Abstract
Objective
Ploidy analysis of Feulgen-thionin stained cervical cytology specimens has been shown to detect cases of high grade cervical dysplasia. However, ploidy analysis alone cannot always distinguish between cells with abnormal DNA content and normal cycling cells. We sought to use double staining with anti-Ki-67 immunocytochemistry to improve ploidy analysis.
Study Design
Cervical cytology specimens from 49 patients with various diagnoses, mostly dysplasias, from a previous study were used. Samples were double stained with Feulgen-thionin and anti-Ki-67 immunocytochemistry. Ki-67-negative cells were non-cycling, so non-diploid Ki-67-negative cells were likely truly abnormal cells.
Results
The area under the receiver operating characteristic curve for the ability to identify high-grade dysplasias was 0.73 for double staining and 0.74 for thionin-only ploidy analysis on cytospin specimens. At 90% specificity, sensitivities for double staining and thionin alone were 45% and 32%, respectively, but the difference was not statistically significant.
Conclusion
Double staining with Feulgen-thionin and anti-Ki-67 immunocytochemistry does not improve the ability of ploidy analysis of cervical cytology specimens to separate high- and low-grade dysplasias, but our insights into the technical aspects of double staining, especially the effects of antigen retrieval, give hope that this technique could be applied to other immunocytochemical stains that would have a greater ability to improve ploidy analysis.
Keywords: Ploidy, Early cancer detection, Cervical cancer, Quantitative image cytometry, Proliferation, Immunocytochemistry, Heat-mediated antigen retrieval
Introduction
Cervical cancer is the third most commonly diagnosed cancer in females globally.1 While screening programs based on the Papanicolaou (Pap) smear have significantly reduced mortality due to cervical cancer in industrialized nations,1–3 more than 85% of cases today arise in low-resource settings, making cervical cancer the second-leading cause of cancer death among women in developing countries.1,2 This presents a distinct challenge to establishing cervical screening programs where they are needed most, as screening programs based on the Pap smear require an extensive and costly infrastructure, in addition to significant training and skill to interpret the patient slides. Potential alternatives include visual inspection with acetic acid4 and human papillomavirus (HPV) testing.5–9 Unfortunately, visual inspection methods continue to rely on adequate training of practitioners while rollout of HPV testing programs in low-resource settings has been hindered by cost and logistics.10 Moreover, high-risk HPV testing has a higher sensitivity but lower specificity for detecting high-grade cervical precancers than conventional cytology11 and low-resource settings are particularly sensitive to the follow up costs of false positive cases.
We have previously shown that ploidy analysis using Feulgen-thionin staining performs comparably with conventional cytology and HPV testing for detecting cervical high-grade lesions.12 Further study suggests that ploidy and HPV mRNA may be independent predictors of cervical dysplasia.13
However, the amount of DNA present within the nucleus of a normal cycling cell changes as it progresses through the cell cycle. A normal cycling cell can be diploid, tetraploid, or somewhere in between. Frankly abnormal cells (>2.5 times the normal complement of DNA) are rare and occur in widely disparate and very low frequencies, even in high grade squamous intraepithelial lesions (HGSIL).14–16 Hence, ploidy might be an improved biomarker for cervical cancer screening if normal dividing cells could be distinguished from abnormal non-cycling cells by using an immunostain for Ki-67 as a marker of cell proliferation. Ki-67 is an antigen expressed in the nuclei or on chromosome surfaces during all active phases of the cell cycle (ie. all except G0).17 As such, it has been used for many years as a proliferation marker and in the assessment of many cancers,17–21 including cervical cancer.22–25
Previous attempts to simultaneously determine DNA content and assess proliferation status in the same cell have relied heavily on fluorescent labels detected by flow cytometry, in which abnormal cell identification is hindered by the uncertainty of only individual cell passage through the flow cytometer, which can mistakenly detect signals from non-cellular material, especially when trying to detect a relatively rare event.26,27 We instead propose to use absorbance stains on slide-mounted samples. Absorbance stains are permanent and less costly to image, as a simple light microscope will suffice. A slide-based assay would enable the study of a wider range of sample types without picking up signals from non-cellular material. By double staining cervical cytological specimens, normal cycling cells can be removed from the analysis, focussing on those cells whose abnormal DNA content might be indicative of large-scale chromosomal mutations associated with precancerous changes. Hence, we hypothesize that by studying Ki-67-negative cells only, ploidy analysis can be a better indicator of high-risk dysplastic lesions than ploidy analysis on cycling and non-cycling cells combined.
Materials and Methods
Cell culture
Cell culture was performed to generate large numbers of cytology slides for protocol optimization. HL-60 acute promyelocytic leukemia and H460 large cell lung cancer cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in Iscove’s Modified Dulbecco’s Medium and RPMI, respectively, supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cells were incubated at 37°C in an atmosphere of 95% air and 5% carbon dioxide. To generate HL-60 slides, autoclaved, uncharged, pre-cleaned glass slides were placed in square culture dishes, 3 per dish, and covered with 15mL of cell suspension at 5×105 cells/mL in growth medium. To each dish was added 15µL of 1mg/mL phorbol 12-myristate 13-acetate (Sigma-Aldrich Canada, Oakville, ON, Canada) solution in ethanol, causing the cells to adhere to the slides.28 After an additional 48 hours of growth, the slides were rinsed, fixed in Sed-Fix® (Surgipath, Richmond, IL, USA) for 40 minutes, and allowed to dry overnight. Before use in any staining procedures, slides were cleared of dried fixative by immersion in ethanol for 20 minutes at room temperature followed by thorough air-drying. H460 slides were made by fixing a cell suspension (via trypsinization) of 4×104cells/mL in 10% buffered formalin for 10 minutes at room temperature. 250µL of this suspension was cytospun directly to each slide.
Cell lines were chosen for convenience and because HL-60 slides prepared as described were used routinely in our laboratory as a control for batch-to-batch variation in Feulgen-thionin staining. Staining of cell lines was used to optimize staining and imaging protocols only and no attempt was made to glean information about cancer biology from these results.
Patient samples
Specimens collected from forty-nine cervical cytology brushings representing a range of dysplastic grades from a previous study12 were used in this work. Approval was granted by the Internal Review Boards at M. D. Anderson Cancer Center, the University of Texas Health Science Center, the Lyndon Baines Johnson Hospital Health District, British Columbia Cancer Agency (BCCA), and the University of British Columbia. In the previous study, brushings were collected and fixed in PreservCyt (Hologic Inc, Bedford, MA, USA) and used to generate slides using the ThinPrep method (Hologic Inc). The residual material was stored at 1°C in a cold room before being used for the present study. As many of the vials contained pieces of tissue and other debris that might confound cytological analysis, the specimens were vortexed and allowed to settle for 15 minutes on ice before use. Samples were taken from the supernatant, post-fixed 10 minutes at room temperature with 10% buffered formalin, and cytospun on to new slides in duplicate. Within a day, one slide was stained with Feulgen-thionin only as a control, while the other was double stained.
Immunocytochemistry
Concentrate buffer solutions for antigen retrieval (pH 6 and 9) were obtained from Vector Laboratories (Burlingame, CA, USA) and used at 1:100 dilution. Bovine serum albumin (BSA), SIGMAFAST™ 3,3’-diaminobenzidine (DAB) chromogen tablets, and HRP-conjugated rabbit anti-mouse secondary antibody were obtained from Sigma-Aldrich Canada. Anti-Ki-67 monoclonal antibody (clone MIB-1) and serum-free protein block were purchased from Dako Canada (Mississauga, ON, Canada). All antibodies were diluted in 1% BSA in phosphate-buffered saline (PBS) just prior to use.
Antigen retrieval was performed using the microwave method, followed by cooling for 20 minutes. For patient samples, pH 9 buffer was used for 22.5 minutes, consistent with the vendor’s recommendations for the anti-Ki-67 primary antibody; various conditions were tried in the optimization experiments with cell lines. Blocking steps were 15 minutes with 3% v/v H2O2 in methanol for endogenous peroxidase, 5 minutes with 1% BSA and 0.1% Triton® X-100 in PBS for permeabilization, and 30 minutes with protein block for non-specific binding. Antibody incubations were one hour at room temperature for primary and 30 minutes for secondary (diluted 1:800), followed by 7 minutes with the DAB chromogen solution. After a thorough rinse, slides were dehydrated through graded alcohols, cleared in xylene, and coverslipped with Cytoseal™ mounting medium (Fisher Scientific Canada, Ottawa, ON, Canada).
