Abstract
Background:
The purpose of this multicenter Spanish study was to evaluate the response to immediate-release methylphenidate by children and adults diagnosed with attention-deficit/hyperactivity disorder (ADHD), as well as to obtain information on current therapy patterns and safety characteristics.
Methods:
This multicenter, observational, retrospective, noninterventional study included 730 patients aged 4–65 years with a diagnosis of ADHD. Information was obtained based on a review of medical records for the years 2002–2006 in sequential order.
Results:
The ADHD predominantly inattentive subtype affected 29.7% of patients, ADHD predominantly hyperactive-impulsive was found in 5.2%, and the combined subtype in 65.1%. Overall, a significant lower Clinical Global Impression (CGI) score and mean number of DSM-IV TR (Diagnostic and Statistical Manual of Mental Disorders Fourth Edition, Text Revision) symptoms by subtype were found after one year of treatment with immediate-release methylphenidate; CGI decreased from 4.51 to 1.69, symptoms of inattention from 7.90 to 4.34, symptoms of hyperactivity from 6.73 to 3.39, and combined subtype symptoms from 14.62 to 7.7. Satisfaction with immediate-release methylphenidate after one year was evaluated as “very satisfied” or “satisfied” by 86.90% of the sample; 25.75% of all patients reported at least one adverse effect. At the end of the study, 41.47% of all the patients treated with immediate-release methylphenidate were still receiving it, with a mean time of 3.80 years on therapy.
Conclusion:
Good efficacy and safety results were found for immediate-release methylphenidate in patients with ADHD.
Keywords: attention deficit hyperactivity disorder, ADHD, pharmacologic treatment, methylphenidate, satisfaction
Background
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common neurobehavioral disorders of childhood, may continue to show symptoms in adolescence and adult life, and is characterized by symptoms of inattention and/or hyperactivity-impulsivity. Children with ADHD may experience significant adaptation problems because their functional level and behavior may not correspond to their chronological age or expected development level.1,2
Estimated prevalence rates in the general population vary from 3% to 7%. The condition is more frequent in boys than in girls (proportion varies between 9:1 and 2.5:1 according to the population studied), even though increasing numbers of girls affected by ADHD are being identified. ADHD is a chronic disease, the symptoms of which can persist into adulthood and become lifelong.3–5
The diagnosis of ADHD requires a complete medical evaluation to detect specific symptoms that have to be present in consistent contexts and with a persistent level of deterioration.6 The presence of symptoms is directly obtained from the child, parents, and teachers. Multiple scales have been created to identify specific symptoms in order to diagnose ADHD, but most of the criteria are similar to those of the DSM-IV TR (Diagnostic and Statistical Manual of Mental Disorders Fourth Edition, Text Revision).6 ADHD is classified into three subtypes, ie, ADHD predominantly inattentive (10%–15% of patients), ADHD predominantly hyperactive-impulsive (5%), and ADHD combined type (80%).7–9 Pharmacologic treatment with stimulants usually has a direct effect of reducing overactivity and increasing attention,10 with these effects being evident within a short time. Because of its efficacy and safety profile, methylphenidate is the most commonly used stimulant in ADHD.10,11
The aim of this retrospective study was to evaluate the response of patients diagnosed with ADHD to immediate-release methylphenidate (MPH-IR), and to obtain information on current treatment patterns and any adverse effects during follow-up. Improvement in quality of life for patients and their families was also evaluated.
Materials and methods
Patients
Patients of both genders, aged 4–65 years, diagnosed with ADHD according to DSM-IV TR criteria, having an intelligence quotient higher than 80, and treated with MPH-IR at the start of follow-up were included in this study. Information on treatment patterns from January 2002 to December 2006 was collected from patient medical records in a sequential order. Patients whose response to MPH-IR could not be evaluated and those participating in other clinical trials were excluded.
Design and treatments
This observational, retrospective, noninterventional, multicenter study was carried out in the neurology, clinical neurophysiology, pediatric, and psychiatry departments of 25 Spanish centers. The study was conducted in accordance with the recommendations of the Declaration of Helsinki, the Guidelines for Good Clinical Practice, the International Guidelines for Ethical Review of Epidemiological Studies (1991), and the Spanish Epidemiological Society.12–14 The study was submitted to the corresponding ethics committee for approval and following the Spanish Law 15/1999 on Personal Character Data Protection concerning confidentiality of patient data.
Measures
Baseline data included demographic information and the clinical history of ADHD, ie, date of diagnosis, number of ADHD symptoms according to DSM-IV TR subtypes, comorbidities, associated DSM-IV TR disorders, current methylphenidate therapy profile (including previous treatments, methylphenidate dosage and frequency of administration, and modifications of ADHD therapy in the year following baseline), and other related symptoms. The Clinical Global Impression (CGI) score was also collected as a reference for severity of illness and global improvement, whether or not these were related to therapy.
