Abstract
Bioactivity-directed fractionation of the organic extracts of two filamentous fungi of the Bionectriaceae, strains MSX 64546 and MSX 59553 from the Mycosynthetix library, led to the isolation of a new dimeric epipolythiodioxopiperazine alkaloid, verticillin H (1), along with six related analogues, Sch 52900 (2), verticillin A (3), gliocladicillin C (4), Sch 52901 (5), 11′-deoxyverticillin A (6), and gliocladicillin A (7). The structures of compounds 1–7 were determined by extensive NMR and HRMS analyses, as well as by comparisons to the literature. All compounds (1–7) were evaluated for cytotoxicity against a panel of human cancer cell lines, displaying IC50 values ranging from 1.2 µM to 10 nM. Compounds 1–5 were examined for activity in the NF-κB assay, where compounds 2 and 3 revealed activity in the sub-micromolar range. Additionally, compounds 1, 3, and 4 were tested for EGFR inhibition using an enzymatic assay, while compound 3 was examined against an overexpressing EGFR+ve cancer cell line.
Keywords: fungus, epipolythiodioxopiperazine alkaloids, cytotoxicity, NF-κB, EGFR
INTRODUCTION
Dimeric epipolythiodioxopiperazine (ETP) alkaloids are bioactive secondary metabolites reported to have potent cytotoxic1–10 and antibacterial activities,11–16 along with antiparasitic,17 nematicidal,18 antiviral,19 and immunosuppressive properties.20 This class of compounds has been isolated largely from several terrestrial and marine fungi, including species of the Leptosphaeria, Chaetomium, Tilachlidium, Verticillium, Gliocladium, and Penicillium genera.21 The densely functionalized and stereochemically-complex core of these alkaloids, coupled with their potent biological activities, presents an attractive target for drug leads. Although the ETP alkaloids have been known for over four decades, with chatecocin22 and verticillin A16, 23, 24 first described in 1970, only 23 other natural analogues have been described since then. The first total synthesis (11,11'-dideoxyverticillin A),25 which stymied chemists for nearly 40 years, was hailed in Science.26 Ongoing studies by this same group27 resulted in a synthetic strategy to generate the di-, tri-, and tetrasulfides, (+)-chaetocin A, (+)-chaetocin C, and (+)-12,12′-dideoxychetracin A, respectively, with complete stereochemical control in thiolation and precision in the degree of sulfidation. The mechanism of action for the potent cytotoxicity of the ETPs has not been fully elucidated. However, several different activities have been described, including: generation of reactive oxygen species (ROS) through oxidation of the polysulfide bridge,2 intracellular induction of the c-fos proto-oncogene,28 inhibition of the epidermal growth factor receptor (EGFR) tyrosine kinase activity,3 and more recently, apoptosis activation by BNIP3 (19 kDa interacting protein-3) upregulation and/or tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) sensitizer.29 Interest in these compounds remains high, due to both their potent biological activities and interesting chemistry.
As part of ongoing studies to discover new anticancer drug leads from filamentous fungi,30, 31 the organic-soluble extracts of solid substrate fermentation of fungi of the Bionectriaceae, cultures MSX 64546 and MSX 59553 from the Mycosynthetix library, exhibited cytotoxic activity against MCF-7 (breast), H460 (large cell lung), and SF-268 (astrocytoma) human cell lines that was indicative of IC50 values < 2 µg mL−1 for the crude extracts. Preliminary LC-HRMS dereplication procedures suggested the presence of the known ETP, verticillin A (3), as well as characteristic fragmentation patterns for related and possibly new ETPs, both of which likely contributed to the cytotoxicity of the extracts. Therefore, bioactivity-directed fractionations were initiated using H460 cancer cells to monitor the purifications, and this led to the isolation of a new ETP, verticillin H (1), along with six known verticillin-type compounds, Sch 52900 (2), verticillin A (3), gliocladicillin C (4), Sch 52901 (5), 11′-deoxyverticillin A (6), and gliocladicillin A (7). All pure isolates were assessed for cytotoxicity against additional cancer cell lines. Compounds 1–5 were examined in an NF-κB inhibition assay, while compounds 1, 3, and 4 were tested in vitro for EGFR inhibition using an enzymatic assay, and 3 was examined against an overexpressing EGFR+ve cancer cell line.