Thionin staining
Thionin acetate powder was obtained from Sigma-Aldrich. All solutions required for thionin staining were prepared the day before use. To make approximately 250mL of the thionin staining solution, 0.125g thionin was added to 110mL deionized water and boiled for 5 minutes. After cooling to room temperature, 32.5mL 1N hydrochloric acid, 110mL tert-butanol, and 2.175g sodium bisulphite were added. The mixture was stirred for one hour, allowed to stand overnight, and filtered immediately prior to use.
All steps were performed at 23–24°C inside a temperature-controlled water bath. All steps were separated by thorough deionized water washes. The slides were post-fixed in Böhm-Sprenger fixative (methanol, formalin, and acetic acid, in a 16:3:1 volume ratio) for one hour, hydrolyzed for one hour in 5N hydrochloric acid, immersed in the thionin staining solution for one hour, and rinsed thrice in a bisulphite rinse solution (0.5% sodium bisulphite (w/v) in 0.05N hydrochloric acid), each separated by water rinses. After a final thorough wash, the slides were dehydrated through three changes of ethanol, 30 seconds each, cleared in xylene, and coverslipped before imaging. The hydrolysis period was varied in some experiments, so when multiple slides were stained on the same run with different hydrolysis times, the slides with the longest hydrolysis time were started first, with the other slides joining in such a manner that the hydrolysis period for all slides ended together. All thionin staining runs included at least one HL-60 slide that was to be stained with only thionin and hydrolyzed for 60 minutes to act as a run control.
Double staining with thionin and immunocytochemistry
When immunocytochemistry (ICC) was performed first, the procedure for immunostaining was followed as above and the slides were left overnight fully coverslipped. The following day, the slides were decoverslipped in xylene and rehydrated through graded alcohols, ending in several water washes before proceeding with the thionin staining.
When thionin staining was performed before ICC, the thionin procedure was followed as described up to the final rinse before dehydration with ethanol. The slides were then placed in PBS overnight. The slides were rinsed briefly with deionized water before performing immunocytochemistry.
The majority of the double staining of patient samples was done over 3 batches, each comprising a mix of dysplastic grades and control slides.
Imaging and analysis
Thionin-stained cells were imaged and analyzed using the automated Cyto-Savant™ image cytometer (Oncometrics Inc, Vancouver, Canada).29,30 The system was programmed to collect a random sampling of about 7000–10000 cells. All objects were subjected to a classification tree to sort objects into different classes; the only class of objects used in our analysis are the epithelial cells.31 As the Feulgen-thionin stain is stoichiometric for DNA, DNA content is proportional to the integrated optical density (IOD) of the cell. Each cell’s ploidy status was assessed by normalizing the cell’s IOD against the mean IOD of the sample’s diploid cell population, as determined from a frequency histogram of the nuclear IODs.32 Diploid cells were assigned a DNA index of 1.0, alternatively denoted 2c.
For double stained slides, imaging was performed after thionin staining. As the imaging system was monochrome, there was no way of determining a cell’s immunostaining status directly from the system’s output. However, cells could be manually revisited under the microscope by selecting them from the image gallery of cells automatically collected, allowing a human operator to manually assess each cell’s immunocytochemical staining status. Hence, Ki-67-positive cells must be captured by the cytometer in order for them to be counted in our analysis.
Results
As this work used residual specimens, summary data on the study population have previously been published.12 Moreover, the results from previously performed ploidy analyses, the cytological and histological diagnoses, and the HPV test results were all available. A subset of 49 specimens was selected from this set (Table I). Using moderate dysplasia and worse as the threshold for defining a high-grade lesion, the study samples were from 29 low-grade (LGSIL) or negative and 20 high-grade dysplasia cases (HGSIL), as determined previously by histopathology.12 HPV status had previously been determined by the Hybrid Capture II (HC II) test12 and those positive for both low-risk and high-risk strains were counted as high-risk for the purpose of this study.
Table I.
Patient specimens classified by conventional cytology and histopathology. Highlighting and italicized font denote those cases classified as positive for high-grade dysplasia. All others were treated as negative.
| Cytology | Histology | ||||
|---|---|---|---|---|---|
| Negative | Atypia/HPV | LG-SIL/CIN1 | HG-SIL/CIN2+ | Total | |
| Negative/atypia | 7 | 11 | 1 | 5 | 24 |
| LG-SIL | 0 | 0 | 2 | 6 | 8 |
| HG-SIL | 0 | 0 | 5 | 8 | 13 |
| No diag | 0 | 3 | 0 | 1 | 4 |
| Total | 7 | 14 | 8 | 20 | 49 |
Destaining of thionin by antigen retrieval
It was quickly discovered that heat-mediated antigen retrieval would destain thionin stained samples. Alternatively, the images and coordinates of the thionin stained cells could be stored on a computer before performing ICC. The ICC staining could then be matched up with the stored thionin data. However, even 15 minutes of Feulgen hydrolysis was sufficient to render the Ki-67 antigen undetectable by ICC. Hence, it was determined that thionin staining must follow immunocytochemical staining.
Reduction of thionin staining intensity after immunocytochemical staining due to antigen retrieval
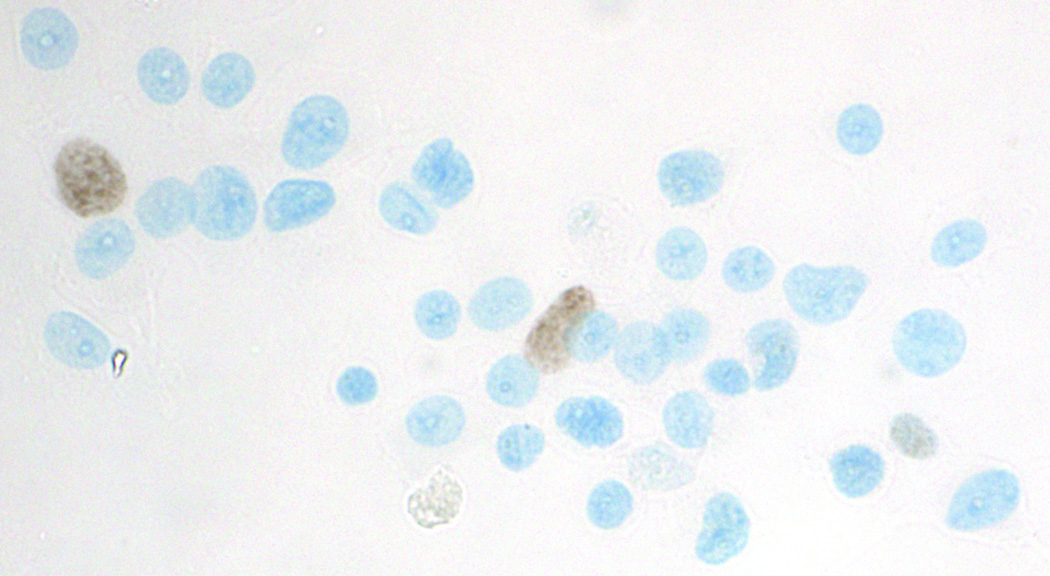
In our hands, the MIB-1 monoclonal antibody used to detect Ki-67 required antigen retrieval. Omitting this step consistently resulted in a complete abrogation of staining. Initial tests of thionin staining following immunocytochemistry revealed that while double staining was attainable (Figure 1), there was a significant reduction in thionin staining intensity compared to thionin staining alone, as reflected in the mean IOD of the diploid histogram peak.
Figure 1.
Successful double staining of HL-60 cells with anti-Ki-67 immunocytochemistry (brown) and Feulgen-thionin for DNA (blue). Compared with regular thionin-only staining, however, thionin intensity was noticeably weaker and reaction conditions needed to be re-optimized. Antibodies were diluted 1:100, antigen retrieval conditions were 10.5 minutes in pH6 citrate buffer, and the microscope image was obtained under 40X objective.
