Abstract
The metalloproteases meprin α and meprin β exhibit structural and functional features that are unique among all extracellular proteases. Although meprins were discovered more than 30 years ago, their precise substrates and physiological roles have been elusive. Both enzymes were originally found to be highly expressed in kidney and intestine, which focused research on these particular tissues and associated pathologies. Only recently it has become evident that meprins exhibit a much broader expression pattern, implicating functions in angiogenesis, cancer, inflammation, fibrosis and neurodegenerative diseases. Different animal models, as well as proteomics approaches for the identification of protease substrates, have helped to reveal more precise molecular signalling events mediated by meprin activity, such as activation and release of pro-inflammatory cytokines. APP (amyloid precursor protein) is cleaved by meprin β in vivo, reminiscent of the β-secretase BACE1 (β-site APP-cleaving enzyme 1). The subsequent release of Aβ (amyloid β) peptides is thought to be the major cause of the neurodegenerative Alzheimer's disease. On the other hand, ADAM10 (a disintegrin and metalloprotease domain 10), which is the constitutive α-secretase, was shown to be activated by meprin β, which is itself shed from the cell surface by ADAM10. In skin, both meprins are overexpressed in fibrotic tumours, characterized by massive accumulation of fibrillar collagens. Indeed, procollagen III is processed to its mature form by meprin α and meprin β, an essential step in collagen fibril assembly. The recently solved crystal structure of meprin β and the unique cleavage specificity of these proteases identified by proteomics will help to generate specific inhibitors that could be used as therapeutics to target meprins under certain pathological conditions.
Keywords: cancer, fibrosis, inflammation, meprin, metalloprotease, neurodegeneration, proteomics
Abbreviations: Aβ, amyloid β; AD, Alzheimer’s disease; ADAM, a disintegrin and metalloprotease domain; AIEC, adherent-invasive Escherichia coli; AKI, acute kidney injury; APP, amyloid precursor protein; BACE1, β-site APP-cleaving enzyme 1; BMP-1, bone morphogenetic protein-1; CD, Crohn’s disease; CF, cystic fibrosis; DSS, dextran sodium sulfate; ECM, extracellular matrix; EGF, epidermal growth factor; EGFR, EGF receptor; ERK1/2, extracellular-signal-regulated kinase 1/2; IBD, inflammatory bowel disease; IL, interleukin; IR, ischaemia/reperfusion; KLK, kallikrein-related peptidase; MAM, meprin A5 protein tyrosine phosphatase μ; MBP, mannan-binding protein; MMP, matrix metalloproteinase; SNP, single nucleotide polymorphism; TAILS, terminal amine isotopic labelling of substrates; TGFα, transforming growth factor α; TRAF, tumour-necrosis-factor-receptor-associated factor; UC, ulcerative colitis; VEGF-A, vascular endothelial growth factor A
MEPRIN METALLOPROTEASES: OLD ENZYMES NEWLY DISCOVERED
Proteolysis is an irreversible post-translational modification that affects every single cell of an organism. Over the last decades proteolytic enzymes have been identified to be master switches in the regulation of the immune system, in neuronal development and neurodegeneration, and in apoptosis and cancer progression. Substrates define protease roles. Therefore the identification of single enzymes, their substrates and inhibitors is crucial to understand complex molecular events responsible for pathophysiological conditions.
It was in the early 1980s when Erwin Sterchi and Judith Bond found unexpected proteolytic activity in the intestine of patients after pancreas surgery and in mouse kidney respectively, which led to the identification of a novel metalloprotease, later called meprin (metalloprotease from renal tissue) [1–4]. Shortly thereafter, two genes from different chromosomes were identified encoding for the proteases meprin α and meprin β [5–10]. Meprin metalloproteases belong to the astacin family of zinc endopeptidases and the metzincin superfamily [11]. Hence they are phylogenetically related to MMPs (matrix metalloproteinases) and ADAMs (a disintegrin and metalloprotease domain), proteases that are mostly associated with cancer and metastasis, as well as inflammation [12,13]. MMPs and ADAMs have been intensively studied and many physiological substrates, inhibitors and regulatory molecules have been identified that demonstrate the biological importance of these enzymes [14–16]. In contrast, for a long time the physiological functions of meprin α and meprin β were (and in many cases still are) ambiguous. Owing to their strong expression in rodent kidney and intestine [1,17–20], research has focused on the organ-specific activity of these proteases. A breakthrough in understanding the biological activity of meprins was the generation of meprin α- and meprin β-knockout mice [21–23]. These animals showed clear immunological phenotypes and later on meprins were identified as susceptibility genes in IBD (inflammatory bowel disease) [24,25]. The identification of meprins in human skin revealed strong evidence for different functions of these proteases in epidermal homoeostasis [26,27]. Meprin α expressed in basal epidermis promotes cell proliferation, whereas meprin β induces cell death in terminally differentiated keratinocytes. The knockdown of meprin expression in zebrafish embryos revealed an important contribution of meprin α in angiogenesis, as demonstrated by reduced blood vessel formation in the morpholino-injected animals [28]. The knockdown of meprin β led to a general failure in organogenesis, resulting in the death of the embryos between days 1 and 3 post-fertilization. The zebrafish revealed a broad expression pattern for meprins, and opened up new perspectives for research on the human orthologues [29]. In particular, proteomics approaches have helped to identify specific substrates of human meprin α and meprin β and have revealed important contributions of the enzymes in collagen assembly and fibrosis, and in neurodegenerative processes such as AD (Alzheimer's disease).
Structure leads function
Meprins are complex and highly glycosylated multi-domain enzymes that require post-translational modifications to reach full activity. They are expressed as proteolytically inactive zymogens that require the removal of their N-terminal propeptides by other proteases. Several serine proteases have been identified as doing this job [30–34], e.g. members of the tissue KLKs (kallikrein-related peptidases).
Meprins belong to the astacin family of metalloproteases, comprising only six members in humans [35]. These enzymes are characterized by a conserved zinc-binding motif (HExxHxxGxxHxxxRxDR) and by a sequence in close proximity to the active-site cleft, the so called Met-turn, that includes a tyrosine residue as a fifth zinc ligand. Within the astacin family, meprins exhibit a unique domain composition.
Human meprin α and β, encoded on chromosomes 6 and 8 respectively, comprise an N-terminal signal peptide directing the polypeptide chain to the endoplasmic reticulum, an N-terminal propeptide, an astacin-like protease domain, a MAM (meprin A5 protein tyrosine phosphatase μ) domain and a TRAF (tumour-necrosis-factor-receptor-associated factor) domain, both of which are known to mediate protein–protein interactions, an EGF (epidermal growth factor)-like domain, a transmembrane domain, and a C-terminal cytosolic tail (Figure 1A).
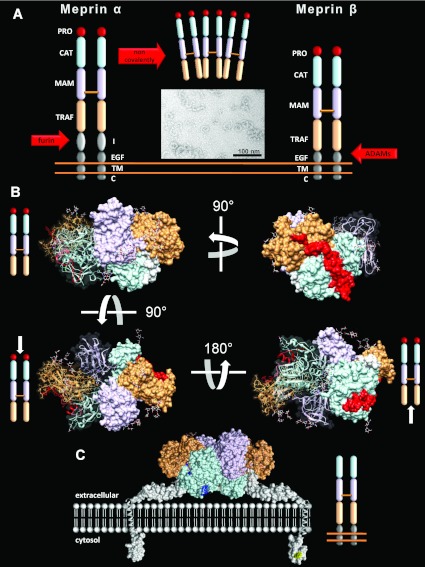
Figure 1. Oligomeric structure of human meprins.
(A) Meprin α and meprin β are multi-domain enzymes, building dimers linked by one intermolecular disulfide bond between the MAM domains. Meprins are expressed as zymogens with a propeptide (PRO) N-terminal to the protease domain (CAT) that must be cleaved off proteolytically to gain full activity. Only meprin α contains an inserted domain (I) which is cleaved by furin during the secretory pathway, resulting in secretion of the protein and further oligomerization. Meprin α is the largest secreted protease known, up to 6 mDa in size, as visualized by electron microscopy of purified recombinant enzyme. Meprin β predominantly remains membrane-bound, but can be shed from the cell surface by ectodomain shedding through ADAM10 and ADAM17 activity. C, cytosolic; TM, transmembrane. (B) Crystal structure of the ectodomain of dimeric human meprin β in different orientations (PDB codes 4GWN and 4GWM). One monomer is displayed with a transparent surface, revealing the ribbon structure. The colour code of the single domains correspond to (A). (C) Model of the membrane-bound form of meprin β and its orientation at the cell surface. EGF-like, transmembrane and cytosolic domains are displayed in grey, and phosphorylation sites are in yellow. Positively charged residues within the active-site cleft of the catalytic domain are shown in dark blue (see also Figure 2F and Supplementary Movie S1 at http://www.biochemj.org/bj/450/bj4500253add.htm). For structural details see [41].
A major difference between both enzymes is the so called ‘inserted’ domain in meprin α, which includes a furin cleavage site. This results in the loss of the EGF-like transmembrane and cytosolic domains, and to subsequent release of the enzyme into the extracellular environment. Meprin α further oligomerizes non-covalently, building huge complexes in the mega-Dalton range [32,36]. This makes it the largest secreted protease known to date. Meprin β in contrast is predominantly tethered to the cell membrane as a dimeric type I integral protein, but can be shed from the surface by ADAM10 and ADAM17 [37,38].
For the rodent enzymes, it has been observed that, when co-expressed, meprin α and meprin β can also be organized as hetero-oligomers [36]. This led to the nomenclature meprin A, comprising meprin α homo-oligomers, as well as meprin α/β hetero-oligomers, and meprin B, a synonym for meprin β dimers [39,40]. However, such hetero-oligomers have not been identified in humans and, with respect to the recently solved crystal structure [41] (see Supplementary Movie S1 at http://www.biochemj.org/bj/450/bj4500253add.htm), it seems unlikely that human meprin α and meprin β could assemble this way (Figures 1B and 1C). Hence, at least for the human enzymes, we strongly encourage the sole use of the terms meprin α and meprin β.
It became evident early on that both meprins were capable of forming homodimers linked by disulfide bridges. This was simply deduced from non-reducing SDS/PAGE. For a long time, however, it was not clear which and how many cysteine residues were involved in forming intermolecular S–S bonds, depending on whether it was meprin α or meprin β or which species was under investigation [42–45]. Finally, by solving the crystal structure of the ectodomain of dimeric human meprin β it turned out that only one disulfide bridge between the MAM domains is sufficient to do the job [41]. Interstingly, this particular S–S bond occurs in a disordered region within the crystal structure of meprin β, indicating more a stabilizing role than an indispensible structural feature required for proper folding.