RESULTS AND DISCUSSION
Identification of ETPs
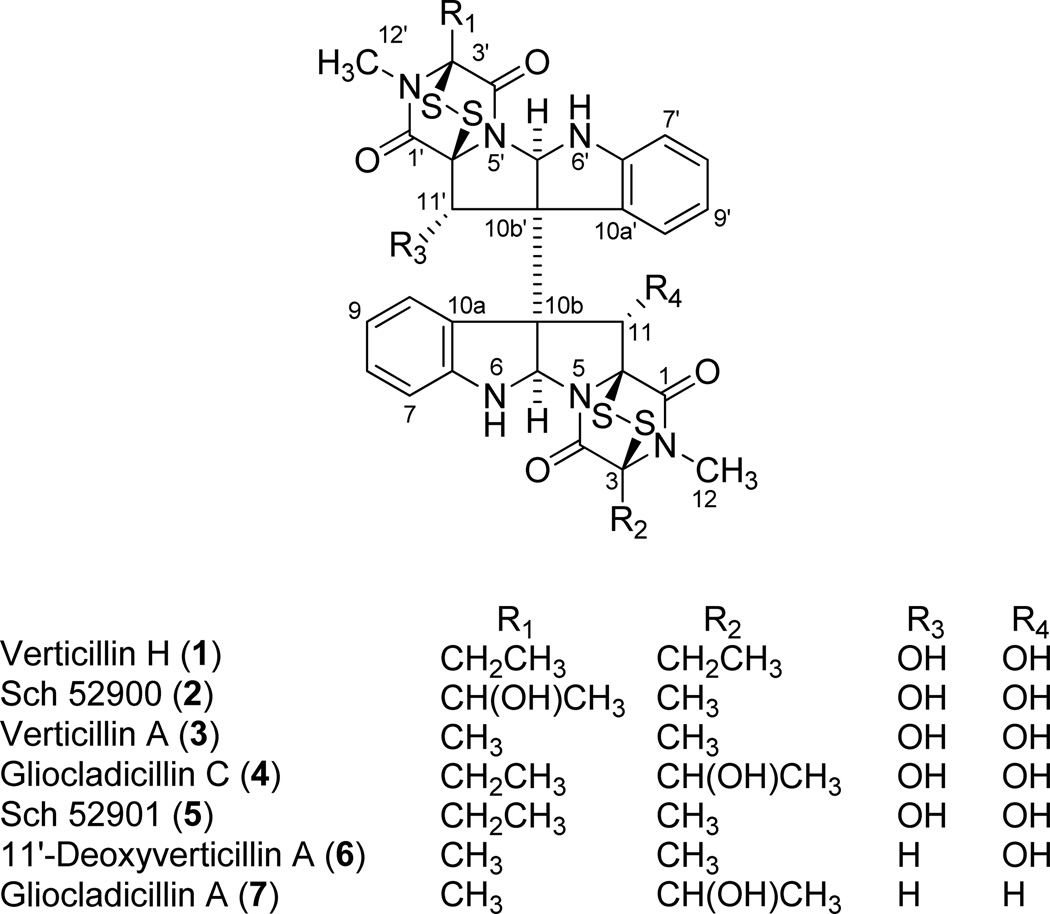
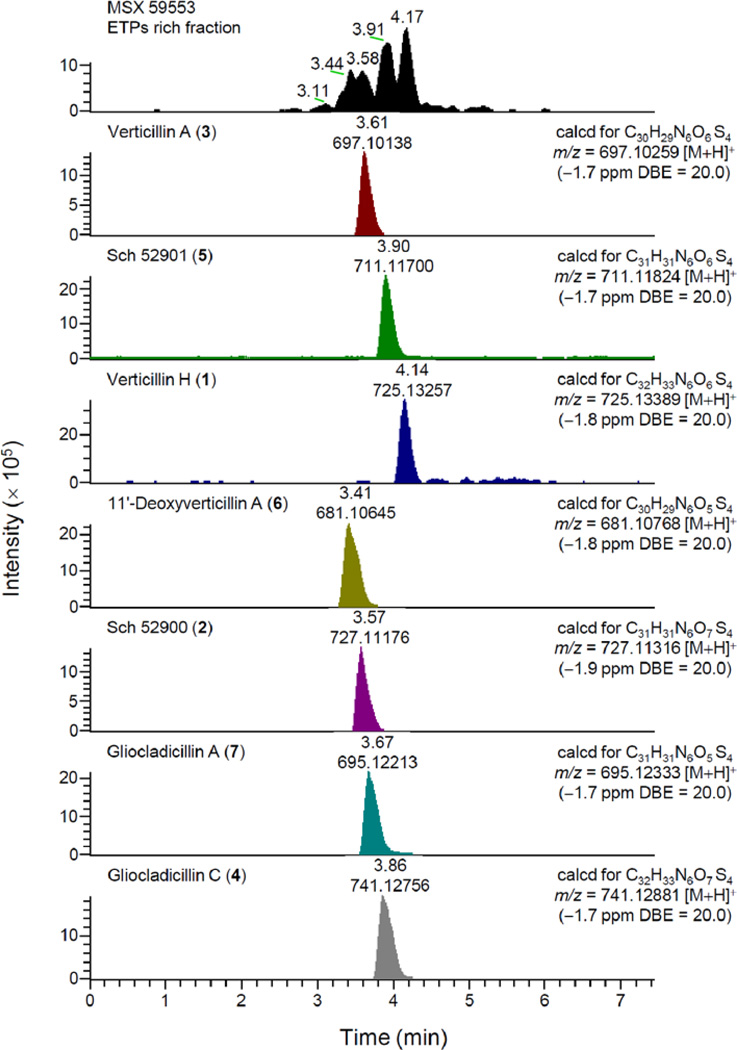
Bioactivity-directed purification of the organic extracts of fungal strains MSX 64546 and MSX 59553 led to the isolation of seven cytotoxic ETPs (1–7; see Figure 1), including the new compound, verticillin H (1). An LC-HRMS dereplication procedure (Figure 2), which utilized molecular formula, exact mass, and UV maxima (204, 240, and 301 nm) as search criteria in the Dictionary of Natural Products and SciFinder databases, coupled with NMR analysis of pure isolates (Table S1), confirmed that compounds 2–7 belong to the ETP class of fungal natural products. The data for the known compounds matched the literature for Sch 52900 (2),28 verticillin A (3),23 gliocladicillin C (4),32 Sch 52901 (5),28 11′-deoxyverticillin A (6),9 and gliocladicillin A (7);1 Table S1 compiles the 1H and 13C data for these compounds in CDCl3.
Figure 1.
Structure of compounds (1–7) isolated from fungi MSX 64546 and MSX 59553.
Figure 2.
LC-UV-MS dereplication for compounds 1–7 and the ETPs rich fraction from fungus MSX 59553.