A year after treatment, in addition to baseline information, satisfaction with current treatment was evaluated on a five-point scale (1, very satisfied; 2, satisfied; 3, neutral, neither satisfied or dissatisfied; 4, dissatisfied; and 5, very dissatisfied). Information on continuity of treatment with MPH-IR, use of concomitant medication, and adverse events were obtained. All parameters were assessed globally and by subgroup of age (<6 years, 6–16 years, and 17–65 years).
Statistical analysis
The information collected was processed using SAS version 9.1 software (SAS, Cary, NC, USA). The main statistical analysis was of a descriptive nature. Categorical variables are presented according to the number and percentage of patients for each category. Continuous variables are expressed as the mean, standard deviation, median, inferior and superior quartile, and minimal and maximal values.
The 95% confidence intervals are presented as appropriate. No interpolation or extrapolation methods were used to assign missing values. Contingence tables were constructed for cross-analysis of data and parametric and nonparametric tests, depending on the results. Univariate, bivariate, and stratified methods of analysis were used. An exploratory analysis was undertaken to analyze the relationship between outcomes of interest and the different data recorded throughout the study. Safety analyses were performed on the safety dataset, defined as all patients who took at least one dose of MPH-IR, whether or not the inclusion criteria were met. Safety outcomes included adverse events and serious adverse events reported, and their recurrence, duration, and relationship with the study drug.
The main variable, ie, number of DSM-IV TR symptoms, was evaluated at the start and end of treatment with MPH-IR using inference statistic techniques to identify any significant changes between number of symptoms (Student’s t-test for paired samples) and any influence of a number of factors on the probability of response to treatment.
Results
Demographic and anamnesis characteristics
From a total of patients, 598 were evaluable according to the inclusion criteria. The safety population included all 730 patients identified in the study. Patients were classified into three categories by age, ie, <6 years (5.7%), 6–16 years (88.1%), and 17–65 years (6.2%). The clinical and demographic characteristics of the patients are listed in Table 1. Their mean age was approximately 11 years and 80.87% were males.
Table 1.
Baseline demographic and clinical data at the beginning of immediate-release methylphenidate treatment
| Total (n = 598) | <6 years (n = 34) | 6–16 years (n = 525) | 17–65 years (n = 37) | |
|---|---|---|---|---|
| Age (years), mean (SD) | 10.85 (6.33) | 5.27 (0.49) | 9.77 (2.37) | 31.26 (10.37) |
| Height (m), mean (SD) | 1.37 (0.16) | 1.11 (0.08) | 1.37 (0.14) | 1.69 (0.11) |
| Weight (kg), mean (SD) | 35.50 (14.23) | 20.81 (3.80) | 35.24 (11.88) | 72.87 (25.01) |
| SBP (mmHg), mean (SD) | 103.8 (13.69) | 93.50 (8.02) | 103.2 (12.80) | 123.1 (16.61) |
| DBP (mmHg), mean (SD) | 65.43 (12.72) | 56.83 (11.38) | 65.66 (12.57) | 71.15 (13.59) |
| Gender, n (%) | ||||
| Male | 482 (80.87) | 31 (91.18) | 423 (80.57) | 28 (75.68) |
| Female | 114 (19.13) | 3 (8.82) | 102 (19.43) | 9 (24.32) |
| Total | 596 (100.0) | 34 (100.0) | 525 (100.0) | 37 (100.0) |
| ADHD subtype, n (%) | ||||
| Predominantly inattentive | 177 (29.7) | 3 (8.8) | 165 (31.4) | 9 (24.3) |
| Predominantly hyperactive-impulsive | 31 (5.2) | 5 (14.7) | 23 (4.4) | 3 (8.1) |
| Combined | 388 (65.1) | 26 (76.5) | 337 (64.2) | 25 (67.6) |
| Familial history, n (%) | ||||
| Yes | 195 (32.61) | 7 (20.59) | 172 (32.64) | 16 (43.24) |
| No | 403 (67.39) | 27 (79.41) | 355 (67.36) | 21 (56.76) |
| Total | 598 (100.0) | 34 (100.0) | 527 (100.0) | 37 (100.0) |
Abbreviations: ADHD, attention deficit hyperactivity disorder; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
Based on DSM-IV TR criteria, the ADHD predominantly inattentive subtype accounted for 29.7% (<6 years, 8.8%; 17–65 years, 31.4%; 17–65 years, 24.3%), predominantly hyperactive-impulsive subtype for 5.2% (<6 years,14.7%; 17–65 years, 4.4%; 17–65 years, 8.1%), and combined subtype for 65.1% (<6 years, 76.5%; 17–65 years, 64.2%; 17–65 years, 67.6%) of patients.
