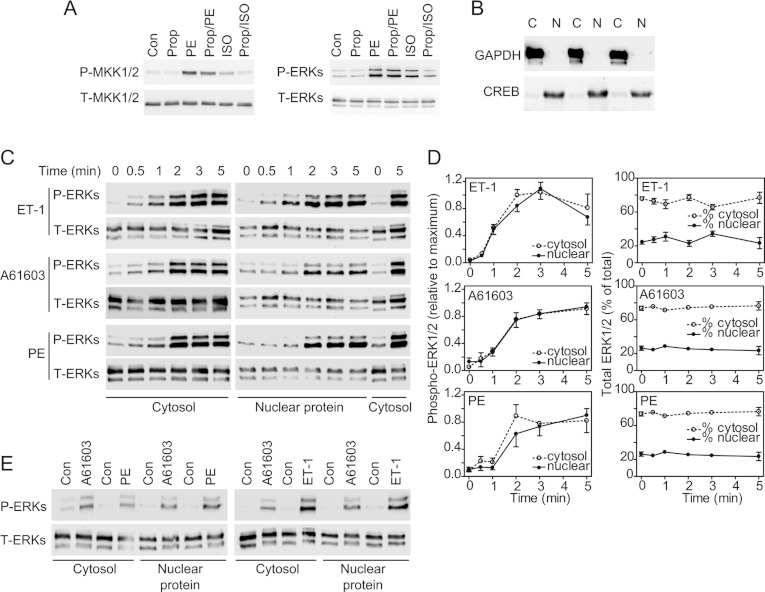
Figure 1. Nuclear signalling of ERK1/2 in cardiomyocytes.
(A) Cardiomyocytes were unstimulated (Con), exposed to 100 μM PE, 50 μM isoprenaline (ISO; 5 min) or to 20 μM propranolol (Prop; 15 min), or exposed to propranolol (10 min) before addition of PE (Prop/PE) or isoprenaline (Prop/ISO) (5 min). Samples were immunoblotted for phospho-MKK1/2 (P-MKK1/2), total MKK1/2 (T-MKK1/2), phospho-ERK1/2 (P-ERKs) and total ERK1/2 (T-ERKs). The experiment was repeated with similar results. (B) Immunoblots of GAPDH or CREB in cytosolic (C) and NPE (N) fractions from cardiomyocytes. (C and E) Cardiomyocytes were unstimulated (Con), or exposed to 100 nM ET-1, 100 μM PE or 50 nM A61603 for the times indicated (C) or for 5 min (E). Fractions were immunoblotted for phospho-ERK1/2 or total ERK1/2. Blots are representative of at least three experiments with different cardiomyocyte preparations. (D) Densitometric analysis of the blots in (C). The graphs on the left-hand side show data normalized to maximum values. The graphs on the right-hand show the relative percentage in each fraction. Results are means±S.E.M. for three independent myocyte preparations.
Soluble cytosolic proteins (e.g. GAPDH) were separated from an NPE fraction containing transcription factors [e.g. CREB (cAMP-response-element-binding protein); Figure 1B] and the levels of activated or total ERK1/2 were determined by immunoblotting. Phospho-ERK1/2 were detected in the cytosolic and NPE fractions following stimulation with each agonist (Figures 1C–1E). ET-1 promoted maximal phosphorylation of ERK1/2 within 2–3 min with similar profiles of activation in the cytosolic or NPE fractions (Figures 1C and 1D). Since MKK1/2 (the upstream kinases for ERK1/2) localize to the cytoplasm [24], this suggests that trafficking of activated ERK1/2 between compartments is rapid. Quantitative assessment indicated that 24±2% (mean±S.E.M., n=3) of the total cellular ERK1/2 protein was present in the NPE fractions of unstimulated cells and this did not change significantly following stimulation (Figure 1C). The time course for activation of ERK1/2 by A61603 or PE was delayed relative to and was less than that induced by ET-1 (Figures 1C–1E). A61603 and PE induced similar responses, indicating that PE signals predominantly through α1A-ARs to ERK1/2.
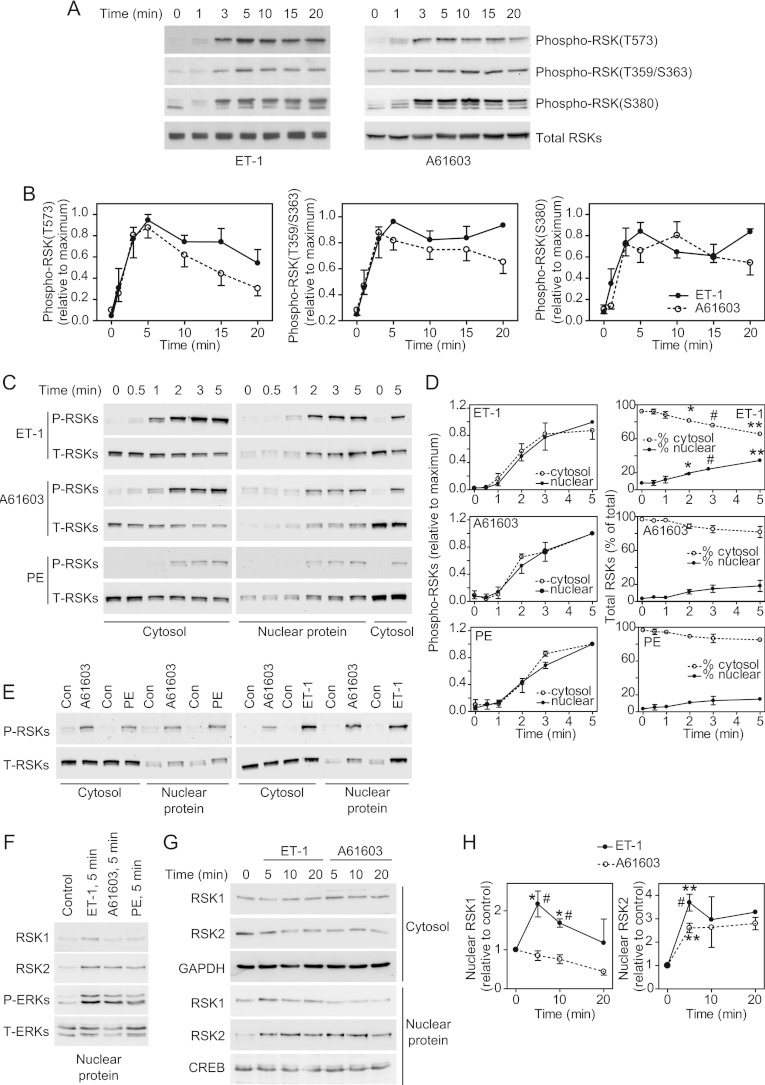
ERK1/2 activate RSKs by phosphorylation of Thr573 in the C-terminal kinase domain with additional phosphorylation of Thr359/Ser363 [4]. The C-terminal kinase causes autophosphorylation of Ser380 (residue numbers relate to RSK1). ET-1 and A61603 promoted phosphorylation of all of these sites with maximal phosphorylation at ~5 min (Figures 2A and 2B). The profiles for phosphorylation were similar with both agonists. Although the degree of phosphorylation of Thr573 started to decline from ~10 min, phosphorylation of Thr359/Ser363 and Ser380 was sustained over at least 20 min. As with phospho-ERK1/2, phospho-RSKs [assessed by immunoblotting with antibodies against phospho-RSK(Thr573)] were detected in the cytosolic and NPE fractions following stimulation with ET-1, PE or A61603. The time course indicated that phosphorylation of RSKs (Figures 2C and 2D) was delayed relative to ERK1/2 (Figures 1C and 1D) as expected. Only 4–8% of the total RSKs were present in the NPE fractions of unstimulated cells (Figure 2D). This increased to 34±2% following stimulation with ET-1 with a lesser increase induced by A61603 (18±7%) or PE (15±2%). The time course for translocation of total RSKs to the nucleus coincided with RSK phosphorylation, suggesting that the events are associated. ET-1 promoted greater activation of RSKs than A61603 or PE (Figure 2E). RSK1 and RSK2 are the preponderant isoforms in cardiomyocytes [25]. Although all three agonists stimulated nuclear accumulation of RSK2, only ET-1 increased nuclear localization of RSK1 (Figures 2E–2H). Thus differential signalling through RSKs may influence gene expression profiles.
Figure 2. Phosphorylation and nuclear signalling of RSKs in cardiomyocytes.