Structure elucidation of verticillin H (1)
The HRMS data of compound 1 yielded a molecular formula C32H32N6O6S4. The IR data suggested the presence of carbonyl (1673 cm−1), hydroxy (3402 cm−1), and aromatic (1685 and 1429 cm−1) moieties. The UV spectrum of 1 exhibited absorption maxima at 204, 240, and 301 nm, as observed with similar epipolythiodioxopiperazine analogues.9, 15, 23, 24 Although the 13C NMR spectrum showed resonances for 16 carbons (Table 1), inspection of the HRMS data, which suggested 32 carbons, revealed a symmetrical dimeric structure. Detailed analysis of HMBC and HSQC data for compound 1 (Table 1) showed similarities to verticillin A (3); however, the molecular weight of 1 was 28 greater than that of 3. This suggested the presence of an additional methylene unit in each half of the molecule, which was evidenced by the resonances at δH 2.4, 2.1 (for H2-13/H2-13') that were coupled to a methyl doublet of doublets at δH 1.2 (H3-14/H3-14'). In addition, HMBC correlations of the methyl proton signals H3-14/H3-14' with the quaternary carbon C-3/C-3' (δC 77.0), the secondary carbon C-13/C-13' (δC 24.6), and the amide carbonyl C-4/C-4' (δC 161.5), established the connection of the ethyl side chain to position C-3 and C-3' in each monomeric unit. In turn, these two units were connected via the C-10b to C-10b' bond by virtue of HMBC correlations of the methine proton H-5a/H-5a' (δH 5.7) with the quaternary carbons C-6a/C-6a' (δC 148.9), C-10b/C-10b' (δC 65.8), and C-11a/C-11a' (δC 77.5), respectively; such a connection was consistent with other ETP alkaloids in the literature.9, 11, 15, 24 All other NMR data and HMBC correlations for 1 were consistent with the structural assignment (Figure 1), and compound 1 was ascribed the trivial name verticillin H. The absolute configuration of 1 was determined by comparison of the optical rotation values and CD spectra with those reported for several verticillin analogues.9, 11, 15
Table 1.
NMR data for compound 1 [in CDCl3, 500 (1H) and 125 (13C) MHz, chemical shifts in δ, coupling constants in Hz]
| Position | δC | δH, Mult.(J in Hz) | HMBC (H → C) |
|---|---|---|---|
| 1, 1′ | 167.3 | – | – |
| 3, 3′ | 77.0 | – | – |
| 4, 4′ | 161.5 | – | – |
| 5a, 5a′ | 82.1 | 5.7, s | 6a, 10a, 10b, 11a |
| 6a, 6a′ | 148.9 | – | – |
| 7, 7′ | 110.9 | 6.7, d (8.0) | 8, 9, 10a |
| 8, 8′ | 130.1 | 7.1, ddd (8.0, 7.6, 1.1) | 6a, 10 |
| 9, 9′ | 120.5 | 6.8, ddd (8.0, 7.6, 1.1) | 7, 8, 10a |
| 10, 10′ | 128.3 | 7.8, d (7.6) | 6a, 10a |
| 10a, 10a′ | 129.5 | – | – |
| 10b, 10b′ | 65.8 | – | – |
| 11, 11′ | 83.3 | 5.2, s | 5a, 10b |
| 11a, 11a′ | 77.5 | – | – |
| 12, 12′ | 28.1 | 3.0, s | 1, 3 |
| 13,13′ | 24.6 | a 2.4, b 2.1, dd (14.5,7.3) | 3, 4, 14 |
| 14,14′ | 9.9 | 1.2, d (7.3) | 3, 13 |
| OH-11, OH-11′ | 5.1, s | – | |
| NH-6, NH-6′ | 5.6, s | 5a |
Abbreviations: Mult., multiplicity.
Biological activity
Inhibition of cancer cell proliferation in vitro
All isolates (1–7) were evaluated for cytotoxic activity against HT-29, H460, SF-268, MCF-7, and MDA-MB-435 cell lines (see Table 2). Previously, Erkel et al.20 reported the cytotoxicity of 2 against HL60 (leukemia) cells; Katagiri et al.23 described the effect of 3 against the Ehrlich ascites carcinoma in mice and HeLa cells; and Che et al.32 showed that 4 exhibited high cytotoxic activity on A549 (adenocarcinoma) and HepG2 (hepatocellular carcinoma) human cell lines. However, this is the first report of the simultaneous evaluation of 1–7 against all the cancer cell lines listed above. In general, all of the compounds displayed potent cytotoxicity, approaching the activity of the positive controls. However, as suggested by Gardiner et al.,21 side chain modifications did not change the cytotoxicity of these compounds drastically.
Table 2.
Cytotoxicity against a panel of human tumor cell lines and NF-κB inhibitory activity of compounds isolated from MSX 64546 and MSX 59553
| Compound | Cytotoxic activity | NF-κB inhibitory activity |
||||
|---|---|---|---|---|---|---|
| IC50 values (in µM)a | ||||||
| HT-29 | H460 | SF-268 | MCF-7 | MDA- MB-435 |
IC50 values (in µM) | |
| Verticillin H (1) | 0.04 | 0.30 | 0.33 | 0.49 | 0.10 | >10 |
| Sch 52901 (2) | 0.01 | 0.29 | 0.37 | 0.58 | 0.48 | 0.5 |
| Verticillin A (3) | 0.02 | 0.20 | 0.25 | 0.37 | 0.07 | 0.1 |
| Gliocladicillin C (4) | 0.03 | 0.52 | 0.38 | 0.61 | 0.08 | >10 |
| Sch 52900 (5) | 0.19 | 1.20 | 0.75 | 1.11 | 0.03 | >10 |
| 11′-Deoxyverticillin (6) | NTb | 0.01 | 0.04 | 0.03 | NT | NT |
| Gliocladicillin A (7) | NT | 0.03 | 0.09 | 0.09 | NT | NT |
| Camptothecinc | NT | 0.01 | 0.01 | 0.13 | NT | NT |
| Silvestrolc | 0.01 | NT | NT | NT | 0.01 | NT |
| Rocaglamidec | NT | NT | NT | NT | NT | 0.075 |
IC50 values were determined as the concentration required to reduce cellular staining with sulforhodamine B by 50% relative to untreated controls following 72 h of continuous exposure.
Indicates ′not tested′.
Positive controls.