Comorbid DSM-IV TR disorders were identified in the total patient population and for subgroups by age. Anxiety disorder was diagnosed in 13.21% of all patients (<6 years, 8.8%; 6–16 years, 13.3%; 17–65 years, 16.2%); oppositional defiant disorder in 22.1% (<6 years, 38.2%; 6–16 years, 22.4%; 17–65 years, 2.70%); learning disorder in 47.3% (<6 years, 44.1%; 6–16 years, 50.5%; 17–65 years, 5.4%); conduct disorder in 28.6% (<6 years, 38.24%; 6–16 years, 29.8%; 17–65 years, 2.7%); depressive disorder in 6.35% (<6 years, 0%; 6–16 years, 5.7%; 17–65 years, 21.6%); tics in 6.86% (<6 years, 5.9%; 6–16 years, 7.0%; 17–65 years, 5.41%); and substance abuse disorder in 1.84% (<6 years, 0%; 6–16 years, 0%–0.38%; 17–65 years, 24.3%). Other associated symptoms were apathy (8.5%, all aged 6–16 years) and anhedonia (3.85%, all aged 6–16 years).
Information on the presence of ADHD-associated disorders, in accordance with DSM-IV TR criteria, was also collected. Developmental coordination disorder was identified in 11.87% of patients (<6 years, 5.9%; 6–16 years, 13.1%), development dysphasia in 9.87% (<6 years, 5.9%; 6–16 years, 10.8%); Tourette’s disorder in 0.84% (<6 years, 2.9%; 6–16 years, 0.57%; 17–65 years, 22.7%), and generalized epilepsy in 1.67% (<6 years, 8.8%; 6–16 years, 1.3%).
Mean patient age at the start of treatment was 10.85 years (<6 years, mean 5.27 years; 6–16 years, mean 9.77 years; 17–65 years, mean 31.26 years), with a mean interval of 2.59 months between diagnosis and start of medication (<6 years, 1.06 months; 6–16 years, 2.74 months; 17–65 years, 1.89 months) and a mean time on treatment of 9.85 months (<6 years, 11.01 months; 6–16 years, 9.93 months; 17–65 years, 7.35 months).
Treatment characteristics
The mean starting dose of MPH-IR was 18.11 mg/day (11.84 mg/day for patients aged < 6 years, 17.04 mg/day for those aged 6–16 years, and 39.05 mg/day for those aged 17–65 years). For patients who were still receiving methylphenidate after one year, the mean dose was 22.84 mg/day (19.58 mg/day for those aged < 6 years; 22.18 mg/day for those aged 6–16 years; 60 mg/day for those aged 17–65 years). There were significant statistical differences on average doses between the reported at baseline and those that correspond to follow up visits (1 month, 3 months, 6 months and a year).
Dose modifications were required after one month of therapy in 25.08% of all patients (<6 years, 17.65%; 6–16 years, 24.29%; 17–65 years, 43.24%). This change was necessary after 6 months for 30.43% of patients (<6 years, 26.5%; 6–16 years, 30.55%; 17–65 years, 32.4%) and after one year for 34.45% of patients (<6 years, 20.59%; 6–16 years, 32.83%; 17–65 years, 70.27%). The most frequent reason for modification of therapy after one and 6 months was uptitration of the dose (67.33% and 46.70%, respectively). After one year, a 30,58% of all dose changes were related to inefficacy of current dose. However, for a 54.85% there were not specific reasons registered.
Treatment evaluation
A total of 365 (61.04%) patients completed one year of treatment with MPH-IR (<6 years, 70.6%; 6–16 years, 63%; 17–65 years, 24.3%). Evaluation based on CGI score was performed by a specialist prior to and one year after starting treatment with MPH-IR. On this basis, 69.86% of patients were considered to be “moderately-severely ill” and 0.27% were considered to be “normal-doubtfully ill” at the beginning of therapy. After one year, 2.46% of patients were considered to be “moderately-severely ill”, and 63.29% were considered to be “normal-doubtfully ill”.
