Abstract
The selection of chemotherapy drugs is based on the cytotoxicity to specific tumor cell types and the relatively low toxicity to normal cells and tissues. However, the toxicity to normal cells poses a major clinical challenge, particularly when malignant cells have acquired resistance to chemotherapy. This drug resistance of cancer cells results from multiple factors including individual variation, genetic heterogeneity within a tumor, and cellular evolution. Much progress in the understanding of tumor cell resistance has been made in the past 35 years, owing to milestone discoveries such as the identification and characterization of ABC transporters. Nonetheless, the complexity of the genetic and epigenetic rewiring of cancer cells makes drug resistance an equally complex phenomenon that is difficult to overcome. In this review, we discuss how the remarkable changes in the levels of glucose, IGF-I, IGFBP-1 and in other proteins caused by fasting have the potential to improve the efficacy of chemotherapy against tumors by protecting normal cells and tissues and possibly by diminishing multidrug resistance in malignant cells.
Keywords: Fasting, Starvation, Cancer, Multidrug resistance, Differential stress resistance
1. Dietary restriction, nutrient signaling, and stress resistance
Dietary restriction triggers highly conserved survival mechanisms that enhance the protection of organisms ranging from bacteria, yeast, worms, flies, and mice, to non-human primates against various types of stress and/or disease. This counterintuitive effect is mediated in part by the reduction of conserved nutrient-signaling pathways that include several mitogenic components, especially the IGF-I receptor and its downstream effectors (Fontana et al., 2010; Guarente and Kenyon, 2000; Kenyon, 2010; Longo, 1999; Longo and Finch, 2003). In this section, studies that establish the link between starvation conditions and the conserved nutrient-signaling pathways in eukaryotes with enhanced cellular protection in a variety of organisms will be discussed (Fig. 1).
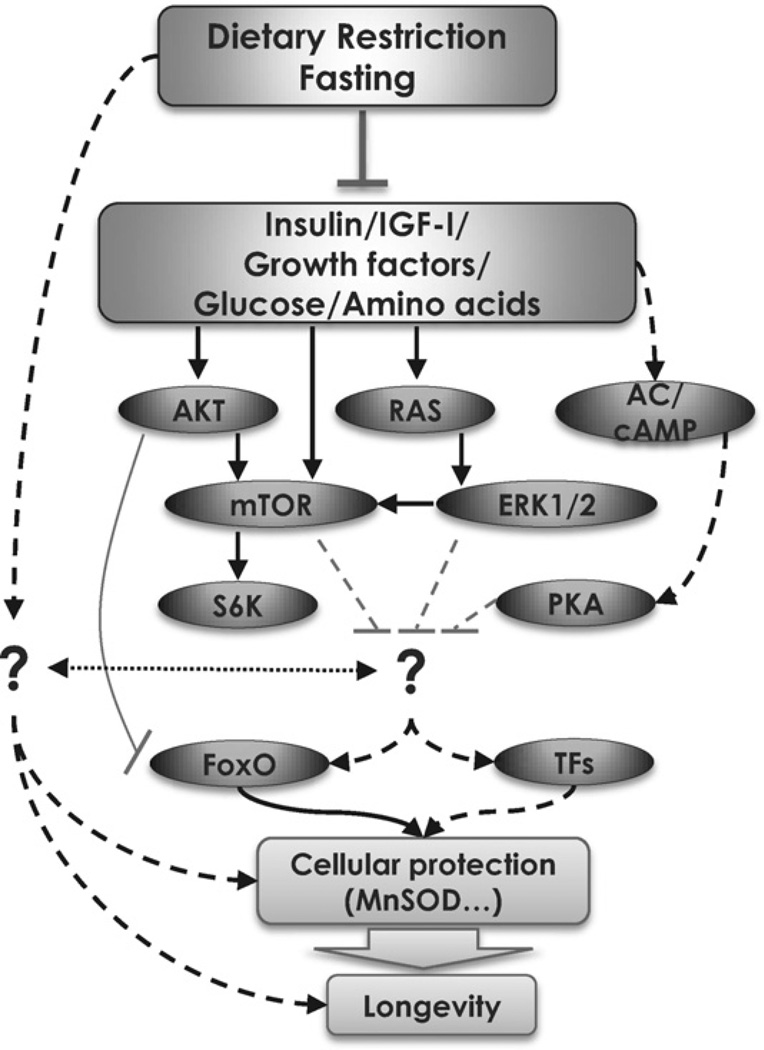
Fig. 1.
The conserved role of IGF-I in lifespan regulation and stress resistance. The insulin/IGF-I signaling pathway plays a major role in regulating lifespan and cellular stress resistance. Deficiencies in these pathways have been shown to convey protection to the cell/organism against multiple toxins.
In Escherichia coli, carbon-starvation promotes the synthesis of stress resistance proteins, including heat-shock proteins, as determined by 2-D gel electrophoresis analysis (Matin, 1991). In fact, glucose or nitrogen starvation enhances the resistance of E. coli to heat (57 °C) or H2O2 (15 mM) (Jenkins et al., 1988). Furthermore, glucose restriction not only increases the expression of alternate carbon/energy and nitrogen sources, but it also increases components of the stress response regulons (Hua et al., 2004; Ihssen and Egli, 2004; Wick et al., 2001). Similar effects were reported in Salmonella typhimurium (Seymour et al., 1996). In many bacterial species, starvation induces the stationary-phase specific sigma factor RpoS expression, which in turn controls a large set of stress defense genes, in particular oxidative stress (McDougald et al., 2002; O’Neal et al., 1994).
In the yeast Saccharomyces cerevisiae, incubation in reduced glucose (2.0–0.5 g/L) promotes markedly increased resistance to oxidative stress, and starvation achieved by incubation in water promotes a major increase in life span as well as protection from oxidative insults and heat shock (Longo et al., 1997; Wei et al., 2008). Also, deficiency in the nutrient-sensing Ras/cAMP/PKA and/or the Tor/S6K pathways (Thevelein and de Winde, 1999; Wei et al., 2008) more than doubles lifespan and protects against a variety of stresses (Fabrizio et al., 2001, 2003; Longo and Finch, 2003; Wei et al., 2009). The stress responsive transcription factors Msn2/Msn4 and Gis1, and the genes regulated by them, mediate much of this protection, as yeast that lack these factors no longer exhibit enhanced stress resistance (Longo et al., 1997; Wei et al., 2008). Down-regulation of these pro-growth pathways and up-regulation of the Msn2/4 and Gis1 transcription factors also represents a major component responsible for the effects of dietary restriction (DR) and starvation on stress resistance and longevity (Wei et al., 2008). Among the genes regulated by these transcription factors that contribute to stress resistance are heat shock proteins, MnSOD, and metabolic enzymes that generate glycerol (Wei et al., 2009).
In worms, starvation conditions also enhance protection against oxidative stress. When grown under DR, achieved by feeding the animals with diluted bacteria, worms increase their resistance to paraquat (Greer et al., 2007). Every 2-day fasting also increases resistance to oxidative and heat stress, and extends the lifespan of worms by up to 56% via modulation of the RHEB-1 and TOR signaling pathway, both of which are linked to the FOXO transcription factor homolog DAF-16 (Honjoh et al., 2009; Weinkove et al., 2006). Conversely, excessive glucose shortens the lifespan of worms, in part, by decreasing DAF-16 activity (Lee et al., 2009). Further, AGE-1 (PI3K homolog) and DAF-2 (insulin/IGF-I receptor homolog) mutants live up to twice as long by decreasing AKT-1/2 signaling, and by activating the transcription factor DAF-16/FoxO, and promoting resistance to oxidative and ER stress (Henis-Korenblit et al., 2010; Hsu et al., 2003; Johnson, 1990; Paradis et al., 1999). AGE-1 deficiency confers increased survival when challenged with 3 mM H2O2 (9-fold), paraquat (5-fold), and 4-hydroxynonenal (4-HNE; 4.4-fold) (Ayyadevara et al., 2008; Larsen, 1993; Morris et al., 1996). These longevity mutations are also associated with the induction of stress resistance transcription factors, mitochondrial superoxide dismutase (MnSOD), and HSPs in worms (McColl et al., 2010).