Cleavage specificity of meprins and substrate identification
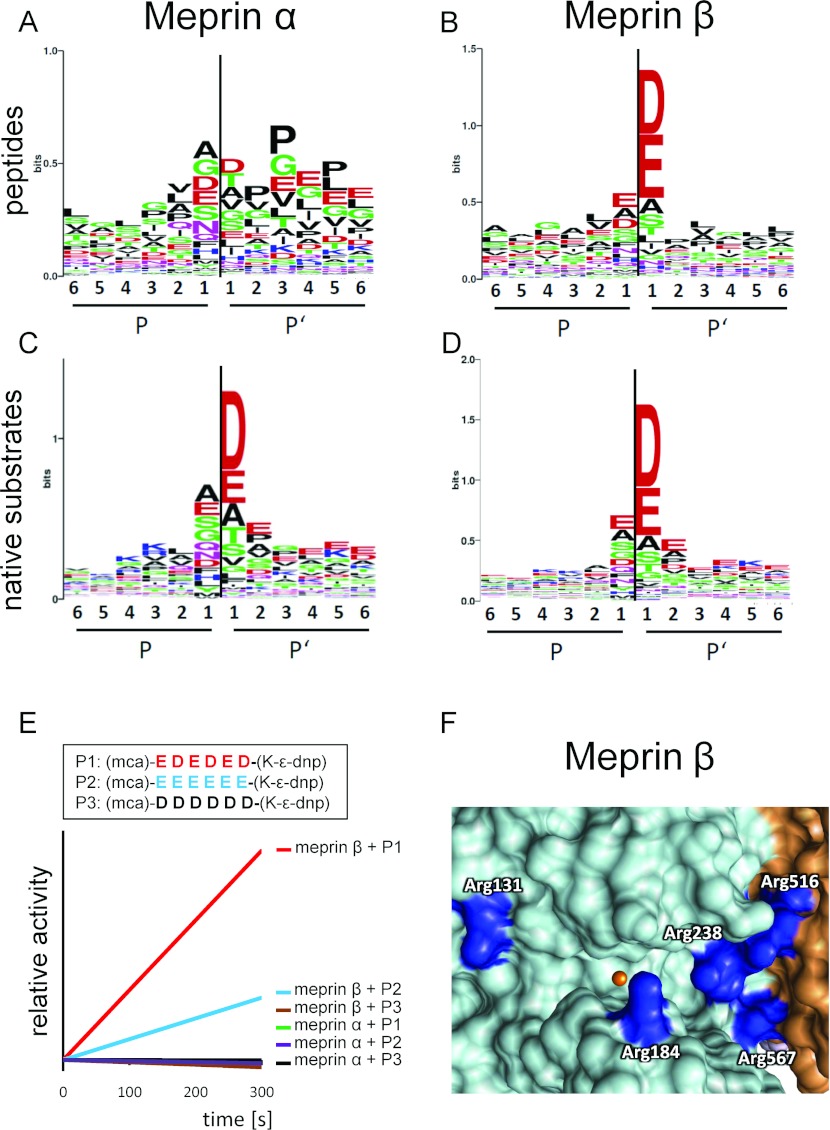
In view of the rather complex structure of both meprins, it is most likely that exosites play an important role in substrate recognition. On the other hand, cleavage specificity determines the capability to hydrolyse peptide bonds in a defined amino acid sequence. To investigate the preferences of meprin α and meprin β for certain amino acid residues around the cleavage site, the proteomics approaches PICS (proteomic identification of protease cleavage sites) [46–49] and TAILS (terminal amine isotopic labelling of substrates) [50–53] were employed. Using a human peptide library, we found a striking preference for negatively charged residues at the P1′ position for meprin β, which was also detectable for meprin α, although significantly less pronounced [54] (Figures 2A and 2B). This was concordant with the few cleavage sites previously determined for meprins [55,56]. However, when we analysed cleavage events in native proteins, a remarkably increased preference for aspartate and glutamate residues was identified for meprin α as well (Figures 2C and 2D). This striking cleavage specificity was found to be characteristic of both meprin α and meprin β, and also of the other astacin proteases BMP-1 (bone morphogenetic protein-1) and ovastacin [54]. Apart from these, no further extracellular proteases share this particular substrate motif, suggesting that many cleavage events seen in vivo exhibiting negatively charged amino acid residues in P1′ might be due to meprin activity, or related family members. Indeed meprin β was even capable of completely cleaving acidic peptide sequences (Figure 2E), revealing an outstanding feature among all extracellular proteases. The structure of active human meprin β exhibits several positively charged arginine residues within the active-site cleft, which probably interact with the negatively charged residues of the cleaved protein (Figure 2F). This knowledge might help development of highly specific inhibitors targeting meprin metalloproteases under certain pathological conditions.
Figure 2. Cleavage specificity of meprin α and meprin β.
With the help of proteomics approaches based on peptide libraries and native substrates, the cleavage specificity of human meprins has been determined. Results are displayed in the WebLogo style, where the most preferred amino acid residues are shown for positions P6 to P6′. The height of the single letter code corresponds to its occurrence. Colour-coding: acidic residues are in red, basic residues are in blue, polar residues (including tyrosine and glycine) are in green and hydrophobic residues (including alanine and proline) are in black. The black line indicates the cleavage site. WebLogo ordinates are scaled in bits as described previously [118]. Relative abundance of amino acid residues around the cleavage sites of meprin α and meprin β derived from peptide libraries (A and B) and native substrates (C and D). Both proteases prefer negatively charged amino acids at the P1′ position. (E) Incubation of fluorigenic substrates consisting of only aspartate and glutamate residues demonstrates the ability of meprin β to cleave completely acidic peptides. Quenched fluorigenic peptides (P1, P2 and P3) were incubated with human meprin α or meprin β and the relative amount of product (y axis) was calculated with regard to fluorescence intensity at 405 nm with an excitation at 320 nm, and plotted against time in seconds (x axis). dnp, 2,4-dinitrophenyl; Mca, (7-methyloxycoumarin-4-yl) acetyl. For detailed information see [54]. (F) The unique specificity of meprin β is based on structural features of the active-site cleft. Positively charged arginine (Arg) residues (dark blue) can interact with negatively charged amino acid residues of the substrate. Remarkably, Arg516 and Arg567, both part of the TRAF domain (brown), are probably involved in substrate binding, thus building an extended active-site cleft. The catalytic zinc is shown in orange. Numbering of amino acids is based on UniProt.
To identify natural substrates for human meprin α and meprin β we employed TAILS, a proteomics approach that enriches for N-terminal peptides of proteins and cleavage fragments. A total of 151 new extracellular substrates were identified with high confidence, including growth factors, matrix proteins, inhibitors and proteases [37]. Several of these have been validated in follow-up studies in vitro and/or in vivo and by Edman sequencing reported in the present review. The majority of N-termini identified exhibited aspartate and glutamate residues around the cleavage sites, highlighting the unique specificity of meprins. The TAILS approached enabled us to identify a network of complex proteolytic events in one go, the protease web, as nicely demonstrated by the cleavage of APP (amyloid precursor protein) by meprin β. Meprin β itself is shed from the cell surface by ADAM10 and, conversely, ADAM10, the constitutive α-secretase, can be activated by meprin β. This has an impact on the generation of neurotoxic Aβ (amyloid β) peptides, the main cause for the progression of AD.
The identification of the meprin α and meprin β degradomes reveals important and unexpected biological functions mediated by the activity of these metalloproteases. This is a key step in the understanding of related pathological conditions, and subsequently also important for drug development.
Synthetic and endogenous inhibitors
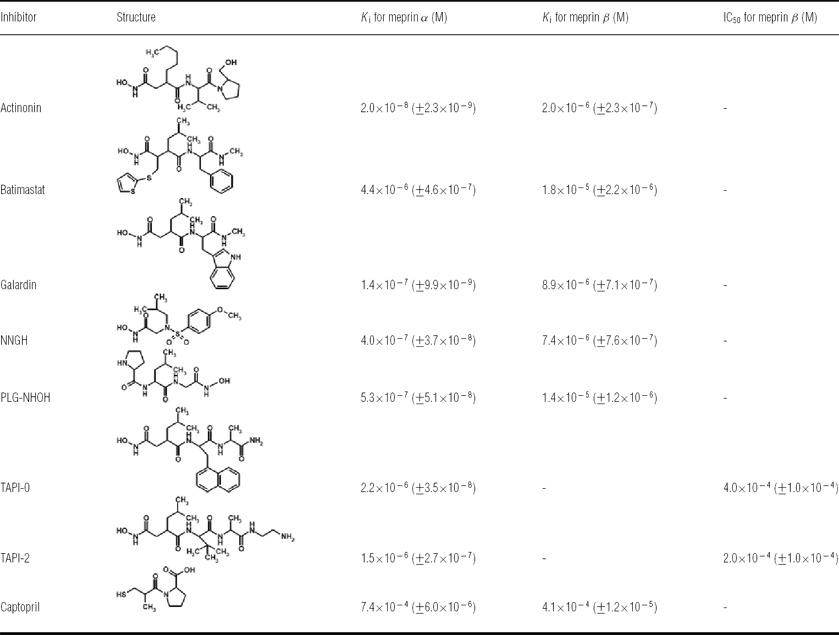
Regulation of meprin metalloproteases occurs at the transcriptional level [57,58], via activation [30] or inhibition by specific inhibitors [55]. Until recently, only synthetic compounds had been identified as inhibitors of meprin α and meprin β, of which the hydroxamate actinonin was shown to be the most potent molecule, with inhibition constants (Ki) in the nanomolar and low micromolar range for meprin α and β respectively [31] (Table 1). Here, the recently solved crystal structure of human meprin β [41] and the intensively studied cleavage specificity [54] will help to design highly specific small-molecule inhibitors. However, although synthetic inhibitors, such as zinc-chelating hydroxamates, might be useful as therapeutics [59], these are not physiological regulators of meprin activity. In vivo, endogenous protein inhibitors, such as TIMPs (tissue inhibitors of metalloproteases), are needed for protease regulation. MBP (mannan-binding protein) has been identified as an inhibitor of meprins in rats [60], but this inhibitory capacity could not be verified for the human enzymes [61]. However, the authors of that paper [60] demonstrated in a second study that meprins in complex with MBP initiate complement activation through the lectin pathway, and may cause acute renal failure as shown in mice [62].
