Abstract
This article summarizes the author's research on fish oil derived n-3 fatty acids, plasma membrane organization and B cell function. We first cover basic model membrane studies that investigated how docosahexaenoic acid (DHA) targeted the organization of sphingolipid-cholesterol enriched lipid microdomains. A key finding here was that DHA had a relatively poor affinity for cholesterol. This work led to a model that predicted DHA acyl chains in cells would manipulate lipid-protein microdomain organization and thereby function. We then review how the predictions of the model were tested with B cells in vitro followed by experiments using mice fed fish oil. These studies reveal a highly complex picture on how n-3 fatty acids target lipid-protein organization and B cell function. Key findings are: 1) n-3 fatty acids target not just the plasma membrane but also endomembrane organization; 2) DHA, but not eicosapentaenoic acid (EPA), disrupts microdomain spatial distribution (i.e. clustering), 3) DHA alters protein lateral organization and 4) changes in membrane organization are accompanied by functional effects on both innate and adaptive B cell function. Altogether, the research over the past ten years has led to an evolution of the original model on how DHA reorganizes membrane microdomains. The work raises the intriguing possibility of testing the model at the human level to target health and disease.
Fish oil contains biologically active n-3 polyunsaturated fatty acids (PUFA), which have tremendous potential for the treatment of a wide variety of human afflictions associated with chronic inflammation [1]. One focus area of fish oil research is on underlying mechanisms by which n-3 fatty acids regulate the function of immune cells. This article briefly covers the author's research (S.R.S) over the past decade, which has focused on the mechanisms by which n-3 PUFAs reorganize plasma membrane microdomains and thereby cellular function.
The inception of the DHA-lipid microdomain model
In the late 1990s, there was a wealth of data emerging about how the n-3 PUFA docosahexaenoic acid (DHA) could manipulate the molecular organization of the plasma membrane. Several excellent reviews by Stillwell and Wassall summarize the basic effects of DHA on membrane properties that were revealed over the course of several years [2, 3]. These studies showed DHA influenced membrane microviscosity (the most commonly used endpoint in many studies even today), permeability, phospholipid flip-flop, curvature stress, elasticity, protein conformation and microdomain formation [2].
The lipid raft model, that aimed to explain compartmentalization of cell signaling, also emerged in the late 1990s [4]. This model proposed that liquid-ordered sphingolipid-cholesterol enriched domains coalesced to sequester protein clusters and thereby regulate signaling. A central component of the model was that rafts accumulated saturated acyl chains [4]. Thus, it was timely to address if DHA acyl chains, which are highly disordered, could target the organization of sphingolipid/cholesterol enriched liquid-ordered domains [3].
In the lab of Bill Stillwell, we collaborated with several groups to address the question of how would DHA impact the formation of raft domains. We conducted a series of studies using pressure-area isotherms, differential scanning calorimetry, detergent extraction, 2H NMR spectroscopy and X-ray diffraction in model membranes [5–9], which provided a highly controlled system to study the molecular behavior of DHA acyl chains. A key finding was that phosphatidylethanolamines (PE) containing DHA had a poor affinity for cholesterol relative to other unsaturated fatty acids [9]. Based on these studies, we proposed a model in which a DHA-containing PE would avoid steric interactions with cholesterol to form a distinct non-raft domain. This model did not address how a phosphatidylcholine (PC) containing DHA or other n-3 PUFAs would interact with sphingomyelin and cholesterol. This is an essential point that has recently been addressed that I will come back to at the end of the article.
There were three major predictions of the DHA-lipid microdomain model that were proposed in 2004 [8]: 1) In cells, DHA should largely avoid cholesterol-enriched lipid rafts; 2) the incorporation of DHA into the nonraft phase should disrupt the spatial distribution of select proteins; 3) the culmination of changes in lipid and protein organization would result in downstream functional changes. I sought to test these predictions as a post-doctoral fellow with a focus on establishing functional relevance first (prediction #3).
