Abstract
This report describes a case of liver failure secondary to pancreatoduodenectomy and rapid recovery following treatment. A 68-year-old woman with cancer on the ampulla of Vater underwent surgery for pancreatoduodenectomy. The patient developed liver failure 3 months postsurgically. She was hospitalized after presenting with jaundice, hypoalbuminemia and decreased serum zinc. Computed tomography (CT) of the abdomen showed a reduction in CT attenuation values postoperatively. We suspected fatty liver due to impaired absorption caused by pancreatoduodenectomy. We initiated treatment with branched-chain amino acids and a zinc formulation orally. Trace elements were administered intravenously. Two months after treatment, there was a noticeable improvement in CT findings. The patient's jaundice and hypoalbuminemia prompted a liver biopsy, which led to a diagnosis of non-alcoholic steatohepatitis.
Key words: Pancreatoduodenectomy, Liver failure, Zinc, Fatty liver, Non-alcoholic steatohepatitis
Introduction
Fatty liver often develops in patients with obesity, diabetes and dyslipidemia. Simple fatty liver sometimes progresses to non-alcoholic steatohepatitis (NASH). A two-hit mechanism has been proposed to explain the progression of NASH to fatty liver [1]. NASH often progresses to cirrhosis and liver cancer associated with underlying inflammation and fibrosis [2]. Fatty liver can also be caused by kwashiorkor [3]. Malnutrition caused by fatty liver is considered to be a consequence of kwashiorkor, which has been reported to occur as a result of chronic hunger [3]. It has been reported that kwashiorkor can occur secondarily to gastrectomy [4], ulcerative colitis [5] and type 2 diabetes [6].
A case of fatty liver after pancreatoduodenectomy has been described previously. Jaundice due to steatohepatitis following pancreatoduodenectomy is a rare occurrence [7, 8, 9, 10, 11]. Our patient progressed rapidly to liver failure and jaundice subsequent to NASH. Fatal liver failure sometimes develops in patients with NASH. Sakada et al. [12] reported a case of NASH associated with anorexia nervosa which resulted in fatal hepatic failure. Acute liver failure occurred due to malnutrition related to NASH. The patient died as a result of liver failure despite receiving total parenteral nutrition and artificial hepatic support.
The patient in our case report was promptly treated with pancreatic enzymes, branched-chain amino acids (BCAA) and trace elements, and as a result the patient's blood test results and computed tomography (CT) attenuation values improved.
Case Report
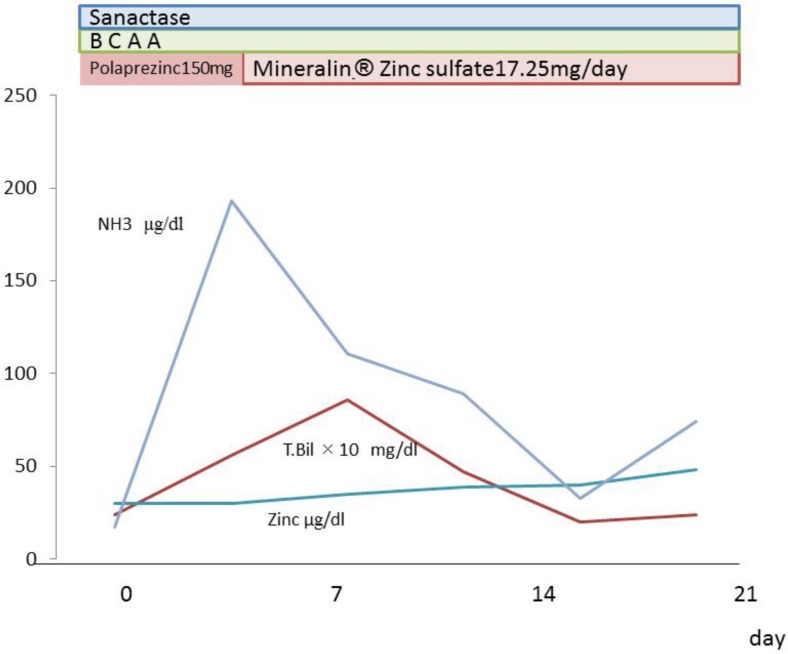
The patient was a 68-year-old woman who underwent pancreatoduodenectomy for cancer on the ampulla of Vater. After being discharged from the hospital 1 month after the operation, the patient developed ascites and was rehospitalized. Jaundice and edema subsequently developed and the patient's performance dropped from grade 1 to grade 3. A diagnosis of acute liver failure was made. The patient was 145 cm in height, weighed 78 kg and had no history of alcohol or tobacco use or known allergies. Physical examination revealed a blood pressure of 96/51 mm Hg, a pulse rate of 101 bpm and a body temperature of 36.2°C. Blood laboratory findings showed mild elevation of aspartate aminotransferase (85 IU/l) and alanine aminotransferase. Platelet count was normal (17.8 × 104/mm3), albumin levels were low (1.3 mg/dl), prothrombin time was decreased by 41%, ammonia was increased to 193 µg/dl, and total bilirubin rose to 5.6 mg/dl. The liver disease was classified as Child-Pugh class C (table 1; fig. 1).