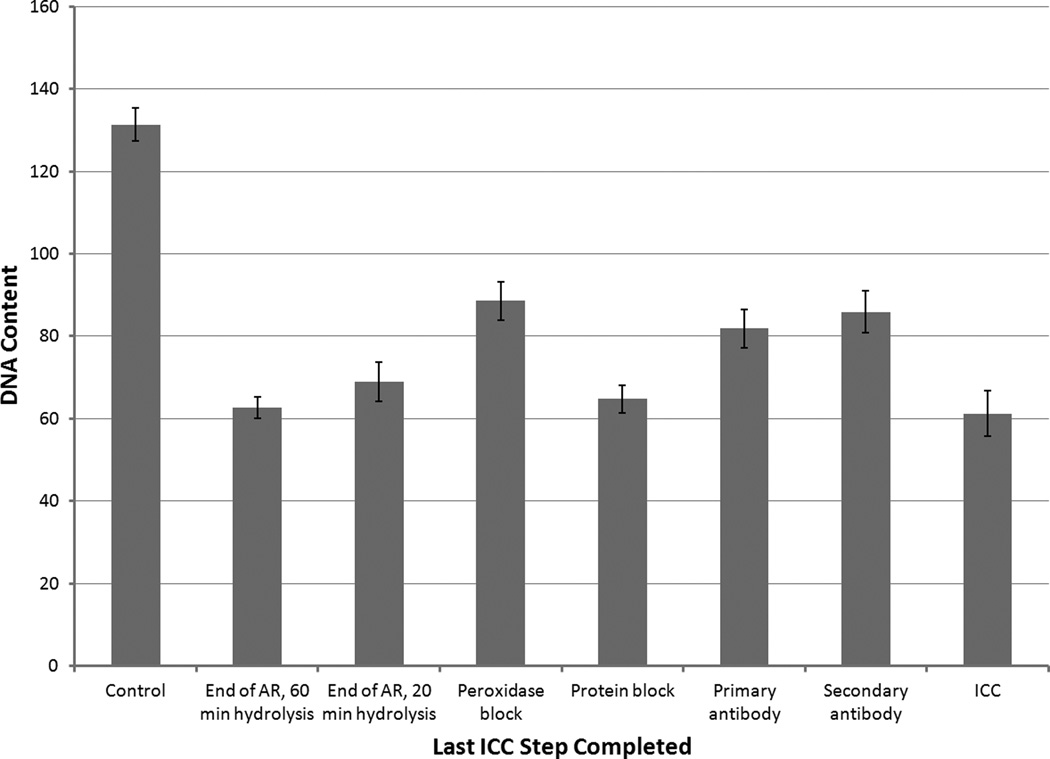
To determine whether this was due to the antigen retrieval step, a series of slides was tested in which immunocytochemistry was stopped at various steps in the protocol before thionin staining. Figure 2 shows that the greatest reduction in thionin staining intensity occurred after antigen retrieval and that subsequent immunocytochemical steps did not significantly alter the intensity of thionin staining beyond this initial reduction. We have also observed qualitatively weaker nuclear staining/fluorescence with hematoxylin or 4',6-diamidino-2-phenylindole (DAPI), both of which bind to DNA, after any procedures involving antigen retrieval.
Figure 2.
Aborted double staining test in which HL-60 slides were treated first with an aborted ICC protocol, placing the slide into PBS after the step indicated. Slides were then subjected to Feulgen-thionin staining the next day. Control slide had no ICC steps performed whatsoever. Two slides were stopped after antigen retrieval, but were hydrolyzed for different durations during Feulgen staining, as indicated. All other Feulgen-stained slides were hydrolyzed for 20 minutes. Antibodies were diluted 1:100 and antigen retrieval conditions were 10.5 minutes in pH6 citrate buffer. In cultured HL-60 slides, the Ki-67 positivity rate is too low to significantly alter IOD means and CVs. Error bars show standard deviations.
Optimization of hydrolysis time
As antigen retrieval is required for MIB-1 staining, attempts were made to minimize its impact on thionin stain intensity by altering the hydrolysis time in the Feulgen-thionin staining procedure. A series of slides was treated with a mock immunocytochemical stain followed by Feulgen-thionin with various hydrolysis times, from 0 to 80 minutes, in 10-minute intervals. The primary antibody was replaced with just the diluent, ensuring that no immunostained cells would confound the automated imaging analysis. The DNA histograms were plotted and we sought to maximize the mean DNA amount of the diploid peak while minimizing the corresponding coefficient of variation (CV). There was some variability between runs, with optimal hydrolysis times ranging between 20 and 40 minutes. For patient samples, 40 minutes was used as it was found to be the most consistent.
Image cytometry
DNA histograms of thionin-only HL-60 slides typically had a diploid peak around 130–150 units with a CV of 2–4%. Histogram bins were 5 units wide. Diploid and tetraploid peaks were considered to consist of all bins within approximately 2.5 standard deviations of the corresponding mean.
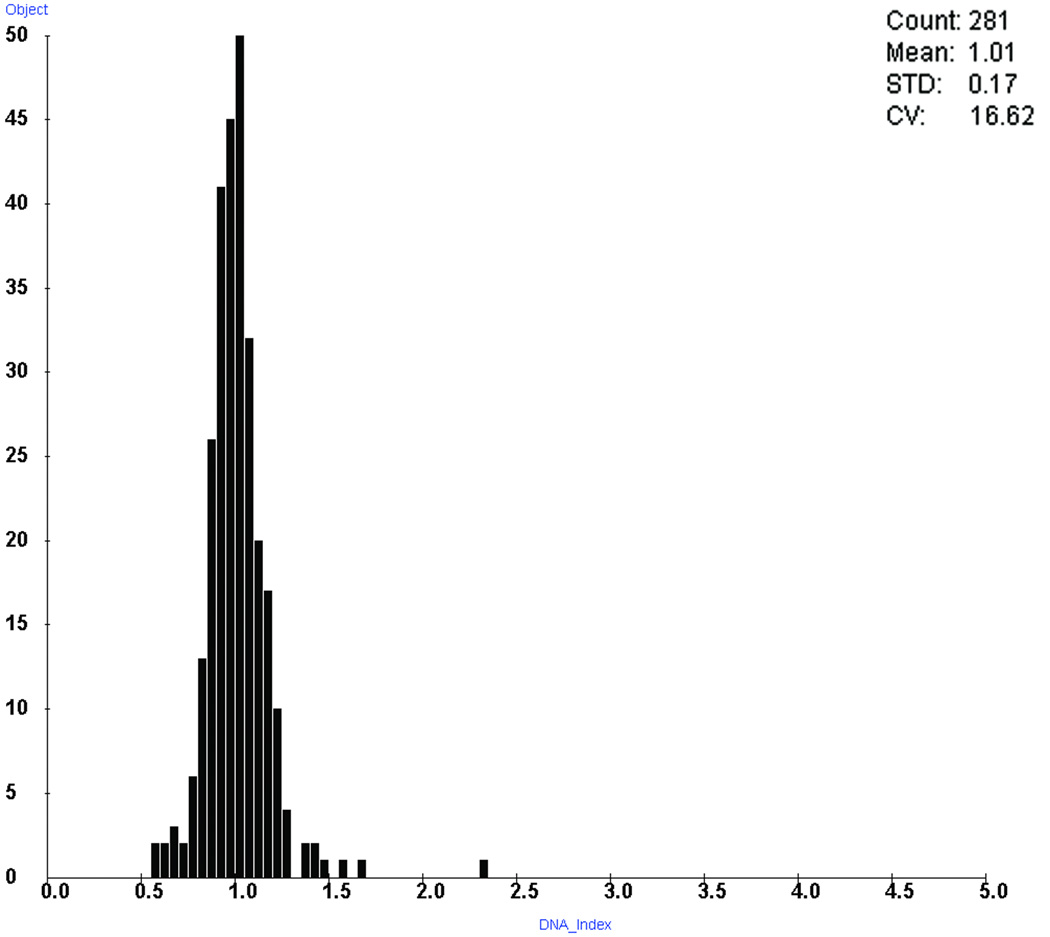
Even after optimization, the reduction in thionin staining intensity due to ICC persisted. Double stained cells in the patient samples had diploid nuclear IODs averaging 56% of the corresponding HL-60 thionin-only staining control, while the thionin-only patient slides averaged 89%. In other words, after using thionin-only HL-60 slides to adjust for batch-to-batch variations in staining intensity, the double stained slides averaged IODs of only 63% of those seen in thionin-only patient slides. As well, CVs were wider, as double stained slides with more than 50 imaged cells showed a median CV of 11.8% (range 7.3%–25%), compared to a median CV of 4.3% (range 2.8%–11.7%) for thionin-only slides. For comparison, the corresponding ThinPrep slides prepared from these samples for the previous study had a median CV of 4.3% (range 2.7%–12.2%). Figure 3 shows a typical DNA histogram of Ki-67-negative cells from a patient who was negative for dysplasia.