Translation to in vitro reveals the complexity of DHA's impact on the B cell
In 2004, a few labs were reporting that n-3 PUFAs administered in the mouse diet or in culture resulted in uptake of EPA and/or DHA into detergent resistant membranes (DRMs), a very crude approximation of lipid rafts in cells [10, 11]. More importantly, it was reported that changes in DRM composition were accompanied by suppression in downstream signaling and subsequent proliferation and activation of CD4+ T cells [12] [13]. Therefore, it was timely to start an investigation of the effects of DHA on B cells, a cell type poorly studied with n-3 PUFAs.
In the lab of Michael Edidin, I initially aimed to test the effects of treating human B lymphoblasts with DHA in order to study protein function in the endoplasmic reticulum. Unexpectedly, while optimizing methods of fatty acid administration to cells, we discovered that overnight treatment with 50–100PM DHA suppressed the ability of these cells to be lysed by alloreactive CD8+ T cells relative to a control [14]. The data were highly consistent with studies showing n-3 PUFAs could suppress antigen presentation by other cell types in vitro [15] and ex vivo [16]. This fulfilled prediction #3 of the model that DHA should have an impact on cellular function, as one would expect. However, it was unclear what the underlying mechanisms were, which would end up addressing predictions 1 and 2 of the proposed model.
Two major mechanisms were driving the functional response of DHA on B cells, which were beyond simple changes in membrane composition and microviscosity. One, DHA lowered surface levels of the major histocompatibility complex (MHC) class I, the molecules on the B cell surface that present antigen to cognate CD8+ T cells [14]. The reduction in MHC class I surface expression was due to DHA targeting endomembrane MHC class I forward traffic from the ER to the Golgi. Second, DHA diminished the ability of B cells to physically conjugate to the T cells. This event is dependent on the formation of the immunological synapse, which requires the appropriate spatio-temporal organization of key plasma membrane antigen presenting, co-stimulatory, and adhesion proteins [17].
There were several limitations of our study, which had to be addressed in order to move the field forward. One, the effects were not just limited to DHA. Arachidonic acid also had an effect, which raised the possibility that this was a general effect of PUFAs [14]. Second, it was entirely plausible that the observations were due to the concentrations of fatty acid and/or the methods of administering the fatty acids. Indeed, we showed that administration of esterified DHA phospholipid vesicles to B cells gave different results on MHC class I surface expression than administration of fatty acid-BSA complexes [18]. Thus, the next major move was to translate the work from model membranes and cells to other in vitro models and more importantly into animals. Therefore, in my own lab starting in 2008, we set up new experiments in cell culture and in animals, relying heavily on appropriate imaging approaches for the study of membrane domains.
DHA but not EPA targets lipid and protein spatial distribution
The first set of studies continued the in vitro work on MHC class I. Here we discovered that treatment of EL4 cells (an immortal cell line) with DHA, but not EPA, diminished the clustering of cholera-toxin induced lipid microdomains [19]. On a micron scale, DHA appeared to increase the size of the microdomains. Furthermore, we found that DHA but not EPA treatment modified the lateral organization of MHC class I, which suggested an additional mechanism by which DHA could be regulating antigen presentation. These studies also opened the door to differences in the molecular organization of EPA vs. DHA.
We further addressed the potential in vitro mechanisms by which DHA could be disrupting lipid microdomain clustering. One possibility was that DHA could be manipulating microdomain clustering by directly incorporating into the domains. Indeed, the Wassall lab (with whom we collaborated) demonstrated in model membranes that PCs containing DHA, more than EPA, incorporated directly into nanoscale sphingolipids/cholesterol domains and thereby manipulated domain size [20]. In cells, we have reported that DHA incorporated into DRMs and had no effect on nonraft organization as measured by staining of the nonraft probe FAST-DiI [21]. Given the limitations of the DRM method of isolation, more direct evidence has emerged from lipidomic analysis showing EPA can incorporate directly into ordered regions of the Jurkat immunological synapse [22]. Thus, prediction #2 that DHA should largely avoid rafts was not entirely consistent with measurements in cells and in model membranes.
We have also addressed if DHA is disrupting lipid microdomain clustering by targeting cholesterol lateral distribution. We discovered trends suggesting DHA was pushing cholesterol molecules from detergent resistant to detergent soluble membranes (DSM) [19, 23]. However, a recent study showed that DHA treatment at a high dose moved cholesterol from DRMs to DSMs in SH-SY5Y cells [24]. More direct evidence has emerged with staining of cholesterol and changes in its lateral distribution upon DHA treatment in HB4a cells [25].