Table 1.
Blood test findings on admission
| White blood cells | 8,510/μl |
| Hemoglobin | 9.0 g/dl |
| Platelets | 17.8×104/μl |
| Prothrombin time | 41% |
| Albumin | 1.3 mg/dl |
| Total bilirubin | 5.6 mg/dl |
| Aspartate aminotransferase | 85 IU/l |
| Alanine aminotransferase | 42 IU/l |
| Alkaline phosphatase | 609 IU/l |
| Lactate dehydrogenase | 231 IU/l |
| Blood urea nitrogen | 10.3 mg/dl |
| Creatinine | 0.65 mg/dl |
| Zinc | 30 μg/dl |
| Ammonia | 190 μg/dl |
| C-reactive protein | 2.0 mg/dl |
| Hepatitis B surface antigen | (–) |
| Hepatitis C virus antibody | (–) |
| Antinuclear antibody | (–) |
| Antimitochondrial membrane (M2) | (–) |
Fig. 1.
Laboratory data after admission. We administrated Sanactase M, BCAA and zinc sulfate. Serum zinc levels subsequently increased and jaundice and ammonia levels improved.
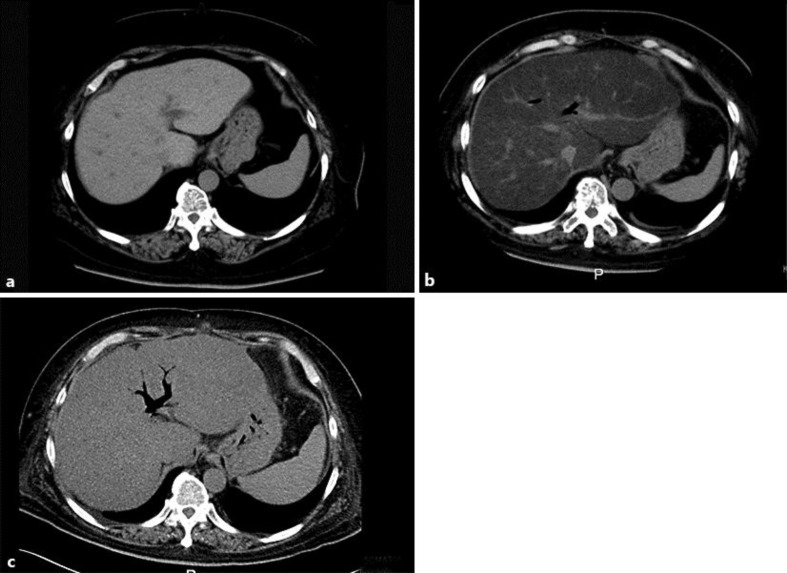
The average of the preoperative CT values for the right hepatic lobe, left hepatic lobe, hepatic portal region and abdominal region was 62.1 Hounsfield units (HU) (fig. 2a). Three months after the operation, the mean CT score was 1.0 HU. Absorption, as indicated by the mean CT score, was substantially lower postoperatively (fig. 2b). The change in CT attenuation values indicated that the patient's fat accumulation increased. Test results for hepatitis B surface antigen, hepatitis C virus antibodies, antimitochondrial antibody and antinuclear antibody were all negative. Viral hepatitis, autoimmune hepatitis and primary biliary cirrhosis were all ruled out.
Fig. 2.
a Abdominal CT before surgery; the average for the right hepatic lobe, left hepatic lobe, hepatic portal region and abdominal region was 62.1 HU. b Abdominal CT 3 months after surgery; the average CT attenuation value was 1.0 HU, which was lower than before surgery. c Abdominal CT 5 months after surgery; the average CT attenuation value was 32 HU, which was higher than 3 months after surgery.