Transcription Factor NF-κB assay
Phal et al.33 reported the inhibition of NF-κB in cells by glyotoxin, the first monomeric ETP characterized from Gliocladium fimbriatum.21 The inhibitory effect of this compound was attributed to a possible interaction within an essential thiol residue of the cysteine rich extracellular domains of the protein.21, 33 Since NF-κB factor is an integral part of the inflammatory immune response and controls expression of some cytokines, its inhibition may account for the cytotoxic and immunosuppressive properties of dimeric ETPs. Compounds 1–5 were examined for NF-κB inhibitory activity (Table 2). Compounds 2 and 3 inhibited the specific binding ability of activated p65 subunits of NF-κB in the nucleus of HeLa cells with IC50 values of 0.5 and 0.1 µM, respectively, being approximately a half-order of magnitude less potent than the positive control (rocaglamide). These results were in agreement with the recently reported effect of verticillin A (3) as a sensitizer of human colon carcinoma cells to TRAIL-induced apoptosis.29
Epidermal growth factor receptor activity
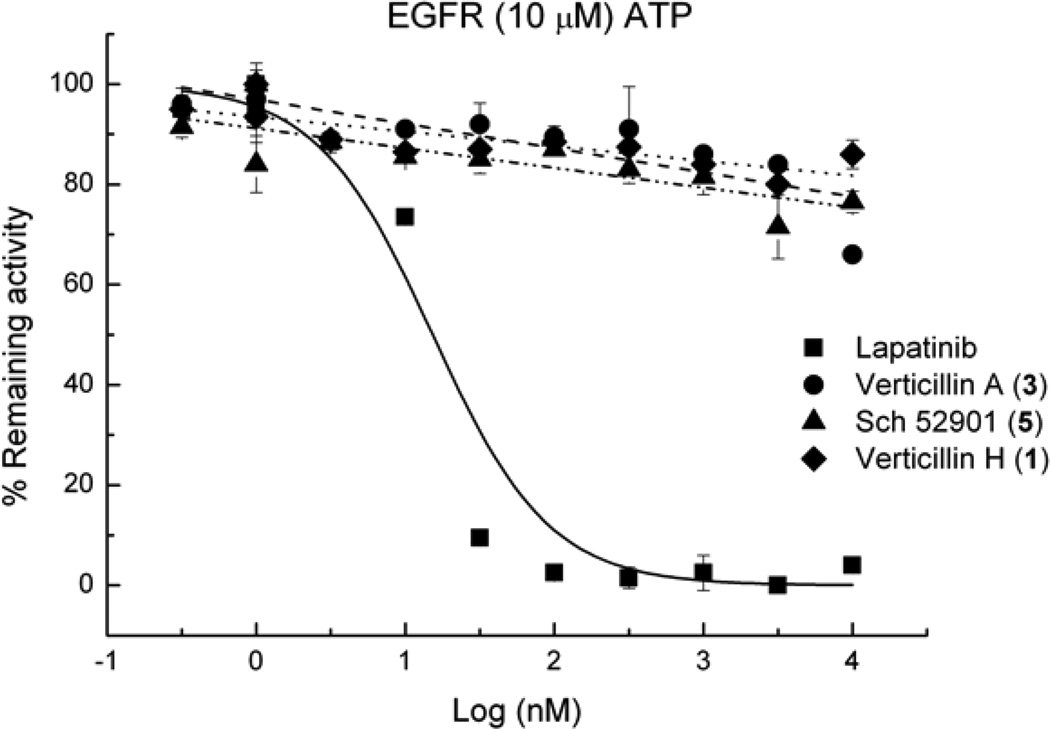
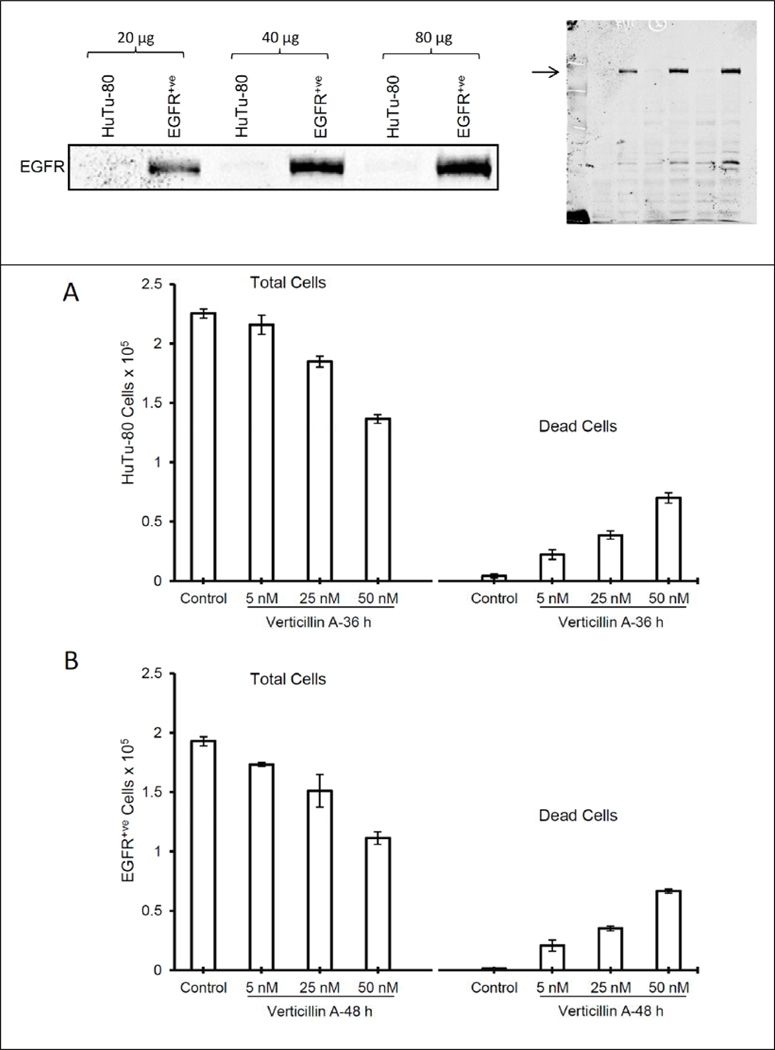
11,11′-Dideoxyverticillin, a structurally-related ETP isolated from the traditional Chinese medicinal herb Shiraia bambusicola,3 showed tyrosine kinase inhibitory activity in a cell-free ELISA assay against the epidermal growth factor receptor (EGFR), the vascular endothelial growth factor receptor-1/fms-like tyrosine kinase-1 (VEGFR-1/Flt-1), and the human epidermal growth factor receptor-2 (HER2/ErbB-2), with activity in the nM range for the former two and µM range for the latter. To the best of our knowledge, those studies represent the first report of enzymatic inhibition of dimeric ETPs. Based on those results, compounds 1, 3, and 4 were tested for EGFR inhibition using a luminescence kinase assay, but none of these substances were found to be active (IC50 values >10 µM; positive control lapatinib, IC50 13 nM; Figure 3). To further assess whether these compounds exert their cytotoxic activity in an EGFR-dependent manner by targeting EGFR expression/activity in a cellular system, compound 3 was tested for cytotoxicity against EGFR+ and EGFR− (EGFR+ve and HuTu-80, respectively) cell lines. Figure 4 (top panel) shows Western immunoblot data that indicates no EGFR protein versus very high EGFR protein levels in HuTu-80 and EGFR+ve cells, respectively. However, such a large difference in EGFR levels among the two cell lines did not result in any biological differences in the activity of compound 3 in these two cell lines (Figure 4; lower panels). Together, these observations suggest that the agent has strong cytotoxic activity independent of cellular EGFR levels. We have not identified 11,11′-dideoxyverticillin in the cultures we have studied to date. Hence, we have not been able to determine if the literature data are reproducible.