(A) Cardiomyocytes were exposed to ET-1 or A61603 for the times indicated. Total extracts were immunoblotted with antibodies against total RSKs or RSKs phosphorylated on Thr573, Thr359/Ser363 or Ser380. Blots are representative of at least three experiments with independent myocyte preparations. (B) Densitometric analysis of the blots in (A). Data were normalized to the maximum values. Results are means±S.E.M. for three (ET-1) or five (A61603) experiments with different cardiomyocyte preparations. (C and E) Cardiomyocytes were unstimulated (Con), or exposed to ET-1, PE or A61603 for the times indicated (C) or for 5 min (E). Cysotolic and nuclear proteins were immunoblotted with antibodies against phospho-RSK(Thr573) (P-RSKs) or total RSKs (T-RSKs). Blots are representative of at least three experiments with different cardiomyocyte preparations. (D) Densitometric analysis of the blots in (C). The graphs on the left-hand side show data normalized to the maximum values. The graphs on the right-hand side show the relative percentage in each fraction. Results are means±S.E.M. for three experiments with different cardiomyocyte preparations. *P<0.05, #P<0.01 and **P<0.001 relative to zero time. (F) Immunoblots of nuclear proteins following stimulation with ET-1, A61603 or PE (5 min) using goat polyclonal antibodies against RSK1 (sc-231) or RSK2 (sc-1430), or antibodies against phospho-ERK1/2 (P-ERKs) or total ERK1/2 (T-ERKs). The experiment was repeated twice with different cardiomyocyte preparations with similar results. (G) Immunoblots of nuclear proteins following stimulation with ET-1 or A61603 using rabbit polyclonal antibodies against RSK1 (#9333) or RSK2 (#9340) or antibodies against GAPDH or CREB. The experiment was repeated twice with different cardiomyocyte preparations with similar results. (H) Densitometric analysis of the data from (F) and (G). Results are means±S.E.M. for at least three observations with different cardiomyocyte preparations. *P<0.01 and **P<0.001 relative to zero time; #P<0.01 relative to A61603 at the same time.
Signalling to gene expression via RSKs
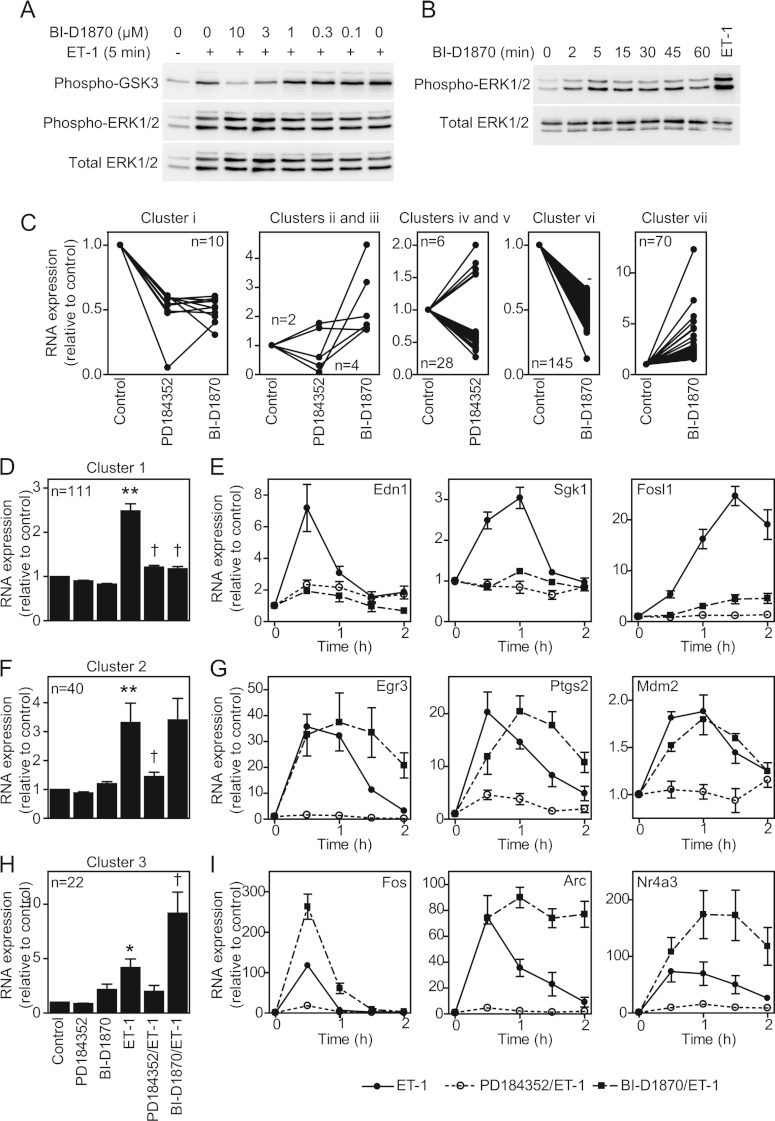
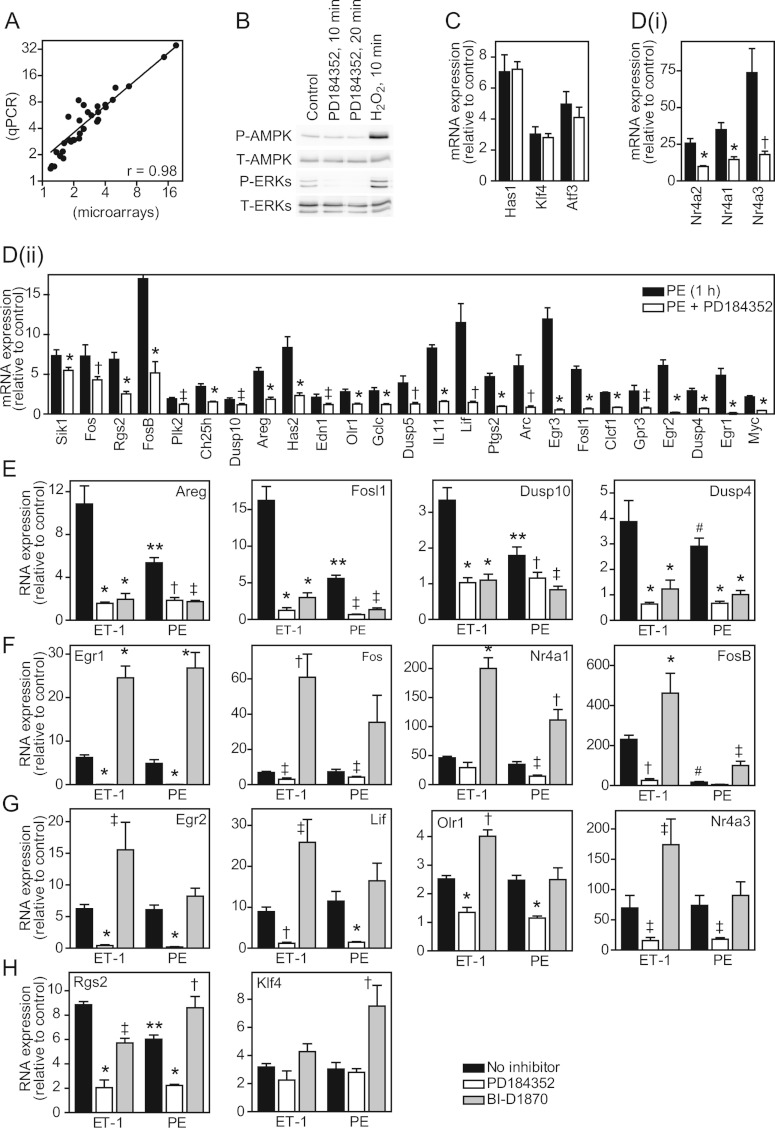
To determine the contribution of RSKs to the changes in gene expression that occur in cardiomyocytes, we focused on ET-1 and compared the effects of 2 μM PD184352 (which inhibits ERK1/2 activation [26]) with 10 μM BI-D1870, a selective inhibitor of RSKs [27]. The effects of BI-D1870 on the phosphorylation of GSK3α/β (glycogen synthase kinase 3α/β; established RSK substrates [27]) demonstrated that 10 μM was the lowest effective concentration for inhibition of RSKs in cardiomyocytes (Figure 3A). BI-D1870 did not affect activation of ERK1/2 by ET-1, but (as in other cells [27]) BI-D1870 alone activated ERK1/2, albeit to a lesser degree than ET-1 (Figure 3B). Others report that BI-D1870 partially inhibits the activation of PKB (protein kinase B) in the context of insulin signalling in some cells [28]. However, PKB is not activated to any significant extent by ET-1 [7] or α1-AR agonists (results not shown) in cardiomyocytes. Cardiomyocytes were exposed to inhibitors alone or ET-1 for 1 h in the absence or presence of inhibitors. Transcriptomic changes were determined using Affymetrix rat exon 1.0 ST arrays. PD184352 alone promoted the down-regulation of 42 RNAs (clusters i, iii and v) and up-regulation of eight RNAs (clusters ii and iv) (Figure 3C and Supplementary Table S2 at http://www.biochemj.org/bj/450/bj4500351add.htm). BI-D1870 had a greater effect promoting the down-regulation of 155 RNAs (clusters i and vi) and the up-regulation of 76 RNAs (clusters ii, iii and vii).
Figure 3. Regulation of cardiomyocyte gene expression by ERK1/2 compared with RSKs.