Table 1. Synthetic inhibitors of human meprin α and meprin β.
The most potent small-molecule inhibitors of meprin metalloproteases are hydroxamates, chelating the catalytic zinc in the active-site cleft. Displayed are the inhibition constants (Ki), revealing actinonin as the most effective molecule, inhibiting meprin α in the nanomolar range and meprin β in the lower micromolar range. Values are means±S.D. For detailed descriptions and further meprin-inhibiting compounds, see [31].
 |
To look for endogenous meprin inhibitors, we coupled recombinant human meprins to activated Sepharose and isolated specific ligands from human plasma. Fetuin-A, a negative acute-phase plasma protein, was thus identified and has further been demonstrated to inhibit the proteolytic activity of meprin α and meprin β [61]. Fetuin-A was additionally found in the proteomics approach that was used for the identification of specific meprin substrates [37], supporting our previous study. Interestingly, in this proteomics screen, cystatin C was also identified, and turned out to be an inhibitor of human meprin α, but not meprin β [37]. Both fetuin-A and cystatin C are members of the cystatin superfamily of protease inhibitors, thus pointing to a common role in the regulation of meprin metalloproteases by this particular group of proteins. This is further supported by nephrosin inhibitor, which is a fetuin-A homologue from carp that exhibits strong inhibitory capacity towards both human meprin proteases [61].
MEPRIN ACTIVITY IN HEALTH AND DISEASE
Inflammation
Chronic IBD, such as CD (Crohn's disease) and UC (ulcerative colitis) are chronic inflammations of the gastrointestinal tract, characterized by a diffuse leucocyte infiltration of the intestinal mucosa and a deregulation of the mucosal immune system [63]. To date, little is known about the development of these diseases, but recent genetic approaches have identified several susceptibility genes [64,65]. The human metalloproteases meprin α (MEP1A) and meprin β (MEP1B) are such candidate genes [24,25,66]. Banerjee et al. [24,25] showed that MEP1A is genetically associated with IBD, on the basis of SNPs (single nucleotide polymorphisms) in UC patients. During intestinal inflammation, mRNA expression of MEP1A in the epithelium is directly down-regulated, mediated by the homeobox transcription factor CDX2 [24,67]. In the ileal mucosa of CD patients, mRNA levels of meprin β are decreased as well [68]. In these patients, ileal lesions are colonized by pathogenic AIEC (adherent-invasive Escherichia coli) bacteria [69]. Interestingly, meprins impair the ability of AIEC bacteria to adhere and to invade epithelial cells. Hence, decreased expression of meprin β leads to an increase in AIEC invasion, underscoring the role for meprins in IBD. Along with these studies, mice lacking meprin α showed a more severe inflammation and intestinal injury in a DSS (dextran sodium sulfate)-induced colitis model than wild-type animals [66,70]. However, the substrates responsible for this phenotype remain elusive. There is evidence that meprins might modulate the immune environment by processing and activating pro-inflammatory cytokines, such as ILs (interleukins) which are also associated with IBD. Meprins are able to generate biologically active IL-1β and IL-18, which are released in response to tissue injury and inflammation [37,66,71,72]. Although both meprins process IL-1β, only meprin β has been shown to activate IL-18. Hence, DSS-treated meprin β-knockout mice exhibit a reduced activation of the pro-inflammatory IL-18 and are therefore less susceptible to intestinal inflammation compared with wild-type mice. The pro-inflammatory role of meprin β was further supported by the finding that this protease mediates intestinal leucocyte infiltration, in accordance with its ability to cleave adhesion molecules and components of the ECM (extracellular matrix) [23,31,73,74].
Previous studies provide evidence that meprin α induces inflammation by transactivation of the EGFR (EGF receptor) through the release of its ligands TGFα (transforming growth factor α) and EGF from the cell surface [34,75,76]. In colon carcinoma cells (Caco-2), meprin α is activated by plasmin which, in turn, is activated by the fibroblast-derived urokinase-type plasminogen activator [33]. Thereafter, meprin α is able to release soluble EGF and TGFα, consequently activating the EGFR and ERK1/2 (extracellular-signal-regulated kinase 1/2) signalling cascade in a ligand-dependent manner [76]. Further investigations are required to see if the release of EGF and TGFα is also influenced through meprin β-mediated ADAM10 activation [37]. ADAM10 is well known to shed both EGFR ligands and hence might be involved in meprin-mediated signalling. The activation of EGFR and ERK1/2 finally leads to an increased cell proliferation and cell migration, indicating meprin α to be involved in the spreading of cancer cells. Additionally, EGFR transactivation via the release of TGFα by meprin α was demonstrated in human bronchial epithelial cells (16HBE14o), where meprin α is activated by the pro-inflammatory serine protease neutrophil elastase [34,77]. Soluble TGFα binds and activates the EGFR/TLR4 (Toll-like receptor 4) signalling cascade. Subsequently, this leads to an increased secretion of IL-8, a neutrophil chemoattractant implicated in chronic inflammatory diseases such as CF (cystic fibrosis) [77,78]. Interestingly, expression of meprin α in mice suffering from CF is increased compared with healthy animals, indicating that this particular protease is a potential therapeutic target in inflammatory lung disease.
Kidney and nephritis
In mice and rats, meprin α and meprin β make up to 5% of total brush-border membrane proteins [79], implicating an important physiological relevance of these proteases in rodent kidney. It has been found that both increased and decreased expression of meprins is associated with kidney diseases. The mouse strains C3H/He and CBA/Ca exclusively express meprin β, but no meprin α, in proximal tubular cells [80]. Interestingly, these mice developed less severe forms of kidney disease after injury compared with mouse strains with normal meprin α levels. This was the first indication of a role for meprin α in acute renal failure [81–83]. Indeed, further studies supported this observation [84–87]. Whereas meprin α is secreted into the lumen of the proximal tubule, meprin β is tethered to the apical plasma membrane [84]. During IR (ischaemia/reperfusion) injury and cisplatin-induced AKI (acute kidney injury) in rodents, meprin α and meprin β undergo redistribution from apical brush-border membranes of the proximal tubule to the basolateral tubular basement membrane [74,85,86]. IR is known to initiate an inflammatory response leading to rapid tissue damage [87]. Hence, increased meprin activity at the basement membrane leads to degradation of the renal tubular laminin–nidogen complex and other components of the basement membrane, and to the cleavage of cell-adhesion molecules (E-cadherin and tenascin-C), consequently injuring the tubular basement membrane and leading to leucocyte infiltration [31,73,74,84,88]. Moreover, meprins are able to induce a pro-inflammatory response by activating or releasing cytokines such as IL-1β, IL-18 or TGFα [66,76,86].
Interestingly, mice lacking meprin α and meprin β are significantly protected against renal IR injury and bladder inflammation. Meprin α-knockout mice exhibit less renal damage compared with wild-type mice, and meprin β-deficient mice show lower levels of the inflammatory marker IL-6 and decreased leucocyte infiltration after renal injury [85,89]. A number of studies have addressed the role of meprins as diagnostic markers and possible novel targets for therapeutic treatment of renal diseases [90–92]. Administration of the potent meprin inhibitor actinonin in rats prevents renal pathology, supporting the contribution of meprins to the pathogenesis of IR-induced renal injury [93,94]. In a sepsis-induced AKI mouse model, this particular inhibitor significantly decreased the initial peritubular capillary dysfunction, as well as the subsequent tubular injury [59].
Interestingly, variations in the structural or regulatory regions of the MEP1B gene have revealed a link to diabetic nephropathy [81]. When analysing the nucleotide sequence of the MEP1B gene from Pima Indians, nine significant SNPs in MEP1B were found, which might influence transcription or trafficking of the enzyme [58]. Diabetic nephropathy finally results in fibrosis, a pathological condition also observed in dermal skin, when meprins are overexpressed by fibroblasts, as described below.
Neurobiology and AD
Recent advances in proteomic techniques have provided a variety of new putative substrates of meprins, including APP. Proteolytic processing of APP is proposed to have a pathological effect on the progression of AD, since the accumulation of amyloid plaques in the brains of patients with AD is characteristic of the disease pathology [95]. Aβ peptides are generated during the amyloidogenic pathway when the β-secretase BACE1 (β-site APP-cleaving enzyme 1) cleaves APP, generating the soluble APP fragment (sAPPβ) and the membrane-bound C-terminal 99 amino acids of APP (C99) [96]. Subsequently, C99 is processed by γ-secretase, generating a small intracellular fragment and the neurotoxic C-terminal Aβ peptide predominantly consisting of either 40 or 42 amino acids. These Aβ peptides form aggregates, which are assumed to be the main cause of AD. Recently, Bien et al. [97] reported that meprin β initially sheds APP, releasing different Aβ species with several cleavage sites identical with or proximal to the known β-secretase cleavage site [97]. In fact, overexpressed meprin β, in contrast with the β-secretase BACE1, generates N-terminally truncated Aβ variants containing alanine at the P1′ position (Aβ2–40) (see Supplementary Movie S1), as found in cerebrospinal fluids [98,99]. The overexpression of meprin β in AD brains suggests a role for this metalloprotease in the accumulation of Aβ peptides [97]. However, further studies and appropriate mouse models are necessary to investigate the contribution of meprin β in AD. Additionally, meprin β processes the N-terminus of APP in vivo releasing N-terminal fragments of 11 and 22 kDa (APP11 and APP22) [100]. The 11 kDa peptide was previously observed in human brain lysates [101], suggesting the physiological relevance of meprin β in neurobiology (Figure 3).
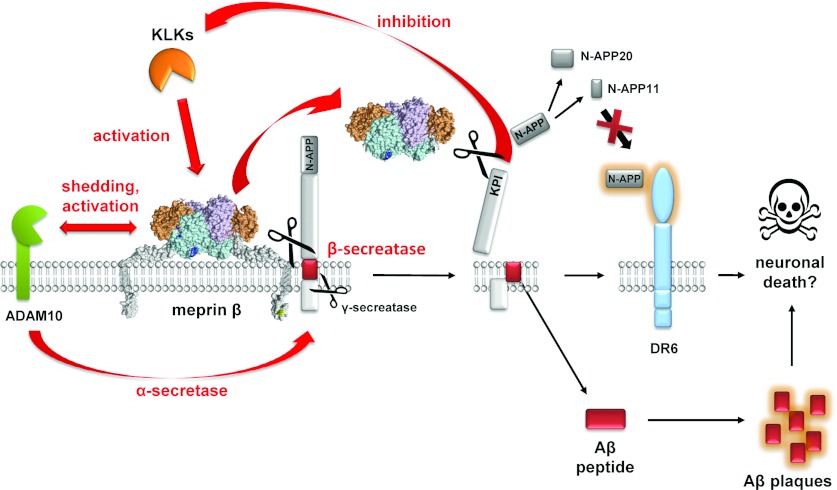
Figure 3. Proteolytic interactions responsible for the shedding of APP by meprin β.