The fruit fly Drosophila melanogaster is generally fed a mixture of sucrose (carbon source) and yeast (protein source) in laboratory settings. Moderate DR (3% sucrose and 3% yeast vs the standard 10% sucrose and 10% yeast) but also amino acid restriction enhances the protection against oxidative damage following chronic hypoxia (Vigne and Frelin, 2006, 2007). Furthermore, a close to starvation condition, induced by feeding 1% sucrose and 1% yeast, but not moderate DR, protects flies from anoxia/reoxygenation injury (Vigne et al., 2009). Starvation-dependent protection against oxidative stress in flies is mediated by d4E-BP, which acts downstream of the PI3K/Akt/dFOXO3 pathway (Tettweiler et al., 2005), and also binds to eIF4E and represses translation, a mechanism consistent with the necessity of stressed cells to divert energy from growth to protection. Also, the insulin-like receptor (InR) and its downstream substrate chico regulate longevity and stress resistance. Chico was first identified as the insulin receptor substrate that regulates cell size and metabolism (Cicchetti et al., 2009), and loss of its activity increases lifespan and provides some resistance to paraquat (Clancy et al., 2001; Giannakou and Partridge, 2007). In flies, there are 7 genes encoding for insulin-like ligands (Drosophila insulin-like peptides; DILP 1–7). The reduction of DILPs increases lifespan and resistance against paraquat and heat (Ikonen et al., 2003).
Laboratory rodents also display increased stress resistance in response to DR. Mice under a 30–50% decreased dietary intake showed diminished levels of age-dependent lipid (Chipalkatti et al., 1983; De et al., 1983; Koizumi et al., 1987), protein (Dubey et al., 1996; Lass et al., 1998), and DNA oxidation (Chung et al., 1992; Kang et al., 1998). As discussed in more detail later, in mice, fasting for 48–60 h increases protection of 3 different strains of mice from etoposide, a drug known to promote oxidative stress, with remarkable improvement in survival compared to its normally fed counterparts (Raffaghello et al., 2008). In addition, 72-h of fasting protects the outbred CD-1 mice from lethal doses of doxorubicin, a drug also known to cause death by oxidative stress induced cardiotoxicity (Lee et al., 2010). Studies also show that fasting protects against ischemia reperfusion injury (IRI), a pathological condition initiated by a lack of blood flow (ischemia) and followed by restoration of blood flow (reperfusion) that causes further damage by inappropriate activation of cellular oxidases and subsequently by inflammatory proteins (Friedewald and Rabb, 2004), in rat brain (Go et al., 1988; Marie et al., 1990), mouse kidney and liver (Mitchell et al., 2010; van Ginhoven et al., 2009), and in human liver (van Ginhoven et al., 2009). Also, fasting following traumatic brain injury proved to be neuroprotective, resulting in reduced oxidative damage and improved cognitive function (Davis et al., 2008). Furthermore, it has been reported that intermittent fasting (IF), during which mice are fed every other day, can protect heart and brain cells against injury and improve functional outcome in animal models of myocardial infarction and stroke (Ahmet et al., 2005; Mattson and Wan, 2005; Wan et al., 2010). Deficiency in certain amino acids has also been shown to promote protection in mice. For instance, methionine restriction dramatically increases the hepatic resistance against acetaminophen-induced oxidative damage, and increases maximum lifespan and reduces age-dependent deterioration (Miller et al., 2005).
In mice, the GH/IGF-I axis and its downstream effectors, many being orthologs of the lower eukaryotes’ proteins mentioned above, regulate stress resistance and lifespan (Bonkowski et al., 2009; Brown-Borg et al., 1996; Coschigano et al., 2000; Holzenberger et al., 2003; Migliaccio et al., 1999; Selman et al., 2009; Yan et al., 2007). Notably, it has recently been shown that the PI3K pathway, which represents a major arm of IGF-I signaling, is important in determining the sensitivity of tumors to DR, and mutations in the pathway may influence the response of cancers to DR. A recent study has shown that dietary-restriction-resistant tumors harbor a mutation that causes PI3K to be constitutively active. Replacing the mutant PI3K allele with a wild-type PI3K, or reintroducing PTEN expression in a PTEN-null cancer, was sufficient to reverse the resistance to DR (Kalaany and Sabatini, 2009) in agreement with the starvation-dependent differential stress resistance of normal and cancer or cancer-like cells to chemotherapy demonstrated in both yeast cells and mammalian cells and discussed in more details later in the review (Lee et al., 2010; Raffaghello et al., 2008).
Consistent with the effect of DR and starvation in reducing growth factor signaling, fibroblasts isolated from mice deficient in the GH/IGF-I axis are resistant to oxidative stress (H2O2, paraquat), UV, genotoxins (methylmethanesulfonate; MMS), heat, and cadmium (Murakami, 2006; Salmon et al., 2005). Conversely, exposure of murine hepatocytes to GH or IGF-I or the overexpression of GH in transgenic mice reduces the levels of superoxide dismutases and catalase activity (Brown-Borg and Rakoczy, 2000; Brown-Borg et al., 2002). Similarly, IGF-I attenuates cellular stress response and the expression of stress response proteins HSP72 and heme-oxygenase in rats (Sharma et al., 2000). Modulation of the downstream elements of the IGF-I receptor (IGF-IR) alter stress resistance. For instance, MEK/ERK1/2 inhibition protects neurons against oxidative stress (Li et al., 2008), persistent PI3K-mTORC1 signaling decreases cellular stress resistance (Sun et al., 2011), and the inhibition of PI3K protected murine microglia against oxygen-glucose deprivation (OGD) stress (Chern et al., 2011) and also protected PC12 cells from serum-deprivation induced death (Guillon-Munos et al., 2005).
With special regards to cancer, GH/IGF-I axis alterations independent of nutrition and its dysfunction have been well documented. For instance, an unbalanced relationship between GH and IGF-I, as well as its binding protein IGFBP-3 has been suggested to play a major role in tumor biology (Luo et al., 2005; Mazzoccoli et al., 1999, 2010). However, the isolated reduction of circulating IGF-I levels alone, achieved by hepatic igf1 gene deletion, has been shown to be insufficient in retarding prostate cancer progression leading to the suggestion that GH levels also need to be reduced (Anzo et al., 2008). However, IGF-I and the IGF-I receptor (IGF-IR) are well known to play a major role in the progression of a variety of tumors (Anzo et al., 2008; Braconi et al., 2008; Gong et al., 2009; Pollak et al., 2004).
2. Dietary restriction, nutrient signaling, and cellular detoxification
Drug metabolism can be largely divided into 3 phases: phase I (redox and hydrolysis), phase II (conjugation), and phase III (transport). Phase III transporters include the ABCB1 pumps which are discussed in more detail later. Diet and nutrient signaling pathways have profound effects on phase I enzymes, which consist primarily of cytochrome P450 (CYP) superfamily proteins (Yang et al., 1992) and phase II proteins including enzymes regulated by Keap1-Nrf2 (Kohle and Bock, 2007) such as UDP-glucuronosyltransferases (UGT) (Innocenti et al., 2002; Tukey and Strassburg, 2000), NAD(P)H:quinone oxidoreductase (NQO), superoxide dismutase (SOD) (Zhu et al., 2005) and glutathione-S-transferase (GST) (Belinsky and Jaiswal, 1993; Kong et al., 2001; Townsend and Tew, 2003). Notably, the Nrf2 pathway has been shown to mediate the anticarcinogenic effects of DR, but not its longevity effects, suggesting that it may function only to regulate specific systems activated by DR including those involved in cellular stress resistance (Choi et al., 2007). In fact, Nrf2 also mediates endothelial protection against oxidative stress induced by resveratrol, a natural polyphenol that promotes protection and longevity in model organisms (Zhai et al., 2005). As discussed above, longevity and stress resistance is heavily regulated by the insulin/IGF-I pathway, and certain downstream elements including ERK, JNK, p38 MAPK, and PI3K, which are known to regulate several detoxification enzymes (Xu et al., 2005). Fasting, DR, and the long-lived and stress resistant mutants discussed above appear to have potent detoxification effects although the mechanisms are poorly understood (Fig. 2). In worms, the extremely resistant spore-like dauer larvae and mutations in Daf-2, the worm ortholog of the insulin/IGF-I receptor up-regulate cytochrome P450, UGT, the small heat shock protein/α-crystallins, and GST (McElwee et al., 2004). Also, they exhibit increased ROS detoxification via the cytosolic antioxidant SOD enzyme (Vanfleteren, 1993; Vanfleteren and De Vreese, 1995) and the mitochondrial MnSOD (Honda and Honda, 1999; McElwee et al., 2003; Murphy et al., 2003).