(A) Cardiomyocytes were exposed to ET-1 (5 min) in the presence of indicated concentrations of BI-D1870. Samples were immunoblotted for phospho-GSK3α/β, phospho-ERK1/2 or total ERK1/2. The experiment was repeated with similar results. (B) Cardiomyocytes were exposed to 10 μM BI-D1870 for the times indicated or ET-1 (5 min). Samples were immunoblotted for phospho- or total ERK1/2. The experiment was repeated with similar results. (C) Cardiomyocytes were unstimulated (control), or exposed to PD184352 or BI-D1870 (70 min). RNA expression was determined using Affymetrix rat exon 1.0 ST arrays. RNAs regulated by PD184352 and/or BI-D1870 alone were clustered according to inhibition or enhancement. Mean expression (n=3) relative to the controls is shown for each RNA (numbers in each cluster are indicated). (D, F and H) Cardiomyocytes were unstimulated (control), exposed to PD184352, BI-D1870 or ET-1 alone (1 h), or exposed to ET-1 in the presence of PD184352 or BI-D1870. RNA expression was determined using Affymetrix rat exon 1.0 ST arrays. RNAs up-regulated by ET-1 were clustered according to effects of each inhibitor (numbers in each cluster are shown). Results are means±S.E.M. for RNAs in each cluster relative to controls. *P<0.001 and **P<0.01 relative to the control; †P<0.001 relative to ET-1. (E, G and I) Cardiomyocytes were exposed to ET-1 in the absence/presence of PD184352 or BI-D1870 and mRNA expression for selected transcripts analysed by qPCR. Results are means±S.E.M. (n=4).
To determine the role of RSKs in regulating the ET-1-responsive transcriptome, we selected 213 RNAs up-regulated by ET-1 after 1 h (excluding six with >3-fold change with BI-D1870 alone). The effects of PD184352 or BI-D1870 on the change induced by ET-1 was determined statistically (FDR<0.05) and on the condition of ≥20% modulation of the response allowing for inhibitor baseline effects (Supplementary Table S3 at http://www.biochemj.org/bj/450/bj4500351add.htm). The microarray data were validated by qPCR for selected mRNAs studying the effects of inhibitors over 0.5, 1, 1.5 and 2 h. The largest cluster (111 RNAs, cluster 1) contained RNAs whose up-regulation was inhibited by PD184352 or BI-D1870 (Figure 3D), indicating that the signal from ERK1/2 to RNA expression is mediated by RSKs. For the 11 mRNAs we validated, up-regulation by ET-1 was consistently inhibited by either drug at all times [Figure 3E and results not shown for Areg (amphiregulin), Dusp (dual-specificity phosphatase) 4, Dusp10, Ets1 (v-ets erythroblastosis virus E26 oncogene homologue 1), Has (hyaluronan synthase) 2, Hmgcr (3-hydroxy-3-methylglutaryl-CoA reductase), Klf (Krüppel-like factor) 5 and Ripk (receptor-interacting serine-threonine kinase)]. The second largest group (40 RNAs, cluster 2) contained RNAs inhibited by PD184352 with no significant effect of BI-D1870 (Figure 3F). For the seven mRNAs selected for validation, up-regulation by ET-1 was consistently inhibited by PD184352 and there was no significant change with BI-D1870 at 1 h [Figure 3G and results not shown for Fosb (FBJ murine osteosarcoma viral oncogene homologue B), Klf4, Rnd3 (Rho family GTPase 3) and Plk2 (polo-like kinase 2)]. However, for most [e.g. Egr (early growth response) 3 and Ptgs2 (prostaglandin-endoperoxide synthase 2)], there was enhancement of the ET-1 response with BI-D1870 at the later times. For these RNAs, the primary signal for up-regulation is mediated by ERK1/2 independently of RSKs, although RSKs may negatively influence the response at the later times. The third significant group (22 RNAs, cluster 3) contained RNAs for which BI-D1870 enhanced the response to ET-1, whereas PD184352 inhibited the response (Figure 3H, cluster 3). For the six mRNAs validated from cluster 3, up-regulation by ET-1 was consistently inhibited by PD184352 and BI-D1870 enhanced the response [Figure 3I and results not shown for Egr2, Lif (leukaemia inhibitory factor) and Nr4a (nuclear receptor subfamily 4, group A)3]. Other RNAs did not cluster into significant groups. Overall, ~51% of the mRNAs up-regulated by ET-1 required RSKs for up-regulation, whereas ~29% required ERK1/2, but not RSKs, for up-regulation. Approximately half of the latter mRNAs showed enhanced expression following RSK inhibition.
Regulation of cardiomyocyte RNA expression by α1-AR agonists
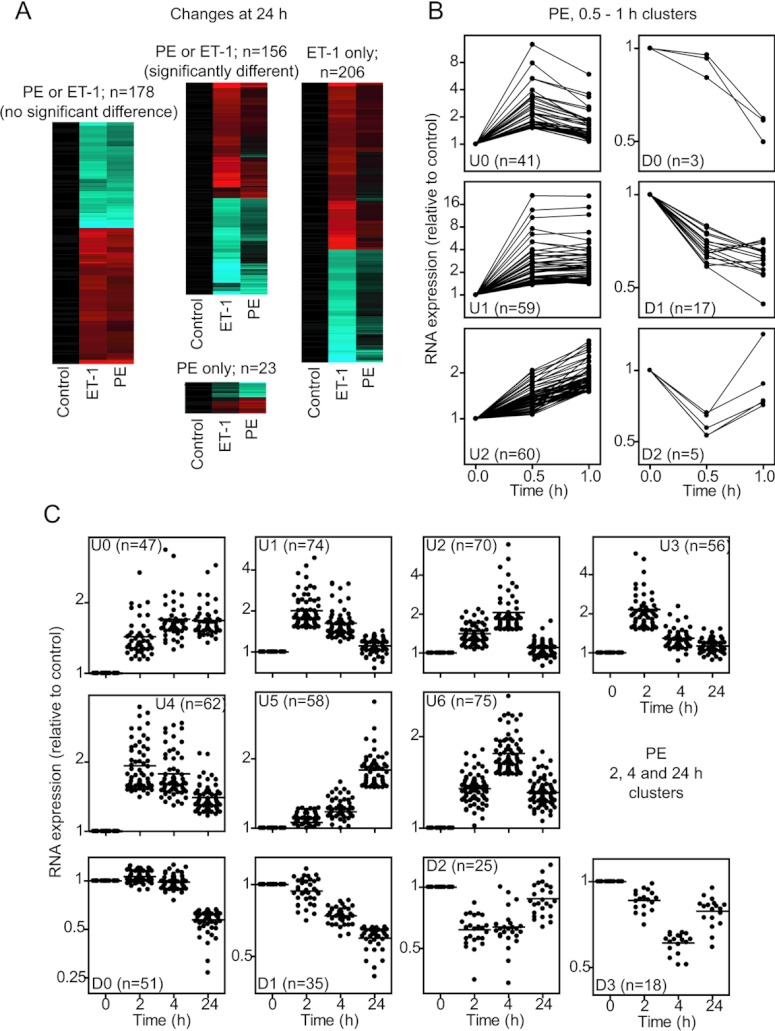
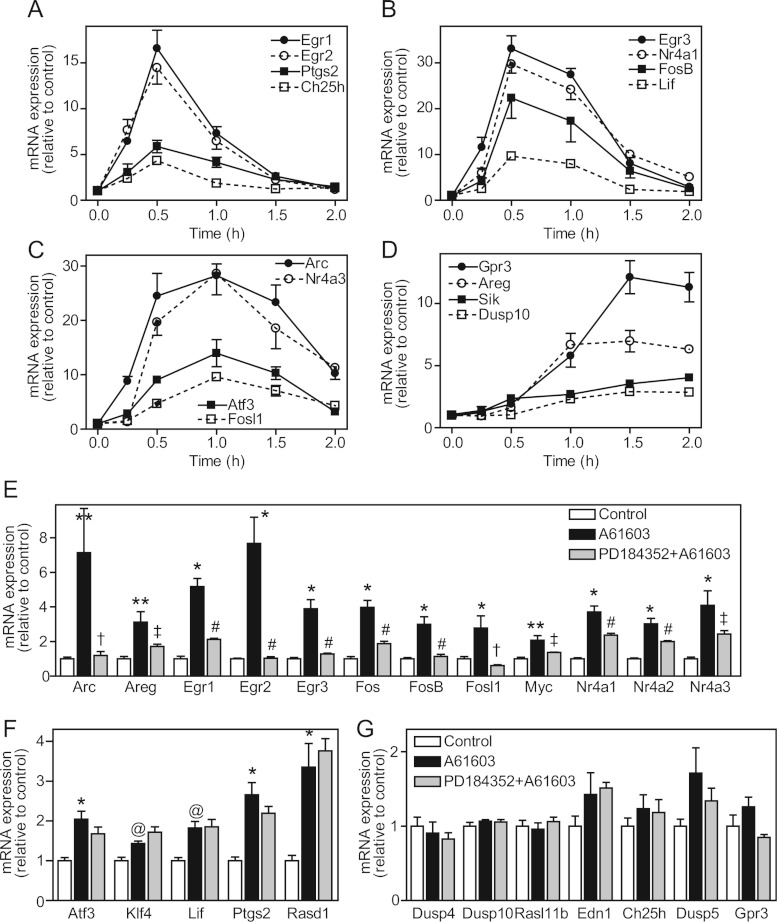
Our previous studies of the regulation of gene expression in cardiomyocytes focused on ET-1. However, α1-AR agonists also promote cardiomyocyte growth [17] whilst activating ERK1/2 to a lesser degree than ET-1 (Figure 1). To compare the effects of α1-AR stimulation with ET-1, we initially used Affymetrix rat expression 230 2.0 arrays for transcriptomic profiling of the response to PE over a prolonged time course (2, 4 and 24 h). We identified 385 and 120 RNAs as significantly up- or down-regulated respectively (Supplementary Table S4 at http://www.biochemj.org/bj/450/bj4500351add.htm), substantially less than the response to ET-1 (827 RNAs up-regulated and 598 RNAs down-regulated; Supplementary Table S5 at http://www.biochemj.org/bj/450/bj4500351add.htm; the previously reported data for ET-1 [14] were reanalysed in the present study in parallel with the data for PE). After 24 h, 178 RNAs were significantly and similarly changed by either agonist, 156 RNAs exhibited significantly different responses to the two agonists, 23 RNAs were significantly changed only with PE, and 206 RNAs were significantly changed with ET-1 (Figure 4A and Supplementary Table S6 at http://www.biochemj.org/bj/450/bj4500351add.htm). Thus, although there is overlap in response, cardiomyocyte hypertrophy induced by ET-1 or PE is associated with different gene expression profiles. This presumably reflects variation in the early phase gene expression. We therefore examined the earliest phase of gene expression (0.5 and 1 h) induced by PE compared with ET-1 using more comprehensive Affymetrix rat exon 1.0 ST arrays. The overall response to PE at 0.5–1 h was less than that induced by ET-1 with up-regulation of 159 (compare with 285 for ET-1) RNAs and down-regulation of 25 (compare with 93 for ET-1) RNAs (Supplementary Tables S7 and S8 at http://www.biochemj.org/bj/450/bj4500351add.htm). As for ET-1 [14], PE-responsive RNAs were temporally regulated (Figures 4B and 4C).