The cartoon summarizes recent findings showing that meprin β generates Aβ peptides [97] and specific N-terminal APP fragments [100]. We further identified a proteolytic interaction between meprin β and ADAM10 [37], the constitutive α-secretase. Shedding by ADAM10 leads to soluble meprin β, which is no longer capable of cleaving at the β-secretase site, but still releases N-APP fragments that exhibit no neurotoxic capacity and are rather protective against death receptor 6 (DR6)-mediated neuronal death. Human tissue KLKs are activators of meprin β.
Alternatively, during the non-amyloidogenic pathway, the α-secretase ADAM10 sheds APP within the Aβ domain, liberating the soluble ectodomain sAPPα, thus preventing the formation and aggregation of neurotoxic Aβ peptides [102,103] Interestingly, meprin β has been shown to activate the α-secretase ADAM10 through cleavage of the propeptide [37]. ADAM10 is synthesized as a zymogen and requires proteolytic activation. Although the propeptide is cleaved by proprotein convertase PC7 and furin during the secretory pathway, it remains non-covalently bound to the catalytic domain of ADAM10 and acts as an inhibitor [104]. The second cleavage event by meprin β releases the propeptide leading to an increase in ADAM10 activity. However, whether this meprin β-mediated activation of the α-secretase ADAM10 has any protective function in the progression of AD is not yet clear. One might assume that increased ADAM10 activity mediated by meprin β decreases the amount of Aβ. However, it was also shown by a neuronal knockout of ADAM10 that Aβ levels were significantly lower in the absence of this particular protease [105]. Still little is known about the regulation of the Aβ generation and the balance between α- and β-secretase activities in AD. Meprin β indeed is involved in the modulation of APP in vivo and might therefore be a promising therapeutic target in AD.
Angiogenesis and cancer
Previous studies have addressed the role of meprin α in angiogenesis [28,106]. Angiogenesis is the physiological process of new blood vessel formation from pre-existing vessels and is therefore a key step in organ development and homoeostasis. However, the imbalance in angiogenesis contributes to numerous pathologies, such as cancer and inflammation [107]. Interestingly, morpholino knockdowns of meprin α in zebrafish embryos revealed a severe phenotype with a dramatically reduced vascular system [28] (Figure 4A) resembling the phenotype of VEGF-A (vascular endothelial growth factor A) morphants [108,109]. Hence, meprin α is a pro-angiogenic enzyme important for blood vessel formation during embryogenesis. In a rat aortic ring assay, the application of meprin α increased outgrowth, length and branching of capillaries [106]. The molecular mechanism behind this function might involve proteolytic activation and/or release of pro-angiogenic growth factors, such as VEGF-A and CTGF (connective tissue growth factor), both identified as meprin substrates [37]. Human meprin α cleaves VEGF-A in vitro, generating proteolytic fragments as found in wild-type zebrafish [28] (Figure 4B). Besides its contribution in the regulation of angiogenesis, meprin α has been described to be involved in cardiovascular homoeostasis by enzymatic cleavage of the 32-amino acid BNP (B-type natriuretic peptide) (BNP1–32) in vitro and in vivo, leading to its reduced bioactivity [110].
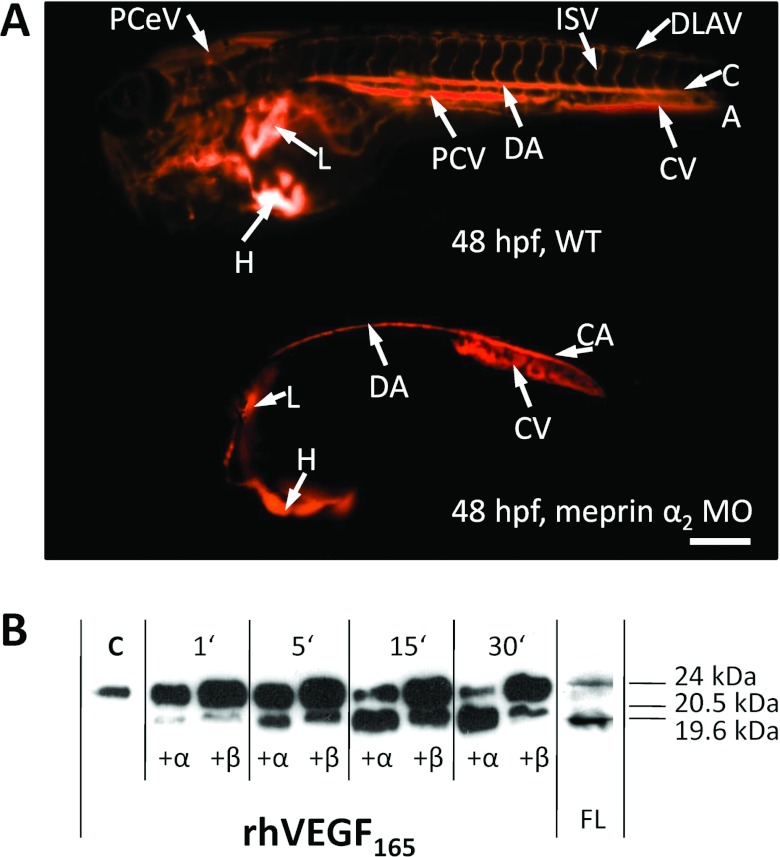
Figure 4. Meprin α promotes blood vessel formation.
To analyse the biological function of meprins, morpholino knockdowns of meprin α1, meprin α2 and meprin β were done in zebrafish embryos. (A) Microangiography with fluorigenic TRITC (tetramethylrhodamine β-isothiocyanate)–Dextran revealed severe defects in the formation of blood vessels when meprin α2 was reduced. Compared with wild-type (WT) embryos, meprin α2 morphants (MO) showed nearly complete absence of the head vascular system and the posterior cardinal vein (PCV), indicating a pro-angiogenic function of this protease. CA, caudal artery; CV, caudal vein; DA, dorsal aorta; DLAV, dorsal longitudinal anastomotic vessel; H, heart; hpf, hours post-fertilization; ISV, intersegmental vessels; L, liver; PCeV, posterior cerebral vein; PCV, posterior cardinal vein. Scale bar is 250 μm. Reproduced from [28], under the terms of the Creative Commons Attribution Licence. (B) Interestingly, processing of the VEGF-A (VEGF-A165) by meprins in vitro resulted in cleavage products comparable with those found in zebrafish lysates (FL). For detailed information see [28].
Consistent with the described pro-angiogenic activity, meprin α is expressed in several different tumours, e.g. breast and colon carcinoma [57,111]. In cultured colon carcinoma cells (Caco-2) meprin α is secreted not only apically, but also basolaterally leading to an accumulation of meprin α activity in the tumour stroma [111]. Hence, the proteolytic potential of meprin α is directed towards the ECM, where it can contribute to the breakdown of stromal structure thereby affecting proliferation and migration of tumour cells into the surrounding tissue. This is supported by cell-culture experiments with actinonin-treated human breast carcinoma cells, which resulted in decreased invasiveness in vitro [57]. Additionally, Lottaz et al. [106] demonstrated that meprin α directly promotes cell migration, although the pro-angiogenic effect of meprin α might be even more important for cancer pathology. The processing of growth factors and cytokines, especially with regard to the pro-angiogenic function of meprin α, should be pursued futher in future studies. Previously, it was demonstrated that both meprins are capable of processing procollagen III, thereby enhancing fibril assembly, a role which could be contrary to metastasis [26] (see the section below). Meprin α expression differs between different carcinomas, with quite low levels in ovarian cancer compared with gastrointestinal carcinomas [106,112]. Hence, the role of meprins in cancer development, progression and metastasis seems to be more complex than the ECM-degrading function previously proposed for certain MMPs.