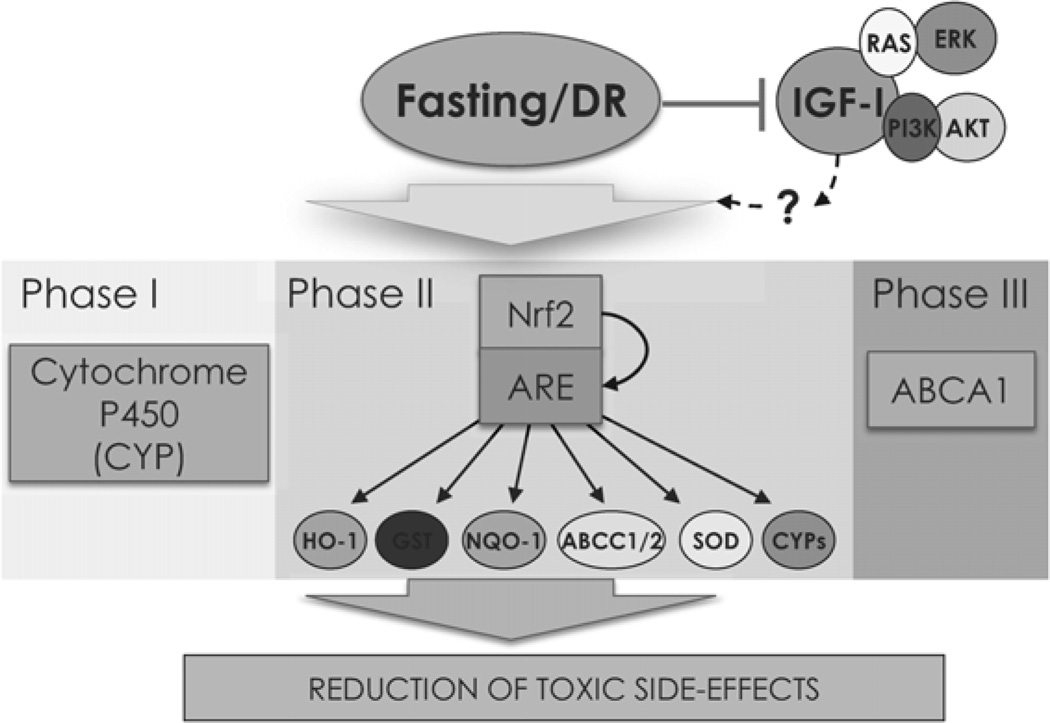
Fig. 2.
Fasting and DR modulate drug metabolism. Fasting and DR have been shown to affect all 3 major phases of drug metabolism. Phase I involves redox and hydrolysis and is mediated by the cytochrome P450 family. Phase II are usually detoxification processes that involves conjugation steps. Genes under this category are regulated by the stress responsive master transcription factor Nrf2. Proteins classified as phase III are involved in transport, such as members of the ABC superfamily of transporters.
DR, in worms, up-regulates the stress-responsive cytoprotective transcription factor SKN-1 (Nrf2 ortholog) which is required for its longevity (Bishop and Guarente, 2007). In flies, cytochrome P450 activity increases following 30 days of DR (Pletcher et al., 2005), whereas in mice, fasting for 48 h modulates hepatic expression of several ABC transporters and cytochrome P450s (Bauer et al., 2004). Similar to worms, DR induces Nrf2 activity in mice, which has been shown to be responsible for most of its anticarcinogenic effect (Martin-Montalvo et al., 2011).
IGF-I signaling has also been shown to be involved in the regulation of Nrf2. PI3K inhibitors block nuclear translocation of Nrf2 and induction of stress proteins (Kang et al., 2002; Nakaso et al., 2003; Shen et al., 2004). Also, a recent report suggests that Nrf2 and IGF-I are involved in cooperative effects (Wang et al., 2011). In human cancer cells, mutations in the coding region of NRF2 cause constitutive induction of drug efflux pumps and cytoprotective enzymes (Shibata et al., 2008) such as NQO (Belinsky and Jaiswal, 1993). Further, IGF-I, and its downstream elements such as RAS/ERK (Nakamura et al., 2006) and PI3K/AKT (Abdul-Ghani et al., 2006; Han et al., 2007; Tazzari et al., 2007) promote MDR, in part, by modulating ABC transporter expression. Notably, DR increases Nrf2 in normal cells (Martin-Montalvo et al., 2011) and thus has the potential to be an important mediator of the differential effects of DR in the protection of normal and cancer cells (Lee and Longo, 2011; Raffaghello et al., 2008). Considering that fasting reduces IGF-I levels and promotes enhanced protection to chemotherapy in normal cells (Lee and Longo, 2011; Lee et al., 2010), it will be important to understand the link between different types of dietary restriction, growth signaling and detoxification enzymes.
3. Dietary restriction and MDR
Resistance to multiple categories of chemotherapy, also known as multidrug resistance (MDR), is a complex hurdle in cancer treatment. The mechanistic details behind multidrug resistance (MDR) are still under active investigation, but members of the ATP binding cassette (ABC) transporter superfamily, that often act as cellular efflux pumps for a wide range of chemotherapeutic compounds, have well-established roles (Calcagno et al., 2007; Gottesman et al., 2002). There are at least 48 MDR genes described, of which the ABCB1 gene, encoding an efflux transporter P-glycoprotein (PGP) or MDR1, is probably the most understood, closely followed by ABCC1 (MRP1), and ABCG2 (MXR and BCRP) (Gillet et al., 2004).
It appears that fasting for 24 h in 12–16 week old mice does not alter hepatic expression of many ABC family members, including Abcb1, but it induces the expression of Abca1 (2.3-fold), involved in cholesterol transport, and Abcg8 (1.8-fold), involved in sterol transport (van den Bosch et al., 2007). The ATP-binding cassette transporter A1 (ABCA1) has also been shown to promote MDR in addition to its more regular role as a regulator of hepatic cholesterol levels (Gillet et al., 2004; Iwasaki et al., 2010). Because malignant cells respond poorly or are indifferent to fasting, whereas normal cells rapidly adapt by regulating a large number of genes, the up-regulation of MDR proteins in the liver during fasting may contribute to reducing toxic side-effects. However, another study reports that ABCA1 expression can be repressed by a short fasting (20 h) in 6–8 week old mice (Rodgers and Puigserver, 2007). Thus additional studies on the effect of longer periods of fasting which are effective in promoting multi-stress resistance (48–72 h, see following section) on ABC transporter expression and host protection are necessary.
In an effort to overcome MDR, Gottesman and colleagues developed multidrug resistant bone marrow cells by transfecting them with vectors carrying the MDR1 cDNA allowing the use of unusually high doses of chemotherapy. High-dose chemotherapy, accompanied by bone-marrow transplant, is used to overcome MDR by overwhelming the PGP pump and increasing intracellular drug concentration (Makatsoris and Seiden, 1997; Patel and Rothenberg, 1994). The possibility that fasting may contribute to the differential chemotherapy protection by inducing specific MDR proteins in a variety of normal cells including bone marrow cells, but not cancer cells, remains to be tested (Lee et al., 2010; Raffaghello et al., 2008; Safdie et al., 2009).
Clinical approaches to overcome MDR in cancer cells include the use of chemotherapeutics that are not substrates of ABC transporters (e.g. cyclophosphamide, 5-fluorouracil, and Herceptin) and also combination therapy with non-toxic compounds that inhibit ABC transporters, also known as MDR inhibitors, MDR modulators, MDR reversal agents or chemosensitizers (McHugh and Callaghan, 2008; Ozben, 2006; Polgar and Bates, 2005). However, clinical data show that most inhibitors (first, second, and third generation MDR inhibitors) are not providing major improvements over the chemotherapy alone, suggesting that new combination therapies with wider effects may be required (Dong and Mumper, 2010; Hall et al., 2009; McHugh and Callaghan, 2008; Ozben, 2006). Because starvation conditions affect so many protective systems, it will be important to combine fasting with lower and non-toxic doses of these MDR inhibitors to test the hypothesis that this combination may reduce the toxic side-effects in normal tissues while reversing the resistance of cancer cells.
4. Starvation (STS) and differential stress resistance (DSR)
A concern arising from the fasting-based methods to protect normal cells is the possibility that malignant cells may also be protected. However, fundamental differences between normal and malignant cells are the basis for the starvation-dependent differential stress resistance (DSR) hypothesis, which proposes that specific starvation conditions will protect normal cells against chemotherapy without interfering with the killing of malignant cells (Lee et al., 2010; Raffaghello et al., 2008) (Fig. 3). In fact, we have recently shown that fasting alone can cause cytotoxicity of many different types of tumors, which further increases when combined with chemotherapy (Lee et al., 2012). Cancer cells have undergone a series of genetic and epigenetic alterations. Two of the major alterations in cell physiology that dictate malignant growth are: (1) self-sufficiency in growth signal, and (2) insensitivity to anti-growth signals (Hanahan and Weinberg, 2000). In fact, the great majority of normal cells are unable to grow in the absence of growth factors, whereas cancer cells express oncogenes that can continue to relay the effect of growth factors even in their absence (Hanahan and Weinberg, 2000).