Figure 3.
Inhibitory effects of compounds 1, 3 and 5 and the positive control, lapatinib, on EGFR activity.
Figure 4.
EGFR level of expression on HuTu-80 and EGFR+ve cell lines and cytotoxicity after treatment with compound 3 [HuTu-80 (A) and EGFR+ve (B)].
CONCLUSIONS
Using bioactivity-directed fractionation and a LC-UV-HRMS dereplication protocol, one new compound, along with six known ETPs were isolated from the organic extracts of two fungi of the Bionectriaceae (strains MSX 64546 and MSX 59553). The isolated ETPs were potently cytotoxic against a panel of human cell lines in culture. Compounds 1–5 were tested for NF-κB inhibitory activity, with compounds 2 and 3 having IC50 values in the sub-micromolar range. Based on literature precedence, some of the isolates were examined for activity vs EGFR; however, no activity was observed when tested against both enzyme-based and cell-based assays. Regardless, the potent cytotoxicity of these compounds, which was on the same order of magnitude as the positive control, camptothecin, coupled with the promising NF-κB activity, warrants further exploration of the ETPs.
MATERIALS AND METHODS
General
Optical rotations, UV spectra, IR spectra, and CD spectra were obtained on a Rudolph Research Autopol III polarimeter (Rudolph Research, Hackettstown, NJ, USA), a Varian Cary 100 Bio UV-vis spectrophotometer (Varian Inc., Walnut Creek, CA, USA), a Perkin-Elmer Spectrum One with Universal ATR attachment (Perkin-Elmer, Waltham, MA, USA), and an Olis DSM 17 CD spectrophotometer (Olis, Bogard, GA, USA), respectively. NMR experiments were conducted in CDCl3 using a JEOL ECA-500 (operating at 500 MHz for 1H and 125 MHz for 13C; JEOL Ltd., Tokyo, Japan). HRESIMS data were measured using an electrospray ionization (ESI) source coupled to a Q-TOF Premier mass spectrometer (Waters Corp., Milford, MA, USA) or a LTQ Orbitrap XL system (Thermo Fisher Scientific, San Jose, CA, USA) in both positive and negative ionization modes and by either direct injection or via a liquid chromatographic/autosampler system that consisted of a Acquity UPLC system (Waters Corp.). Flash chromatography was conducted using a CombiFlash Rf system using a RediSep Rf Si-gel Gold column (both from Teledyne-Isco, Lincoln, NE, USA). HPLC was carried out on Varian Prostar HPLC systems equipped with Prostar 210 pumps and a Prostar 335 photodiode array detector, with data collected and analyzed using Galaxie Chromatography Workstation software (version 1.9.3.2, Varian Inc.). For preparative HPLC, a Synergi Max-RP (4 µm; 250 × 21 mm; Phenomenex, Torrance, CA, USA) or a YMC ODS-A (5 µm, 250 × 20 mm; Waters Corp.) column was used. For semipreparative HPLC, a YMC ODS-A (5 µm, 250 × 10 mm; Waters Corp.) column was used. A Gemini-NX (5 µm; 250 × 4.6 mm; Phenomenex) column was used for analytical HPLC. For UPLC analysis, a BEH C18 (1.7 µm; 50 × 2.1 mm; Waters Corp.) column was used.
LC-UV-HRMS Dereplication Procedure
UPLC-HRESIMS conditions
Sample concentration, 1.0 mg mL−1 MeOH-Dioxane (1:1) solution; mobile phase (UPLC Acquity): gradient elution of CH3CN–0.1% aqueous formic acid (0–1.0 min, 20:80; 1.0–5.0 min, from 20:80 to 100% CH3CN and hold for 2 min); UV detection wavelengths, 210 and 254 nm; flow rate, 0.3 mL/min; injection volume, 3 µL. ESI conditions (Thermo LTQ Orbitrap XL): capillary temperature, 275 °C; sheath gas, 15; auxiliary gas, 5; sweep gas, 2; source voltage, 4.50 kV; capillary voltage, 46 V; tube lens, 115 V. Mass range was set to optimally pass ions from m/z 100–2000. Data were acquired in centroid mode during the LC run.
Data analysis
Using a combination search of proposed molecular formulas, exact mass, and UV maxima corresponding to the major peaks in the Dictionary of Natural Products (Chapman and Hall, London, UK) and SciFinder databases (Chemical Abstracts Service, Columbus, OH, USA), the samples with unknown molecular formulas were designated for further purification.