Skin and fibrosis
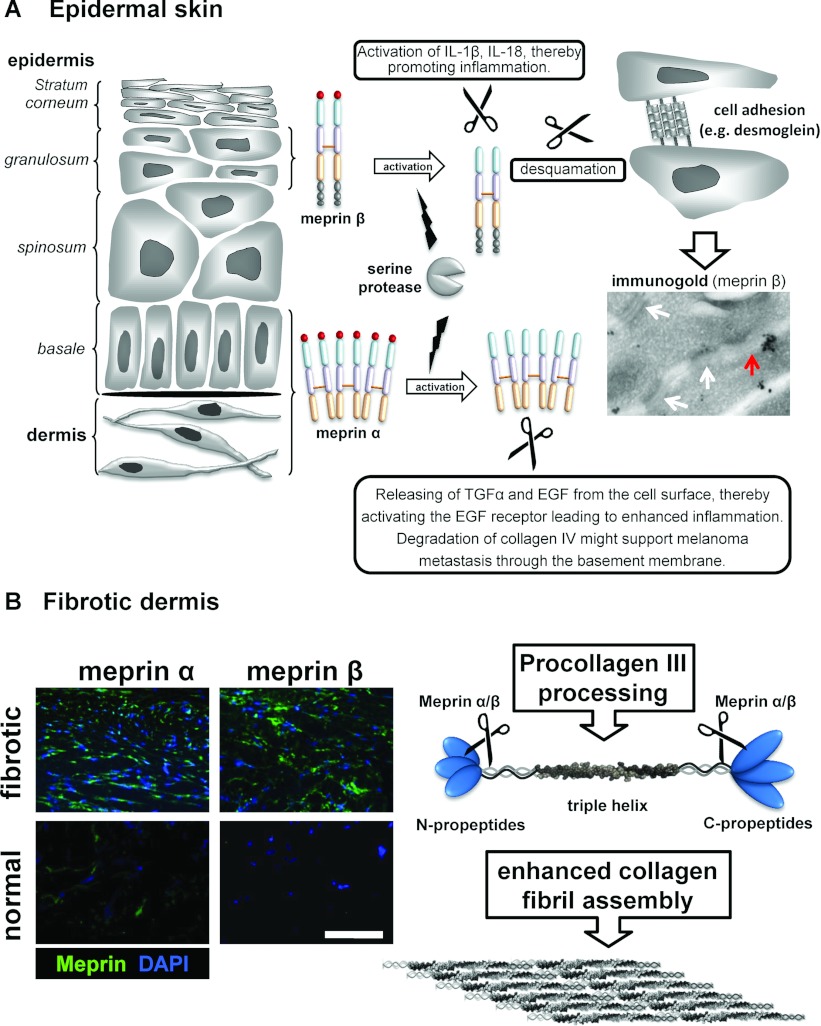
The skin is the largest organ of the body and protects it against external physical, chemical and biological influences due to its elaborate organization in distinct layers [113]. Meprin α and meprin β are constitutively expressed in human epidermis, albeit in different cell layers due to their different roles in keratinocyte differentiation [27]. Meprin α is exclusively expressed in the stratum basale, where it might be activated by plasmin by the removal of the propeptide [30,33] and where it promotes proliferation of human keratinocytes. In vitro studies suggest a contribution of meprin α in cell migration by processing several components of the basal lamina such as collagen IV, fibronectin, laminin and nidogen-1 [31,37,84], thus allowing cells to detach from the underlying basement membrane and to migrate into the upper granular layers of the epidermis. During hyperproliferation, as in psoriasis vulgaris and in Netherton syndrome, meprin α expression is shifted from the basolateral to the upper layers of the epidermis where it is probably activated by KLK5 [30]. Meprin β, in turn, is expressed in the stratum granulosum, the layer of the epidermis where the cornified envelope is formed and the epidermal barrier is established [27]. Here, promeprin β can be activated by KLK4, 5 and 8, all of these being expressed in the upper layers of the epidermis [30]. Once activated, meprin β induces terminal differentiation and might therefore be involved in cornification, a unique process of cell death in which a water-impermeable barrier is formed. Concomitant with its expression in the granular layer, meprin β has been proposed to be involved in desquamation by cleaving cell-adhesion molecules such as E-cadherin and desmogleins [37,114] (Figure 5A). Moreover, the role of meprin β in desquamation is supported by its ability to process proKLK7 only two amino acids N-terminal from the activation site, subsequently leading to an accelerated activation of proKLK7 [30]. Additionally, meprin β cleaves a range of serine protease inhibitors such as LEKTI (lymphoepithelial Kazal-type-related inhibitor), SLPI (antileucoproteinase) and elafin, which then can directly modulate KLK7 activity [37]. In the same study, we could also show that meprin β activates ADAM10, an enzyme known to be essential for epidermal homoeostasis [115].
Figure 5. Activity of human meprin α and meprin β in skin homoeostasis and fibrosis.
(A) Meprin α and meprin β are expressed in separate layers of human epidermis, in the stratum basale and the stratum granulosum respectively. Meprin β was shown to be involved in terminal differentiation of keratinocytes, whereas meprin α promotes cell proliferation by activating the EGFR. A proteomics screen identified desmogleins as substrates of meprin β [37], adhesion molecules that are cleaved during desquamation. Immunogold staining of human epidermis revealed co-localization of meprin β (black dots) and desmoglein-containing desmosomes (white arrows). The red arrow indicates possible degradation of desmosomal proteins by meprin β. (B) In the dermis both meprins are overexpressed in fibroblasts of fibrotic skin, as demonstrated by immunofluorescence microscopy [26]. Here, meprins might contribute to the increased formation of fibrillar collagen. In vitro it was shown that meprins can cleave off the N- and C-propeptides of procollagen III, thereby releasing mature triple helical collagen. Further studies are required to validate this observation in vivo. DAPI, 4′,6-diamidino-2-phenylindole.
Further investigations with regard to the physiological role of meprin α and β in skin have led to the finding that both meprins are overexpressed in human dermal fibroblasts of fibrotic tumours (keloids) compared with normal skin [26] (Figure 5B). The dermis consists mainly of connective tissue and is the site of collagen synthesis. Dermal collagens, mainly types I and III, are the major components of the connective tissue, responsible for tensile strength and stability. Previously, meprin α and meprin β have been shown to cleave the C-propeptides from the fibrillar procollagen III in vitro, the initial step in collagen fibril formation [116,117]. Interestingly, meprins process type III procollagen at exactly the same site and even more efficient than the procollagen C proteinase BMP-1, suggesting that meprins are involved in procollagen turnover and might contribute to collagen assembly in skin. Whether meprins are procollagen proteinases in vivo has yet to be shown.
SUMMARY
Proteolytic enzymes are important regulators in health and disease. Understanding the orchestration of proteases, substrates, inhibitors and other regulatory molecules, in one word ‘the protease web’, is crucial for the treatment of associated pathological conditions. Among all 553 human proteases, the metalloproteases meprin α and meprin β exhibit unique structural and functional properties. Recent studies revealed structural details of meprin β, which explain the molecular basis for the unique cleavage specificity, with high preference for acidic amino acid residues in the P1′ position, reflected by native substrates. Proteomics analyses enabled the identification of more than 100 new meprin substrates and revealed a possible link to neurodegeneration with regard to the release of Aβ peptides by meprin β. Cleavage of pro-inflammatory cytokines by meprins and genetic studies demonstrated contribution of these proteases to the progression of IBD. This was further validated in DSS-induced colitis in meprin α- and meprin β-knockout mice. The knockdown of meprin expression in zebrafish revealed important functions of these enzymes during embryonic development. Meprin α, for instance, was shown to be a pro-angiogenic enzyme, and therefore might contribute to tumour progression in colon carcinoma, where the protease is highly expressed. Further studies, including appropriate animal models, will elucidate the precise molecular pathways mediated by meprin α and meprin β activity in health and disease. The generation of specific meprin inhibitors is a crucial goal to develop potential therapeutics for the treatment of meprin-associated pathologies.
The present review summarizes recent findings in meprin research in health and disease and describes the multiple molecular pathways mediated by meprin activity. It is also an invitation to scientists not in the field to re-examine their data for possible meprin activity, something that might have been overlooked due to the still orphan status of these fascinating metalloproteases.
Online data
ACKNOWLEDGEMENTS
We thank numerous scientific colleagues, in particular Judith S. Bond and Erwin E. Sterchi, for their contributions to meprin research. We also thank Xavier Gomis-Rüth for providing PDB files of membrane-bound meprin β and David S. Hulmes for critical reading and editing of the paper before submission.
FUNDING
The work of the authors is supported by the Deutsche Forschungsgemeinschaft (DFG) [grant numbers BE 4086/1-2 and SFB877 ‘Proteolysis as a Regulatory Event in Pathophysiology’ project A9], and by the Cluster of Excellence ‘Inflammation at Interfaces’.
References
- 1.Beynon R. J., Shannon J. D., Bond J. S. Purification and characterization of a metallo-endoproteinase from mouse kidney. Biochem. J. 1981;199:591–598. doi: 10.1042/bj1990591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes K., Ingram J., Kenny A. J. Proteins of the kidney microvillar membrane. Structural and immunochemical properties of rat endopeptidase-2 and its immunohistochemical localization in tissues of rat and mouse. Biochem. J. 1989;264:335–346. doi: 10.1042/bj2640335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sterchi E. E., Green J. R., Lentze M. J. Non-pancreatic hydrolysis of N-benzoyl-L-tyrosyl-p-aminobenzoic acid (PABA-peptide) in the human small intestine. Clin. Sci. 1982;62:557–560. doi: 10.1042/cs0620557. [DOI] [PubMed] [Google Scholar]
- 4.Sterchi E. E., Green J. R., Lentze M. J. Nonpancreatic hydrolysis of N-benzoyl-L-tyrosyl-p-aminobenzoic acid (PABA peptide) in the rat small intestine. J. Pediatr. Gastroenterol. Nutr. 1983;2:539–547. doi: 10.1097/00005176-198302030-00024. [DOI] [PubMed] [Google Scholar]
- 5.Bond J. S., Beynon R. J. Mammalian metalloendopeptidases. Int. J. Biochem. 1985;17:565–574. doi: 10.1016/0020-711x(85)90287-3. [DOI] [PubMed] [Google Scholar]
- 6.Bond J. S., Beynon R. J. The astacin family of metalloendopeptidases. Protein Sci. 1995;4:1247–1261. doi: 10.1002/pro.5560040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bond J. S., Beynon R. J., Reckelhoff J. F., David C. S. Mep-1 gene controlling a kidney metalloendopeptidase is linked to the major histocompatibility complex in mice. Proc. Natl. Acad. Sci. U.S.A. 1984;81:5542–5545. doi: 10.1073/pnas.81.17.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorbea C. M., Marchand P., Jiang W., Copeland N. G., Gilbert D. J., Jenkins N. A., Bond J. S. Cloning, expression, and chromosomal localization of the mouse meprin β subunit. J. Biol. Chem. 1993;268:21035–21043. [PubMed] [Google Scholar]
- 9.Jiang W., Sadler P. M., Jenkins N. A., Gilbert D. J., Copeland N. G., Bond J. S. Tissue-specific expression and chromosomal localization of the α subunit of mouse meprin A. J. Biol. Chem. 1993;268:10380–10385. [PubMed] [Google Scholar]
- 10.Hahn D., Illisson R., Metspalu A., Sterchi E. E. Human N-benzoyl-L-tyrosyl-p-aminobenzoic acid hydrolase (human meprin): genomic structure of the α and β subunits. Biochem. J. 2000;346:83–91. [PMC free article] [PubMed] [Google Scholar]