Figure 3.
A typical DNA histogram of Ki-67-negative cells taken from a patient who was negative for dysplasia. Double staining was used to identify and remove Ki-67-positive cells. Cells were binned according to DNA index, where a value of 1.0 corresponds to the mean of the diploid peak.
Cervical cytology samples
Due to the weaker thionin staining after ICC, a lot fewer imaged cells were kept as they were too faint to be recognized as cells. In order to preserve statistical significance, a minimum cell count threshold of 50 was set for all patient slides. This resulted in about 12% of double stained slides being excluded, while none of the thionin-only slides were excluded.
To assess the ability of double staining to detect the HGSIL, patients were classified based on diagnostic data from their prior study involvement. In that study, patients had conventional cytology and histopathological diagnoses of colposcopically directed biopsy. Based on these two diagnoses, presence of moderate dysplasia or worse in either defined the patient as positive for high-grade dysplasia (HGSIL). This is the same criterion for treatment at the BCCA. Patients with other observed dysplasias were considered LGSIL, while those without dysplasia were classified as normal.
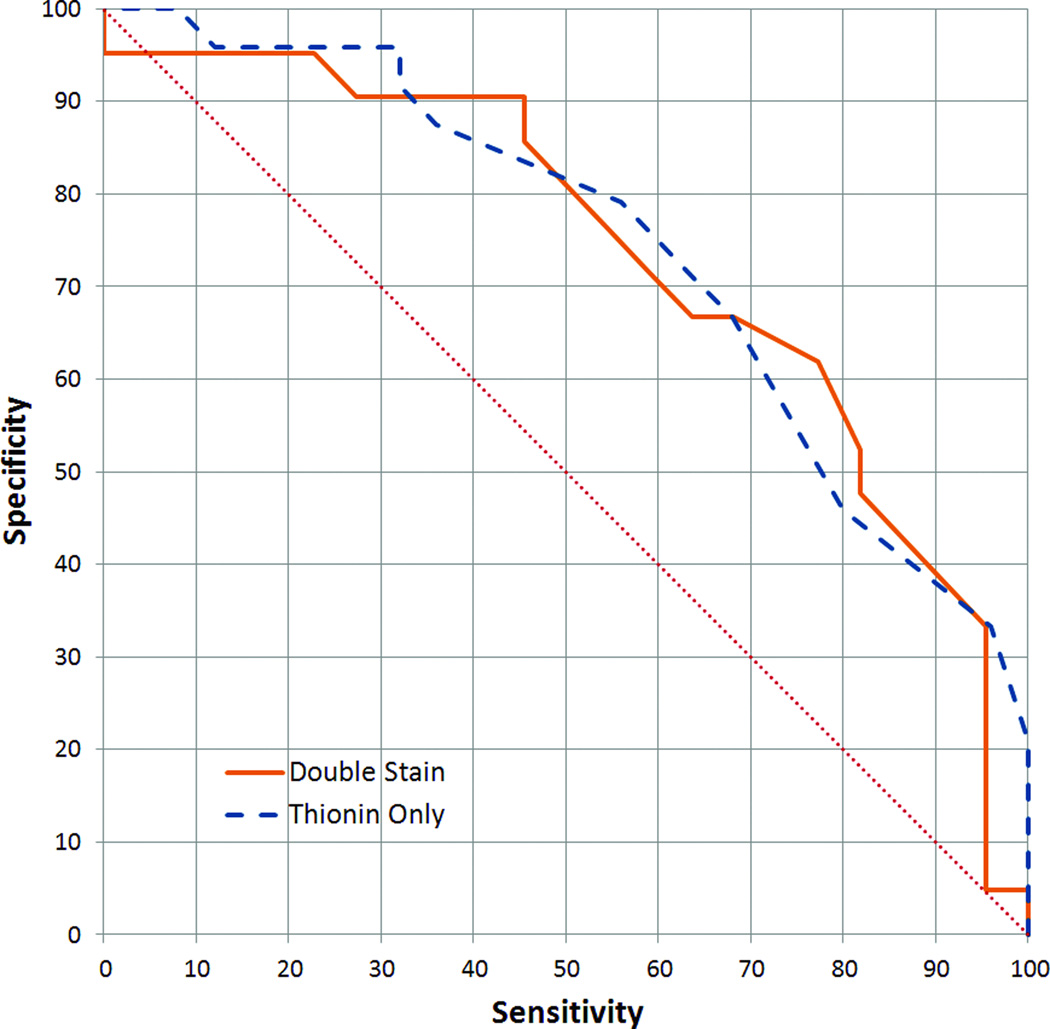
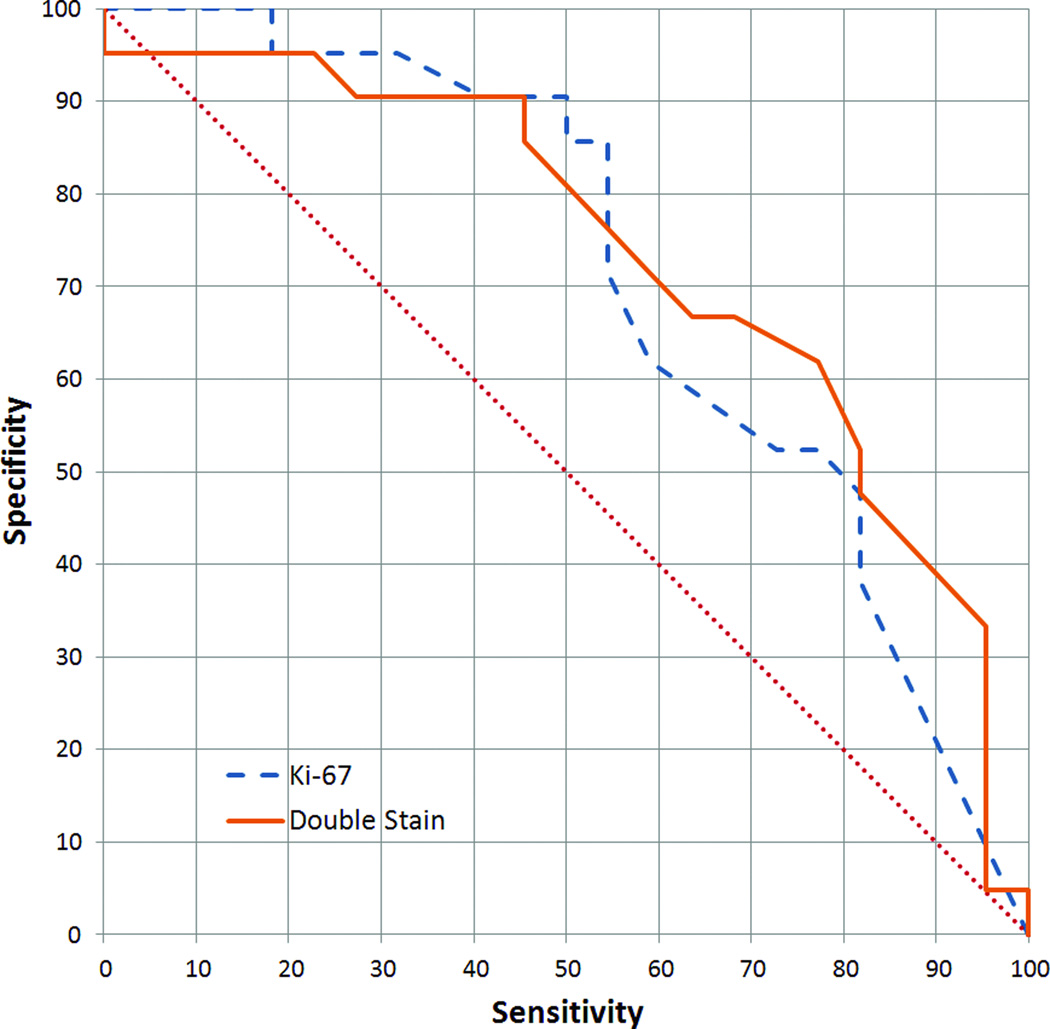
A critical test of the double staining method was whether the proportion of non-diploid cells became a better indicator of high-grade dysplasia once double staining was used to remove proliferating cells. In double stained samples for the analyses described in this section, only Ki-67-negative cells were considered. The diploid-exceeding rate is calculated by dividing the number of cells with greater than diploid DNA content (ie. >2.5 standard deviations above the diploid mean) by the number of all Ki-67-negative cells. In the thionin-only samples, the diploid-exceeding rate was determined from all imaged cells. Using the definitions of high-grade dysplasia given above, receiver operating characteristic (ROC) curves for both these analyses could be constructed. Figure 4 shows that the two analyses gave similar results, with areas under the curve (AUC) of 0.73 and 0.74 (approximated by trapezoidal rule) for double stained and thionin-only, respectively.