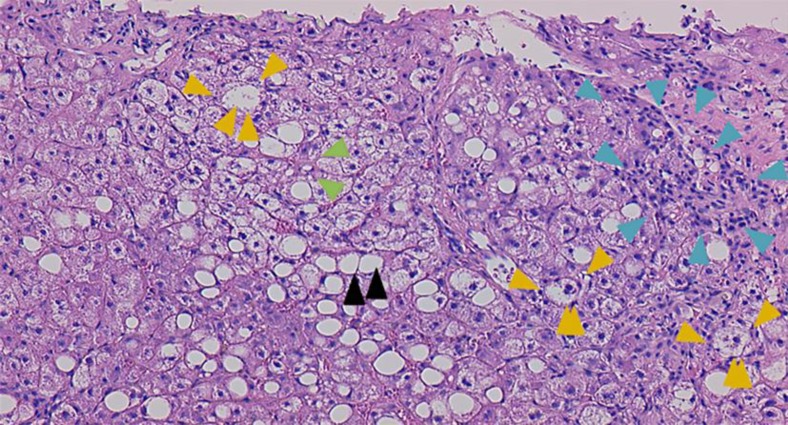
Based on the findings above, we suspected secondary kwashiorkor [2] due to impaired absorption caused by the pancreatoduodenectomy. A diagnosis of liver failure was made and measures were taken to control abdominal dropsy. BCAA and Sanactase M were administered orally. The patient's serum zinc levels decreased to 29 μg/dl. A zinc formulation (Polaprezinc, 150 mg) was administered orally. We then administered zinc sulfate (17.25 mg) by intravenous injection (Mineralin®, 97 μg/dl zinc). The serum zinc level then rose to 48 μg/dl, serum albumin increased to 2.7 g/dl, prothrombin time increased by 62%, NH3 decreased to 33 µg/dl, total bilirubin rose to 1.0 mg/dl, and a Child-Pugh A score was achieved. The patient was in good general condition and was discharged (fig. 1). The mean CT value improved to 32 HU (fig. 2c). Figure 3 shows a microscopic image of a liver section obtained following biopsy on day 35 of hospitalization. Lipid droplets and inflammatory cell infiltration of large lipid droplets were evident, and the portal areas appeared to be expanded due to infiltration of lymphocytes and fibrous tissue. Hepatocellular ballooning was evident. The liver showed extensive sinusoidal fibrosis. Based on these findings, a diagnosis of NASH was made.
Fig. 3.
Pathological examination of liver biopsy (HE stain, ×100). The liver parenchyma shows a necrotic lesion accompanied by fibrosis and large droplets of fat (black arrowheads). The portal area was expanded due to infiltration of lymphocytes and fibrous tissue (blue arrowheads). No changes were seen in the bile ducts. Hepatocellular ballooning was evident (orange arrowheads). There was also mild to moderate fibrosis of the hepatic sinusoids (green arrowheads).
Discussion
The case reported herein provides valuable insight into the pathophysiology of postoperative NASH. NASH can occur as a result of both malnutrition and overnutrition. Prior to surgery, the patient's BMI was 30.1 and no evidence of liver dysfunction or fatty liver was present based on blood test and abdominal CT scan results. Postoperatively, the patient developed low albumin levels and edema. She also developed dysfunctional absorption subsequent to invasive surgery, and hypoalimentation was suspected.
The liver failure experienced by our patient, who had low caloric intake and developed jaundice, hypoalbuminemia and hyperammonemia, could have been due to kwashiorkor [3]. It has been reported that kwashiorkor can occur secondarily to gastrectomy [4]. The presence of secondary kwashiorkor can be indicative of blind loop formation and exocrine pancreatic failure, and is associated with inadequate protein ingestion [4]. It has recently been reported that steatohepatitis occurs in 20–40% of patients who undergo pancreatoduodenectomy [7, 8, 9, 10, 11]. Pancreatoduodenectomy can result in deficiencies of vitamins and trace elements such as zinc, which can lead to fatty liver disease [7, 8, 9, 10, 11]. Abdominal CT scan results were also improved.
Exocrine function is lost as a result of pancreatectomy, and in the presence of intestinal malabsorption, the patient rapidly progressed to severe liver failure. The liver biopsy findings in our case included significant fat infiltration of the liver parenchyma and indicated that the liver failure was caused by rapid NASH. This finding, in combination with the time at which progression occurred, makes our patient a very rare case.