- 11.Stocker W., Zwilling R. Astacin. Methods Enzymol. 1995;248:305–325. doi: 10.1016/0076-6879(95)48021-8. [DOI] [PubMed] [Google Scholar]
- 12.Saftig P., Reiss K. The ‘A Disintegrin And Metalloproteases’ ADAM10 and ADAM17: novel drug targets with therapeutic potential? Eur. J. Cell Biol. 2011;90:527–535. doi: 10.1016/j.ejcb.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Scheller J., Chalaris A., Garbers C., Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32:380–387. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Fanjul-Fernandez M., Folgueras A. R., Cabrera S., Lopez-Otin C. Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta. 2010;1803:3–19. doi: 10.1016/j.bbamcr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Overall C. M., Blobel C. P. In search of partners: linking extracellular proteases to substrates. Nat. Rev. Mol. Cell Biol. 2007;8:245–257. doi: 10.1038/nrm2120. [DOI] [PubMed] [Google Scholar]
- 16.Overall C. M., Kleifeld O. Tumour microenvironment–opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi T., Fukase M., Kido H., Sugimoto T., Katunuma N., Chihara K. Meprin is predominantly involved in parathyroid hormone degradation by the microvillar membranes of rat kidney. Life Sci. 1994;54:381–386. doi: 10.1016/0024-3205(94)00795-0. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi T., Fukase M., Sugimoto T., Kido H., Chihara K. Purification of meprin from human kidney and its role in parathyroid hormone degradation. Biol. Chem. 1994;375:821–824. [PubMed] [Google Scholar]
- 19.Yamaguchi T., Kido H., Kitazawa R., Kitazawa S., Fukase M., Katunuma N. A membrane-bound metallo-endopeptidase from rat kidney: its immunological characterization. J. Biochem. 1993;113:299–303. doi: 10.1093/oxfordjournals.jbchem.a124042. [DOI] [PubMed] [Google Scholar]
- 20.Addison M. L., Minnion J. S., Shillito J. C., Suzuki K., Tan T. M., Field B. C., Germain-Zito N., Becker-Pauly C., Ghatei M. A., Bloom S. R., Murphy K. G. A role for metalloendopeptidases in the breakdown of the gut hormone, PYY 3-36. Endocrinology. 2011;152:4630–4640. doi: 10.1210/en.2011-1195. [DOI] [PubMed] [Google Scholar]
- 21.Sun Q., Jin H. J., Bond J. S. Disruption of the meprin α and β genes in mice alters homeostasis of monocytes and natural killer cells. Exp. Hematol. 2009;37:346–356. doi: 10.1016/j.exphem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norman L. P., Jiang W., Han X., Saunders T. L., Bond J. S. Targeted disruption of the meprin β gene in mice leads to underrepresentation of knockout mice and changes in renal gene expression profiles. Mol. Cell. Biol. 2003;23:1221–1230. doi: 10.1128/MCB.23.4.1221-1230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crisman J. M., Zhang B., Norman L. P., Bond J. S. Deletion of the mouse meprin β metalloprotease gene diminishes the ability of leukocytes to disseminate through extracellular matrix. J. Immunol. 2004;172:4510–4519. doi: 10.4049/jimmunol.172.7.4510. [DOI] [PubMed] [Google Scholar]
- 24.Banerjee S., Oneda B., Yap L. M., Jewell D. P., Matters G. L., Fitzpatrick L. R., Seibold F., Sterchi E. E., Ahmad T., Lottaz D., Bond J. S. MEP1A allele for meprin A metalloprotease is a susceptibility gene for inflammatory bowel disease. Mucosal Immunol. 2009;2:220–231. doi: 10.1038/mi.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee S., Jin G., Bradley S. G., Matters G. L., Gailey R. D., Crisman J. M., Bond J. S. Balance of meprin A and B in mice affects the progression of experimental inflammatory bowel disease. Am. J. Physiol. Gastroinstest. Liver Physiol. 2011;300:G273–G282. doi: 10.1152/ajpgi.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kronenberg D., Bruns B. C., Moali C., Vadon-Le Goff S., Sterchi E. E., Traupe H., Bohm M., Hulmes D. J., Stocker W., Becker-Pauly C. Processing of procollagen III by meprins: new players in extracellular matrix assembly? J. Invest. Dermatol. 2010;130:2727–2735. doi: 10.1038/jid.2010.202. [DOI] [PubMed] [Google Scholar]
- 27.Becker-Pauly C., Howel M., Walker T., Vlad A., Aufenvenne K., Oji V., Lottaz D., Sterchi E. E., Debela M., Magdolen V., et al. The α and β subunits of the metalloprotease meprin are expressed in separate layers of human epidermis, revealing different functions in keratinocyte proliferation and differentiation. J. Invest. Dermatol. 2007;127:1115–1125. doi: 10.1038/sj.jid.5700675. [DOI] [PubMed] [Google Scholar]
- 28.Schutte A., Hedrich J., Stocker W., Becker-Pauly C. Let it flow: morpholino knockdown in zebrafish embryos reveals a pro-angiogenic effect of the metalloprotease meprin α2. PLoS ONE. 2010;5:e8835. doi: 10.1371/journal.pone.0008835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schutte A., Lottaz D., Sterchi E. E., Stocker W., Becker-Pauly C. Two α subunits and one β subunit of meprin zinc-endopeptidases are differentially expressed in the zebrafish Danio rerio. Biol. Chem. 2007;388:523–531. doi: 10.1515/BC.2007.060. [DOI] [PubMed] [Google Scholar]
- 30.Ohler A., Debela M., Wagner S., Magdolen V., Becker-Pauly C. Analyzing the protease web in skin: meprin metalloproteases are activated specifically by KLK4, 5 and 8 vice versa leading to processing of proKLK7 thereby triggering its activation. Biol. Chem. 2010;391:455–460. doi: 10.1515/BC.2010.023. [DOI] [PubMed] [Google Scholar]
- 31.Kruse M. N., Becker C., Lottaz D., Kohler D., Yiallouros I., Krell H. W., Sterchi E. E., Stocker W. Human meprin α and β homo-oligomers: cleavage of basement membrane proteins and sensitivity to metalloprotease inhibitors. Biochem. J. 2004;378:383–389. doi: 10.1042/BJ20031163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker C., Kruse M. N., Slotty K. A., Kohler D., Harris J. R., Rosmann S., Sterchi E. E., Stocker W. Differences in the activation mechanism between the α and β subunits of human meprin. Biol. Chem. 2003;384:825–831. doi: 10.1515/BC.2003.092. [DOI] [PubMed] [Google Scholar]
- 33.Rosmann S., Hahn D., Lottaz D., Kruse M. N., Stocker W., Sterchi E. E. Activation of human meprin-α in a cell culture model of colorectal cancer is triggered by the plasminogen-activating system. J. Biol. Chem. 2002;277:40650–40658. doi: 10.1074/jbc.M206203200. [DOI] [PubMed] [Google Scholar]
- 34.Bergin D. A., Greene C. M., Sterchi E. E., Kenna C., Geraghty P., Belaaouaj A., Taggart C. C., O’Neill S. J., McElvaney N. G. Activation of the epidermal growth factor receptor (EGFR) by a novel metalloprotease pathway. J. Biol. Chem. 2008;283:31736–31744. doi: 10.1074/jbc.M803732200. [DOI] [PubMed] [Google Scholar]
- 35.Gomis-Ruth F. X., Trillo-Muyo S., Stocker W. Functional and structural insights into astacin metallopeptidases. Biol. Chem. 2012;393:1027–1041. doi: 10.1515/hsz-2012-0149. [DOI] [PubMed] [Google Scholar]
- 36.Bertenshaw G. P., Norcum M. T., Bond J. S. Structure of homo- and hetero-oligomeric meprin metalloproteases. Dimers, tetramers, and high molecular mass multimers. J. Biol. Chem. 2003;278:2522–2532. doi: 10.1074/jbc.M208808200. [DOI] [PubMed] [Google Scholar]
- 37.Jefferson T., auf dem Keller U., Bellac C., Metz V. V., Broder C., Hedrich J., Ohler A., Maier W., Magdolen V., Sterchi E., et al. The substrate degradome of meprin metalloproteases reveals an unexpected proteolytic link between meprin β and ADAM10. Cell. Mol. Life Sci. 2012 doi: 10.1007/s00018-012-1106-2. doi:10.1007/s00018-012-1106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahn D., Pischitzis A., Roesmann S., Hansen M. K., Leuenberger B., Luginbuehl U., Sterchi E. E. Phorbol 12-myristate 13-acetate-induced ectodomain shedding and phosphorylation of the human meprin β metalloprotease. J. Biol. Chem. 2003;278:42829–42839. doi: 10.1074/jbc.M211169200. [DOI] [PubMed] [Google Scholar]
- 39.Norman L. P., Matters G. L., Crisman J. M., Bond J. S. Expression of meprins in health and disease. Curr. Top. Dev. Biol. 2003;54:145–166. doi: 10.1016/s0070-2153(03)54008-x. [DOI] [PubMed] [Google Scholar]
- 40.Bond J. S., Beynon R. J. Meprin: a membrane-bound metalloendopeptidase. Curr. Top. Cell. Regul. 1986;28:263–290. doi: 10.1016/b978-0-12-152828-7.50009-3. [DOI] [PubMed] [Google Scholar]
- 41.Arolas J. L., Broder C., Jefferson T., Guevara T., Sterchi E. E., Bode W., Stocker W., Becker-Pauly C., Gomis-Ruth F. X. Structural basis for the sheddase function of human meprin β metalloproteinase at the plasma membrane. Proc. Natl. Acad. Sci. U.S.A. 2012;109:16131–16136. doi: 10.1073/pnas.1211076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker-Pauly C., Bruns B. C., Damm O., Schutte A., Hammouti K., Burmester T., Stocker W. News from an ancient world: two novel astacin metalloproteases from the horseshoe crab. J. Mol. Biol. 2009;385:236–248. doi: 10.1016/j.jmb.2008.10.062. [DOI] [PubMed] [Google Scholar]
- 43.Ishmael S. S., Ishmael F. T., Jones A. D., Bond J. S. Protease domain glycans affect oligomerization, disulfide bond formation, and stability of the meprin A metalloprotease homo-oligomer. J. Biol. Chem. 2006;281:37404–37415. doi: 10.1074/jbc.M602769200. [DOI] [PubMed] [Google Scholar]
- 44.Ishmael F. T., Shier V. K., Ishmael S. S., Bond J. S. Intersubunit and domain interactions of the meprin B metalloproteinase. Disulfide bonds and protein-protein interactions in the MAM and TRAF domains. J. Biol. Chem. 2005;280:13895–13901. doi: 10.1074/jbc.M414218200. [DOI] [PubMed] [Google Scholar]
- 45.Ishmael F. T., Norcum M. T., Benkovic S. J., Bond J. S. Multimeric structure of the secreted meprin A metalloproteinase and characterization of the functional protomer. J. Biol. Chem. 2001;276:23207–23211. doi: 10.1074/jbc.M102654200. [DOI] [PubMed] [Google Scholar]