Again, it is important to note how the recent studies highlight the complexity of the cell. Originally, we had observed that DHA esterified to PEs avoided cholesterol, yet in several cell types, DHA can incorporate into raft-like domains. On the other hand, recent work in model membranes shows that DHA esterified to PCs can incorporate into rafts, consistent with our work with EL4 cells and that of others [20]. Thus, it appears that DHA behaves differently when esterified to a PC versus a PE.
Fish oil targets B cell lipid spatial distribution and cellular function at the animal level
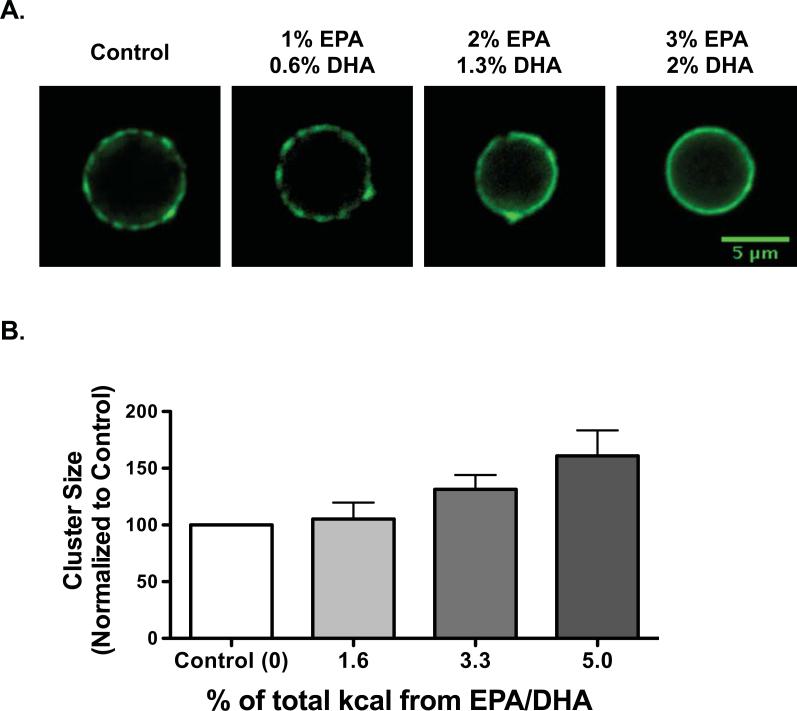
We have conducted studies with C57BL/6 mice to determine how fish oil targets splenic B220+ B cell microdomain organization and function. We have found that lipid microdomain clustering, induced by cholera toxin cross-linking, is diminished with fish oil [21, 23]. A re-analysis of the data suggest that the effects of n-3 fatty acids are dose dependent (Fig. 1A). On a micron scale, the microdomains appear to be larger in response to increasing levels of EPA/DHA (Fig. 1B). Mechanistically, we have shown, similar to our cell culture studies, that fish oil is not targeting non-raft distribution but selectively targeting lipid microdomain distribution. Furthermore, we have demonstrated that the effects on microdomain clustering are specific for fish oil since administration of n-6 PUFA diets had no effect [21].
Figure 1. N-3 fatty acids diminish the clustering of murine B cell cholera-toxin induced lipid microdomains.
(A) Samples images demonstrating cross-linked GM1 lipid microdomains on the surface of splenic B220+ B cells isolated from mice fed differing diets. Domains were visualized with cholera toxin subunit B-FITC. The percentage of total kcal from EPA and DHA are indicated. (B) The size of the clusters increases with increasing levels of EPA and DHA. The cluster sizes are normalized to the control since the data are pooled from separate studies [21, 23]. It is important to note that although microdomain size increases on a micron scale, it remains unclear what the impact of the diet is on a nanometer scale.