Figure 4. Regulation of the cardiomyocyte transcriptome by PE.
Cardiomyocytes were exposed to PE or ET-1 for the times indicated. RNA expression profiling was performed using Affymetrix rat 230 2.0 microarrays (A and C) or Affymetrix rat exon 1.0 ST arrays (B). (A) Heatmaps for transcriptional changes at 24 h clustered according to similarity of response to PE compared with ET-1 (range: cyan=−2.5; black=0; red=2.5; log2 scale). Results are means for n=3 independent hybridizations of different samples each prepared from three cardiomyocyte preparations. (B) k-Means clustering of mRNAs regulated by PE at 0.5 or 1 h. (C) k-Means clustering of RNAs regulated by PE at 2, 4 or 24 h. (B and C) Results (means for n=3 hybridizations) are shown for each RNA. Numbers of transcripts in each cluster are in parentheses.
We selected 34 mRNAs (~25% of protein-encoding transcripts significantly up-regulated by PE over 0.5–1 h) for validation experiments and further study by qPCR (Table 1). Overall, the correlation between microarray and qPCR data was high with a linear regression coefficient of 0.98 (Figure 5A). We used 2 μM PD184352 to determine the role of ERK1/2 activation in the response to PE at 1 h. In some, although not all, cells PD184352 may alter the energy balance and activate AMP kinase [29,30]. In cardiomyocytes we did not detect phosphorylation (i.e. activation) of AMPKα (AMP-activated protein kinase α) by 2 μM PD184352, although AMPKα phosphorylation was increased by 0.5 mM H2O2 (Figure 5B). As expected, PD184352 reduced the degree of ERK1/2 phosphorylation below basal levels. Of the 31 mRNAs significantly up-regulated by PE at 1 h, only Has1 and Klf4 were insensitive to PD184352 and Atf3 (activating transcription factor 3) was inhibited to a small non-significant degree (Figure 5C), whereas increased expression of 28 was significantly inhibited by PD184352 (Figures 5D, i and 5D, ii). Thus ERK1/2 signalling plays a significant role in the PE-responsive cardiomyocyte transcriptome. We compared the effects of PD184352 or BI-D1870 on the up-regulation of selected mRNAs by PE with ET-1 (Figures 5E–5H). For all mRNAs studied the inhibition of the response by PD184352 was similar with either stimulus, whereas the relative effect of BI-D1870 varied. For some the relative inhibition (e.g. Areg, Fosl1, Dusp10 and Dusp4; Figure 5E) or enhancement (e.g. Egr1, Fos, Nr4a1 and FosB; Figure 5F) by BI-D1870 was similar. However, for seven of the 28 RNAs the effects of the inhibitors were qualitatively different for the two agonists. For Egr2, Lif, Olr1 [oxidized low-density lipoprotein (lectin-like) receptor 1], Nr4a3 and Nr4a2, up-regulation of mRNA expression by ET-1 was enhanced by BI-D1870 with no significant effect of BI-D1870 on the response to PE (Figure 5G and results not shown for Nr4a2). For Rgs2 (regulator of G-protein signalling 2, 24 kDa) and Klf4, up-regulation of mRNA expression by PE, but not ET-1 was enhanced by BI-D1870 (Figure 5H). These data indicate that differential activation of RSKs contributes to the different RNA expression profiles induced in cardiomyocytes by ET-1 compared with α1-ARs and are consistent with differential regulation of nuclear RSKs, either because of the different relative degrees of stimulation (Figure 2E) or selective nuclear translocation of RSK2, but not RSK1, by α1-AR agonists (Figures 2F–2H).
Table 1. RNA expression data for PE and A61603.
Cardiomyocytes were exposed to PE or A61603 for 0.5 or 1 h. RNA was extracted and analysed using Affymetrix microarrays or by qPCR. Results are expressed relative to the controls. Microarray results are means for n=3. The qPCR data are means±S.E.M. (n=3). Gene symbols are provided; accession numbers are provided in Supplementary Table S1(B) at http://www.biochemj.org/bj/450/bj4500351add.htm Arc, activity-regulated cytoskeleton-associated protein; Ch25h, cholesterol 25-hydroxylase; Clcf1, cardiotrophin-like cytokine factor 1; Edn1, endothelin 1; Gclc, glutamate-cysteine ligase, catalytic subunit; Gpr3, G-protein-coupled receptor 3; Rasd1, RAS, dexamethasone-induced 1; Rasl11b, RAS-like, family 11, member B.
| PE (microarrays) | PE (qPCR) | A61603 (qPCR) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.5 h | 1 h | 1 h | 0.5 h | 1 h | ||||
| Gene symbol | Mean | Mean | Mean | S.E.M. | Mean | S.E.M. | Mean | S.E.M. |
| Arc | 2.67 | 2.76 | 6.05 | 1.38 | 24.50 | 4.16 | 28.25 | 2.14 |
| Areg | 1.33 | 1.79 | 5.36 | 0.48 | 1.62 | 0.14 | 6.67 | 0.92 |
| Atf3 | 3.30 | 3.45 | 4.96 | 0.81 | 9.04 | 1.12 | 13.96 | 2.49 |
| Ch25h | 3.44 | 2.31 | 3.44 | 0.35 | 4.32 | 0.43 | 1.84 | 0.07 |
| Clcf1 | 1.63 | 1.63 | 2.68 | 0.11 | 2.42 | 0.23 | 3.36 | 0.69 |
| Dusp10 | 1.16 | 1.61 | 1.79 | 0.24 | 1.03 | 0.02 | 2.30 | 0.18 |
| Dusp4 | 1.53 | 2.04 | 2.91 | 0.32 | 1.40 | 0.02 | 2.16 | 0.24 |
| Dusp5 | 2.35 | 2.51 | 3.90 | 0.89 | 3.05 | 0.33 | 2.82 | 0.32 |
| Edn1 | 2.22 | 1.38 | 2.11 | 0.38 | 2.66 | 0.16 | 2.04 | 0.21 |
| Egr1 | 3.17 | 2.29 | 4.87 | 0.87 | 16.59 | 1.99 | 7.30 | 0.74 |
| Egr2 | 5.15 | 3.45 | 6.08 | 0.73 | 14.46 | 1.77 | 6.47 | 0.90 |
| Egr3 | 6.46 | 6.82 | 11.93 | 1.42 | 33.12 | 2.82 | 27.44 | 1.37 |
| Fos | 7.34 | 2.51 | 7.29 | 1.41 | 44.57 | 3.62 | 6.43 | 0.18 |
| FosB | 3.49 | 2.64 | 16.99 | 3.23 | 22.34 | 4.43 | 17.29 | 4.54 |
| Fosl1 | 2.08 | 2.98 | 5.58 | 0.46 | 4.69 | 0.14 | 9.60 | 0.61 |
| Gclc | 1.45 | 1.99 | 2.91 | 0.41 | 1.40 | 0.10 | 2.26 | 0.27 |
| Gpr3 | 1.36 | 1.83 | 2.89 | 0.72 | 1.91 | 0.03 | 5.79 | 0.90 |
| Has1 | 3.37 | 3.42 | 7.05 | 1.10 | 4.19 | 0.42 | 2.78 | 0.21 |
| Has2 | 3.84 | 4.77 | 8.35 | 1.37 | 2.10 | 0.21 | 4.76 | 0.78 |
| IL11 | 2.15 | 2.25 | 8.29 | 0.42 | 4.37 | 0.61 | 5.50 | 1.35 |
| Klf4 | 2.19 | 2.28 | 3.03 | 0.47 | 1.78 | 0.04 | 1.93 | 0.16 |
| Lif | 7.45 | 5.07 | 11.49 | 2.38 | 9.61 | 0.33 | 7.93 | 0.60 |
| Myc | 1.35 | 1.59 | 2.15 | 0.14 | 2.07 | 0.07 | 2.69 | 0.07 |
| Nr4a1 | 19.17 | 19.23 | 34.94 | 4.81 | 29.73 | 1.93 | 24.18 | 2.20 |
| Nr4a2 | 13.16 | 14.77 | 25.77 | 3.11 | 2.70 | 0.26 | 2.23 | 0.35 |
| Nr4a3 | 10.40 | 11.64 | 73.74 | 16.49 | 19.66 | 2.38 | 28.59 | 3.88 |
| Olr1 | 1.55 | 1.90 | 2.79 | 0.34 | 1.60 | 0.07 | 2.30 | 0.20 |
| Pim1 | 1.75 | 1.21 | 1.38 | 0.19 | 2.00 | 0.12 | 0.98 | 0.07 |
| Plk2 | 1.64 | 1.50 | 1.91 | 0.17 | 2.10 | 0.11 | 2.50 | 0.17 |
| Ptgs2 | 5.23 | 3.38 | 4.70 | 0.43 | 5.86 | 0.68 | 4.15 | 0.54 |
| Rasd1 | 2.41 | 1.24 | 1.47 | 0.15 | 1.55 | 0.24 | 0.86 | 0.13 |
| Rasl11b | 1.62 | 1.32 | 1.46 | 0.18 | 1.99 | 0.19 | 1.78 | 0.07 |
| Rgs2 | 4.93 | 4.09 | 6.87 | 0.88 | 2.95 | 0.23 | 4.62 | 0.36 |
| Sik1 | 3.43 | 4.05 | 7.33 | 0.74 | 2.35 | 0.05 | 2.69 | 0.13 |
Figure 5. Role of ERK1/2 compared with RSK signalling in the RNA expression response to PE.