Producing Organisms and Fermentations
Mycosynthetix fungal strains 64546 and 59553 were isolated by Dr. Barry Katz in May of 1992 and January of 1992 from leaf litter and leaves, respectively. DNA analyses were performed by MIDI Labs (Newark, DE, USA), and the D2 variable region of the large subunit rRNA was sequenced and compared with their database, which suggested that these fungi belong to the Bionectriaceae family (order Hypocreales); the sequences were deposited in Genbank (accession numbers JQ749727 and JQ749725 for MSX 64546 and MSX 59553, respectively). The cultures were stored on malt extract slants and transferred periodically. Fresh cultures were grown on similar slants, and a piece of each culture was transferred individually to a medium containing 2% soy peptone, 2% dextrose and 1% yeast extract (YESD media). Following incubation (7 days) at 22°C with agitation, the cultures were used to inoculate 50 mL of rice media, prepared using rice to which was added a vitamin solution and twice the volume of rice with H2O, in 250 mL Erlenmeyer flasks. These were incubated at 22°C until the cultures showed good growth (~14 days) to generate the screener cultures. The scale-up cultures were treated in a similar manner but grown in 2.8-L Fernbach flasks (Corning, Inc., Corning, NY, USA) containing 150 g rice and 300 mL H2O. These were inoculated using the seed cultures grown in the YESD media and were incubated at 22°C for 14 days.
Extraction and Isolation
To the small scale cultures on rice were added 60 mL of 1:1 MeOH-CHCl3, and the mixtures were shaken for 16 h on a reciprocating shaker. The solutions were filtered, and equal volumes of H2O and CHCl3 were added to the filtrate to bring the total volume to 250 mL. The solutions were stirred vigorously for 1 h, partitioned in a separatory funnel, and the bottom, organic layers were concentrated by rotary evaporation to dryness. Then, each extract was defatted by stirring vigorously for 1 h in a mixture of 50 mL MeOH, 50 mL CH3CN and 100 mL hexane and then partitioned in a separatory funnel. The bottom layers were collected and evaporated to dryness. The scale-up solid fermentations on rice were extracted using the same procedures, except that the extraction volumes were adjusted as follow: 500 mL of 1:1 MeOH-CHCl3 mixture, equal volumes of H2O and CHCl3 to a total volume of 2 L, and a mixture of 150 mL MeOH, 150 mL CH3CN and 200 mL hexane for the defatting process. Each defatted large scale extract (602 mg and 3.2 g for MSX 64546 and MSX 59553, respectively) was adsorbed onto a minimal amount of Celite 545 (Acros Organics, Geel, Belgium) and dried with mixing via a mortar and pestle. The materials were fractionated separately at 40 mL/min on a RediSep Rf Gold Si-gel column (40 g), first with 100% hexanes for 0.7 column volumes (CV) followed by a gradient of 100% hexanes to 100% CHCl3 over 8.9 CV. The elution continued with 100% CHCl3 for 7.4 CV, then with a gradient of MeOH in CHCl3 (0–2% over 9.7 CV, then 2–5% over 5.2 CV, then 5–10% over 5.2 CV, then 10–20% over 3.7 CV, then 20–100% over 2.2 CV. MeOH (100%) was finally held for a further 6.7 CV. Fractions were collected every 25 mL and pooled according to UV and ELSD profiles. From MSX 64546, compounds 1–5 were present in fractions 19–29, which were combined and evaporated (229.8 mg; IC50 values < 2.0 µg mL−1 against H460 cells). This pooled fraction was then subjected to preparative HPLC (Synergi Max-RP column, 20–100% CH3CN in 0.1% aqueous formic acid over 30 min; fractions collected every 0.5 min). Further purification of the subsequent fractions by semipreparative HPLC (various ratios of CH3CN in 0.1% aqueous formic acid) afforded compounds 1 (1.6 mg), 2 (0.8 mg), 3 (2.3 mg), 4 (2.5 mg), and 5 (4.9 mg). From MSX 59553, compounds 1, 3, and 5 were present in fractions 34–56, while compounds 2, 4, 6, and 7 were present in fractions 57–61. These two sets of fractions were combined and evaporated individually (108.5 and 132.7 mg, respectively; IC50 values < 2.0 µg mL−1 against H460 cells). The combined pool of the former (fractions 34–56) was then subjected to preparative HPLC (YMC ODS-A, 20–100% CH3CN in 0.1% aqueous formic acid over 30 min; fractions collected every 0.5 min). Further purification of the subsequent fractions by semipreparative HPLC (various ratios of CH3CN in 0.1% aqueous formic acid) afforded compounds 1 (3.0 mg), 3 (2.1 mg), and 5 (5.5 mg). The combined pool of the latter (fractions 57–71) was subjected to preparative HPLC using the same conditions and column. Further purification of the subsequent fractions by semipreparative HPLC (various ratios of CH3CN in 0.1% aqueous formic acid) afforded compounds 2 (8.5 mg), 4 (15.5 mg), 6 (6.0 mg), and 7 (11.8 mg).
Verticillin H (1)
Compound 1 was isolated as a white amorphous solid (4.6 mg); [α]D25 +440 (c 0.1 CHCl3); UV (MeOH) λmax (log ε) 204 (4.79), 240 (2.84), and 301 (3.79) nm; IR (diamond) νmax 3522, 2939, 1685, 1429, and 1376 cm−1; CD (MeOH) λmax (Δε) 235 (+62.1), 270 (−5.9), 304 (+8.6), and 373 (−1.2) nm; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz), see Table 1 and Supplementary Information (Figures S1–S6); HRESIMS (positive mode) m/z 725.1326 [M+H]+ (calcd for C32H33N6O6S4, 725.1339), (negative mode) m/z 723.1189 [M–H]− (calcd for C32H31N6O6S4, 723.1193).