Surprisingly, using polarization imaging, we discovered that fish oil has a greater ordering effect upon formation of lipid microclusters relative to no clustering [23]. These results are in agreement with studies with CD4+ T cells to show that n-3 PUFAs target microdomain size and order in the immunological synapse [26–28]. This revealed that DHA can actually exert an ordering effect on the membrane upon formation of lipid microdomains. However, the probe used to measure molecular order has its own limitations given that it detects solvent relaxation, so the probe may be simply reporting on the headgroup region rather than acyl chain order. Therefore, in a recent study, we used a custom synthesized DHA-labeled fluorophore to show that when DHA incorporates into an ordered lipid microdomain, the DHA acyl chain increases its order and lowers its rotational diffusion [29]. We are now pursuing additional studies on molecular order and the impact of disrupting the lipid environment on the organization of signaling proteins in B cells.
We have uncovered two different functional outcomes of fish oil on B cell function ex vivo. We first discovered that a high dose of fish oil increased B cell activation in response to the T-independent antigen lipopolysaccharide (LPS), a peptidoglycan from the outer membrane of Gram-negative bacteria [30]. We initially interpreted our data to imply that fish oil's enhancement of B cell activation was simply a consequence of a high dose of n-3 PUFAs, which had negative effects such as increased body weight and reduced locomotor activity [31]. Very recently, we reproduced the results with a lower dose of fish oil on B cell activation with LPS [23]. The dose modeled the equivalent of a human consuming ~4 grams per day, which is achievable as an over-the-counter supplement and currently in use pharmacologically to suppress elevated triglycerides. These results are in agreement with emerging data that fish oil has immune enhancing properties. For example, a very recent study showed that treating human B cells with DHA-derived metabolites increased antibody production [32]. Also, Arnadottir et al. showed fish oil suppressed the proportion of classical monocytes in blood of healthy mice but actually enhanced their number upon LPS induced inflammation [33]. Thus, fish oil may have immune enhancing effects upon inflammation that lead to resolution of inflammation.
Interestingly, although fish oil enhanced an innate B cell response, we also observed that B cell antigen presentation was suppressed, highly consistent with the aforementioned in vitro studies [23]. Specifically, B cells isolated from mice fed fish oil did not efficiently activate naïve CD4+ T cells. This raises the intriguing possibility that fish oil has both immune enhancing and immunosuppressive effects on B cells, which appears to be dependent on the type of antigen and underlying mechanisms of action associated with the receptors for the antigens. We are now focused on addressing how fish oil impacts several aspects of innate and adaptive B cell function ex vivo and in vivo.
Conclusions
Since the inception of the DHA-lipid microdomain model, our lab and many others have made tremendous progress in establishing the mechanism by which DHA targets the biophysical organization of the membrane. A summary of key concepts from these studies, in addition to model systems and methods used, are provided in Table 1. More work is required to obtain a complete picture on how DHA, unlike EPA, by targeting the clustering of membrane microdomains, is able to alter protein lateral distribution and ultimately exert immunomodulatory effects. By having a solid mechanistic foundation, we aim to translate the model into humans in order to effectively implement EPA and DHA into the clinic for treating specific ailments and for making sound dietary recommendations for the general public.
Table 1.
Summary of key concepts, model systems, and methods used to study how n-3 PUFAs reorganize lipid-protein microdomain distribution. The concepts are presented in chronological order.
| Key concepts | Model System | Methods |
|---|---|---|
| Lipid Environment | ||
| • DHA has a poor affinity for cholesterol | Model membranes - DHA esterified to PE | NMR, X-ray diffraction, DSC, pressure-area isotherms |
| • DHA incorporates into nonraft-like liquid-disordered domains | Model membranes - DHA esterified to PE | NMR, detergent extraction, DSC, atomic force imaging |
| • DHA incorporates directly into DRMs | In vitro, ex vivo | Detergent extraction |
| • Fish oil or EPA/DHA do not target nonraft organization | In vitro, ex vivo | Confocal & FRET imaging |
| • Fish oil targets lipid microdomain size on a micron scale and molecular order | Ex vivo | Confocal & polarization imaging |
| • DHA has a tendency to push cholesterol from DRMs to DSMs | In vitro, ex vivo | Detergent extraction, imaging |
| • DHA, but not EPA, targets micron and nanoscale lipid microdomain size and order | In vitro, model membranes | NMR, confocal and polarization imaging |
| • DHA, but not EPA, incorporates into liquid-ordered domains | Model membranes - DHA esterified to PC | NMR |
| Protein Environment | ||
| • Mice fed fish oil or fat-1 transgenic mice display a change in organization of key proteins (e.g. PKCθ) in the immunological synapse | In vitro, ex vivo | Confocal imaging |
| • DHA, but not EPA, moves MHC class I from nonraft to raft-like microdomains | In vitro | Confocal and FRET imaging |
Acknowledgments
The research was supported in part by a grant from the NIH (R15AT006152) to S.R.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author disclosures: no conflicts of interest.