- 46.Schilling O., Overall C. M. Proteome-derived, database-searchable peptide libraries for identifying protease cleavage sites. Nat. Biotechnol. 2008;26:685–694. doi: 10.1038/nbt1408. [DOI] [PubMed] [Google Scholar]
- 47.Schilling O., Overall C. M. Proteomic discovery of protease substrates. Curr. Opin. Chem. Biol. 2007;11:36–45. doi: 10.1016/j.cbpa.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 48.Schilling O., auf dem Keller U., Overall C. M. Protease specificity profiling by tandem mass spectrometry using proteome-derived peptide libraries. Methods Mol. Biol. 2011;753:257–272. doi: 10.1007/978-1-61779-148-2_17. [DOI] [PubMed] [Google Scholar]
- 49.Schilling O., Huesgen P. F., Barre O., auf dem Keller U., Overall C. M. Characterization of the prime and non-prime active site specificities of proteases by proteome-derived peptide libraries and tandem mass spectrometry. Nat. Protoc. 2011;6:111–120. doi: 10.1038/nprot.2010.178. [DOI] [PubMed] [Google Scholar]
- 50.Kleifeld O., Doucet A., Prudova A., auf dem Keller U., Gioia M., Kizhakkedathu J. N., Overall C. M. Identifying and quantifying proteolytic events and the natural N terminome by terminal amine isotopic labeling of substrates. Nat. Protoc. 2011;6:1578–1611. doi: 10.1038/nprot.2011.382. [DOI] [PubMed] [Google Scholar]
- 51.Kleifeld O., Doucet A., auf dem Keller U., Prudova A., Schilling O., Kainthan R. K., Starr A. E., Foster L. J., Kizhakkedathu J. N., Overall C. M. Isotopic labeling of terminal amines in complex samples identifies protein N-termini and protease cleavage products. Nat. Biotechnol. 2010;28:281–288. doi: 10.1038/nbt.1611. [DOI] [PubMed] [Google Scholar]
- 52.auf dem Keller U., Prudova A., Gioia M., Butler G. S., Overall C. M. A statistics-based platform for quantitative N-terminome analysis and identification of protease cleavage products. Mol. Cell. Proteomics. 2010;9:912–927. doi: 10.1074/mcp.M000032-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.auf dem Keller U., Schilling O. Proteomic techniques and activity-based probes for the system-wide study of proteolysis. Biochimie. 2010;92:1705–1714. doi: 10.1016/j.biochi.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 54.Becker-Pauly C., Barre O., Schilling O., auf dem Keller U., Ohler A., Broder C., Schutte A., Kappelhoff R., Stocker W., Overall C. M. Proteomic analyses reveal an acidic prime side specificity for the astacin metalloprotease family reflected by physiological substrates. Mol. Cell. Proteomics. 2011;10:M111.009233. doi: 10.1074/mcp.M111.009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sterchi E. E., Stocker W., Bond J. S. Meprins, membrane-bound and secreted astacin metalloproteinases. Mol. Aspects Med. 2008;29:309–328. doi: 10.1016/j.mam.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bertenshaw G. P., Turk B. E., Hubbard S. J., Matters G. L., Bylander J. E., Crisman J. M., Cantley L. C., Bond J. S. Marked differences between metalloproteases meprin A and B in substrate and peptide bond specificity. J. Biol. Chem. 2001;276:13248–13255. doi: 10.1074/jbc.M011414200. [DOI] [PubMed] [Google Scholar]
- 57.Matters G. L., Manni A., Bond J. S. Inhibitors of polyamine biosynthesis decrease the expression of the metalloproteases meprin α and MMP-7 in hormone-independent human breast cancer cells. Clin. Exp. Metastasis. 2005;22:331–339. doi: 10.1007/s10585-005-0660-5. [DOI] [PubMed] [Google Scholar]
- 58.Red Eagle A. R., Hanson R. L., Jiang W., Han X., Matters G. L., Imperatore G., Knowler W. C., Bond J. S. Meprin β metalloprotease gene polymorphisms associated with diabetic nephropathy in the Pima Indians. Hum. Genet. 2005;118:12–22. doi: 10.1007/s00439-005-0019-7. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z., Herzog C., Kaushal G. P., Gokden N., Mayeux P. R. Actinonin, a meprin A inhibitor, protects the renal microcirculation during sepsis. Shock. 2011;35:141–147. doi: 10.1097/SHK.0b013e3181ec39cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirano M., Ma B. Y., Kawasaki N., Okimura K., Baba M., Nakagawa T., Miwa K., Oka S., Kawasaki T. Mannan-binding protein blocks the activation of metalloproteases meprin α and β. J. Immunol. 2005;175:3177–3185. doi: 10.4049/jimmunol.175.5.3177. [DOI] [PubMed] [Google Scholar]
- 61.Hedrich J., Lottaz D., Meyer K., Yiallouros I., Jahnen-Dechent W., Stocker W., Becker-Pauly C. Fetuin-A and cystatin C are endogenous inhibitors of human meprin metalloproteases. Biochemistry. 2010;49:8599–8607. doi: 10.1021/bi1004238. [DOI] [PubMed] [Google Scholar]
- 62.Hirano M., Ma B. Y., Kawasaki N., Oka S., Kawasaki T. Role of interaction of mannan-binding protein with meprins at the initial step of complement activation in ischemia/reperfusion injury to mouse kidney. Glycobiology. 2012;22:84–95. doi: 10.1093/glycob/cwr107. [DOI] [PubMed] [Google Scholar]
- 63.Neuman M. G. Immune dysfunction in inflammatory bowel disease. Transl. Res. 2007;149:173–186. doi: 10.1016/j.trsl.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 64.Franke A., McGovern D. P., Barrett J. C., Wang K., Radford-Smith G. L., Ahmad T., Lees C. W., Balschun T., Lee J., Roberts R., et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat. Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGovern D. P., Gardet A., Torkvist L., Goyette P., Essers J., Taylor K. D., Neale B. M., Ong R. T., Lagace C., Li C., et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat. Genet. 2010;42:332–337. doi: 10.1038/ng.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banerjee S., Bond J. S. Prointerleukin-18 is activated by meprin β in vitro and in vivo in intestinal inflammation. J. Biol. Chem. 2008;283:31371–31377. doi: 10.1074/jbc.M802814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coskun M., Olsen A. K., Holm T. L., Kvist P. H., Nielsen O. H., Riis L. B., Olsen J., Troelsen J. T. TNF-α-induced down-regulation of CDX2 suppresses MEP1A expression in colitis. Biochim. Biophys. Acta. 2012;1822:843–851. doi: 10.1016/j.bbadis.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 68.Vazeille E., Bringer M. A., Gardarin A., Chambon C., Becker-Pauly C., Pender S. L., Jakob C., Muller S., Lottaz D., Darfeuille-Michaud A. Role of meprins to protect ileal mucosa of Crohn's disease patients from colonization by adherent-invasive E. coli. PLoS ONE. 2011;6:e21199. doi: 10.1371/journal.pone.0021199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boudeau J., Glasser A. L., Masseret E., Joly B., Darfeuille-Michaud A. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect. Immun. 1999;67:4499–4509. doi: 10.1128/iai.67.9.4499-4509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahn S. H., Shah Y. M., Inoue J., Morimura K., Kim I., Yim S., Lambert G., Kurotani R., Nagashima K., Gonzalez F. J., Inoue Y. Hepatocyte nuclear factor 4α in the intestinal epithelial cells protects against inflammatory bowel disease. Inflamm. Bowel Dis. 2008;14:908–920. doi: 10.1002/ibd.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herzog C., Haun R. S., Kaushal V., Mayeux P. R., Shah S. V., Kaushal G. P. Meprin A and meprin α generate biologically functional IL-1β from pro-IL-1β. Biochem. Biophys. Res. Commun. 2009;379:904–908. doi: 10.1016/j.bbrc.2008.12.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herzog C., Kaushal G. P., Haun R. S. Generation of biologically active interleukin-1β by meprin B. Cytokine. 2005;31:394–403. doi: 10.1016/j.cyto.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Ambort D., Brellier F., Becker-Pauly C., Stocker W., Andrejevic-Blant S., Chiquet M., Sterchi E. E. Specific processing of tenascin-C by the metalloprotease meprinbeta neutralizes its inhibition of cell spreading. Matrix Biol. 2009;29:31–42. doi: 10.1016/j.matbio.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 74.Oneda B., Lods N., Lottaz D., Becker-Pauly C., Stocker W., Pippin J., Huguenin M., Ambort D., Marti H. P., Sterchi E. E. Metalloprotease meprin β in rat kidney: glomerular localization and differential expression in glomerulonephritis. PLoS ONE. 2008;3:e2278. doi: 10.1371/journal.pone.0002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choudry Y., Kenny A. J. Hydrolysis of transforming growth factor-α by cell-surface peptidases in vitro. Biochem. J. 1991;280:57–60. doi: 10.1042/bj2800057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Minder P., Bayha E., Becker-Pauly C., Sterchi E. E. Meprin α transactivates the epidermal growth factor receptor (EGFR) via ligand shedding, thereby enhancing colorectal cancer cell proliferation and migration. J. Biol. Chem. 2012;287:35201–35211. doi: 10.1074/jbc.M112.368910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cosgrove S., Chotirmall S. H., Greene C. M., McElvaney N. G. Pulmonary proteases in the cystic fibrosis lung induce interleukin 8 expression from bronchial epithelial cells via a heme/meprin/epidermal growth factor receptor/Toll-like receptor pathway. J. Biol. Chem. 2011;286:7692–7704. doi: 10.1074/jbc.M110.183863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Richman-Eisenstat J. B., Jorens P. G., Hebert C. A., Ueki I., Nadel J. A. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am. J. Physiol. 1993;264:L413–L418. doi: 10.1152/ajplung.1993.264.4.L413. [DOI] [PubMed] [Google Scholar]
- 79.Craig S. S., Reckelhoff J. F., Bond J. S. Distribution of meprin in kidneys from mice with high- and low-meprin activity. Am. J. Physiol. 1987;253:C535–C540. doi: 10.1152/ajpcell.1987.253.4.C535. [DOI] [PubMed] [Google Scholar]
- 80.Beynon R. J., Bond J. S. Deficiency of a kidney metalloproteinase activity in inbred mouse strains. Science. 1983;219:1351–1353. doi: 10.1126/science.6338590. [DOI] [PubMed] [Google Scholar]