In patients aged < 6 years, 83.33% versus 8.33%, respectively, were deemed to be in the “moderate-severely ill” CGI category before and after one year of treatment with MPH-IR; the corresponding figures were 68.37% versus 1.81% for those aged 6–16 years and 88.9% versus 0% for those aged 17–65 years (Table 2). Of the 233 patients who did not continue with MPH-IR beyond one year, 51.93% changed to extended-release methylphenidate (MPH-ER; <6 years, 10%; 6–16 years, 49.7%; and 17–65 years, 82.1%). The principal reasons for the change from MPH-IR to MPH-ER were “other reasons” (all ages, 55.65%; 6–16 years, 46.8%; 17–65 years, 95%), inefficacy (all ages, 22.61%; 6–16 years, 27.66%), and usual procedure (all ages, 20.87%; 6–16 years, 25.53%).
Table 2.
Clinical Global Impression score and number of symptoms (DSM-IV TR) by ADHD subtypes at baseline and after one year of immediate-release methylphenidate
| Total | <6 years | 6–16 years | 17–65 years | |
|---|---|---|---|---|
| CGI score, mean (SD) | ||||
| At baseline | 4.51 (1.01) | 4.5 (1.41) | 4.52 (0.97) | 4.44 (1.13) |
| After one year | 1.67 (0.91)* | 1.83 (1.13)* | 1.66 (0.89)* | 1.2 (0.45) |
| DSM-IV TR number of symptoms by ADHD subtypes, mean (SD) | ||||
| Predominantly inattentive baseline | 7.90 (1.33) | 7.71 (1.92) | 7.93 (1.27) | 7.22 (3.02) |
| Predominantly inattentive after one year | 4.34 (1.56)* | 4.31 (2.21)* | 4.34 (1.52)* | – |
| Predominantly hyperactive-impulsive baseline | 6.73 (2.43) | 7.58 (1.91) | 6.71 (2.42) | 5.00 (2.96) |
| Predominantly hyperactive-impulsive after one year | 3.39 (1.99)* | 3.69 (2.32)* | 3.37 (1.98)* | – |
| Combined baseline | 14.62 (2.98) | 15.29 (3.06) | 14.63 (2.95) | 12.22 (2.95) |
| Combined type after one year | 7.70 (3.06)* | 8.00 (3.87)* | 7.68 (3.02)* | – |
Note:
Statistically significant between baseline and after one year of treatment (P < 0.0001).
Abbreviations: ADHD, attention deficit hyperactivity disorder; CGI, Clinical Global Impression; DSM-IV TR, Diagnostic and Statistical Manual of Mental Disorders Fourth Edition, Text Revision; SD, standard deviation.
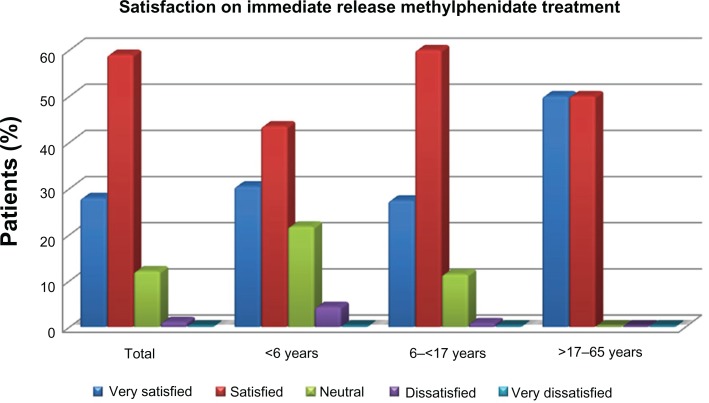
After one year of treatment with MPH-IR, 86.9% were “very satisfied” or “satisfied” with therapy (<6 years, 87.8%; 6–16 years, 73.91%). Patients who changed to MPH-ER during follow-up reported themselves as being “very satisfied” or “satisfied” in 65.69% of cases (6–16 years, 61.6%; 17–65 years, 87.5%) after a mean 5.59 months of treatment. Satisfaction with MPH-IR therapy is shown in Figure 1.
Figure 1.
Evaluation of satisfaction with immediate-release and extended-release methylphenidate treatment.
At the end of the study, 41.47% of all patients were still receiving MPH-IR (<6 years 47.1%; 6–16 years, 42.5%; 17–65 years, 21.6%). For these patients (n = 248), the mean duration of MPH-IR therapy was 3.73 years (<6 years, 3.73 years; 6–16 years, 3.80 years; 17–65 years, 3.92 years).
Concomitant medication
Of all the evaluable patients (n = 598), 25.92% received other medication concomitantly with MPH-IR, with 17.06% having at least one concomitant medication. Of all the age groups, those aged < 6 years showed the highest concomitant medication use (29.41%, Table 3).
Table 3.