References
- [1].Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br J Clin Pharm. 2012 doi: 10.1111/j.1365-2125.2012.04374.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem. Phys Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- [3].Wassall SR, Brzustowicz MR, Shaikh SR, Cherezov V, Caffrey M, Stillwell W. Order from disorder, corralling cholesterol with chaotic lipids. The role of polyunsaturated lipids in membrane raft formation. Chem. Phys Lipids. 2004;132:79–88. doi: 10.1016/j.chemphyslip.2004.09.007. [DOI] [PubMed] [Google Scholar]
- [4].Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- [5].Shaikh SR, Dumaual AC, Jenski LJ, Stillwell W. Lipid phase separation in phospholipid bilayers and monolayers modeling the plasma membrane. Biochim. Biophys. Acta. 2001;1512:317–328. doi: 10.1016/s0005-2736(01)00335-2. [DOI] [PubMed] [Google Scholar]
- [6].Shaikh SR, Brzustowicz MR, Gustafson N, Stillwell W, Wassall SR. Monounsaturated PE does not phase-separate from the lipid raft molecules sphingomyelin and cholesterol: role for polyunsaturation? Biochemistry. 2002;41:10593–10602. doi: 10.1021/bi025712b. [DOI] [PubMed] [Google Scholar]
- [7].Shaikh SR, Cherezov V, Caffrey M, Stillwell W, Wassall SR. Interaction of cholesterol with a docosahexaenoic acid-containing phosphatidylethanolamine: trigger for microdomain/raft formation? Biochemistry. 2003;42:12028–12037. doi: 10.1021/bi034931+. [DOI] [PubMed] [Google Scholar]
- [8].Shaikh SR, Dumaual AC, Castillo A, LoCascio D, Siddiqui RA, Stillwell W, Wassall SR. Oleic and docosahexaenoic acid differentially phase separate from lipid raft molecules: a comparative NMR, DSC, AFM, and detergent extraction study. Biophys. J. 2004;87:1752–1766. doi: 10.1529/biophysj.104.044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shaikh SR, Cherezov V, Caffrey M, Soni SP, LoCascio D, Stillwell W, Wassall SR. Molecular organization of cholesterol in unsaturated phosphatidylethanolamines: X-ray diffraction and solid state 2H NMR reveal differences with phosphatidylcholines. J. Am. Chem. Soc. 2006;128:5375–5383. doi: 10.1021/ja057949b. [DOI] [PubMed] [Google Scholar]
- [10].Fan Y-Y, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) Polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J. Nutr. 2003;133:1913–1920. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- [11].Stulnig TM, Huber J, Leitinger N, Imre E-M, Angelisová P, Nowotny P, Waldhäusl W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J. Biol. Chem. 2001;276:37335–37340. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- [12].Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J Immunol. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- [13].Kim W, Khan NA, McMurray DN, Prior IA, Wang N, Chapkin RS. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog. Lipid. Res. 2010;49:250–261. doi: 10.1016/j.plipres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shaikh SR, Edidin M. Immunosuppressive effects of polyunsaturated fatty acids on antigen presentation by human leukocyte antigen class I molecules. J. Lipid Res. 2007;48:127–138. doi: 10.1194/jlr.M600365-JLR200. [DOI] [PubMed] [Google Scholar]
- [15].Zeyda M, Säemann MD, Stuhlmeier KM, Mascher DG, Nowotny PN, Zlabinger GJ, Waldhäusl W, Stulnig TM. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-κB Activation. J. Biol. Chem. 2005;280:14293–14301. doi: 10.1074/jbc.M410000200. [DOI] [PubMed] [Google Scholar]
- [16].Sanderson P, MacPherson GG, Jenkins CH, Calder PC. Dietary fish oil diminishes the antigen presentation activity of rat dendritic cells. J. Leuk. Biol. 1997;62:771–777. doi: 10.1002/jlb.62.6.771. [DOI] [PubMed] [Google Scholar]