(A) Cardiomyocytes were exposed to PE (1 h). RNA expression was determined by qPCR or microarrays. Linear regression analysis was performed to compare mRNA expression determined by qPCR (ordinate) compared with microarrays (abscissa) (axes are on a log2 scale). The regression coefficient (r) is 0.98. Results are means (relative to controls) for n=3 different cardiomyocyte preparations. (B) Cardiomyocytes were unstimulated, or exposed to 2 μM PD184352 or 0.5 mM H2O2 for the times indicated. Total extracts were immunoblotted with antibodies against phospho-AMPKα (P-AMPK), total AMPKα (T-AMPK), phospho-ERK1/2 (P-ERKs) or total ERK1/2 (T-ERKs). The experiment was repeated with similar results. (C and D) Cardiomyocytes were exposed to PE in the absence (closed bars) or presence (open bars) of PD184352 for 1 h. RNA expression was determined by qPCR. Data were normalized to GAPDH and then to the controls. Results are means±S.E.M. (n=3). *P<0.001, †P<0.01 and ‡P<0.05 relative to PE alone. (E–H) Cardiomyocytes were exposed to ET-1 or PE (1 h) in the absence (black bars) or presence of PD184352 (white bars) or BI-D1870 (grey bars). Expression of selected mRNAs was measured by qPCR. Results are means±S.E.M. for three (Rgs2 and Olr1) or four (other mRNAs) experiments with different cardiomyocyte preparations. mRNAs are grouped according to relative effects of the inhibitors with each agonist. *P<0.001, †P<0.01 and ‡P<0.05 relative to the respective agonist alone; **P<0.001 and #P<0.05 relative to ET-1.
To determine the specific role of α1A-ARs in regulating cardiomyocyte gene expression, cardiomyocytes were exposed to A61603 (50 nM) and expression of the panel of 34 mRNAs studied for validation of the PE response was examined by qPCR. All were up-regulated by A61603 over 0.5–1 h (Figures 6A–6D and Table 1). The relative increases in expression of four were significantly greater and five were significantly less than PE. However, the increase in expression of the remaining 25 mRNAs was within 1.5-fold that induced by PE. Thus the principal effect of PE on cardiomyocyte gene expression is mediated through α1A-ARs, although other receptors may modulate the response of selected mRNAs. As with PE and ET-1, there was temporal regulation of gene expression by A61603 with some mRNAs showing maximal stimulation at 0.5 h (Figure 6A), some at 0.5–1 h (Figure 6B) and some over 0.5–1.5 h (Figure 6C), whereas other mRNAs were not up-regulated until later times (Figure 6D). To confirm the extent to which the response in neonatal cardiomyocytes is representative of the intact heart, we perfused adult rat hearts ex vivo with A61603 in the absence or presence of PD184352 and examined the mRNA expression of a subset of 25 mRNAs (for reasons unknown, but possibly because of effects on the sinoatrial node or because of effects on the vasculature, 5 or 10 μM BI-D1870 had a significant adverse effect on cardiac contractility in the perfused heart system, so we were unable to study its effects on mRNA expression). As expected with a mixed population of unsynchronized cells, the degree of up-regulation of all mRNAs in perfused hearts was less, but 17 were significantly up-regulated (four showed a small non-significant increase), the response of 13 of which was inhibited by PD184352 (Figure 6E) consistent with the cardiomyocyte response to PE (Figures 5C and 5D). Five were not sensitive to PD184352 (Figure 6F), the greatest anomalies being Lif and Ptgs2 that were sensitive to PD184352 inhibition in cardiomyocytes exposed to PE (Figure 5C). Three showed no change in expression in perfused hearts (Figure 6G), but these are delayed and/or low level responses and the lack of synchronization of the cells may be a factor. Overall the early gene expression response to α1A-AR stimulation in adult intact rat hearts is largely representative of that of neonatal rat cardiomyocytes in primary culture.
Figure 6. Regulation of cardiomyocyte and cardiac mRNA expression by A61603.
(A–D) Cardiomyocytes were exposed to A61603 for the times indicated and mRNA expression was measured by qPCR. Results expressed relative to controls are means±S.E.M. for four independent experiments. Expression profiles are grouped according to temporal regulation. (E–G) Adult rat hearts were perfused retrogradely under control conditions (white bars), with A61603 alone (black bars) or with A61603 in the presence of PD184352 (grey bars). (E) mRNAs significantly up-regulated by A61603 with significant inhibition by PD184352. (F) mRNAs significantly up-regulated by A61603 without significant inhibition by PD184352. (G) mRNAs not significantly up-regulated by A61603. Results are means±S.E.M. for at least four hearts per condition. *P<0.001, **P<0.01 and @P<0.05 relative to control; #P<0.001, †P< 0.01 and ‡P<0.05 relative to A61603 alone.