Concomitant medication
| Total | <6 years | 6–16 years | 17–65 years | |
|---|---|---|---|---|
| Patients with concomitant medication, n (%) | ||||
| Yes | 132 (22.07) | 10 (29.41) | 114 (21.63) | 8 (21.62) |
| No | 466 (77.93) | 24 (70.59) | 413 (78.37) | 29 (78.38) |
| Total | 598 (100.00) | 34 (100.00) | 527 (100.00) | 37 (100.00) |
| Concomitant medications by patient, n (%) | ||||
| None | 466 (77.93) | 24 (70.59) | 413 (78.37) | 29 (78.38) |
| One medication | 102 (17.06) | 8 (23.53) | 89 (16.89) | 5 (13.51) |
| Two medications | 24 (4.01) | 2 (5.88) | 20 (3.80) | 2 (5.41) |
| Three or more medications | 6 (1.00) | – | 5 (0.95) | 1 (2.70) |
| Total | 598 (100.00) | 34 (100.00) | 527 (100.00) | 37 (100.00) |
Safety
Of all patients included in the safety analysis (n = 730), at least one adverse event was reported for 25.75% (Table 4). The most common adverse events in all age groups (Table 5) were appetite and nutrition disorders (29.91% of all specified adverse events; <6 years, 3.85%; 6–16 years, 25.21%; 17–65 years, 0.85%).
Table 4.
Safety evaluation
| Total | <6 years | 6–16 years | 17–65 years | |
|---|---|---|---|---|
| Patients with AEs, n (%) | ||||
| Yes | 188 (25.75) | 20 (42.44) | 157 (24.80) | 11 (25.00) |
| No | 542 (74.25) | 27 (57.45) | 476 (75.20) | 33 (75.00) |
| Total | 730 (100.00) | 47 (100.00) | 633 (100.00) | 44 (100.00) |
| AEs by patient, n (%) | ||||
| None | 542 (74.24) | 27 (57.45) | 476 (75.20) | 33 (75.00) |
| 1 AE | 97 (13.29) | 7 (14.89) | 84 (13.27) | 6 (13.64) |
| 2 AEs | 52 (7.12) | 9 (19.15) | 39 (6.16) | 4 (9.09) |
| 3 or more AEs | 39 (5.34) | 4 (8.51) | 34 (5.37) | 1 (2.27) |
| Total | 730 (100.00) | 47 (100.00) | 633 (100.00) | 44 (100.00) |
Abbreviation: AE, adverse event.
Table 5.
Most commonly reported adverse events
| Total | <6 years | 6–16 years | 17–65 years | |
|---|---|---|---|---|
| Appetite and nutrition disorders | 70 (29.91) | 9 (3.85) | 59 (25.21) | 2 (0.85) |
| Sleep disorders | 35 (14.96) | 2 (0.85) | 31 (13.25) | 2 (0.85) |
| Physical activity modifications | 17 (7.26) | – | 16 (6.84) | 1 (0.43) |
| Personality and behavior disorders | 15 (6.41) | 1 (0.43) | 14 (5.98) | – |
| Nonspecific Pathogen infections (n) | 13 (5.56) | 1 (0.43) | 12 (5.13) | – |
| Headaches | 12 (5.13) | 1 (0.43) | 10 (4.27) | 1 (0.43) |
| Gastrointestinal symptomatology | 9 (3.85) | 3 (1.28) | 6 (2.56) | – |
| Other less frequent AEs | 63 (26.92) | 6 (2.56) | 51 (21.79) | 6 (2.56) |
| Total | 234 (100) | 23 (9.83) | 199 (85.03) | 12 (5.12) |
Abbreviation: AEs, adverse events.
Discussion
ADHD may cause difficulties at school, in the work place, and in the social environment. Subjects with ADHD in this study were of the predominantly inattentive subtype (29.7%), predominantly hyperactive-impulsive subtype (5.2%), and combined subtype (65.1%).
ADHD has been traditionally considered a childhood disorder. However, long-term follow-up studies have demonstrated the persistence of symptoms in many adults diagnosed with ADHD in childhood.2,15–18 A meta-analysis of follow-up ADHD studies reported that 15% of all cases show persistence of symptoms beyond childhood, and 40%–50% of patients continue to have a partial diagnostic status and impairment.19,20
A meta-analysis of studies of the prevalence of ADHD in adults reports a negative association with increasing age, conditioned by the gender composition of the sample.21 There is a clear predominance of ADHD in males, who have a 57% possibility of inheritance.22 In the present study, 81% of patients diagnosed to have ADHD were male, but this tendency decreased with increasing age (<6 years, 91%; 17–65 years, 76%). Adults with ADHD had a significantly higher number of first-degree relatives with ADHD than those without (28% versus 5%), and this finding is consistent with a recent report of strong familial clustering of ADHD in first-degree relatives of adults with ADHD in comparison with a control group.23 In that report, 32.6% had a family history of ADHD.