Cytotoxicity Assay
The cytotoxicity measurements against the MCF-734 human breast carcinoma (Barbara A Karmanos Cancer Center), NCI-H46035 human large cell lung carcinoma [HTB-177, American Type Culture Collection (ATCC)], SF-26836 human astrocytoma (NCI Developmental Therapeutics Program), HT-2937 human colorectal adenocarcinoma (HTB-38, ATCC) and the MDA-MB-43538 human melanoma (HTB-129, ATCC) cell lines were performed as described in detail previously.39
NF-κB p65 Assay
An ELISA based NF-κB inhibitory assay was performed as described previously.39 Rocaglamide (Enzo Life Sciences, Inc., Farmingdale, NY, USA) was used as a positive control (IC50 value of 0.075 µM).
Epidermal Growth Factor Receptor Enzymatic Assay
Measurement of EGFR tyrosine kinase inhibitor activity for compounds 1–7 was performed by BPS Biosciences (San Diego, CA, USA). Detailed experimental descriptions are provided in the Supporting Information as well as via protocols available at http://www.bpsbioscience.com.
EGFR+ve Cell Assay
Human EGFR positive (EGFR+ve) and EGFR negative (HuTu-80) cells were cultured under standard conditions, and total cell lysates were prepared from sub-confluent cultures and analyzed by standard immunoblotting for total EGFR levels as described earlier.40 In other studies, both cell lines were treated with indicated concentrations of the agent and after desired time, both total and dead cell numbers were determined using a hemocytometer, using methods described previously.40
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by grants P01 CA125066 and R01 CA102514 (to RA) from the National Cancer Institute/National Institutes of Health, Bethesda, MD, USA. Mycology technical support was provided by Maurica Lawrence. The high resolution mass spectrometry data were acquired at the David H Murdock Research Institute, Kannapolis, NC, USA and the Triad Mass Spectrometry Laboratory at the University of North Carolina at Greensboro. EGFR tyrosine kinase assays were performed by BPS Biosciences (San Diego, CA, USA). We thank Mallikarjuna Gu in the Agarwal laboratory for his technical assistance in conducting the EGFR+ve cell assays.
Footnotes
Supporting Information. Experimental protocol for the EGFR tyrosine kinase assay, 1D and 2D NMR spectra for verticillin H (1), and NMR data for known compounds 2–7. Supplementary Information accompanies the paper on The Journal of Antibiotics website (http://www.nature.com/ja).
References
- 1.Chen Y, et al. Ecology-based screen identifies new metabolites from a Cordyceps-colonizing fungus as cancer cell proliferation inhibitors and apoptosis inducers. Cell Prolif. 2009;42:838–847. doi: 10.1111/j.1365-2184.2009.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isham CR, et al. Chaetocin: a promising new antimyeloma agent with in vitro and in vivo activity mediated via imposition of oxidative stress. Blood. 2007;109:2579–2588. doi: 10.1182/blood-2006-07-027326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang YX, et al. 11,11'-Dideoxy-verticillin: a natural compound possessing growth factor receptor tyrosine kinase-inhibitory effect with anti-tumor activity. Anti-Cancer Drugs. 2005;16:515–524. doi: 10.1097/00001813-200506000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi C, et al. Leptosins I and J, cytotoxic substances produced by a Leptosphaeria sp. Physico-chemical properties and structures. J. Antibiot. 1994;47:1242–1249. doi: 10.7164/antibiotics.47.1242. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi C, et al. Leptosins, antitumor metabolites of a fungus isolated from a marine alga. J. Chem. Soc., Perkin Trans. 1. 1994:1859–1864. [Google Scholar]
- 6.Takahashi C, et al. Potent cytotoxic metabolites from a Leptosphaeria species. Structure determination and conformational analysis. Tetrahedron. 1995;51:3483–3498. [Google Scholar]
- 7.Chen Y, et al. Antiangiogenic activity of 11,11′-dideoxyverticillin, a natural product isolated from the fungus Shiraia bambusicola. Biochem. Biophys. Res. Commun. 2005;329:1334–1342. doi: 10.1016/j.bbrc.2005.02.115. [DOI] [PubMed] [Google Scholar]
- 8.Saito T, et al. Chetracin A and chaetocins B and C, three new epipolythiodioxopiperazines from Chaetomium spp. Chem. Pharm. Bull. 1998;36:1942–1956. [Google Scholar]
- 9.Son BW, Jensen PR, Kauffman CA, Fenical W. New cytotoxic epidithiodioxopiperazines related to verticillin A from a marine isolate of the fungus Penicillium. Nat. Prod. Lett. 1999;13:213–222. [Google Scholar]
- 10.Feng Y, Blunt JW, Cole AL, Munro MH. Novel cytotoxic thiodiketopiperazine derivatives from a Tilachlidium sp. J. Nat. Prod. 2004;67:2090–2092. doi: 10.1021/np030326w. [DOI] [PubMed] [Google Scholar]
- 11.Zheng CJ, Park SH, Koshino H, Kim YH, Kim WG. Verticillin G a new antibacterial compound from Bionectra byssicola. J. Antibiot. 2007;60:61–64. doi: 10.1038/ja.2007.8. [DOI] [PubMed] [Google Scholar]
- 12.Zheng CJ, Kim CJ, Bae KS, Kim YH, Kim WG. Bionectins A–C, epidithiodioxopiperazines with anti-MRSA activity, from Bionectra byssicola F120. J. Nat. Prod. 2006;69:1816–1819. doi: 10.1021/np060348t. [DOI] [PubMed] [Google Scholar]
- 13.Bertinetti BV, Rodriguez MA, Godeas AM, Cabrera GM. 1H,1'H-[3,3']biindolyl from the terrestrial fungus Gliocladium catenulatum. J. Antibiot. 2010;63:681–683. doi: 10.1038/ja.2010.103. [DOI] [PubMed] [Google Scholar]
- 14.Argoudelis AD. Melinacidins 2, 3 and 4, new "3,6-epidithiadiketopiperazine" antibiotics. J. Antibiot. 1972;25:171–178. doi: 10.7164/antibiotics.25.171. [DOI] [PubMed] [Google Scholar]