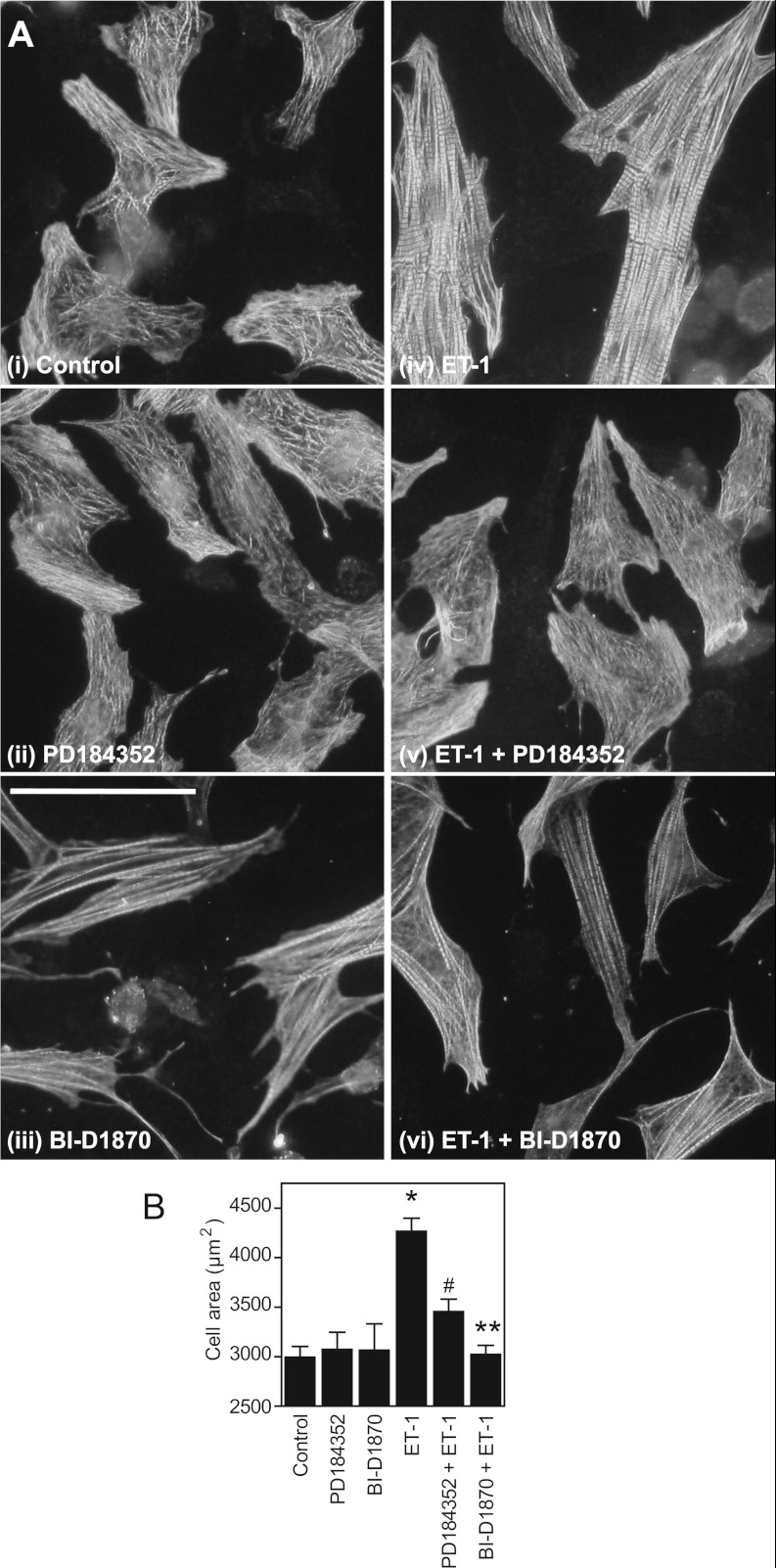
RSKs are required for the increase in surface area induced by ET-1 in cardiomyocytes
In neonatal cardiomyocytes, ET-1 increases myofibrillar organization and content, in addition to increasing cell surface area. We compared the effects of PD184352 with BI-D1870 on cardiomyocyte morphology by immunostaining with antibodies against troponin T, a component of the myofibrillar apparatus (Figure 7). PD184352 alone increased disruption of myofibrillar organization compared with the unstimulated cells, whereas BI-D1870 appeared to enhance myofibrillar organization to some degree. Neither drug alone significantly affected the cell surface area. ET-1 increased cardiomyocyte surface area, a response that was reduced by PD184352 and inhibited by BI-D1870. Notably, PD184352 particularly disrupted the myofibrillar organization induced by ET-1.
Figure 7. RSKs are required for the increase in size induced by ET-1.
(A) Representative images of cardiomyocytes that were unstimulated (i, Control), or exposed (24 h) to PD184352 (ii), BI-D1870 (iii), ET-1 (iv), or ET-1 plus PD184352 (v) or BI-D1870 (vi). Cells were immunostained for troponin T. The experiment was performed four times with similar results. Scale bar=100 μm. (B) Cardiomyocyte surface area was measured for each condition, the average values being taken from each experiment as a single observation. Results are means±S.E.M. (n=4). *P<0.001 relative to the control, **P<0.001 relative to ET-1 and #P<0.01 relative to ET-1.
DISCUSSION
Regulation of cardiomyocyte gene expression by ET-1 compared with α1-ARs
Our previous transcriptomics studies focused on ET-1 and the role of ERK1/2 signalling [13–15,20] because maximally effective concentrations activate the entire pool of ERK1/2 in cardiomyocytes (thus a maximal transcriptomics response should be obtained), and rapid (within 2 min) nuclear localization of phosphorylated ERK1/2 was detectable by immunostaining [12]. In contrast, α1-AR agonists such as PE do not activate the entire pool of ERK1/2 and we could not detect a significant nuclear signal for phospho-ERK1/2 using immunostaining (results not shown). We now attribute this latter issue to the lower signal being below the threshold for detection by immunostaining, since nuclear phospho-ERK1/2 is detected in response to PE or A61603 by immunoblotting (Figure 1). Additional concerns for studies with PE related to possible contradictory signals between different α1-AR subtypes and even whether there may be interaction with β-ARs. However, the β-AR antagonist propranolol did not enhance the activation of ERK1/2 by PE (Figure 1A), indicating that the reduced level of activation did not result from an inhibitory input from β-ARs. Furthermore, activation profiles for ERK1/2 and RSKs by PE and A61603 (highly selective for α1A-ARs) were similar (Figures 1 and 2), and mRNA expression studies conducted with PE and A61603 were largely consistent (Figures 5 and 6, and Table 1). We therefore conclude that the responses to PE are mediated primarily through α1A-ARs.
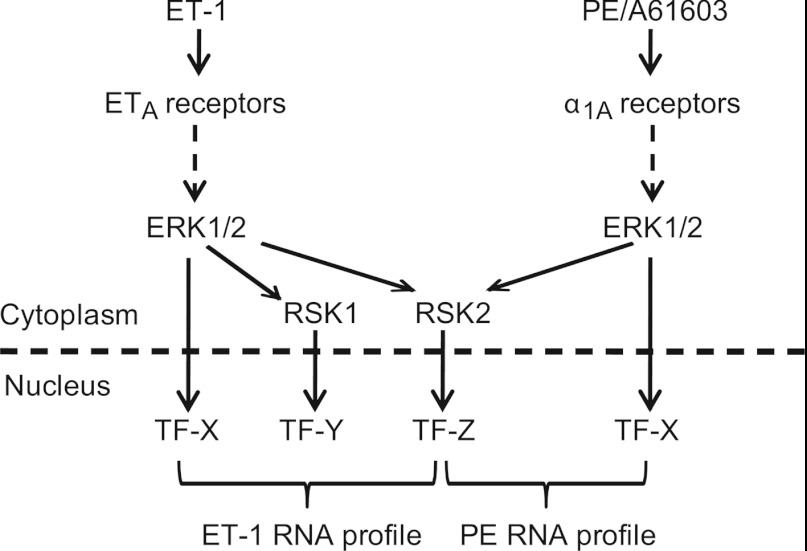
ET-1 and α1A-AR agonists each activate Gq-protein-coupled receptors, signalling through protein kinase C and Ras to the ERK1/2 cascade [17]. It is not clear, therefore, why gene expression profiles induced by the two agonists, although overlapping, are quite distinct (Figure 4 and Supplementary Tables S4–S8). Our data indicate that this does not result from differential ERK1/2 signalling in the cytoplasmic and nuclear fractions (Figures 1C and 1D). Instead, with RSKs playing such a prominent role in the transcriptomic response to ET-1 (Figure 3 and Supplementary Table S3), differential regulation of nuclear RSK1 by ET-1 and α1-AR agonists (Figures 2F–2H) seems the most likely reason for the divergent profiles (Figure 8). Further studies are necessary to dissect the downstream events and identify the specific transcriptional regulators involved in each response. The reason why PE and A61603 fail to stimulate nuclear translocation of RSK1 also remains to be investigated. One tantalizing observation is that ET-1 promotes accumulation of catalytically active monophosphothreonyl-ERK1/2 in addition to the classical dually phosphorylated form [12]. Since the phospho-tyrosine residue is important in substrate recognition, the substrate specificity of monophosphothreonyl-ERK1/2 may differ from the dually phosphorylated form. Alternatively or additionally, constraints with respect to location within the cytoplasm may influence signalling specificity. Finally, it is possible that a threshold effect might operate whereby lower levels of ERK1/2 activation are sufficient to activate RSK2, but an increased level of activity is required for RSK1.
Figure 8. Schematic diagram of the signal from ET-1 or PE/A61603 via ERK1/2 to cardiomyocyte gene expression.
ET-1, signalling through ETA (endothelin receptor type A) receptors, activates ERK1/2. The nuclear signal is propagated via ERK1/2, RSK1 and RSK2 which phosphorylate and regulate different panels of transcription factors (TFs). PE or A61603 signal through α1A-ARs to activate ERK1/2 and the nuclear signal is propagated via ERK1/2 and RSK2, but not RSK1. This divergence contributes to the overlapping, although distinct, transcriptomic profiles induced in cardiomyocytes by these stimuli.
Nuclear trafficking of ERK1/2 and RSKs
In other cells, activation of ERK1/2 is associated with net nuclear accumulation. ERK1/2 do not possess a classic nuclear localization signal, and it is still unclear how ERK1/2 migrate into the nucleus. Passive constitutive flux clearly plays a role [24], potentially modulated by dimerization and/or scaffolding proteins that retain inactive ERK1/2 in the cytoplasm [31]. Phosphorylation of ERK1/2 on additional sites may also be important [32] and, in the heart, autophosphorylation of ERK2(Thr188) may promote nuclear localization [33]. Unexpectedly, our immunoblotting experiments failed to reveal a net translocation of total ERK1/2 to the nuclear compartment in response to ET-1 or α1-AR agonists, despite accumulation of phosphorylated ERK1/2 (Figures 1C–1E). This is unlikely to result from poor quality preparations either of cardiomyocytes or of nuclear proteins since we detect nuclear accumulation of RSKs (Figure 2). Thus our data are consistent with a model of constitutive, rapid and passive flux of ERK1/2 in and out of the nucleus and there could be separate pools of ERK1/2, one of which is directed to the nucleus, whereas another is anchored in the cytoplasm. We propose that the difference between cardiomyocytes and other cells most likely reflects the relative concentrations and precise components of the anchoring and shuttling machinery, all of which remain to be defined. Unlike ERK1/2, we detected very low levels of RSKs in the nuclear compartment of unstimulated cells, with net translocation of total RSKs to the nucleus following stimulation (Figures 2C–2E). This is consistent with a model of cytoplasmic ERK1/2 phosphorylating cytoplasmic RSKs, enhancing their nuclear translocation. Our observations are in agreement with recent studies in other cells which indicate that activation of RSKs reveals a bipartite nuclear localization signal resulting in nuclear translocation [34].