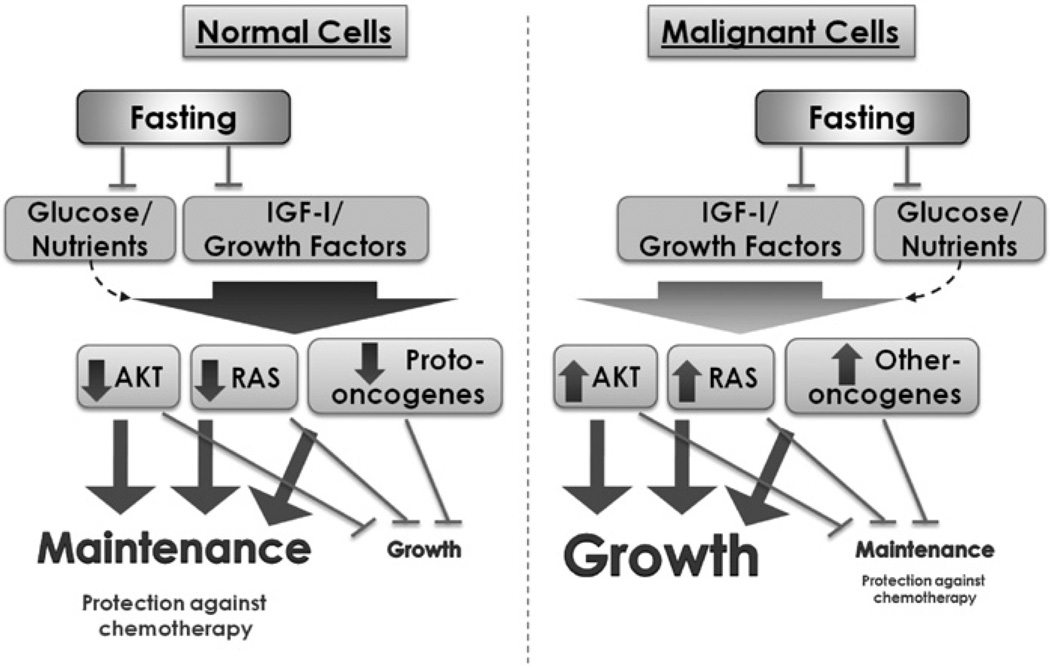
Fig. 3.
A model for DSR in response to fasting. In a variety of normal cells, downstream elements of the IGF-I and other growth factors pathways, including the Akt, Ras and other proto-oncogenes, can be down-regulated in response to the reduction in growth factors caused by starvation. This down-regulation can block/reduce growth and promotes protection. By contrast, oncogenic mutations render tumor cells less responsive to fasting due to their constitutive pro-growth mode and relative independence from anti-growth signals. Constitutive activation of one of the pro-growth pathways may be sufficient for the continued growth. Therefore, cancer cells fail to or only partially respond to starvation conditions and continue to promote growth instead of entering a protected mode.
Such “independence” is in many cases caused by the autocrine production of growth factors, or by mutations that cause the constitutive activation of membrane receptors, or of intracellular signal transduction proteins. Some of the extracellular factors overproduced by cancer cells include PDGF, VEGF and IGF-I (Hanahan and Weinberg, 2000). Perhaps the most important contribution to the “growth factors independence of cancer cells” comes instead from intracellular alterations, particularly in genes coding for components of the Ras/Raf/MAPK and the PTEN/PI3K/AKT pathways. Altered forms of Ras that can render the cell growth independent from extracellular signals are found in approximately a quarter of all cancers (Medema and Bos, 1993) and half of colon cancers (Kinzler and Vogelstein, 1996). The interaction between Ras and PI3K may further stimulate growth and also activate anti-apoptotic pathways in the cancer cells (Castellano and Downward, 2011). Another highly relevant feature of cancer cells is the ability to “disobey” anti-growth orders. p53 or genes upstream or downstream of it, are well-conserved coordinators of stress and metabolic response which are inactivated in the great majority of cancers (Junttila and Evan, 2009; Vousden and Ryan, 2009). For example, mutations in the MDM2-p53 and Rb-E2F pathways contribute to cancer progression by allowing malignant cells to override cell cycle check points (Polager and Ginsberg, 2009). The retinoblastoma protein (Rb) is one of the central negative regulators of proliferation in normal cells (Weinberg, 1995). PTEN, which negatively regulates the PI3K/AKT/mTOR pathway, is another tumor suppressor that is frequently inactivated in human cancers (Hollander et al., 2011).
The constant or at least frequent proliferation of cancer cells demands entirely different cellular metabolic requirements, since macronutrients are utilized to provide both cellular infrastructure as well as energy. For instance, the Warburg effect represents an important portion of such cellular reprogramming by shifting malignant cells to a more aerobic glycolysis mode that requires increased glucose consumption not resulting in oxidative phosphorylation; i.e., mitochondrial respiration does not increase proportionally to glucose uptake. It has been proposed that the excess glucose consumed by malignant cells is channeled through alternate pathways with the purpose of producing the biomass (nucleotides, lipids, amino acids, etc.) required for successful cell division (Vander Heiden et al., 2009). We now understand that oncogenic mutations (e.g. PI3K/Akt and p53) do not simply promote mitogenesis, but, in fact, frequently regulate cellular metabolism (Vander Heiden et al., 2009). However, as discussed above, normal cells, unlike cancer cells, rapidly adapt to fasting, and shift the finite cellular energy to focus on cellular protection with the intention of maximizing the protection of its macromolecules and organelles and survival. Because severe starvation conditions have been frequently encountered by organisms ranging from bacteria to humans, normal cells of any type recognize the starvation environment and respond in a highly coordinated manner. Because down-regulation of proto-oncogenes plays such a central role in this starvation response, the oncogenes in cancer cells prevent this response (Fig. 3). This remarkable characteristic is fundamental in causing a fasting-dependent protection of normal but not malignant cells. In addition, fasting reduces nutrient availability, in particular that of glucose, which is the preferential carbon source for cancer cells, but also causes major changes in ketone bodies, insulin, IGF-I, IGFBP-1, and a variety of other molecules and factors which generates an environment detrimental to many cancer cell types (Lee et al., 2012).
5. Enhancing cancer treatment by fasting induced DSR: translational potential
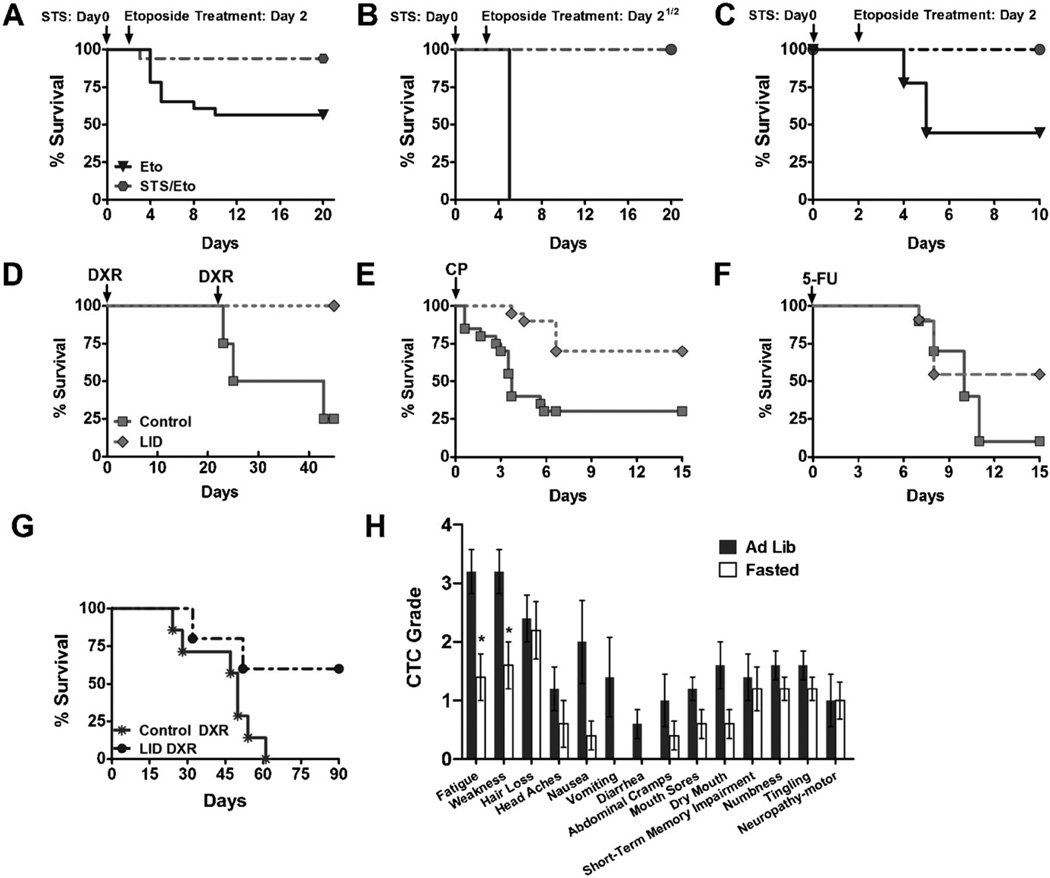
Chemotherapy is the most widely adopted strategy for the treatment of a wide range of cancers (Chabner and Roberts, 2005) but the toxicity of the treatment makes it only partially effective particularly with advanced malignancies. As discussed earlier, fasting has been recently demonstrated to selectively protect normal cells and organisms from chemotherapy toxicity, while simultaneously sensitizing tumors (Raffaghello et al., 2008; Lee et al., 2012) (Fig. 4A–C). In the original study, different strains of mice were allowed to consume only water for 48–60 h (short-term starvation: STS) before treatment with high doses of etoposide, a widely used chemotherapeutic drug that damages DNA by multiple mechanisms and displays a generalized toxicity profile ranging from myelosuppression to liver and neurologic damage. Mice fed ad libitum, showed signs of etoposide-induced toxicity including reduced mobility, ruffled hair, and hunched back posture, whereas mice fasted prior to chemotherapy showed no visible signs of stress or pain (Raffaghello et al., 2008). In addition, 43% of control animals, and only one in the fasted group died from acute etoposide toxicity (Raffaghello et al., 2008). In order to evaluate the DSR against chemotherapy of normal and malignant cells, a metastatic model was established by intravenously injecting mice with neuroblastoma cells. Tumor-bearing mice were then fasted for 48-h prior to etoposide treatment. Fasting protected mice from etoposide without interfering with its killing of tumor cells (Raffaghello et al., 2008). Further, fasting has been recently shown to sensitize 15 of 17 cell lines tested to the widely used chemotherapy drug doxorubicin. Also, fasting alone retarded tumor progression, which was significantly enhanced when combined with chemotherapy in mouse models of various cancers (Lee et al., 2012).