According to previous reports, non-alcoholic fatty liver disease and NASH following pancreatoduodenectomy can be improved by the administration of pancreatic enzymes [10]. After switching the patient's treatment from oral to intravenous zinc, serum zinc levels rose rapidly and ammonia levels declined. The latter result is consistent with a study by Marchesini et al. [13], which showed that administration of zinc sulfate can reduce blood ammonia levels in liver cirrhosis patients, and with the known role of zinc in ammonia metabolism [14]. In urgent cases such as the one described in our report, pancreatic enzymes, BCAA and trace elements should be administered promptly.
Author Contributions
Kazushige Nirei contributed equally to this work; Norikazu Ogihara, Wataru Kawamura and Woodea Kang as for the discussion. Kazushige Nirei and Mitsuhiko Moriyama wrote this paper.
References
- 1.Day CP, James OF. Steatohepatitis: a tale of two ‘hits’? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 2.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams CD. Kwashiorkor. A nutritional disease of children associated with a maize diet. Lancet. 1935;16:1151. [PMC free article] [PubMed] [Google Scholar]
- 4.Krikler DM, Schrire V. Kwashiorkor in an adult due to an intestinal blind loop. Lancet. 1958;i:510–511. doi: 10.1016/s0140-6736(58)90815-8. [DOI] [PubMed] [Google Scholar]
- 5.Mellinkoff SM. Temporary redness of the hair in ulcerative colitis. Am J Dig Dis. 1957;12:738–742. doi: 10.1007/BF02239418. [DOI] [PubMed] [Google Scholar]
- 6.Schaffner F. Nonalcoholic fatty liver. In: Berk JE, editor. Bockus Gastroenterology. ed 4. Philadelphia: WB Saunders; 1985. pp. 3049–3061. [Google Scholar]
- 7.Kato H, Isaji S, Azumi Y, Kishiwada M, Hamada T, Mizuno S, Usui M, Sakurai H, Tabata M. Development of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) after pancreatoduodenectomy: proposal of a postoperative NAFLD scoring system. J Hepatobiliary Pancreat Sci. 2010;17:296–304. doi: 10.1007/s00534-009-0187-2. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka N, Horiuchi A, Yokoyama T, et al. Pancreatic exocrine insufficiency: a rare cause of nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:245–246. doi: 10.1111/j.1572-0241.2007.01562_7.x. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka N, Horiuchi A, Yokoyama T, Kawa S, Kiyosawa K. Clinical characteristics of de novo nonalcoholic fatty liver disease following pancreatoduodenectomy. J Gastroenterol. 2011;46:758–768. doi: 10.1007/s00535-011-0370-5. [DOI] [PubMed] [Google Scholar]
- 10.Murata Y, Mizuno S, Kato H, Kishiwada M, Ohsawa I, Hamada T, Usui M, Sakurai H, Tabata M, Nishimura K, Fukutome K, Isaji S. Nonalcoholic steatohepatitis (NASH) after pancreatoduodenectomy: association of pancreatic exocrine deficiency and infection. Clin J Gastroenterol. 2011;4:242–248. doi: 10.1007/s12328-011-0226-9. [DOI] [PubMed] [Google Scholar]
- 11.Kato K, Isaji S, Kawarada Y, Hibasami H, Nakashima K. Effect of zinc administration on pancreatic regeneration after 80% pancreatectomy. Pancreas. 1997;14:158–165. doi: 10.1097/00006676-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Sakada M, Tanaka A, Ohta D, Takayanagi M, Kodama T, Suzuki K, Inoue K, Fujita Y, Maruyama M. Severe steatosis resulted from anorexia nervosa leading to fatal hepatic failure. J Gastroenterol. 2006;41:714–715. doi: 10.1007/s00535-006-1845-7. [DOI] [PubMed] [Google Scholar]
- 13.Marchesini G, Fabbri A, Bianchi G, Brizi M, Zoli M. Zinc supplementation and amino acid-nitrogen metabolism in patients with advanced cirrhosis. Hepatology. 1996;23:1084–1092. doi: 10.1053/jhep.1996.v23.pm0008621138. [DOI] [PubMed] [Google Scholar]
- 14.Jain A, Varma M, Agrawal BK, Jadhav AA. Serum zinc and malondialdehyde concentrations and their relation to total antioxidant capacity in protein energy malnutrition. J Nutr Sci Vitaminol. 2008;54:392–395. doi: 10.3177/jnsv.54.392. [DOI] [PubMed] [Google Scholar]