Figure 4.
Receiver operating characteristic (ROC) curve of the performance of the diploid-exceeding rate in detecting high-grade dysplasias (as defined in the text). Areas under the curve (AUC) were 0.73 and 0.74 (trapezoidal rule) for double stained and thionin-only cytospins, respectively.
In addition to comparing double staining with thionin staining alone, one can also consider using Ki-67 alone as a potential marker for high-grade dysplasia. The Ki-67-positivity rate for the sample was determined by dividing the number of Ki-67 cells imaged by the total of all cells imaged (Ki-67 positive and negative) in a slide. Figure 5 shows that using Ki-67-positivity rate alone performs slightly worse than double staining at detecting high-grade dysplasias, with an AUC of 0.71.
Figure 5.
ROC curve comparing the use of Ki-67-positivity rate and double staining for detecting high-grade dysplasias. Double staining performed better, with an AUC of 0.73, compared to 0.67 for Ki-67 alone.
A common approach to using ploidy measurements for cancer detection is to count the number of 5c exceeding cells. Due to the significant variation in the number of cells on each slide, the percentage of imaged cells with DNA content exceeding 5c (5cER) was used instead. In an analysis of the subset of our previously published results12 corresponding to the samples in the present study, at a 5cER cutoff of 0.2%, sensitivity and specificity were 52% and 92%, roughly in line with our previously reported results where at least five 5c exceeding cells was used as a threshold (Table 4 in Guillaud et al.12). The thionin-only cytospins only had about 60% as many imaged cells per slide (median of 2032 versus 3373 for ThinPrep), resulting in a reduced sensitivity and specificity of 56% and 83%, respectively, when using either a 5cER cutoff of 0.02% or at least one 5c exceeding cell to be considered positive. For the double stained slides, however, a threshold of at least one Ki-67-negative 5c exceeding cell produced a very low sensitivity of 23% (95% specificity), likely because of the low cell count (median of 254 Ki-67-negative imaged cells, ranging from 1–2440). With 250 imaged cells per slide, a 5cER of 0.2% (ie. the ThinPrep threshold) would be equivalent to half a 5c exceeding cell per slide.
Another approach to analyzing the ploidy data is to assess the discriminating ability of the frequency of cells falling within a series of DNA index ranges. The ranges considered were 1.3–1.6, 1.6–1.85, and 1.85–2.15. In all cases, ROC curves for the thionin-only cytospins had AUCs between 0.7–0.8, while double staining performed noticeably worse. Double staining performed best in the near-tetraploid 1.85–2.15 range, with an AUC of 0.65. Double stained slides typically had very few cells in this range (maximum 12). Raising the minimum imaged Ki-67-negative cells per slide threshold for inclusion in this analysis to 150 improves the AUC for the 1.85–2.15 DNA index range to 0.79, virtually identical to thionin-only staining, but at a cost of excluding over 30% of the samples due to inadequate cell count.
Discussion
Automated image cytometry has demonstrated utility for early detection of various cancers.33–39 Our group had previously shown that ploidy analysis using Feulgen-thionin staining performs comparably with conventional cytology and HPV testing for detecting cervical high-grade lesions.12 However, as the ever-changing amount of DNA present within the nucleus of a normal cycling cell might confound a ploidy-based analysis, we sought to determine if double staining with an additional proliferation marker might improve the use of ploidy in detecting high-grade cervical dysplasias.
A previous attempt at Feulgen/Ki-67 double staining used the Feulgen stain as a low-background nuclear counterstain to quantify Ki-67 labelling.40 However, the present study attempts to exploit the quantitative nature of the Feulgen reaction. This was the approach of Oud et al., who used an alkaline phosphatase detection of Ki-67 with the proprietary chromogen CAS Red.41 Their analysis was restricted to cell lines, while we applied this technique to patient samples to see if it would improve the clinical utility of ploidy analysis. Fleskens et al. applied double staining to paraffin sections of oral dysplasias,42 although not in a screening or early detection setting. However, as even the authors themselves point out, direct ploidy analysis of tissue sections remains highly controversial, with studies arguing both for and against it, as it must contend with nuclear truncation and overlap.42
A procedure for optimizing double staining conditions
Our results show that any attempt to double stain with Feulgen-thionin and an immunocytochemical marker must start with the ICC. The harsh conditions of microwave-induced antigen retrieval destained thionin and even a short period of acid hydrolysis as part of Feulgen-thionin staining rendered the Ki-67 antigen undetectable by ICC.
Contrary to the observations of Oud et al.,41 we found that combining immunocytochemical staining with Feulgen-thionin had a significant impact on the intensity of thionin staining. Evidence suggests this is due to our use of antigen retrieval and we further observe that Oud and colleagues did not mention any use of antigen retrieval in their report. (While both Kolles et al.40 and Fleskens et al.42 reported using antigen retrieval, neither group attempted to compare Feulgen stain intensity with and without ICC.) As Feulgen staining involves exposing a cell sample to an acid to hydrolyze off purine bases of DNA to generate reactive sites for the thionin stain,43,44 Feulgen staining intensity might be impacted by any reactions that might also result in hydrolysis of DNA. Antigen retrieval, especially when mediated by heat, is hypothesized to work at least partly through hydrolysis.45 Optimization of thionin staining involves balancing the creation of more abasic reactive sites against the destruction of the DNA backbone (where the shorter segments can be lost to diffusion) using longer and more potent hydrolysis reactions.43,44 It appears antigen retrieval both disrupts this balance and permanently reduces the quantity of DNA available for Feulgen reaction by destroying some of the DNA backbone.
As many antibodies available today require some form of antigen retrieval, our experiences with double staining with MIB-1 might enable countless scientific questions to be answered by simultaneously staining with Feulgen-thionin and any immunostain requiring antigen retrieval. Future investigations may consider, for example, the biological mechanisms by which premalignant cells become aneuploid and the consequences of such transformations. By understanding such mechanisms, biomarkers and interventions may yet be developed that target such abnormalities to help detect or even treat precancerous changes even earlier.
In order to successfully double stain a cytological sample, one must optimize a series of parameters in a specific order. First, immunocytochemical staining conditions must be optimized, with special attention given to antigen retrieval. The mildest form of antigen retrieval required to get an acceptable level of staining should be chosen. Second, Feulgen hydrolysis conditions must be optimized to account for the effects of the antigen retrieval method selected in the first step. This is best achieved by subjecting a series of slides to double staining with mock ICC and various Feulgen acid hydrolysis conditions. Antigen retrieval will generally lead to shorter optimal hydrolysis times and reduced overall Feulgen staining intensity. Finally, imaging and analysis protocols may need to be optimized to handle the lower intensities expected with double stained samples.
Ability to discriminate HGSIL and LGSIL samples
Double staining was found to match thionin staining alone in its ability to discriminate between high- and low-grade cervical dysplasias (Figure 4). In the high-specificity operating range, where HPV testing has typically not fared as well, double staining performs better than thionin alone on cytospins, but Fisher’s exact test showed the sensitivities were not statistically significant at 90% specificity (one-tailed, p=0.26). At this specificity, for the samples available, a test with twice the sensitivity of thionin alone (64% versus 32%) would be statistically distinguishable (p=0.03). Overall, though, double staining failed to show any significant improvement. This is likely due to the low percentages of both Ki-67-positive cells and non-diploid cells in our samples. The median Ki-67-positivity rate across the sample set was 0.4%, while the median rate of cells with greater than diploid DNA content was 3.2%. This meant that even if the Ki-67 staining was contributing information that would allow us to better discriminate between high- and low-grade dysplasias, the effect was so small that it could not be detected with the sample sizes utilized.
A low Ki-67-positivity rate is expected for many low-grade dysplasias.22,46,47 Moreover, cytological specimens are preferentially sampled from the uppermost layers of the epithelium, where proliferation rates are generally substantially lower than in more basal cell layers, except for cancers and the most severe dysplasias.46 Sahebali et al., using Ki-67 immunostaining on cervical cytology, found only about 0.35% average Ki-67 positivity in high grade squamous intraepithelial lesions.22 This is an even lower rate than we observed, underscoring one of the challenges of using these rare cells to improve cancer detection.