Fig. 4.
Fasting selectively protects normal cells from chemotherapy toxicity. (A–C) Mice from 3 different genetic backgrounds were fasted for 48–60 h prior to high-dose etoposide administration. (A) A/J mice were fasted for 48 h. (B) CD-1 mice were fasted for 60 h prior to chemotherapy administration. (C) Athymic Nude mice were fasted for 48 h. (D–F) Transgenic mice with a conditional liver igf1 gene deletion (LID) were treated with (D) 2 cycles of high-dose doxorubicin (DXR), (E) high-dose cyclophosphamide (CP), and (F) high-dose 5-FU. (G) LID mice bearing metastatic melanoma were treated with 2 cycles of high-dose DXR. (H) Patients self-reported side-effects after chemotherapy with or without fasting based on the common toxicity criteria (CTC) outlined by the National Cancer Institute (NCI).
During starvation, several changes in the pro-growth GH/IGF-I axis that optimize survival and maintenance under the new conditions occur. In particular, after a 72-h fast, mice lose approximately 20% of body weight, glucose levels are reduced by 41%, GH levels slightly increase, and IGF-I levels decrease by 70% (Lee et al., 2010). Moreover, the level of IGFBP-1, which regulates the bioavailability of IGF-I by sequestering it and avoiding its interaction with the IGF-I receptor, increases 11.4-fold in starved mice (Lee et al., 2010). Similarly to the results obtained in mice, in humans, glucose and IGF-I levels decrease dramatically in response to a 36–120-h fast despite increased GH secretion (Maccario et al., 2001; Merimee and Fineberg, 1974; Norrelund, 2005; Thissen et al., 1999).
As briefly mentioned earlier, the reduction of IGF-I has been demonstrated to be a mediator of the protection against chemotherapy toxicity in mice that underwent a 48-h fast (Lee et al., 2010). Analogously to starved mice, transgenic mice with a conditional liver igf1 gene deletion (LID), that causes a postnatal 70–80% reduction of circulating IGF-I, display increased resistance to high-doses of various chemotherapeutic drugs such as cyclophosphamide (CP), 5-fluorouracil (5-FU) and doxorubicin (DXR) (Lee et al., 2010) (Fig. 4D–F). Experiments with LID mice intravenously injected with B16 melanoma cells and treated with two cycles of high-dose DXR, showed that decreased IGF-I not only protects the host against the toxicity of chemotherapy but also reduces tumor progression (Lee et al., 2010) (Fig. 4G). Notably, the IGF-I receptor (IGF-IR) is the target of many anti-cancer drugs since its constitutive activation is common in a variety of tumors (Pollak et al., 2004). For example, the down-regulation of IGF-IR results in mammary tumor regression in a majority of the mice without recurrence (Jones et al., 2010). Therefore, IGF-IR-targeting agents including small antagonists and antibodies have entered clinical trials for cancer patients (Gualberto and Pollak, 2009). Although it is still early to draw conclusions regarding efficacy of IGF-IR inhibitors, phase I studies reveal an acceptable safety profile, and recent evidence from a phase II studies suggests that co-administration of an anti-IGF-IR antibody with chemotherapy for non-small-cell lung cancer improves objective response rate and progression-free survival (Gualberto and Pollak, 2009). Notably, the major induction of the IGF-IR blocker IGFBP1 caused by fasting may provide a drug-like effect on the proliferation of IGF-IR-dependent tumors in addition to providing the other benefits described in this review.
A recent study describes 10 cases of patients affected by different types of tumors, ranging from stage II breast cancer, stage IV esophageal, prostate, to lung malignancies, who had voluntarily fasted prior to (48–140 h) and/or following (5–56 h) chemotherapy, under the supervision of their treating oncologists (Safdie et al., 2009) (Fig. 4H). None of these patients, who received different chemotherapy drugs in combination with fasting, reports significant side effects caused by fasting alone other than hunger and light-headedness. In addition, most of the chemotherapy-associated toxicities such as fatigue, weakness, and gastrointestinal side effects, are reported to be significantly reduced by the patients who underwent fasting (Safdie et al., 2009). It is noteworthy that, when it was possible to assess tumor progression by tumor volume or markers, fasting did not appear to interfere with the efficacy of chemotherapy (Safdie et al., 2009). Although these results suggest that fasting in combination with chemotherapy is safe and could reduce common side-effects associated to chemotherapy, only controlled randomized clinical trials can establish its full potential.
There are currently several clinical trials studying fasting in combination with chemotherapy in cancer patients, most of which are still ongoing or ready to recruit participants.
The first clinical trial on a form of fasting in combination with chemotherapy was sponsored by King Fahad Medical City in Riyadh, Saudi Arabia (ClinicalTrials.gov Identifier: NCT00757094), who evaluated the safety of fasting in 12 patients who received chemotherapy during the month of Ramadan. This study has been completed but the results are yet to be published. However, because the fasting period is much shorter (fasting every day but only during daylight hours) than that predicted by animal and preliminary human studies to be effective, and also because patients may tend to overeat to compensate for the short fast, the Ramadan-associated study is not likely to reveal major benefits provided by the altered diet. In July 2009, the University of Southern California (USC)/Norris Comprehensive Cancer Center (ClinicalTrials.gov Identifier: NCT00936364) initiated a clinical trial to test the efficacy and safety of 24–72 h of fasting in combination with platinum-based chemotherapy in patients diagnosed with bladder cancer. A more recent extension will include patients affected by breast, ovarian and lung cancer. This trial is still ongoing. In August 2010, the Mayo Clinic Cancer Center (ClinicalTrials.gov Identifier: NCT01175837) started to enroll, and is still recruiting, patients older than 18 years who are affected by lymphomas and leukemias to fast in combination with chemotherapy. A clinical trial sponsored by Leiden University Medical Center, but not yet open for recruitment (ClinicalTrials.gov Identifier: NCT01304251) will instead study the effects of short-term fasting on tolerance to adjuvant chemotherapy in breast cancer.
Taken together, all the data discussed above suggest that fasting is safe and has the potential to be translated into clinical interventions for the protection of patients against chemotherapy-induced toxicity. Moreover, because of its effects on a variety of proteins and molecules, including IGF-I and glucose, and based on animal studies, fasting also has the potential to affect cancer progression with or without chemotherapy (Lee et al., 2012). However, more animal and cellular studies are needed to understand the role of fasting and of less extreme forms of DR on the differential protection of normal and cancer cells and on the mechanisms mediating these effects. The completion of clinical trials on fasting and cancer treatment will also be essential in understanding its clinical potential.