The significant role of nuclear RSKs in regulating gene expression
Although activated RSKs were shown to translocate to the nucleus in proliferating cells over 20 years ago [35], their contribution to the transcriptomic effects of the ERK1/2 cascade has still not yet been fully evaluated. In the heart, the focus has been almost exclusively on their cytoplasmic role. Thus RSKs are implicated in phosphorylation of Na+/H+ exchanger 1 in the plasma membrane and they phosphorylate cardiac myosin-binding protein C, a regulatory component of the myofibrillar apparatus [36,37]. Other cytoplasmic RSK substrates in cardiomyocytes include the pro-apoptotic protein BAD (Bcl-2/Bcl-xL-antagonist, causing cell death) [25] and GSK3α/β (Figure 3A). To our knowledge, ours is the first study to demonstrate that RSKs play a significant role in the nucleus and in regulating cardiomyocyte gene expression. Interestingly, although the largest proportion of mRNAs up-regulated by ET-1 required RSKs for up-regulation, many other up-regulated mRNAs showed an enhanced response in the presence of BI-D1870. An obvious explanation (since BI-D1870 activates ERK1/2, Figure 3B) is that it is a consequence of enhanced ERK1/2 activity. We think this unlikely because all RNAs in this group should then exhibit similar profiles and this is not the case (Figures 3G and 3I). Secondly, BI-D1870 did not enhance activation of ERK1/2 by ET-1 nor increase the duration of the response (Figure 3A and results not shown). Therefore, although enhanced ERK1/2 signalling induced by BI-D1870 could stimulate RNA expression independently of agonist stimulation (consistent with data in Figure 3C, cluster vii), it cannot account for the enhanced stimulation induced by ET-1. More probably, for some mRNAs, RSKs exert a negative effect on expression, potentially via activation of inhibitory transcriptional regulators, stimulation of microRNAs or enhancement of mRNA degradation.
The role of RSKs in regulating gene expression has been explored in a global context previously in cancer cells in relation to cell migration [38]. That study used artificial and constitutive activation of ERK1/2 over 24 h. The benefits of this approach lie in the highly selective nature of the signal; however, although this occurs in cancer cells, such high level constitutive activation of the pathway is less common in other cells and tissues. In cardiomyocytes exposed to ET-1 [14] or α1-AR agonists (Figures 4 and 6), regulation of early gene expression occurs according to temporal profiles with many RNAs exhibiting only transient expression. This occurs in other systems including (as recent examples) skeletal muscle exposed to insulin [39] and macrophages exposed to pro-inflammatory stimuli [40]. Given the difference in time point studied in our experiments (1 h) and in the cancer cell system (24 h), and the nature of the two cell types, it is perhaps not surprising that there is little overlap in the RSK-responsive genes. However, in both systems and irrespective of the degree of ERK1/2 signalling (i.e. potent activation of the ERK1/2 cascade by ET-1 or lesser activation by α1-AR agonists), RSKs clearly play a major role in regulating gene expression.
Despite the major contribution from RSKs to the transcriptomic changes resulting from ERK1/2 activation, they are not the sole means by which ERK1/2 promote gene expression and there is a divergence, therefore, in the signal immediately after ERK1/2 activation. This appears to be functionally relevant given that inhibition of RSKs with BI-D1870 inhibited the increase in cell surface area induced by ET-1, but not the increase in myofibrillar organization, whereas inhibiting the cascade with PD184352 caused myofibrillar disarray (Figure 7).
Influence of changes in immediate early gene expression on the cardiomyocyte hypertrophic response
The principal aim of the present study was to gain insight into the degree to which RSKs contribute to changes in mRNA expression in cardiomyocyte hypertrophy. However, the genes themselves are of prime importance. With an emphasis on early times following stimulation, we identified different types of modulator that are likely to propagate the hypertrophic response. Thus, within the first few hours, classic immediate early genes encoding transcription factors (e.g. Fos and Egr family members, Atf3 and Myc) were up-regulated, in addition to orphan nuclear receptors whose function remains to be established (e.g. Nr4a family members). Some of these will regulate downstream gene expression, whereas others are required to terminate immediate early gene expression (e.g. Atf3 operates in a negative-feedback loop with Egr1 [20]). We also identified changes in expression of protein kinases [e.g. Sik1 (salt-inducible kinase 1) and Plk2] and phosphatases (e.g. Dusps) indicating that innate signalling networks are undergoing modification. Changes in the expression of secreted growth factors [e.g. Areg, Lif and IL11 (interleukin 11)] and cell surface receptors (e.g. Olr1) are indicative of changes in intercellular signalling. There were also changes in the expression of channels and transporters, in addition to metabolic enzymes, even at early times. Notably, many RNAs encoded hypothetical proteins and proteins of no known function, and the contribution of these to the overall hypertrophic response may be immense.
Clearly it will be necessary to verify the degree to which changes in mRNA expression are recapitulated at the protein level. Nevertheless our data paint a picture of great flux and dynamic change for the cardiomyocyte in this initial phase of the response. This is presumably on-going in vivo, suggesting that there are opportunities for modulating the response. Identifying the optimum points for intervention will require a systems biology approach to develop our understanding of the signalling and gene expression networks within cardiomyocytes and other cardiac cells, in addition to the communication networks that operate between them. In the present study we take the first steps by generating the data required for such an approach.
Online data
AUTHOR CONTRIBUTION
The present study was conceived, directed and supervised by Angela Clerk and Peter Sugden. Emre Amirak conducted the RNA experiments with support from Stephen Fuller. Angela Clerk conducted the immunoblotting experiments with support from Emre Amirak and Stephen Fuller. Angela Clerk analysed the microarray data.
FUNDING
This work was funded by the British Heart Foundation (Clinical Research Fellowship FS/08/073).