Double staining outperformed Ki-67 staining alone in detecting high-grade dysplasias. However, this analysis of Ki-67 staining is far from perfect, hampered by the inherent low rate of Ki-67 staining and our system’s inability to image every individual cell. While these results show promise that double staining is an improvement over Ki-67 staining alone, more work and perhaps an improved imaging system will be needed to show this conclusively.
Any cervical cancer screening technologies are invariably compared to HPV testing. Within our sample set, using the presence of high-risk strains of HPV as the criterion for positivity, HC II testing was found to have a 92% sensitivity and 79% specificity, which is similar to the result previously reported for the full sample population from which our set was derived.12 This is considerably better than double staining (Figure 4) or thionin-only among this sample set.
Although double staining with thionin and anti-Ki-67 immunocytochemistry does not appear to be an improvement over regular thionin staining for the identification of high-grade dysplasias, our results suggest that double staining is a feasible assay that could be extended to other immunocytochemical stains that might demonstrate a greater improvement when paired with thionin. Perhaps some or most of the dysplastic cells are stuck at check points within the non-resting phases of the cell cycle (ie. not in G0) and are therefore seen as cycling cells. A more specific marker for S phase cells (eg. proliferating cell nuclear antigen or cyclin A) or mitotic cells (eg. phosphohistone-H348) might be better in this case, although the increased specificity of the immunostain would require a higher sample cellularity in order to observe any improvement in the ability to discriminate between high- and low-grade dysplasias. Alternatively, ploidy analysis has been shown to complement high-risk HPV testing done in parallel,49 but a double stain approach might prove even more beneficial. Another potential application of double staining could be to restore some of the tissue architectural information that is lost when collecting cytology specimens. An immunostain could be used, for example, to label only basal epithelial cells, enabling a ploidy analysis on a defined subset of the epithelial cells.
Limitations of double staining
While adding anti-Ki-67 ICC does not appear to improve ploidy’s ability to separate high- and low-grade dysplasias over thionin staining alone in cervical dysplasia cytology specimens, double staining remains an intriguing approach to improving ploidy analysis as a screening technique. Double staining with Feulgen-thionin and ICC offers a new approach to studying mechanisms of aneuploidy and possibly novel biomarkers for precancerous changes, but our investigations have revealed several important caveats.
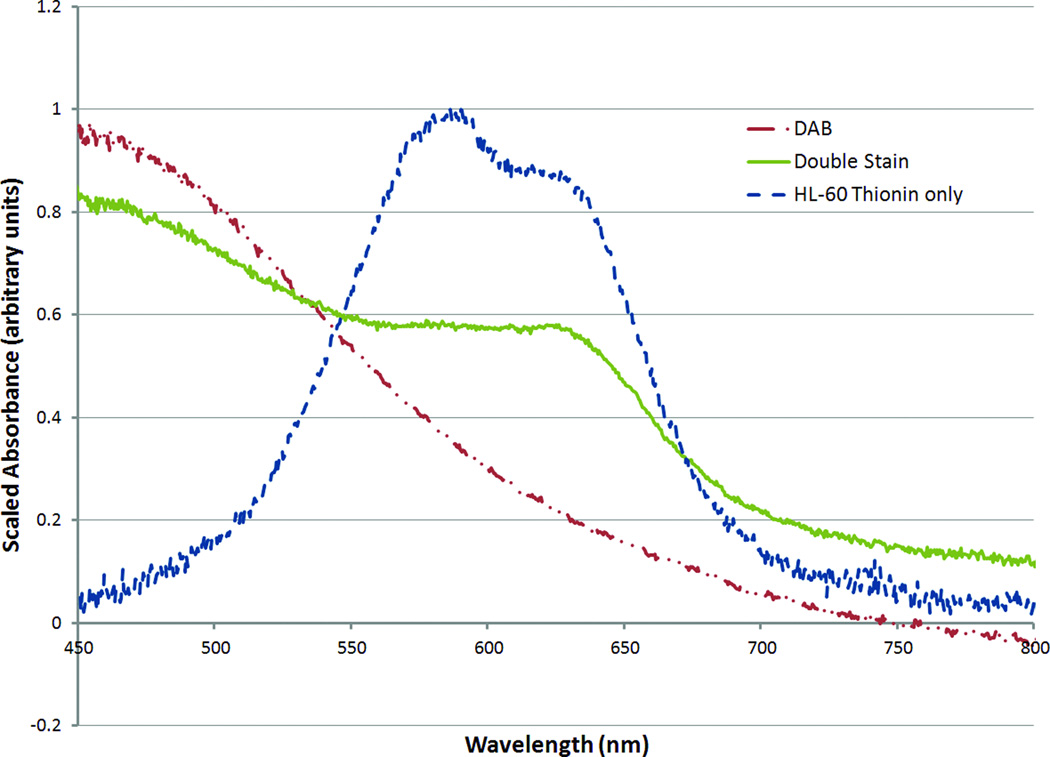
The chromogen we used for ICC was DAB, which is known for its insolubility and general lack of chemical reactivity. Its ubiquity and the ability to perform Feulgen-thionin staining on slides previously stained with hematoxylin and eosin50 or Pap stain51 raise the possibility that previously immunostained specimens might be retrospectively stained with Feulgen-thionin. However, care must be taken when analyzing the results as the Feulgen-thionin stain reacts with deposited DAB stain. To demonstrate this, DAB substrate solution was reacted with HRP-conjugated secondary antibody and allowed to settle and air dry completely, creating a sample of DAB chromogen free of cellular material and any other compounds that might cross-react with Feulgen-thionin staining. After going through the Feulgen-thionin staining procedure, the patch of DAB chromogen demonstrated the colour change characteristic of a reaction with thionin. This was further confirmed via microspectrophotometry,52 as shown in Figure 6.
Figure 6.
Microspectrophotometry data measured from spots of oxidized DAB chromogen deposited on a slide, before and after thionin staining, compared with thionin-stained HL-60 nuclei. Even in the absence of cells, deposited DAB chromogen reacts with thionin, giving a characteristic shoulder in the microspectra.
Analysis of double stained specimens with DAB as the ICC chromogen should therefore be limited to DAB-negative cells. The present analysis fits this requirement, but with the expanding use of colour image analysis, quantitation of the double stained cells may in future become a technical possibility. If analysis of ploidy of immunostain-positive cells is desired, an alternative chromogen should be sought. A washable chromogen could be used as per Fleskens et al.,42 but that would require ensuring every immunostain-positive cell was imaged. Although considered beyond the capabilities of our present system, this type of analysis should be possible with whole slide scanners becoming available for clinical digital pathology.53–55
Double staining also comes with a significant cost. In addition to the time and reagents required to process the samples, the antigen retrieval of ICC significantly widens histogram peaks while reducing the intensity of the thionin stain, making it technically more challenging to ensure accurate and reproducible results in quantitative imaging. The diploid peaks in double staining histograms had larger CVs than typical DNA measurements made by flow or standard image cytometry, but are superior to DNA cytometry measurements of tissue sections.42 Even with 10% CVs, cells with DNA indices greater than 1.25 can still be classified as non-diploid with some confidence, an observation that we had hoped would improve the sensitivity and specificity of ploidy-based detection of high-grade dysplasias. Chromosomal mutations may have given rise to an aneuploid stemline, for example, which manifests itself as a distinct population of mostly Ki-67-negative cells with DNA indices between diploid and tetraploid. Unfortunately, the observed scarcity of cells with DNA indices between 1.25 and 2.5, combined with the reduction in imaged cells per sample as a result of double staining, meant that even if double staining were an improvement, it could not be observed under the present conditions. It might be possible to improve this through the use of more cellular fresh samples.
The fainter Feulgen staining resulting from double staining could be addressed by adjusting the imaging settings. Nevertheless, sampling will undoubtedly be biased in favour of cells with more intense staining, so care must be taken in interpreting and comparing the results. By studying whether the entire double staining analysis would be superior to Feulgen-thionin staining alone, this bias becomes embedded into the reported sensitivities and specificities and does not need to be separately controlled. However, one must be careful not to assume that the Ki-67 positivity “rates” or proportions of aneuploid cells, for example, are absolute and comparable between single stain and double stain procedures. Due to the imaging bias, this is not necessarily the case, so the “rates” are more like scores that correlate with the underlying real rates.