Results from our study showed the most frequent comorbidities to be learning disorders (47.3%), conduct disorders (28.6%), and oppositional defiant disorder (22.1%). These rates are comparable with those found in the 2007 US National Survey of Children’s Health,24 where 46% of children with ADHD had a learning disorder and 27% had a conduct disorder. Compared with the results of that survey, our findings with regard to psychological comorbidity showed lower rates of comorbid anxiety and depression (18% versus 13.2%, respectively, for anxiety; 14% versus 6.2%, respectively, for depression). Other relevant studies have reported comorbid depressive disorder rates of 5%–47% in children and adolescents with ADHD.25–27
Evidence from a meta-analysis of prospective studies in children with ADHD suggests that children with ADHD have a higher risk of developing substance use disorders and cigarette smoking than those without ADHD.28 In this meta-analysis, 24% of subjects aged 17–65 years had a substance abuse disorder.
Several studies have demonstrated the long-term efficacy and tolerability of MPH-IR.29–32 Methylphenidate has been established as an effective agent and is widely used to ameliorate the symptoms of ADHD.33–36 A meta-analysis of randomized controlled trials comparing methylphenidate and placebo in the treatment of ADHD identified a statistically significant beneficial clinical effect of MPH-IR in the short-term treatment of individuals with ADHD aged 18 years and younger.37 MPH-IR also reduces ADHD symptom scales in preschoolers,32 although the effect sizes are smaller than those found in school-aged children.33,37
Improvements in CGI scores after one year of treatment with MPH-IR have been reported in several studies of the effects of long-term treatment on symptom severity and social adjustment in ADHD.37 There was a considerable reduction in CGI score after one year of treatment with MPH-IR in patients previously categorized as moderately to severely ill (from 60% to 2%). Furthermore, treatment with MPH-IR significantly decreased both CGI score and the number of DSM-IV TR symptoms in the study populations, with similar results in comparison with published studies.38–40
It is important to point out the high percentage of patients (61%) who completed one year of treatment with MPH-IR, and it is also remarkable that, of patients who did not continue, a high percentage (52%) changed to MPH-ER.
In our study, global assessment of satisfaction with methylphenidate was high, ranging from 73% to 87%, within the three age groups. In all age groups, patients who changed to MPH-ER were less satisfied than those on MPH-IR. Our results for MPH-ER are not consistent with those in the reference literature in which patients were more satisfied with MPH-ER treatment.41–43
In this study, only 26% of patients experienced an adverse event, with appetite and nutrition disorders being the most frequent. Previous studies and reviews report insomnia, reduced appetite, abdominal pain, weight loss, tics, jitteriness, and headaches as the most common adverse effects of stimulant treatment, and, less frequently, anxiety, dizziness, and drowsiness,44–46 but these are usually tolerable.47 Overall, stimulants are relatively safe medications and the risks of not treating ADHD usually outweighs the risk of using stimulants.47
A limitation of the study is that even when favorable results were found overall in reduction of the number of symptoms and a significant global improvement, these results are only preliminary due to the limited numbers of patients included. In conclusion, patients with ADHD showed a good response to MPH-IR and a good safety profile with this treatment.
Acknowledgments
The authors wish to acknowledge Sergio Peris Cancio, Hospital Univesitario La Fe, Valencia and Covadonga Gonzalvo Rodríguez, Centro de Salud La Felguera, Langreo, Asturias for their collaboration in the study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.American Academy of Pediatrics Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics. 2000;105:1158–1170. doi: 10.1542/peds.105.5.1158. [DOI] [PubMed] [Google Scholar]
- 2.Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157:816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- 3.International Consensus Statement on ADHD Clin Child Fam Psychol Rev. 2002;5:89–111. doi: 10.1023/a:1017494719205. [DOI] [PubMed] [Google Scholar]
- 4.Kutcher S, Aman M, Brooks SJ, et al. International consensus statement on attention deficit hyperactivity disorder (ADHD) and disruptive behaviour disorders (DBDs): clinical implications and treatment practice suggestions. Eur Neuropsychopharmacol. 2004;14:11–28. doi: 10.1016/s0924-977x(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 5.Wilens TE, Faraone SV, Biederman J. Attention-deficit/hyperactivity disorder in adults. JAMA. 2004;292:619–623. doi: 10.1001/jama.292.5.619. [DOI] [PubMed] [Google Scholar]
- 6.Valdizán JR, Izaguerri-Gracia AC. ADHD in adults. Rev Neurol. 2009;48(Suppl 2):S95–S99. [PubMed] [Google Scholar]
- 7.American Psychiatric Association . The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 8.Polanczyk G, Silva de Lima M, Lessa B, Biederman J, Rohde A. The worldwide prevalence of ADHD: a systematic review and meta-regression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 9.Gaub M, Carlson CL. Behavioral characteristics of DSM-IV ADHD subtypes in a school-based population. J Abnorm Child Psychol. 1997;25:103–111. doi: 10.1023/a:1025775311259. [DOI] [PubMed] [Google Scholar]
- 10.Faraone SV, Spencer T, Aleardi M, Pagano C, Biederman J. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2004;24:24–29. doi: 10.1097/01.jcp.0000108984.11879.95. [DOI] [PubMed] [Google Scholar]
- 11.Conners CK, March JS, Frances A, Wells KC, Ross R. The expert consensus guideline series: treatment of attention-deficit/hyperactivity disorder. J Atten Disord. 2001;4(Suppl 1):7–128. [Google Scholar]
- 12.European Medicines Agency Note for guidance on Good Clinical Practice CPMP/ICH/135/95. Jul, 2002.