References
- 1.Turjanski A. G., Vaqué J. P., Gutkind J. S. MAP kinases and the control of nuclear events. Oncogene. 2007;26:3240–3253. doi: 10.1038/sj.onc.1210415. [DOI] [PubMed] [Google Scholar]
- 2.Clerk A., Cullingford T. E., Fuller S. J., Giraldo A., Markou T., Pikkarainen S., Sugden P. H. Signaling pathways mediating cardiac myocyte gene expression in physiological and stress responses. J. Cell. Physiol. 2007;212:311–322. doi: 10.1002/jcp.21094. [DOI] [PubMed] [Google Scholar]
- 3.Cargnello M., Roux P. P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romeo Y., Zhang X., Roux P. P. Regulation and function of the RSK family of protein kinases. Biochem. J. 2012;441:553–569. doi: 10.1042/BJ20110289. [DOI] [PubMed] [Google Scholar]
- 5.Clerk A., Sugden P. H. Signaling through the extracellular signal-regulated kinase 1/2 cascade in cardiac myocytes. Biochem. Cell Biol. 2004;82:603–609. doi: 10.1139/o04-110. [DOI] [PubMed] [Google Scholar]
- 6.Gillespie-Brown J., Fuller S. J., Bogoyevitch M. A., Cowley S., Sugden P. H. The mitogen-activated protein kinase kinase MEK1 stimulates a pattern of gene expression typical of the hypertrophic phenotype in rat ventricular cardiomyocytes. J. Biol. Chem. 1995;270:28092–28096. doi: 10.1074/jbc.270.47.28092. [DOI] [PubMed] [Google Scholar]
- 7.Clerk A., Aggeli I.-K. S., Stathopoulou K., Sugden P. H. Peptide growth factors signal differentially through protein kinase C to extracellular signal-regulated kinases in neonatal cardiomyocytes. Cell. Signalling. 2006;18:225–235. doi: 10.1016/j.cellsig.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Yue T. L., Gu J.-L., Wang C., Reith A. D., Lee J. C., Mirabile R. C., Kreutz R., Wang Y., Maleeff B., Parsons A. A., Ohlstein E. H. Extracellular signal-regulated kinase plays an essential role in hypertrophic agonists, endothelin-1 and phenylephrine-induced cardiomyocyte hypertrophy. J. Biol. Chem. 2000;275:37895–37901. doi: 10.1074/jbc.M007037200. [DOI] [PubMed] [Google Scholar]
- 9.Bueno O. F., De Windt L. J., Tymitz K. M., Witt S. A., Kimball T. R., Klevitsky R., Hewett T. E., Jones S. P., Lefer D. J., Peng C.-F., et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lips D. J., Bueno O. F., Wilkins B. J., Purcell N. H., Kaiser R. A., Lorenz J. N., Voisin L., Saba-El-Leil M. K., Meloche S., Pouysségur J., et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–1941. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 11.Kehat I., Davis J., Tiburcy M., Accornero F., Saba-El-Leil M. K., Maillet M., York A. J., Lorenz J. N., Zimmermann W. H., Meloche S., Molkentin J. D. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ. Res. 2011;108:176–183. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugden P. H., Markou T., Fuller S. J., Tham E. L., Molkentin J. D., Paterson H. F., Clerk A. Monophosphothreonyl extracellular signal-regulated kinases 1 and 2 (ERK1/2) are formed endogenously in intact cardiac myocytes and are enzymically active. Cell. Signalling. 2011;23:468–477. doi: 10.1016/j.cellsig.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy R. A., Kemp T. J., Sugden P. H., Clerk A. Using U0126 to dissect the role of the extracellular signal-regulated kinase 1/2 (ERK1/2) cascade in the regulation of gene expression by endothelin-1 in cardiac myocytes. J. Mol. Cell. Cardiol. 2006;41:236–247. doi: 10.1016/j.yjmcc.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Cullingford T. E., Markou T., Fuller S. J., Giraldo A., Pikkarainen S., Zoumpoulidou G., Alsafi A., Ekere C., Kemp T. J., Dennis J. L., et al. Temporal regulation of expression of immediate early and second phase transcripts by endothelin-1 in cardiomyocytes. Genome Biol. 2008;9:R32. doi: 10.1186/gb-2008-9-2-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall A. K., Barrett O. P., Cullingford T. E., Shanmugasundram A., Sugden P. H., Clerk A. ERK1/2 signaling dominates over RhoA signaling in regulating early changes in RNA expression induced by endothelin-1 in neonatal rat cardiomyocytes. PLoS ONE. 2010;5:e10027. doi: 10.1371/journal.pone.0010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clerk A., Bogoyevitch M. A., Andersson M. B., Sugden P. H. Differential activation of protein kinase C isoforms by endothelin-1 and phenylephrine and subsequent stimulation of p42 and p44 mitogen-activated protein kinases in ventricular myocytes cultured from neonatal rat hearts. J. Biol. Chem. 1994;269:32848–32857. [PubMed] [Google Scholar]
- 17.Sugden P. H., Clerk A. Cellular mechanisms of cardiac hypertrophy. J. Mol. Med. 1998;76:725–746. doi: 10.1007/s001090050275. [DOI] [PubMed] [Google Scholar]
- 18.Clerk A., Fuller S. J., Michael A., Sugden P. H. Stimulation of “stress-regulated” mitogen-activated protein kinases (stress-activated protein kinases/c-Jun N-terminal kinases and p38-mitogen-activated protein kinases) in perfused rat hearts by oxidative and other stresses. J. Biol. Chem. 1998;273:7228–7234. doi: 10.1074/jbc.273.13.7228. [DOI] [PubMed] [Google Scholar]
- 19.Clerk A., Sugden P. H. Cell stress-induced phosphorylation of ATF2 and c-Jun transcription factors in rat ventricular myocytes. Biochem. J. 1997;325:801–810. doi: 10.1042/bj3250801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giraldo A., Barrett O. P., Tindall M. J., Fuller S. J., Amirak E. A., Bhattacharya B. S., Sugden P. H., Clerk A. Feedback regulation by Atf3 in the endothelin-1responsive transcriptome of cardiomyocytes: Egr1 is a principal Atf3 target. Biochem. J. 2012;444:343–355. doi: 10.1042/BJ20120125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen B. C., O’Connell T. D., Simpson P. C. Alpha-1-adrenergic receptors: targets for agonist drugs to treat heart failure. J. Mol. Cell. Cardiol. 2011;51:518–528. doi: 10.1016/j.yjmcc.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clerk A., Pham F. H., Fuller S. J., Sahai E., Aktories K., Marais R., Marshall C. J., Sugden P. H. Regulation of mitogen-activated protein kinases in cardiac myocytes through the small G protein, Rac1. Mol. Cell. Biol. 2001;21:1173–1184. doi: 10.1128/MCB.21.4.1173-1184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knepper S. M., Buckner S. A., Brune M. E., DeBernardis J. F., Meyer M. D., Hancock A. A. A-61603, a potent α1-adrenergic receptor agonist, selective for the alpha 1A receptor subtype. J. Pharmacol. Exp. Ther. 1995;274:97–103. [PubMed] [Google Scholar]
- 24.Adachi M., Fukuda M., Nishida E. Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J. 1999;18:5347–5358. doi: 10.1093/emboj/18.19.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valks D. M., Cook S. A., Pham F. H., Morrison P. R., Clerk A., Sugden P. H. Phenylephrine promotes phosphorylation of Bad in cardiac myocytes through the extracellular signal-regulated kinases 1/2 and protein kinase A. J. Mol. Cell. Cardiol. 2002;34:749–763. doi: 10.1006/jmcc.2002.2014. [DOI] [PubMed] [Google Scholar]
- 26.Davies S. P., Reddy H., Caivano M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sapkota G. P., Cummings L., Newell F. S., Armstrong C., Bain J., Frodin M., Grauert M., Hoffmann M., Schnapp G., Steegmaier M., et al. BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. Biochem. J. 2007;401:29–38. doi: 10.1042/BJ20061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S., Mackintosh C. Differential regulation of NHE1 phosphorylation and glucose uptake by inhibitors of the ERK pathway and p90RSK in 3T3-L1 adipocytes. Cell. Signalling. 2009;21:1984–1993. doi: 10.1016/j.cellsig.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Dokladda K., Green K. A., Pan D. A., Hardie D. G. PD98059 and U0126 activate AMP-activated protein kinase by increasing the cellular AMP:ATP ratio and not via inhibition of the MAP kinase pathway. FEBS Lett. 2005;579:236–240. doi: 10.1016/j.febslet.2004.11.084. [DOI] [PubMed] [Google Scholar]
- 30.Choi K., Mollapour E., Choi J. H., Shears S. B. Cellular energetic status supervises the synthesis of bis-diphosphoinositol tetrakisphosphate independently of AMP-activated protein kinase. Mol. Pharmacol. 2008;74:527–536. doi: 10.1124/mol.107.044628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casar B., Pinto A., Crespo P. ERK dimers and scaffold proteins: unexpected partners for a forgotten (cytoplasmic) task. Cell Cycle. 2009;8:1007–1013. doi: 10.4161/cc.8.7.8078. [DOI] [PubMed] [Google Scholar]
- 32.Chuderland D., Konson A., Seger R. Identification of a general nuclear translocation signal in signaling proteins. Mol. Cell. 2008;31:850–861. doi: 10.1016/j.molcel.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Lorenz K., Schmitt J. P., Schmitteckert E. M., Lohse M. J. A new type of ERK1/2 autophosphorylation cause cardiac hypertrophy. Nat. Med. 2008;15:75–83. doi: 10.1038/nm.1893. [DOI] [PubMed] [Google Scholar]
- 34.Gao X., Chaturvedi D., Patel T. B. Localization and retention of p90 ribosomal S6 kinase 1 in the nucleus: implications for its function. Mol. Biol. Cell. 2012;23:503–515. doi: 10.1091/mbc.E11-07-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen R. H., Sarnecki C., Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol. Cell. Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moor A. N., Fliegel L. Protein kinase-mediated regulation of the Na+/H+ exchanger in the rat myocardium by mitogen-activated protein kinase-dependent pathways. J. Biol. Chem. 1999;274:22985–22992. doi: 10.1074/jbc.274.33.22985. [DOI] [PubMed] [Google Scholar]
- 37.Cuello F., Bardswell S. C., Haworth R. S., Ehler E., Sadayappan S., Kentish J. C., Avkiran M. Novel role for p90 ribosomal S6 kinase in the regulation of cardiac myofilament phosphorylation. J. Biol. Chem. 2011;286:5300–5310. doi: 10.1074/jbc.M110.202713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doehn U., Hauge C., Frank S. R., Jensen C. J., Duda K., Nielsen J. V., Cohen M. S., Johansen J. V., Winther B. R., Lund L. R., et al. RSK is a principal effector of the RAS-ERK pathway for eliciting a coordinate promotile/invasive gene program and phenotype in epithelial cells. Mol. Cell. 2009;35:511–522. doi: 10.1016/j.molcel.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Camillo, B., Irving B. A., Schimke J., Sanavia T., Toffolo G., Cobelli C., Nair K. S. Function-based discovery of significant transcriptional temporal patterns in insulin stimulated muscle cells. PLoS ONE. 2012;7:e32391. doi: 10.1371/journal.pone.0032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatt D. M., Pandya-Jones A., Tong A. J., Barozzi I., Lissner M. M., Natoli G., Black D. L., Smale S. T. Transcript dynamics of proinflammatory genes revealed by sequence analysis of subcellular RNA fractions. Cell. 2012;150:279–290. doi: 10.1016/j.cell.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.