6. Conclusion
In the US, the death rate for heart disease has declined steadily between 1975 and 2007, but cancer death rates have been relatively constant (Siegel et al., 2011). Part of the limited efficacy of cancer treatment is due to the toxicity of chemotherapy to the host but also to the ability of cancer cells to become resistant to many toxic drugs. A variety of novel treatment options are being tested but it will take many years before these treatments are widely adopted and even those therapies which will be shown to be effective for one set of patients or a specific tumor, may not be effective for others. Thus, in addition to more sophisticated and personalized cancer treatments, it is essential to develop treatments whose efficacy is as broad as that of the common chemotherapy drugs but far superior and with greatly reduced side effects. In addition, progress in this direction should not require decades but should attempt to find translational applications in the near future. The emerging understanding of the relationship between starvation/calorie restriction and stress resistance genes and pathways in eukaryotic cells has provided novel strategies to improve cancer treatment focused not only on the killing of tumor cells but also on the protection of normal cells and on how they respond differently to starvation conditions. Considering that MDR and reduced detoxification in cancer cells are recognized as major problems in cancer therapy, particularly in the attempt to achieve cures and not only modest extensions in survival, the understanding of the link between starvation conditions, specific nutrient restricted diets and multidrug resistance and other detoxification systems is undoubtedly important to maximize the differential protection of normal and cancer cells. If fasting affects MDR proteins differentially in normal and cancer cells, it may provide a therapy to reduce MDR and possibly the toxicity of MDR inhibitors.
Acknowledgments
This work was funded in part by NIH/NIA AG20642, AG025135, P01 AG034906, Ted Bakewell (The Bakewell Foundation), the V Foundation for Cancer Research, and a USC Norris Cancer Center pilot grant to VDL. Lizzia Raffaghello is a recipient of a MF AIRC Grant.
References
- Abdul-Ghani R, Serra V, Gyorffy B, Jurchott K, Solf A, Dietel M, Schafer R. The PI3K inhibitor LY294002 blocks drug export from resistant colon carcinoma cells overexpressing MRP1. Oncogene. 2006;25:1743–1752. doi: 10.1038/sj.onc.1209201. [DOI] [PubMed] [Google Scholar]
- Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- Anzo M, Cobb LJ, Hwang DL, Mehta H, Said JW, Yakar S, LeRoith D, Cohen P. Targeted deletion of hepatic Igf1 in TRAMP mice leads to dramatic alterations in the circulating insulin-like growth factor axis but does not reduce tumor progression. Cancer Res. 2008;68:3342–3349. doi: 10.1158/0008-5472.CAN-07-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyadevara S, Alla R, Thaden JJ, Shmookler Reis RJ. Remarkable longevity and stress resistance of nematode PI3K-null mutants. Aging Cell. 2008;7:13–22. doi: 10.1111/j.1474-9726.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- Bauer M, Hamm AC, Bonaus M, Jacob A, Jaekel J, Schorle H, Pankratz MJ, Katzenberger JD. Starvation response in mouse liver shows strong correlation with life-span-prolonging processes. Physiol. Genomics. 2004;17:230–244. doi: 10.1152/physiolgenomics.00203.2003. [DOI] [PubMed] [Google Scholar]
- Belinsky M, Jaiswal AK. NAD(P)H:quinone oxidoreductase1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metastasis Rev. 1993;12:103–117. doi: 10.1007/BF00689804. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R, Spong A, Panici J, Masternak MM, Kopchick JJ, Bartke A. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One. 2009;4:e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Braconi C, Bracci R, Bearzi I, Bianchi F, Sabato S, Mandolesi A, Belvederesi L, Cascinu S, Valeri N, Cellerino R. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann. Oncol. 2008;19:1293–1298. doi: 10.1093/annonc/mdn040. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Exp. Gerontol. 2000;35:199–212. doi: 10.1016/s0531-5565(00)00079-6. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Romanick MA, Kennedy MA. Effects of growth hormone and insulin-like growth factor-1 on hepatocyte antioxidative enzymes. Exp. Biol. Med. (Maywood) 2002;227:94–104. doi: 10.1177/153537020222700203. [DOI] [PubMed] [Google Scholar]
- Calcagno AM, Kim IW, Wu CP, Shukla S, Ambudkar SV. ABC drug transporters as molecular targets for the prevention of multidrug resistance and drug-drug interactions. Curr. Drug Deliv. 2007;4:324–333. doi: 10.2174/156720107782151241. [DOI] [PubMed] [Google Scholar]
- Castellano E, Downward J. RAS interaction with PI3K: more than just another effector pathway. Genes Cancer. 2011;2:261–274. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabner BA, Roberts TG., Jr Timeline: chemotherapy and the war on cancer. Nat. Rev. Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- Chern CM, Liou KT, Wang YH, Liao JF, Yen JC, Shen YC. Andrographolide inhibits PI3K/AKT-dependent NOX2 and iNOS expression protecting mice against hypoxia/ischemia-induced oxidative brain injury. Planta Med. 2011;77:1669–1679. doi: 10.1055/s-0030-1271019. [DOI] [PubMed] [Google Scholar]
- Chipalkatti S, De AK, Aiyar AS. Effect of diet restriction on some biochemical parameters related to aging in mice. J. Nutr. 1983;113:944–950. doi: 10.1093/jn/113.5.944. [DOI] [PubMed] [Google Scholar]
- Choi J, Zhai D, Zhou X, Satterthwait A, Reed JC, Marassi FM. Mapping the specific cytoprotective interaction of humanin with the pro-apoptotic protein bid. Chem. Biol. Drug Des. 2007;70:383–392. doi: 10.1111/j.1747-0285.2007.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MH, Kasai H, Nishimura S, Yu BP. Protection of DNA damage by dietary restriction. Free Radic. Biol. Med. 1992;12:523–525. doi: 10.1016/0891-5849(92)90105-p. [DOI] [PubMed] [Google Scholar]
- Cicchetti F, Drouin-Ouellet J, Gross RE. Environmental toxins and Parkinson’s disease: what have we learned from pesticide-induced animal models? Trends Pharmacol. Sci. 2009;30:475–483. doi: 10.1016/j.tips.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- Davis LM, Pauly JR, Readnower RD, Rho JM, Sullivan PG. Fasting is neuroprotective following traumatic brain injury. J. Neurosci. Res. 2008;86:1812–1822. doi: 10.1002/jnr.21628. [DOI] [PubMed] [Google Scholar]
- De AK, Chipalkatti S, Aiyar AS. Some biochemical parameters of ageing in relation to dietary protein. Mech. Ageing Dev. 1983;21:37–48. doi: 10.1016/0047-6374(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Dong X, Mumper RJ. Nanomedicinal strategies to treat multidrug-resistant tumors: current progress. Nanomedicine (Lond) 2010;5:597–615. doi: 10.2217/nnm.10.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch. Biochem. Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Liou LL, Moy VN, Diaspro A, SelverstoneValentine J, Gralla EB, Longo VD. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics. 2003;163:35–46. doi: 10.1093/genetics/163.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem. Sci. 2007;32:180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Gillet JP, Efferth T, Steinbach D, Hamels J, de Longueville F, Bertholet V, Remacle J. Microarray-based detection of multidrug resistance in human tumor cells by expression profiling of ATP-binding cassette transporter genes. Cancer Res. 2004;64:8987–8993. doi: 10.1158/0008-5472.CAN-04-1978. [DOI] [PubMed] [Google Scholar]
- Go KG, Prenen GH, Korf J. Protective effect of fasting upon cerebral hypoxic–ischemic injury. Metab. Brain Dis. 1988;3:257–263. doi: 10.1007/BF00999535. [DOI] [PubMed] [Google Scholar]
- Gong Y, Yao E, Shen R, Goel A, Arcila M, Teruya-Feldstein J, Zakowski MF, Frankel S, Peifer M, Thomas RK, Ladanyi M, Pao W. High expression levels of total IGF-1R and sensitivity of NSCLC cells in vitro to an anti-IGF-1R antibody (R1507) PLoS One. 2009;4:e7273. doi: 10.1371/journal.pone.0007273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009;28:3009–3021. doi: 10.1038/onc.2009.172. [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Guillon-Munos A, van Bemmelen MX, Clarke PG. Role of phosphoinositide 3-kinase in the autophagic death of serum-deprived PC12 cells. Apoptosis. 2005;10:1031–1041. doi: 10.1007/s10495-005-0741-6. [DOI] [PubMed] [Google Scholar]