- [17].Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristan C, Victora GD, Zanin-Zhorov A, Dustin ML. Functional anatomy of T cell activation and synapse formation. Ann. Rev. Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shaikh SR, Edidin M. Polyunsaturated fatty acids and membrane organization: elucidating mechanisms to balance immunotherapy and susceptibility to infection. Chem. Phys. Lipids. 2008;153:24–33. doi: 10.1016/j.chemphyslip.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shaikh SR, Rockett BD, Salameh M, Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J. Nutr. 2009;139:1632–1639. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- [20].Williams Justin A., Batten Shawn E., Harris M, Rockett Benjamin D., Shaikh Saame R., Stillwell W, Wassall Stephen R. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys. J. 2012;103:228–237. doi: 10.1016/j.bpj.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rockett BD, Franklin A, Harris M, Teague H, Rockett A, Shaikh SR. Membrane raft organization is more sensitive to disruption by (n-3) PUFA than nonraft organization in EL4 and B cells. J. Nutr. 2011;141:1041–1048. doi: 10.3945/jn.111.138750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zech T, Ejsing CS, Gaus K, de Wet B, Shevchenko A, Simons K, Harder T. Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. EMBO J. 2009;28:466–476. doi: 10.1038/emboj.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rockett BD, Teague H, Harris M, Melton M, Williams J, Wassall SR, Shaikh SR. Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. J. Lipid Res. 2012;53:674–685. doi: 10.1194/jlr.M021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Grimm MOW, Kuchenbecker J, Grösgen S, Burg VK, Hundsdörfer B, Rothhaar TL, Friess P, de Wilde MC, Broersen LM, Penke B, Péter M, Vígh L, Grimm HS, Hartmann T. Docosahexaenoic acid reduces amyloid β production via multiple pleiotropic mechanisms. J. Biol. Chem. 2011;286:14028–14039. doi: 10.1074/jbc.M110.182329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ravacci GR, Brentani MM, Tortelli T, et al. Lipid raft disruption by docosahexaenoic acid induces apoptosis in transformed human mammary luminal epithelial cells harboring HER-2 overexpression. J. Nutr. Biochem. 2012 doi: 10.1016/j.jnutbio.2012.02.001. In press. [DOI] [PubMed] [Google Scholar]
- [26].Hou TY, Monk JM, Fan YY, Barhoumi R, Chen YQ, Rivera GM, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress phosphatidylinositol 4,5-bisphosphate-dependent actin remodelling during CD4+ T-cell activation. The Biochem. J. 2012;443:27–37. doi: 10.1042/BJ20111589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yog R, Barhoumi R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress mitochondrial translocation to the immunologic synapse and modulate calcium signaling in T cells. J. Immunol. 2010;184:5865–5873. doi: 10.4049/jimmunol.0904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J. Immunol. 2008;181:6236–6243. doi: 10.4049/jimmunol.181.9.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Teague H, Ross R, Harris M, Mitchell D, Shaikh SR. DHA-fluorescent probe is sensitive to membrane order and reveals molecular adaptation of DHA in ordered lipid microdomains. J. Nutr. Biochem. 2012 doi: 10.1016/j.jnutbio.2012.04.010. In press, doi not yet available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rockett BD, Salameh M, Carraway K, Morrison K, Shaikh SR. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. J. Lipid Res. 2010;51:1284–1297. doi: 10.1194/jlr.M000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Drew Rockett B, Harris M, Raza Shaikh S. High dose of an n-3 polyunsaturated fatty acid diet lowers activity of C57BL/6 mice. Prost. Leukot. Essent. Fatty Acids. 2012;86:137–140. doi: 10.1016/j.plefa.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J. Immunol. 2012;189:1036–1042. doi: 10.4049/jimmunol.1103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Arnardottir HH, Freysdottir J, Hardardottir I. Dietary fish oil decreases the proportion of classical monocytes in blood in healthy mice but increases their Proportion upon Induction of Inflammation. J. Nutr. 2012;142:803–808. doi: 10.3945/jn.111.153221. [DOI] [PubMed] [Google Scholar]