- 15.Joshi BK, Gloer JB, Wicklow DT. New verticillin and glisoprenin analogues from Gliocladium catenulatum, a mycoparasite of Aspergillus flavus Sclerotia. J. Nat. Prod. 1999;62:730–733. doi: 10.1021/np980530x. [DOI] [PubMed] [Google Scholar]
- 16.Minato H, Matsumoto M, Katayama T. Verticillin A, a new antibiotic from Verticillium sp. J. Chem. Soc. D. 1971:44–45. [Google Scholar]
- 17.Watts KR, et al. Assessing the trypanocidal potential of natural and semi-synthetic diketopiperazines from two deep water marine-derived fungi. Bioorg. Med. Chem. 2010;18:2566–2574. doi: 10.1016/j.bmc.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong JY, He HP, Shen YM, Zhang KQ. Nematicidal epipolysulfanyldioxopiperazines from Gliocladium roseum. J. Nat. Prod. 2005;68:1510–1513. doi: 10.1021/np0502241. [DOI] [PubMed] [Google Scholar]
- 19.Ovenden SP, et al. A diketopiperazine dimer from a marine-derived isolate of Aspergillus niger. J. Nat. Prod. 2004;67:2093–2095. doi: 10.1021/np0497494. [DOI] [PubMed] [Google Scholar]
- 20.Erkel G, Gehrt A, Anke T, Sterner O. Induction of differentiation in acute promyelocytic leukemia cells (HL-60) by the verticillin derivative Sch 52900. Z. Naturforsch., C: J. Biosci. 2002;57:759–767. doi: 10.1515/znc-2002-7-834. [DOI] [PubMed] [Google Scholar]
- 21.Gardiner DM, Waring P, Howlett BJ. The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis. Microbiology. 2005;151:1021–1032. doi: 10.1099/mic.0.27847-0. [DOI] [PubMed] [Google Scholar]
- 22.Hauser D, Weber HP, Sigg HP. Isolierung und strukturaufklärung von chaetocin. Helv. Chim. Acta. 1970;53:1061–1073. doi: 10.1002/hlca.19700530521. [DOI] [PubMed] [Google Scholar]
- 23.Katagiri K, Sato K, Hayakawa S, Matsushima T, Minato H. Verticillin A, a new antibiotic from Verticillium sp. J. Antibiot. 1970;23:420–422. doi: 10.7164/antibiotics.23.420. [DOI] [PubMed] [Google Scholar]
- 24.Minato H, Matsumoto M, Katayama T. Studies on the metabolites of Verticillium sp. Structures of verticillins A, B, and C. J. Chem. Soc., Perkin Trans. 1. 1973;17:1819–1825. doi: 10.1039/p19730001819. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Ashenhurst JA, Movassaghi M. Total synthesis of (+)-11,11'-dideoxyverticillin A. Science. 2009;324:238–241. doi: 10.1126/science.1170777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller SJ. Chemistry. Total chemical synthesis peers into the biosynthetic black box. Science. 2009;324:186–187. doi: 10.1126/science.1172081. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Movassaghi M. General approach to epipolythiodiketopiperazine alkaloids: total synthesis of (+)-chaetocins A and C and (+)-12,12'-dideoxychetracin A. J. Am. Chem. Soc. 2010;132:14376–14378. doi: 10.1021/ja106869s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu M, et al. Inhibition of c-fos proto-oncogene induction by Sch 52900 and Sch 52901, novel diketopiperazine produced by Gliocladium sp. J. Antibiot. 1995;48:1440–1445. doi: 10.7164/antibiotics.48.1440. [DOI] [PubMed] [Google Scholar]
- 29.Liu F, et al. Verticillin a overcomes apoptosis resistance in human colon carcinoma through DNA methylation-dependent upregulation of BNIP3. Cancer Res. 2011;71:6807–6816. doi: 10.1158/0008-5472.CAN-11-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orjala J, Oberlies NH, Pearce CJ, Swanson SM, Kinghorn AD. Discovery of potential anticancer agents from aquatic cyanobacteria, filamentous fungi, and tropical plants. In: Tringali C, editor. Bioactive Compounds from Natural Sources. Natural Products as Lead Compounds in Drug Discovery. 2nd edn. London, UK: Taylor & Francis; 2012. pp. 37–63. [Google Scholar]
- 31.Kinghorn AD, et al. Discovery of anticancer agents of diverse natural origin. Pure Appl. Chem. 2009;81:1051–1063. doi: 10.1351/PAC-CON-08-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Che Y, et al. (Institute of Microbiology, Chinese Academy of Sciences, Peop. Rep. China). Method for preparing gliocladicillin C and application thereof. 2009-10077302-101805699. CN patent no. 2009 Feb 17;
- 33.Pahl HL, et al. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-kappaB. J. Exp. Med. 1996;183:1829–1840. doi: 10.1084/jem.183.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soule HD, Vazguez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J. Natl. Cancer Inst. 1973;51:1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 35.Carney DN, Gazdar AF, Bunn PA, Jr, Guccion JG. Demonstration of the stem cell nature of clonogenic tumor cells from lung cancer patients. Stem Cells. 1982;1:149–164. [PubMed] [Google Scholar]
- 36.Rosenblum ML, et al. Stem cell studies of human malignant brain tumors. Part 1: Development of the stem cell assay and its potential. J. Neurosurg. 1983;58:170–176. doi: 10.3171/jns.1983.58.2.0170. [DOI] [PubMed] [Google Scholar]
- 37.Fogh J, Trempe G. Human Tumor Cells In Vitro. New York, USA: Plenum Press; 1975. New human tumor cell lines; pp. 115–159. [Google Scholar]
- 38.Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD. MDA-MB-435 cells are derived from M14 melanoma cells--a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res. Treat. 2007;104:13–19. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- 39.Ayers S, et al. Resorcylic acid lactones with cytotoxic and NF-kappaB inhibitory activities and their structure-activity relationships. J. Nat. Prod. 2011;74:1126–1131. doi: 10.1021/np200062x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deep G, Singh RP, Agarwal C, Kroll DJ, Agarwal R. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2005;25:1053–1069. doi: 10.1038/sj.onc.1209146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.