- 13.International Guidelines for Ethical Review of Epidemiological Studies . Council for the International Organizations of Medical Sciences. Ginebra: 1991. [Google Scholar]
- 14.Tormo MJ, Dal-Ré R, Pérez G. Ethics and epidemiological research: principles, applications and case studies. Barcelona, Spain: Spanish Society of Epidemiology; 1998. [Google Scholar]
- 15.Weiss G, Hechtman L, Milroy T, Perlman T. Psychiatric status of hyperactives as adults: a controlled prospective 15-year follow-up of 63 hyperactive children. J Am Acad Child Adolesc Psychiatry. 1985;24:211–220. doi: 10.1016/s0002-7138(09)60450-7. [DOI] [PubMed] [Google Scholar]
- 16.Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult follow-up of hyperactive children: antisocial activities and drug use. J Child Psychol Psychiatry. 2004;45:195–211. doi: 10.1111/j.1469-7610.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 17.Biederman J, Faraone SV, Spencer TJ, Mick E, Monuteaux MC, Aleardi M. Functional impairments in adults with self-reports of diagnosed ADHD: A controlled study of 1001 adults in the community. J Clin Psychiatry. 2006;67:524–540. doi: 10.4088/jcp.v67n0403. [DOI] [PubMed] [Google Scholar]
- 18.Mannuzza S, Klein RG, Bonagura N, Malloy P, Giampino TL, Addalli KA. Hyperactive boys almost grown up. V. Replication of psychiatric status. Arch Gen Psychiatry. 1991;48:77–83. doi: 10.1001/archpsyc.1991.01810250079012. [DOI] [PubMed] [Google Scholar]
- 19.Boomsma DI, Saviouk V, Hottenga JJ, et al. Genetic epidemiology of attention deficit hyperactivity disorder (ADHD index) in adults. PloS One. 2010;5:e10621. doi: 10.1371/journal.pone.0010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36:159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- 21.Simon V, Czobor P, Balint S, Meszaros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194:204–211. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- 22.Valdizán JR. Evaluation of diagnostic and therapeutic basis of immediate release methylphenidate in attention deficit disorder with hyperactivity. Rev Neurol. 2004;38:501–506. [PubMed] [Google Scholar]
- 23.Antshel KM, Faraone SV, Maglione K, et al. Is adult attention deficit hyperactivity disorder a valid diagnosis in the presence of high IQ? Psychol Med. 2009;39:1325–1335. doi: 10.1017/S0033291708004959. [DOI] [PubMed] [Google Scholar]
- 24.Larson K, Russ SA, Kahn RS, Halfon N. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2001;127:462–470. doi: 10.1542/peds.2010-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pliszka SR. Comorbidity of attention-deficit/hyperactivity disorder with psychiatric disorder: an overview. J Clin Psychiatry. 1998;59(Suppl 7):50–58. [PubMed] [Google Scholar]
- 26.Spencer T, Biederman J, Wilens T, Greene R. Principals and Practice. New York, NY: Oxford University Press; 2003. Pediatric Psychopharmacology. [Google Scholar]
- 27.Wilens TE, Biederman J, Brown S, et al. Psychiatric comorbidity and functioning in clinically referred preschool children an school age youths with ADHD. J Am Acad Child Adolesc Psychiatry. 2002;41:262–268. doi: 10.1097/00004583-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Wilens TE, Martelon MK, Joshi G, et al. Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adolesc Psychiatry. 2011;50:543–553. doi: 10.1016/j.jaac.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein S, Ellison AT, editors. Clinician’s Guide to Adult ADHD: Assessment and Intervention. San Diego, CA: Academic Press; 2002. [Google Scholar]