Moreover, in a cancer screening setting, the diagnostic dysplastic cells are quite rare (typically only a few, if any, are observed per sample).34,56,57 Cytological methods are attractive for cancer screening because they do not rely on the physician knowing the precise location of a suspected lesion, thereby theoretically allowing greater sensitivity over biopsy or direct visualization-based methods. However, as cytological specimens represent an averaging of both diseased and normal cells, detecting the rare dysplastic cells can be quite difficult, a situation that is compounded by the weaker double stain that might lead many cells to be missed by the imaging system.
Conclusion
Future investigations into other ICC markers to be paired with Feulgen-thionin staining for screening or diagnostic purposes will need to show statistically significant improvement at distinguishing cases of different severities to justify the added costs of double staining. While combining Ki-67 with ploidy analysis did not show a statistically significant benefit, an improved sensitivity trend was seen in the high-specificity range of the ROC result. Meanwhile, a protocol has been demonstrated that can be used for countless other ICC markers. Unlike prior attempts at combining ICC with Feulgen staining, we have considered the effects of antigen retrieval, thus expanding the universe of ICC markers that might be suitable for combination with Feulgen-thionin staining. As long as a suitable ICC marker is chosen and proper care is taken in the analysis of double staining data, it seems that combining ICC with Feulgen-thionin staining could prove advantageous.
Acknowledgments
The authors wish to acknowledge the assistance of Priscilla Fung with HL-60 cells and thionin staining, Jagoda Korbelik and Anita Carraro with imaging, as well as Dr. Haishan Zeng and Jianhua Zhao with microspectrophotometry. Funding for this project was provided by the National Institutes of Health (R01-CA-103830 and P01-CA-82710). GL was also supported by scholarships from the Canadian Institutes of Health Research, the Natural Sciences and Engineering Council of Canada, and the Michael Smith Foundation for Health Research.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Mathew A, George PS. Trends in Incidence and Mortality Rates of Squamous Cell Carcinoma and Adenocarcinoma of Cervix - Worldwide. Asian Pac J Cancer Prev. 2009;10:645–650. [PubMed] [Google Scholar]
- 3.Vizcaino AP, Moreno V, Bosch FX, Munoz N, Barros-Dios XM, Borras J, Parkin DM. International trends in incidence of cervical cancer: II. Squamous-cell carcinoma. Int J Cancer. 2000;86:429–435. doi: 10.1002/(sici)1097-0215(20000501)86:3<429::aid-ijc20>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, Fayette JM, Cherian J. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 5.Qiao Y-L, Sellors JW, Eder PS, Bao Y-P, Lim JM, Zhao F-H, Weigl B, Zhang W-H, Peck RB, Li L, Chen F, Pan Q-J, Lorincz AT. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–936. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- 6.Denny L, Kuhn L, Hu CC, Tsai WY, Wright TC., Jr Human papillomavirus-based cervical cancer prevention: long-term results of a randomized screening trial. J Natl Cancer Inst. 2010;102:1557–1567. doi: 10.1093/jnci/djq342. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, Dillner J, Meijer CJ. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26(Suppl 10):K29–K41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, Radberg T, Strander B, Forslund O, Hansson BG, Hagmar B, Johansson B, Rylander E, Dillner J. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst. 2009;101:88–99. doi: 10.1093/jnci/djn444. [DOI] [PubMed] [Google Scholar]
- 9.Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103:368–383. doi: 10.1093/jnci/djq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villa LL. Assessment of new technologies for cervical cancer screening. Lancet Oncol. 2008;9:910–911. doi: 10.1016/S1470-2045(08)70238-9. [DOI] [PubMed] [Google Scholar]
- 11.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 12.Guillaud M, Benedet JL, Cantor SB, Staerkel G, Follen M, MacAulay C. DNA ploidy compared with human papilloma virus testing (Hybrid Capture II) and conventional cervical cytology as a primary screening test for cervical high-grade lesions and cancer in 1555 patients with biopsy confirmation. Cancer. 2006;107:309–318. doi: 10.1002/cncr.21993. [DOI] [PubMed] [Google Scholar]
- 13.Scheurer ME, Guillaud M, Tortolero-Luna G, McAulay C, Follen M, Adler-Storthz K. Human papillomavirus-related cellular changes measured by cytometric analysis of DNA ploidy and chromatin texture. Cytometry B Clin Cytom. 2007;72B:324–331. doi: 10.1002/cyto.b.20173. [DOI] [PubMed] [Google Scholar]
- 14.Bartels PH, Wied GL. Performance testing for automated prescreening devices in cervical cytology. J Histochem Cytochem. 1974;22:660–662. doi: 10.1177/22.7.660. [DOI] [PubMed] [Google Scholar]
- 15.Mariuzzi G, Santinelli A, Valli M, Sisti S, Montironi R, Mariuzzi L, Alberti R, Pisani E. Cytometric evidence that cervical intraepithelial neoplasia I and II are dysplasias rather than true neoplasias. An image analysis study of factors involved in the progression of cervical lesions. Anal Quant Cytol Histol. 1992;14:137–147. [PubMed] [Google Scholar]
- 16.Bibbo M, Bartels PH, Dytch HE, Wied GL. Ploidy patterns in cervical dysplasia. Anal Quant Cytol Histol. 1985;7:213–217. [PubMed] [Google Scholar]
- 17.Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Aleskandarany M, Rakha E, Macmillan R, Powe D, Ellis I, Green A. MIB1/Ki-67 labelling index can classify grade 2 breast cancer into two clinically distinct subgroups. Breast Cancer Res Treat. 2011;127:591–599. doi: 10.1007/s10549-010-1028-3. [DOI] [PubMed] [Google Scholar]
- 19.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 20.Zellweger T, Günther S, Zlobec I, Savic S, Sauter G, Moch H, Mattarelli G, Eichenberger T, Curschellas E, Rüfenacht H, Bachmann A, Gasser TC, Mihatsch MJ, Bubendorf L. Tumour growth fraction measured by immunohistochemical staining of Ki67 is an independent prognostic factor in preoperative prostate biopsies with small-volume or low-grade prostate cancer. Int J Cancer. 2009;124:2116–2123. doi: 10.1002/ijc.24174. [DOI] [PubMed] [Google Scholar]
- 21.Brown DC, Gatter KC. Ki67 protein: The immaculate deception? Histopathology. 2002;40:2–11. doi: 10.1046/j.1365-2559.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 22.Sahebali S, Depuydt CE, Segers K, Vereecken AJ, Van Marck E, Bogers JJ. Ki-67 immunocytochemistry in liquid based cervical cytology: useful as an adjunctive tool? J Clin Pathol. 2003;56:681–686. doi: 10.1136/jcp.56.9.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Niekerk D, Guillaud M, Matisic J, Benedet JL, Freeberg JA, Follen M, MacAulay C. p16 and MIB1 improve the sensitivity and specificity of the diagnosis of high grade squamous intraepithelial lesions: methodological issues in a report of 447 biopsies with consensus diagnosis and HPV HCII testing. Gynecol Oncol. 2007;107:S233–S240. doi: 10.1016/j.ygyno.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 24.McCluggage WG. Immunohistochemistry as a diagnostic aid in cervical pathology. Pathology. 2007;39:97–111. doi: 10.1080/00313020601123961. [DOI] [PubMed] [Google Scholar]
- 25.Gupta N, Srinivasan R, Rajwanshi A. Functional biomarkers in cervical precancer: an overview. Diagn Cytopathol. 2010;38:618–623. doi: 10.1002/dc.21270. [DOI] [PubMed] [Google Scholar]
- 26.Landberg G, Tan EM, Roos G. Flow cytometric multiparameter analysis of proliferating cell nuclear antigen/cyclin and Ki-67 antigen: a new view of the cell cycle. Exp Cell Res. 1990;187:111–118. doi: 10.1016/0014-4827(90)90124-s. [DOI] [PubMed] [Google Scholar]