- 81.Mathew R., Futterweit S., Valderrama E., Tarectecan A. A., Bylander J. E., Bond J. S., Trachtman H. Meprin-α in chronic diabetic nephropathy: interaction with the renin-angiotensin axis. Am. J. Physiol. Renal Physiol. 2005;289:F911–F921. doi: 10.1152/ajprenal.00037.2005. [DOI] [PubMed] [Google Scholar]
- 82.DeGuzman J. B., Speiser P. W., Trachtman H. Urinary meprin-α: a potential marker of diabetic nephropathy. J. Pediatr. Endocrinol. Metab. 2004;17:1663–1666. doi: 10.1515/jpem.2004.17.12.1663. [DOI] [PubMed] [Google Scholar]
- 83.Trachtman H., Valderrama E., Dietrich J. M., Bond J. S. The role of meprin A in the pathogenesis of acute renal failure. Biochem. Biophys. Res. Commun. 1995;208:498–505. doi: 10.1006/bbrc.1995.1366. [DOI] [PubMed] [Google Scholar]
- 84.Walker P. D., Kaushal G. P., Shah S. V. Meprin A, the major matrix degrading enzyme in renal tubules, produces a novel nidogen fragment in vitro and in vivo. Kidney Int. 1998;53:1673–1680. doi: 10.1046/j.1523-1755.1998.00949.x. [DOI] [PubMed] [Google Scholar]
- 85.Bylander J., Li Q., Ramesh G., Zhang B., Reeves W. B., Bond J. S. Targeted disruption of the meprin metalloproteinase β gene protects against renal ischemia-reperfusion injury in mice. Am. J. Physiol. Renal Physiol. 2008;294:F480–F490. doi: 10.1152/ajprenal.00214.2007. [DOI] [PubMed] [Google Scholar]
- 86.Herzog C., Seth R., Shah S. V., Kaushal G. P. Role of meprin A in renal tubular epithelial cell injury. Kidney Int. 2007;71:1009–1018. doi: 10.1038/sj.ki.5002189. [DOI] [PubMed] [Google Scholar]
- 87.Schrier R. W., Wang W., Poole B., Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J. Clin. Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaushal G. P., Walker P. D., Shah S. V. An old enzyme with a new function: purification and characterization of a distinct matrix-degrading metalloproteinase in rat kidney cortex and its identification as meprin. J. Cell Biol. 1994;126:1319–1327. doi: 10.1083/jcb.126.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yura R. E., Bradley S. G., Ramesh G., Reeves W. B., Bond J. S. Meprin A metalloproteases enhance renal damage and bladder inflammation after LPS challenge. Am. J. Physiol. Renal Physiol. 2009;296:F135–F144. doi: 10.1152/ajprenal.90524.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holly M. K., Dear J. W., Hu X., Schechter A. N., Gladwin M. T., Hewitt S. M., Yuen P. S., Star R. A. Biomarker and drug-target discovery using proteomics in a new rat model of sepsis-induced acute renal failure. Kidney Int. 2006;70:496–506. doi: 10.1038/sj.ki.5001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berthier C., Marti H. P. Metzincins, including matrix metalloproteinases and meprin, in kidney transplantation. Swiss Med. Wkly. 2007;137:109S–114S. [PubMed] [Google Scholar]
- 92.Berthier C. C., Lods N., Joosten S. A., van Kooten C., Leppert D., Lindberg R. L., Kappeler A., Raulf F., Sterchi E. E., Lottaz D., Marti H. P. Differential regulation of metzincins in experimental chronic renal allograft rejection: potential markers and novel therapeutic targets. Kidney Int. 2006;69:358–368. doi: 10.1038/sj.ki.5000049. [DOI] [PubMed] [Google Scholar]
- 93.Carmago S., Shah S. V., Walker P. D. Meprin, a brush-border enzyme, plays an important role in hypoxic/ischemic acute renal tubular injury in rats. Kidney Int. 2002;61:959–966. doi: 10.1046/j.1523-1755.2002.00209.x. [DOI] [PubMed] [Google Scholar]
- 94.Takayama J., Takaoka M., Yamamoto S., Nohara A., Ohkita M., Matsumura Y. Actinonin, a meprin inhibitor, protects ischemic acute kidney injury in male but not in female rats. Eur. J. Pharmacol. 2008;581:157–163. doi: 10.1016/j.ejphar.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 95.LaFerla F. M., Oddo S. Alzheimer's disease: Aβ, tau and synaptic dysfunction. Trends Mol. Med. 2005;11:170–176. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 96.Selkoe D. J. Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid β-protein. J. Alzheimers Dis. 2001;3:75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- 97.Bien J., Jefferson T., Causevic M., Jumpertz T., Munter L., Multhaup G., Weggen S., Becker-Pauly C., Pietrzik C. U. The metalloprotease meprin β generates amino terminal-truncated amyloid β peptide species. J. Biol. Chem. 2012;287:33304–33313. doi: 10.1074/jbc.M112.395608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wiltfang J., Esselmann H., Cupers P., Neumann M., Kretzschmar H., Beyermann M., Schleuder D., Jahn H., Ruther E., Kornhuber J., et al. Elevation of β-amyloid peptide 2–42 in sporadic and familial Alzheimer's disease and its generation in PS1 knockout cells. J. Biol. Chem. 2001;276:42645–42657. doi: 10.1074/jbc.M102790200. [DOI] [PubMed] [Google Scholar]
- 99.Bibl M., Gallus M., Welge V., Lehmann S., Sparbier K., Esselmann H., Wiltfang J. Characterization of cerebrospinal fluid aminoterminally truncated and oxidized amyloid-β peptides. Proteomics Clin. Appl. 2012;6:163–169. doi: 10.1002/prca.201100082. [DOI] [PubMed] [Google Scholar]
- 100.Jefferson T., Causevic M., auf dem Keller U., Schilling O., Isbert S., Geyer R., Maier W., Tschickardt S., Jumpertz T., Weggen S., et al. Metalloprotease meprin β generates nontoxic N-terminal amyloid precursor protein fragments in vivo. J. Biol. Chem. 2011;286:27741–27750. doi: 10.1074/jbc.M111.252718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Portelius E., Brinkmalm G., Tran A. J., Zetterberg H., Westman-Brinkmalm A., Blennow K. Identification of novel APP/Aβ isoforms in human cerebrospinal fluid. Neurodegener. Dis. 2009;6:87–94. doi: 10.1159/000203774. [DOI] [PubMed] [Google Scholar]
- 102.Kuhn P. H., Wang H., Dislich B., Colombo A., Zeitschel U., Ellwart J. W., Kremmer E., Rossner S., Lichtenthaler S. F. ADAM10 is the physiologically relevant, constitutive α-secretase of the amyloid precursor protein in primary neurons. EMBO J. 2010;29:3020–3032. doi: 10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Postina R. Activation of α-secretase cleavage. J. Neurochem. 2012;120:46–54. doi: 10.1111/j.1471-4159.2011.07459.x. [DOI] [PubMed] [Google Scholar]
- 104.Moss M. L., Bomar M., Liu Q., Sage H., Dempsey P., Lenhart P. M., Gillispie P. A., Stoeck A., Wildeboer D., Bartsch J. W., et al. The ADAM10 prodomain is a specific inhibitor of ADAM10 proteolytic activity and inhibits cellular shedding events. J. Biol. Chem. 2007;282:35712–35721. doi: 10.1074/jbc.M703231200. [DOI] [PubMed] [Google Scholar]
- 105.Jorissen E., Prox J., Bernreuther C., Weber S., Schwanbeck R., Serneels L., Snellinx A., Craessaerts K., Thathiah A., Tesseur I., et al. The disintegrin/metalloproteinase ADAM10 is essential for the establishment of the brain cortex. J. Neurosci. 2010;30:4833–4844. doi: 10.1523/JNEUROSCI.5221-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lottaz D., Maurer C. A., Noel A., Blacher S., Huguenin M., Nievergelt A., Niggli V., Kern A., Muller S., Seibold F., et al. Enhanced activity of meprin-α, a pro-migratory and pro-angiogenic protease, in colorectal cancer. PLoS ONE. 2011;6:e26450. doi: 10.1371/journal.pone.0026450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 108.Nasevicius A., Larson J., Ekker S. C. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast. 2000;17:294–301. doi: 10.1002/1097-0061(200012)17:4<294::AID-YEA54>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nasevicius A., Ekker S. C. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 110.Boerrigter G., Costello-Boerrigter L. C., Harty G. J., Huntley B. K., Cataliotti A., Lapp H., Burnett J. C., Jr B-type natriuretic peptide 8–32, which is produced from mature BNP 1–32 by the metalloprotease meprin A, has reduced bioactivity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R1744–R1750. doi: 10.1152/ajpregu.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lottaz D., Maurer C. A., Hahn D., Buchler M. W., Sterchi E. E. Nonpolarized secretion of human meprin α in colorectal cancer generates an increased proteolytic potential in the stroma. Cancer Res. 1999;59:1127–1133. [PubMed] [Google Scholar]
- 112.Heinzelmann-Schwarz V. A., Scolyer R. A., Scurry J. P., Smith A. N., Gardiner-Garden M., Biankin A. V., Baron-Hay S., Scott C., Ward R. L., Fink D., et al. Low meprin α expression differentiates primary ovarian mucinous carcinoma from gastrointestinal cancers that commonly metastasise to the ovaries. J. Clin. Pathol. 2007;60:622–626. doi: 10.1136/jcp.2005.034223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002;12:390–399. [PubMed] [Google Scholar]
- 114.Huguenin M., Muller E. J., Trachsel-Rosmann S., Oneda B., Ambort D., Sterchi E. E., Lottaz D. The metalloprotease meprin β processes E-cadherin and weakens intercellular adhesion. PLoS ONE. 2008;3:e2153. doi: 10.1371/journal.pone.0002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weber S., Niessen M. T., Prox J., Lullmann-Rauch R., Schmitz A., Schwanbeck R., Blobel C. P., Jorissen E., de Strooper B., Niessen C. M., Saftig P. The disintegrin/metalloproteinase Adam10 is essential for epidermal integrity and Notch-mediated signaling. Development. 2011;138:495–505. doi: 10.1242/dev.055210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bourhis J. M., Mariano N., Zhao Y., Harlos K., Exposito J. Y., Jones E. Y., Moali C., Aghajari N., Hulmes D. J. Structural basis of fibrillar collagen trimerization and related genetic disorders. Nat. Struct. Mol. Biol. 2012;19:1031–1036. doi: 10.1038/nsmb.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hulmes D. J. Building collagen molecules, fibrils, and suprafibrillar structures. J. Struct. Biol. 2002;137:2–10. doi: 10.1006/jsbi.2002.4450. [DOI] [PubMed] [Google Scholar]
- 118.Crooks G. E., Hon G., Chandonia J. M., Brenner S. E. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.