- Hall MD, Handley MD, Gottesman MM. Is resistance useless? Multidrug resistance and collateral sensitivity. Trends Pharmacol. Sci. 2009;30:546–556. doi: 10.1016/j.tips.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Hong L, Han Y, Wu K, Han S, Shen H, Li C, Yao L, Qiao T, Fan D. Phospho Akt mediates multidrug resistance of gastric cancer cells through regulation of P-gp, Bcl-2 and Bax. J. Exp. Clin. Cancer Res. 2007;26:261–268. [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Henis-Korenblit S, Zhang P, Hansen M, McCormick M, Lee SJ, Cary M, Kenyon C. Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc. Natl. Acad. Sci. U.S.A. 2010;107:9730–9735. doi: 10.1073/pnas.1002575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat. Rev. Cancer. 2011;11:289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- Honjoh S, Yamamoto T, Uno M, Nishida E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature. 2009;457:726–730. doi: 10.1038/nature07583. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Hua Q, Yang C, Oshima T, Mori H, Shimizu K. Analysis of gene expression in Escherichia coli in response to changes of growth-limiting nutrient in chemostat cultures. Appl. Envirson. Microbiol. 2004;70:2354–2366. doi: 10.1128/AEM.70.4.2354-2366.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihssen J, Egli T. Specific growth rate and not cell density controls the general stress response in Escherichia coli. Microbiology. 2004;150:1637–1648. doi: 10.1099/mic.0.26849-0. [DOI] [PubMed] [Google Scholar]
- Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, Nishimoto I, Cohen P. Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13042–13047. doi: 10.1073/pnas.2135111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti F, Grimsley C, Das S, Ramirez J, Cheng C, Kuttab-Boulos H, Ratain MJ, Di Rienzo A. Haplotype structure of the UDP-glucuronosyltransferase 1A1 promoter in different ethnic groups. Pharmacogenetics. 2002;12:725–733. doi: 10.1097/00008571-200212000-00006. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Okabe T, Takara K, Yoshida Y, Hanashiro K, Oku H. Downregulation of lipids transporter ABCA1 increases the cytotoxicity of nitidine. Cancer Chemother. Pharmacol. 2010;66:953–959. doi: 10.1007/s00280-010-1247-7. [DOI] [PubMed] [Google Scholar]
- Jenkins DE, Schultz JE, Matin A. Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J. Bacteriol. 1988;170:3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE. Increased life-span of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science. 1990;249:908–912. doi: 10.1126/science.2392681. [DOI] [PubMed] [Google Scholar]
- Jones RA, Petrik JJ, Moorehead RA. Preneoplastic changes persist after IGF-IR downregulation and tumor regression. Oncogene. 2010;29:4779–4786. doi: 10.1038/onc.2010.231. [DOI] [PubMed] [Google Scholar]
- Junttila MR, Evan GI. p53—a Jack of all trades but master of none. Nat. Rev. Cancer. 2009;9:821–829. doi: 10.1038/nrc2728. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang CM, Kristal BS, Yu BP. Age-related mitochondrial DNA deletions: effect of dietary restriction. Free Radic. Biol. Med. 1998;24:148–154. doi: 10.1016/s0891-5849(97)00204-9. [DOI] [PubMed] [Google Scholar]
- Kang KW, Lee SJ, Park JW, Kim SG. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Mol. Pharmacol. 2002;62:1001–1010. doi: 10.1124/mol.62.5.1001. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Kohle C, Bock KW. Coordinate regulation of Phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochem. Pharmacol. 2007;73:1853–1862. doi: 10.1016/j.bcp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Koizumi A, Weindruch R, Walford RL. Influences of dietary restriction and age on liver enzyme activities and lipid peroxidation in mice. J. Nutr. 1987;117:361–367. doi: 10.1093/jn/117.2.361. [DOI] [PubMed] [Google Scholar]
- Kong AN, Owuor E, Yu R, Hebbar V, Chen C, Hu R, Mandlekar S. Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE) Drug Metab. Rev. 2001;33:255–271. doi: 10.1081/dmr-120000652. [DOI] [PubMed] [Google Scholar]
- Larsen PL. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic. Biol. Med. 1998;25:1089–1097. doi: 10.1016/s0891-5849(98)00144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30:3305–3316. doi: 10.1038/onc.2011.91. [DOI] [PubMed] [Google Scholar]
- Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, Emionite L, de Cabo R, Longo VD. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. 2012 doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009;10:379–391. doi: 10.1016/j.cmet.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD. Mutations in signal transduction proteins increase stress resistance and longevity in yeast, nematodes, fruit flies, and mammalian neuronal cells. Neurobiol. Aging. 1999;20:479–486. doi: 10.1016/s0197-4580(99)00089-5. [DOI] [PubMed] [Google Scholar]
- Longo VD, Ellerby LM, Bredesen DE, Valentine JS, Gralla EB. Human Bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. J. Cell Biol. 1997;137:1581–1588. doi: 10.1083/jcb.137.7.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- Luo SM, Tan WM, Deng WX, Zhuang SM, Luo JW. Expression of albumin, IGF-1, IGFBP-3 in tumor tissues and adjacent non-tumor tissues of hepatocellular carcinoma patients with cirrhosis. World J. Gastroenterol. 2005;11:4272–4276. doi: 10.3748/wjg.v11.i27.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccario M, Aimaretti G, Grottoli S, Gauna C, Tassone F, Corneli G, Rossetto R, Wu Z, Strasburger CJ, Ghigo E. Effects of 36 hour fasting on GH/IGF-I axis and metabolic parameters in patients with simple obesity. Comparison with normal subjects and hypopituitary patients with severe GH deficiency. Int. J. Obes. Relat. Metab. Disord. 2001;25:1233–1239. doi: 10.1038/sj.ijo.0801671. [DOI] [PubMed] [Google Scholar]
- Makatsoris T, Seiden MV. High-dose therapy for ovarian carcinoma. Oncologist. 1997;2:330–339. [PubMed] [Google Scholar]
- Marie C, Bralet AM, Gueldry S, Bralet J. Fasting prior to transient cerebral ischemia reduces delayed neuronal necrosis. Metab. Brain Dis. 1990;5:65–75. doi: 10.1007/BF01001047. [DOI] [PubMed] [Google Scholar]
- Martin-Montalvo A, Villalba JM, Navas P, de Cabo R. NRF2, cancer and calorie restriction. Oncogene. 2011;30:505–520. doi: 10.1038/onc.2010.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A. The molecular basis of carbon-starvation-induced general resistance in Escherichia coli. Mol. Microbiol. 1991;5:3–10. doi: 10.1111/j.1365-2958.1991.tb01819.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J. Nutr. Biochem. 2005;16:129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Mazzoccoli G, Giuliani A, Bianco G, De Cata A, Balzanelli M, Carella AM, La Viola M, Tarquini R. Decreased serum levels of insulin-like growth factor (IGF)-I in patients with lung cancer: temporal relationship with growth hormone (GH) levels. Anticancer Res. 1999;19:1397–1399. [PubMed] [Google Scholar]
- Mazzoccoli G, Vendemiale G, De Cata A, Carughi S, Tarquini R. Altered time structure of neuro-endocrine-immune system function in lung cancer patients. BMC Cancer. 2010;10:314. doi: 10.1186/1471-2407-10-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl G, Rogers AN, Alavez S, Hubbard AE, Melov S, Link CD, Bush AI, Kapahi P, Lithgow GJ. Insulin-like signaling determines survival during stress via posttranscriptional mechanisms in C. elegans. Cell Metab. 2010;12:260–272. doi: 10.1016/j.cmet.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougald D, Gong L, Srinivasan S, Hild E, Thompson L, Takayama K, Rice SA, Kjelleberg S. Defences against oxidative stress during starvation in bacteria. Anton. Leeuw. 2002;81:3–13. doi: 10.1023/a:1020540503200. [DOI] [PubMed] [Google Scholar]
- McElwee J, Bubb K, Thomas JH. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell. 2003;2:111–121. doi: 10.1046/j.1474-9728.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J. Biol. Chem. 2004;279:44533–44543. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- McHugh K, Callaghan R. Clinical trials on MDR reversal agents. In: Colabufo NA, editor. Multidrug Resistance: Biological and Pharmaceutical Advance in the Antitumour Treatment. Research Signpost; 2008. [Google Scholar]
- Medema RH, Bos JL. The role of p21ras in receptor tyrosine kinase signaling. Crit. Rev. Oncog. 1993;4:615–661. [PubMed] [Google Scholar]
- Merimee TJ, Fineberg SE. Growth hormone secretion in starvation: a reassessment. J. Clin. Endocrinol. Metab. 1974;39:385–386. doi: 10.1210/jcem-39-2-385. [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, Chu T, Forrer F, Muller C, de Jong M, van Ijcken W, Ijzermans JN, Hoeijmakers JH, de Bruin RW. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell. 2010;9:40–53. doi: 10.1111/j.1474-9726.2009.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JZ, Tissenbaum HA, Ruvkun G. A phospatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- Murakami S. Stress resistance in long-lived mouse models. Exp. Gerontol. 2006;41:1014–1019. doi: 10.1016/j.exger.2006.06.061. [DOI] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Sato H, Motokura T. Development of multidrug resistance due to multiple factors including P-glycoprotein overexpression under K-selection after MYC and HRAS oncogene activation. Int. J. Cancer. 2006;118:2448–2454. doi: 10.1002/ijc.21691. [DOI] [PubMed] [Google Scholar]
- Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003;546:181–184. doi: 10.1016/s0014-5793(03)00517-9. [DOI] [PubMed] [Google Scholar]
- Norrelund H. The metabolic role of growth hormone in humans with particular reference to fasting. Growth Horm. IGF Res. 2005;15:95–122. doi: 10.1016/j.ghir.2005.02.005. [DOI] [PubMed] [Google Scholar]
- O’Neal CR, Gabriel WM, Turk AK, Libby SJ, Fang FC, Spector MP. RpoS is necessary for both the positive and negative regulation of starvation survival genes during phosphate, carbon, and nitrogen starvation in Salmonella typhimurium. J. Bacteriol. 1994;176:4610–4616. doi: 10.1128/jb.176.15.4610-4616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozben T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett. 2006;580:2903–2909. doi: 10.1016/j.febslet.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun G. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 1999;13:1438–1452. doi: 10.1101/gad.13.11.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NH, Rothenberg ML. Multidrug resistance in cancer chemotherapy. Invest. New Drugs. 1994;12:1–13. doi: 10.1007/BF00873229. [DOI] [PubMed] [Google Scholar]
- Pletcher SD, Libert S, Skorupa D. Flies and their golden apples: the effect of dietary restriction on Drosophila aging and age-dependent gene expression. Ageing Res. Rev. 2005;4:451–480. doi: 10.1016/j.arr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Polager S, Ginsberg D. p53 and E2f: partners in life and death. Nat. Rev. Cancer. 2009;9:738–748. doi: 10.1038/nrc2718. [DOI] [PubMed] [Google Scholar]
- Polgar O, Bates SE. ABC transporters in the balance: is there a role in multidrug resistance? Biochem. Soc. Trans. 2005;33:241–245. doi: 10.1042/BST0330241. [DOI] [PubMed] [Google Scholar]
- Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl. Acad. Sci. U.S.A. 2008;105:8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, Cohen P, Longo VD. Fasting and cancer treatments in humans: a case series report. Aging. 2009;1:988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am. J. Physiol. Endocrinol. Metab. 2005;289:E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour RL, Mishra PV, Khan MA, Spector MP. Essential roles of core starvation-stress response loci in carbon-starvation-inducible cross-resistance and hydrogen peroxide-inducible adaptive resistance to oxidative challenge in Salmonella typhimurium. Mol. Microbiol. 1996;20:497–505. doi: 10.1046/j.1365-2958.1996.5451068.x. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Nyberg F, Gordh T, Alm P, Westman J. Neurotrophic factors influence upregulation of constitutive isoform of heme oxygenase and cellular stress response in the spinal cord following trauma. An experimental study using immunohistochemistry in the rat. Amino Acids. 2000;19:351–361. [PubMed] [Google Scholar]
- Shen G, Hebbar V, Nair S, Xu C, Li W, Lin W, Keum YS, Han J, Gallo MA, Kong AN. Regulation of Nrf2 transactivation domain activity. The differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J. Biol. Chem. 2004;279:23052–23060. doi: 10.1074/jbc.M401368200. [DOI] [PubMed] [Google Scholar]
- Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc. Natl. Acad. Sci. U.S.A. 2008;105:13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- Sun J, Conn CS, Han Y, Yeung V, Qian SB. PI3K-mTORC1 attenuates stress response by inhibiting cap-independent Hsp70 translation. J. Biol. Chem. 2011;286:6791–6800. doi: 10.1074/jbc.M110.172882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazzari PL, Cappellini A, Ricci F, Evangelisti C, Papa V, Grafone T, Martinelli G, Conte R, Cocco L, McCubrey JA, Martelli AM. Multidrug resistance-associated protein 1 expression is under the control of the phosphoinositide 3 kinase/Akt signal transduction network in human acute myelogenous leukemia blasts. Leukemia. 2007;21:427–438. doi: 10.1038/sj.leu.2404523. [DOI] [PubMed] [Google Scholar]
- Tettweiler G, Miron M, Jenkins M, Sonenberg N, Lasko PF. Starvation and oxidative stress resistance in Drosophila are mediated through the eIF4E-binding protein, d4E-BP. Genes Dev. 2005;19:1840–1843. doi: 10.1101/gad.1311805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein JM, de Winde JH. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 1999;33:904–918. doi: 10.1046/j.1365-2958.1999.01538.x. [DOI] [PubMed] [Google Scholar]
- Thissen JP, Underwood LE, Ketelslegers JM. Regulation of insulin-like growth factor-I in starvation and injury. Nutr. Rev. 1999;57:167–176. doi: 10.1111/j.1753-4887.1999.tb06939.x. [DOI] [PubMed] [Google Scholar]
- Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22:7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu. Rev. Pharmacol. Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- van den Bosch HM, Bunger M, de Groot PJ, van der Meijde J, Hooiveld GJ, Muller M. Gene expression of transporters and phase I/II metabolic enzymes in murine small intestine during fasting. BMC Genomics. 2007;8:267. doi: 10.1186/1471-2164-8-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginhoven TM, Mitchell JR, Verweij M, Hoeijmakers JH, Ijzermans JN, de Bruin RW. The use of preoperative nutritional interventions to protect against hepatic ischemia-reperfusion injury. Liver Transpl. 2009;15:1183–1191. doi: 10.1002/lt.21871. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanfleteren JR. Oxidative stress and ageing in Caenorhabditis elegans. Biochem. J. 1993;292:605–608. doi: 10.1042/bj2920605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanfleteren JR, De Vreese A. The gerontogenes age-1 and daf-2 determine metabolic rate potential in aging Caenorhabditis elegans. FASEB J. 1995;9:1355–1361. doi: 10.1096/fasebj.9.13.7557026. [DOI] [PubMed] [Google Scholar]
- Vigne P, Frelin C. A low protein diet increases the hypoxic tolerance in Drosophila. PLoS One. 2006;1:e56. doi: 10.1371/journal.pone.0000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne P, Frelin C. Diet dependent longevity and hypoxic tolerance of adult Drosophila melanogaster. Mech. Ageing Dev. 2007;128:401–406. doi: 10.1016/j.mad.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Vigne P, Tauc M, Frelin C. Strong dietary restrictions protect Drosophila against anoxia/reoxygenation injuries. PLoS One. 2009;4:e5422. doi: 10.1371/journal.pone.0005422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Ryan KM. p53 and metabolism. Nat. Rev. Cancer. 2009;9:691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, Mattson MP. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J. Nutr. Biochem. 2010;21:413–417. doi: 10.1016/j.jnutbio.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Zhou CJ, Song SZ, Chen P, Xu WH, Liu B, Zhu KX, Yu WH, Wu HL, Wang HJ, Lin S, Guo JQ, Qin CY. Evaluation of Nrf2 and IGF-1 expression in benign, premalignant and malignant gastric lesions. Pathol. Res. Pract. 2011;207:169–173. doi: 10.1016/j.prp.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo VD. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA Tor Sch9. PLoS Genet. 2008;4:e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Fabrizio P, Madia F, Hu J, Ge H, Li LM, Longo VD. Tor1/Sch9-regulated carbon source substitution is as effective as calorie restriction in life span extension. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000467. e1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Weinkove D, Halstead JR, Gems D, Divecha N. Long-term starvation and ageing induce AGE-1/PI 3-kinase-dependent translocation of DAF-16/FOXO to the cytoplasm. BMC Biol. 2006;4:1. doi: 10.1186/1741-7007-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick LM, Quadroni M, Egli T. Short- and long-term changes in proteome composition and kinetic properties in a culture of Escherichia coli during transition from glucose-excess to glucose-limited growth conditions in continuous culture and vice versa. Environ. Microbiol. 2001;3:588–599. doi: 10.1046/j.1462-2920.2001.00231.x. [DOI] [PubMed] [Google Scholar]
- Xu C, Li CY, Kong AN. Induction of phase I II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 2005;28:249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- Yan L, Vatner DE, O’Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- Yang CS, Brady JF, Hong JY. Dietary effects on cytochromes P450, xenobiotic metabolism, and toxicity. FASEB J. 1992;6:737–744. doi: 10.1096/fasebj.6.2.1537464. [DOI] [PubMed] [Google Scholar]
- Zhai D, Luciano F, Zhu X, Guo B, Satterthwait AC, Reed JC. Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. J. Biol. Chem. 2005;280:15815–15824. doi: 10.1074/jbc.M411902200. [DOI] [PubMed] [Google Scholar]
- Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005;579:3029–3036. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]