- 27.Shi X, Yuan X, Tao D, Gong J, Hu G. Analysis of DNA ploidy, cell cycle and Ki67 antigen in nasopharyngeal carcinoma by flow cytometry. J Huazhong Univ Sci Technolog Med Sci. 2005;25:198–201. doi: 10.1007/BF02873576. [DOI] [PubMed] [Google Scholar]
- 28.Rovera G, Santoli D, Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979;76:2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garner DM, Harrison A, MacAulay C, Palcic B. Cyto-Savant™ and its use in automated screening of cervical smears. In: Wied GL, Bartels PH, Rosenthal PH, Schenck U, editors. Compendium on the Computerized Cytology and Histology Laboratory. Chicago: Tutorials of Cytology; 1994. pp. 346–352. [Google Scholar]
- 30.Payne PW, Sebo TJ, Doudkine A, Garner D, MacAulay C, Lam S, LeRiche JC, Palcic B. Sputum screening by quantitative microscopy: A reexamination of a portion of the National Cancer Institute Cooperative Early Lung Cancer Study. Mayo Clin Proc. 1997;72:697–704. doi: 10.4065/72.8.697. [DOI] [PubMed] [Google Scholar]
- 31.Palcic B, MacAulay C, Shlien S, Treurniet W, Tezcan H, Anderson G. Comparison of three different methods for automated classification of cervical cells. Anal Cell Pathol. 1992;4:429–441. [PubMed] [Google Scholar]
- 32.Guillaud M, Cox D, Malpica A, Staerkel G, Matisic J, Van Niekirk D, Adler-Storthz K, Poulin N, Follen M, MacAulay C. Quantitative histopathological analysis of cervical intra-epithelial neoplasia sections: methodological issues. Cell Oncol. 2004;26:31–43. doi: 10.1155/2004/238769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cecic IK, Li G, MacAulay C. Technologies supporting analytical cytology: clinical, research and drug discovery applications. J Biophotonics. 2012;5:313–326. doi: 10.1002/jbio.201100093. [DOI] [PubMed] [Google Scholar]
- 34.Tong H, Shen R, Wang Z, Kan Y, Wang Y, Li F, Wang F, Yang J, Guo X the Mass Cervical Cancer Screening Regimen Group. DNA ploidy cytometry testing for cervical cancer screening in China (DNACIC Trial): a prospective randomized, controlled trial. Clin Cancer Res. 2009;15:6438–6445. doi: 10.1158/1078-0432.CCR-09-1689. [DOI] [PubMed] [Google Scholar]
- 35.Sun XR, Wang J, Garner D, Palcic B. Detection of cervical cancer and high grade neoplastic lesions by a combination of liquid-based sampling preparation and DNA measurements using automated image cytometry. Cell Oncol. 2005;27:33–41. doi: 10.1155/2005/981612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li G, Guillaud M, leRiche J, McWilliams A, Gazdar A, Lam S, MacAulay C. Automated sputum cytometry for detection of intraepithelial neoplasias in the lung. Anal Cell Pathol (Amst) 2012;35:187–201. doi: 10.3233/ACP-2012-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiffman M, Solomon D. Screening and Prevention Methods for Cervical Cancer. JAMA. 2009;302:1809–1810. doi: 10.1001/jama.2009.1573. [DOI] [PubMed] [Google Scholar]
- 38.Böcking A, Adler CP, Common HH, Hilgarth M, Granzen B, Auffermann W. Algorithm for a DNA-cytophotometric diagnosis and grading of malignancy. Anal Quant Cytol. 1984;6:1–8. [PubMed] [Google Scholar]
- 39.Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: Summary of published evidence. Chest. 2003;123:115S–128S. doi: 10.1378/chest.123.1_suppl.115s. [DOI] [PubMed] [Google Scholar]
- 40.Kolles H, Forderer W, Bock R, Feiden W. Combined Ki-67 and Feulgen stain for morphometric determination of the Ki-67 labelling index. Histochemistry. 1993;100:293–296. doi: 10.1007/BF00270049. [DOI] [PubMed] [Google Scholar]
- 41.Oud PS, Bauwens A, Nauwelaers FA. Multiparameter absorption measurements in automated microscopy. Simultaneous quantitative determination of DNA and nuclear antigen. Acta Cytol. 1997;41:188–196. doi: 10.1159/000332322. [DOI] [PubMed] [Google Scholar]
- 42.Fleskens SJHM, Takes RP, Otte-Höller I, Van Doesburg L, Smeets A, Speel E-JM, Slootweg PJ, Van Der Laak JAWM. Simultaneous assessment of DNA ploidy and biomarker expression in paraffin-embedded tissue sections. Histopathology. 2010;57:14–26. doi: 10.1111/j.1365-2559.2010.03599.x. [DOI] [PubMed] [Google Scholar]
- 43.Kjellstrand P. Mechanisms of the Feulgen acid hydrolysis. J Microsc. 1980;119:391–396. doi: 10.1111/j.1365-2818.1980.tb04110.x. [DOI] [PubMed] [Google Scholar]
- 44.Schulte EK, Wittekind DH. Standardization of the Feulgen reaction: the influence of chromatin condensation on the kinetics of acid hydrolysis. Anal Cell Pathol. 1990;2:149–157. [PubMed] [Google Scholar]
- 45.Shi SR, Cote RJ, Taylor CR. Antigen retrieval techniques: current perspectives. J Histochem Cytochem. 2001;49:931–937. doi: 10.1177/002215540104900801. [DOI] [PubMed] [Google Scholar]
- 46.Kruse A-J, Baak JPA, de Bruin PC, Jiwa M, Snijders WP, Jan Boodt P, Fons G, Houben PWH, The HS. Ki-67 immunoquantitation in cervical intraepithelial neoplasia (CIN): a sensitive marker for grading. J Pathol. 2001;193:48–54. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH719>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 47.Baak JPA, Kruse A-J, Robboy SJ, Janssen EAM, van Diermen B, Skaland I. Dynamic behavioural interpretation of cervical intraepithelial neoplasia with molecular biomarkers. J Clin Pathol. 2006;59:1017–1028. doi: 10.1136/jcp.2005.027839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- 49.Lorenzato M, Bory JP, Cucherousset J, Nou JM, Bouttens D, Thil C, Dez F, Evrard G, Quereux C, Birembaut P, Clavel C. Usefulness of DNA ploidy measurement on liquid-based smears showing conflicting results between cytology and high-risk human papillomavirus typing. Am J Clin Pathol. 2002;118:708–713. doi: 10.1309/6NXC-V9XD-YM87-8FAE. [DOI] [PubMed] [Google Scholar]
- 50.Chen M. Evaluation of applying Feulgen stain for DNA analysis on destained hematoxylin-eosin-stained cytologic smears. Anal Quant Cytol Histol. 2004;26:255–258. [PubMed] [Google Scholar]
- 51.Schramm M, Wrobel C, Born I, Kazimirek M, Pomjanski N, William M, Kappes R, Gerharz CD, Biesterfeld S, Böcking A. Equivocal cytology in lung cancer diagnosis. Cancer Cytopathol. 2011;119:177–192. doi: 10.1002/cncy.20142. [DOI] [PubMed] [Google Scholar]
- 52.Zeng HS, MacAulay C, Mclean DI, Palcic B. Novel Microspectrophotometer and Its Biomedical Applications. Optical Engineering. 1993;32:1809–1814. [Google Scholar]
- 53.Rocha R, Vassallo J, Soares F, Miller K, Gobbi H. Digital slides: Present status of a tool for consultation, teaching, and quality control in pathology. Pathol Res Pract. 2009;205:735–741. doi: 10.1016/j.prp.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Brachtel E, Yagi Y. Digital imaging in pathology - current applications and challenges. J Biophotonics. 2011 doi: 10.1002/jbio.201100103. Preprint. [DOI] [PubMed] [Google Scholar]
- 55.Pantanowitz L, Valenstein P, Evans A, Kaplan K, Pfeifer J, Wilbur D, Collins L, Colgan T. Review of the current state of whole slide imaging in pathology. J Pathol Inform. 2011;2:36. doi: 10.4103/2153-3539.83746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lorenzato M, Caudroy S, Nou JM, Dalstein V, Joseph K, Bellefqih S, Durlach A, Thil C, Dez F, Bouttens D, Clavel C, Birembaut P. Contribution of DNA ploidy image cytometry to the management of ASC cervical lesions. Cancer. 2008;114:263–269. doi: 10.1002/cncr.23638. [DOI] [PubMed] [Google Scholar]
- 57.Haroske G, Baak JP, Danielsen H, Giroud F, Gschwendtner A, Oberholzer M, Reith A, Spieler P, Bocking A. Fourth updated ESACP consensus report on diagnostic DNA image cytometry. Anal Cell Pathol. 2001;23:89–95. doi: 10.1155/2001/657642. [DOI] [PMC free article] [PubMed] [Google Scholar]