- 30.Greenhill LL, Pliszka S, Dulcan MK, et al. Summary of the practice parameter for the use of stimulant medications in the treatment of children, adolescents and adults. J Am Acad Child Adolesc Psychiatry. 2002;40:1352–1355. doi: 10.1097/00004583-200111000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Findling RL. Evolution of the treatment of attention-deficit/hyperactivity disorder in children: a review. Clin Ther. 2008;30:942–957. doi: 10.1016/j.clinthera.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Greenhill L, Kollins S, Abikoff H, et al. Efficacy and safety of inmmediate-release methylphenidate treatment for preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45:1284–1293. doi: 10.1097/01.chi.0000235077.32661.61. [DOI] [PubMed] [Google Scholar]
- 33.MTA Cooperative Group A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 34.Wilens TE, Biederman J. The stimulants. Psychiatr Clin North Am. 1992;15:191–222. [PubMed] [Google Scholar]
- 35.Flapper BC, Schoemaker MM. Effects of methylphenidate on quality of life in children with both developmental coordination disorder and ADHD. Dev Med Child Neurol. 2008;50:294–299. doi: 10.1111/j.1469-8749.2008.02039.x. [DOI] [PubMed] [Google Scholar]
- 36.Rapport MD, Denney C, DuPaul GJ, Gardner MJ. Attention deficit disorder and methylphenidate: normalization rates, clinical effectiveness, and response prediction in 76 children. J Am Acad Child Adolesc Psychiatry. 1994;33:882–893. doi: 10.1097/00004583-199407000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CMAJ. 2001;165:1475–1488. [PMC free article] [PubMed] [Google Scholar]
- 38.Wender PH, Reimherr FW, Marchant BK, Sanford ME, Czajkowski LA, Tomb DA. A one year trial of methylphenidate in the treatment of ADHD. J Atten Disord. 2011;15:36–45. doi: 10.1177/1087054709356188. [DOI] [PubMed] [Google Scholar]
- 39.Maayan L, Paykina MA, Fried J, Strauss T, Gugga S, Greenhill L. The open-label treatment of attention-deficit/hyperactivity disorder in 4- and 5-year old children with beaded methylphenidate. J Child Adolesc Psychopharmacol. 2009;19:147–153. doi: 10.1089/cap.2008.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conners CK. Attention-deficit/hyperactivity disorder-historical development and overview. J Atten Disord. 2000;3:173–191. [Google Scholar]
- 41.Mulas F, Mattos L, Hernandez-Muela S, R Gandia Updated treatment in attention deficit disorder and hyperactivity: Methylphenidate Extended Release. Rev Neurol. 2005;40(Suppl 1):S49–S55. [PubMed] [Google Scholar]
- 42.Steele M, Weiss M, Swanson J, Wang J, Prinzo RS, Binder CE. A randomized, controlled effectiveness trial of OROS-methylphenidate compared to usual care with immediate-release methylphenidate in attention deficit-hyperactivity disorder. Can J Clin Pharmacol. 2006;13:50–62. [PubMed] [Google Scholar]
- 43.Hoare P, Remschmidt H, Medori R, et al. 12-month efficacy and safety of OROS MPH in children and adolescents with attention-deficit/hyperactivity disorder switched from MPH. Eur Child Adolesc Psychiatry. 2005;14:305–309. doi: 10.1007/s00787-005-0486-3. [DOI] [PubMed] [Google Scholar]
- 44.Wigal T, Greenhill L, Chuang S, et al. Safety and tolerability of methylphenidate in preschool children with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45:1294–1303. doi: 10.1097/01.chi.0000235082.63156.27. [DOI] [PubMed] [Google Scholar]
- 45.Wolraich ML, Doffing MA. Pharmokinetic considerations in the treatment of attention-deficit hyperactivity disorder with methylphenidate. CNS Drugs. 2004;18:243–250. doi: 10.2165/00023210-200418040-00004. [DOI] [PubMed] [Google Scholar]
- 46.Lerner M, Wigal T. Long-term safety of stimulant medications used to treat children with ADHD. Pediatr Ann. 2008;37:37–45. doi: 10.3928/00904481-20080101-11. [DOI] [PubMed] [Google Scholar]
- 47.Merkel RL, Kuchibhatla A. Safety of stimulant treatment in attention deficit hyperactivity disorder: Part I. Expert Opin Drug Saf. 2009;8:655–668. doi: 10.1517/14740330903279956. [DOI] [PubMed] [Google Scholar]